Abstract
Purpose
The objective of this study was to investigate the relationship between diversity of 18F-fluorodeoxyglucose (18F-FDG) uptake of primary tumor in positron emission tomography (PET) and various clinicopathologic factors in breast cancer of same pathologic T1, T2 stage.
Methods
A total of 258 patients with invasive ductal breast cancer were enrolled in this study. All patients underwent 18F-FDG PET-CT before surgery. Patients were divided into two groups according to tumor size based on the pathologic T stage, and maximum standardized uptake value (SUVmax) of 2.5, respectively.
Results
On the univariate analysis, estrogen receptor (ER), tumor size, lymphovascular invasion, p53, pathologic N status (pN) and Nottingham tumor grade (NG) were associated with high SUVmax in T1 and T2 breast cancer. On the multivariate logistic regression, tumor size and NG remained significant variables dividing high and low SUVmax. In the T1 group, ER, p53 and NG were significantly associated with high SUVmax on the univariate analysis. In this group, p53 and NG remained significant variables for dividing high and low SUVmax on the multivariate logistic regression. In the T2 group, only NG was associated with high SUVmax on the univariate analysis.
Conclusions
NG showed an association with 18F-FDG uptake in both T1 and T2 breast cancer independently; however, p53 in T1 breast cancer.
Keywords: F-18 Fluorodeoxyglucose, Positron emission tomography, Breast neoplasms, TNM staging
Introduction
Breast cancer is the most common malignancy among women, and the second most frequent cancer overall [1]. In patients with breast cancer, staging of locally advanced cancer allows appropriate treatment options and offers prognostic information. For this purpose, preoperative 18F-fluorodeoxyglucose (18F-FDG) positron emission tomography-computed tomography (PET-CT) is recommended in patients with breast cancer [2, 3]. Although 18F-FDG PET-CT has proved to be valuable for deciding appropriate treatment options, the degree of 18F-FDG uptake, measured as maximum standardized uptake value (SUVmax), is diverse and highly versatile for evaluating the therapeutic effect and prognosis [4–6].
Hormone receptor, including estrogen receptor (ER) and progesterone receptor (PR), human epidermal growth factor receptor 2 (HER2), p53, Ki67 expression, lymphovascular invasion, surgical margin involvement, tumor size, pathologic N status (pN) and Nottingham tumor grade (NG) are well known clinicopathologic factors that influence the prognosis of breast cancer [3, 7, 8]. Several studies have investigated the associating factors that affect the intensity of 18F-FDG uptake in breast cancer, and have focused on these clinicopathologic factors of breast cancer [9–11]. The relationship between clinicopathologic factors of breast cancer and the intensity of 18F-FDG uptake is not completely consensual; nevertheless, most of previous studies have shown that there is a strong relationship between the tumor size and SUVmax; thus, PET imaging has been reported for hard to detect small breast cancers [9, 12, 13].
In the clinical setting, the intensity of 18F-FDG uptake of breast cancer with similar tumor size was diverse [4, 14–18], and there were some cases of small-sized breast cancer with relatively intense 18F-FDG uptake. For this reason, this study was designed to investigate the relationship between clinicopathologic factors for prediction of the aggressiveness of the primary tumor and the intensity of 18F-FDG in the relatively early T stage of breast cancer. In addition, we also investigated how these prognostic factors influence 18F-FDG uptake in each T1 and T2 stage of breast cancer.
Materials and Methods
Patients
We reviewed the medical records of 327 patients with breast cancer, evaluated at Pusan National University Hospital, who had undergone curative surgery and been confirmed as having invasive ductal carcinoma (IDC) between Jan 2012 and May 2013. Of the 327 potentially eligible patients (51 years; 22–88 years), there were 42 patients with tumors less than 10 mm, 6 patients with pathologic T3 (pT3) tumor, and 16 patients with pT4 tumor; 21 patients who did not have complete postoperative histopathologic data were excluded from the study. Finally, 258 patients were enrolled in this study. Patients were divided into two groups according to the tumor size based on the pathologic T stage. Tumor sizes of 10 mm or less were excluded because of partial volume effect. In T1 the group, patients whose tumors were larger than 10 mm, and 20 mm or less were included; in T2 group patients, whose tumors were larger than 20 mm, and 50 mm or less were included. All patients underwent 18F-FDG PET-CT before surgery. Patients were also divided into low and high SUV groups according to an SUVmax of 2.5 [4]. This retrospective study was reviewed and approved by the Institutional Review Board of Pusan National University Hospital.
18F-FDG PET-CT Imaging and Analysis
All patients fasted for at least 8 h before undergoing 18F-FDG PET-CT. Their median blood glucose level was 93 mg/dl (64-167 mg/dl) before 18F-FDG administration. 18F-FDG PET-CT imaging studies were conducted 60 min after intravenous injection of 18F-FDG. Patients were hydrated with 500 ml of water taken orally before PET-CT imaging. Low-dose CT from the base of the skull to the proximal thighs was performed for the purpose of attenuation correction and precise anatomical localization. Patients were examined using two types of PET-CT scanner. The first was Gemini TF (Philips, Milpitas, CA, USA), which consists of a germanium oxyorthosilicate full-ring PET scanner and a dual-slice helical CT scanner. Thereafter, an emission scan was conducted in the three-dimensional mode. PET data were obtained using a high-resolution whole-body scanner with an axial field of view of 18 cm. The average axial resolution varied between 4.2 mm full-width at half-maximum in the center and 5.6 mm at 10 cm. The average total PET-CT examination time was 30 min. After scatter and decay correction, PET data were reconstructed iteratively with attenuation correction and were reoriented in axial, sagittal, and coronal slices. The row action maximum likelihood algorithm was used for three-dimensional reconstruction. The other PET-CT scanner used was Biograph40 (Siemens, Knoxville, TN, USA). PET data were obtained using a high-resolution whole-body scanner with an axial field of view of 21.6 cm. The average axial resolution varied between 2.0 mm full-width a half-maximum in the center and 2.4 mm at 28 cm. The average total PET-CT examination time was 20 min. Attenuation correction was performed for all patients with iterative reconstruction. PET-CT images were analyzed at three different planes: transverse, coronal, and sagittal.
All PET-CT images were evaluated by two nuclear physicians, blinded to all imaging studies, clinical and pathological results. A volume of interest (VOI) was drawn over each breast cancer. SUVmax of each lesion was measured automatically and semiquantitatively on the workstation.
Clinicopathologic Factors
ER, PR, HER2, p53, Ki67 expression, lymphovascular invasion, surgical margin, tumor size, pN and NG were selected as clinicopathologic factors. Patients were pathologically staged according to American Joint Committee on Cancer 7th edition [19]. Tumor size was estimated by measuring the maximum diameter of the tumor invasive component (millimeters). Pathologic N status was categorized as nodal metastasis positive or negative. NG was determined on the basis of tubular formation, nuclear pleomorphism, and mitotic count. Each of these features was scored from 1 to 3, and then each score was added to give a final total score range from 3 to 9. Lymphovascular invasion or surgical margin involvement was determined on the histopathologic findings of hematoxylin and eosin staining under a high-power-field. ER, PR, HER2, p53 and Ki-67 expression were analyzed immunohistochemically using antibodies prepared against these proteins. Immunohistochemistry staining with the antibody was carried out with suitable positive and negative controls. The results were semi-quantitatively scored on a scale of 0, 1+, 2+ and 3+. The 0 and 1+ were considered negative, and 2+ and 3+ were considered as positive.
Statistical Analysis
The Mann–Whitney test was used to investigate the association between tumor size, Ki67 or NG and SUVmax. Associations between other clinicopathologic factors and SUVmax were examined using the chi-squared test. Logistic regression was performed to examine which clinicopathologic factors can reflect intensity of 18F-FDG uptake. The statistical analyses were performed using MedCalc® (Ostend, Belgium) for windows version 12.7.0.0 and a p value of less than 0.05 was regarded as significant.
Results
The characteristics of patients are shown in Table 1. SUVmax and tumor size ranged from 1.00 to 47.90 and 11 to 48 mm, respectively. Of the 258 patients, 216 patients (83.7 %) were included in the high SUVmax group. ER, PR, HER2 and p53 were found positive in 176 (68.2 %), 162 (62.8 %), 90 (34.9 %) and 76 (29.5 %) of the patients (Table 1).
Table 1.
Patients’ characteristics
| Variables | n (%) | SUVmax (mean ± SD) | |
|---|---|---|---|
| Age (years) | 51 (22–84) | 6.88 ± 5.15 | |
| SUVmax | High (≥2.5) | 216 (83.7) | 7.85 ± 5.09 |
| Low (<2.5) | 42 (16.3) | 1.90 ± 0.41 | |
| Estrogen receptor | + | 176 (68.2) | 5.53 ± 3.67 |
| - | 82 (31.8) | 9.79 ± 6.53 | |
| Progesterone receptor | + | 162 (62.8) | 5.72 ± 4.78 |
| - | 96 (37.2) | 8.85 ± 5.20 | |
| HER2 | + | 90 (34.9) | 7.76 ± 6.10 |
| - | 168 (65.1) | 6.41 ± 4.52 | |
| Tumor size (mm) | 21 (11–48) | ||
| Surgical margin | + | 9 (3.5) | 9.55 ± 5.35 |
| - | 249 (96.5) | 6.80 ± 5.14 | |
| Lymphovascular invasion | + | 88 (34.1) | 7.39 ± 4.37 |
| - | 170 (65.9) | 6.62 ± 5.51 | |
| p53 | + | 76 (29.5) | 8.55 ± 5.94 |
| - | 182 (70.5) | 6.19 ± 4.63 | |
| Nottingham tumor grade | 3-5 | 47 (18.2) | 3.37 ± 2.02 |
| 6-7 | 104 (40.3) | 5.62 ± 3.34 | |
| 8-9 | 107 (41.5) | 9.64 ± 6.09 | |
| Ki-67 (%) | 20 (0–95) | ||
| T stage | 1 | 126 (48.8) | 5.38 ± 5.31 |
| 2 | 132 (51.2) | 8.32 ± 4.57 | |
| N stage | 1, 2, 3 | 119 (46.1) | 7.26 ± 4.37 |
| 0 | 139 (53.9) | 6.56 ± 5.74 | |
HER2 human epidermal growth factor receptor 2, SUVmax maximum standardized uptake value
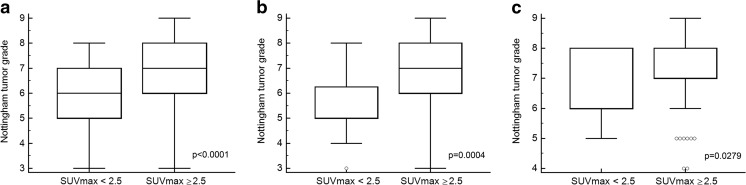
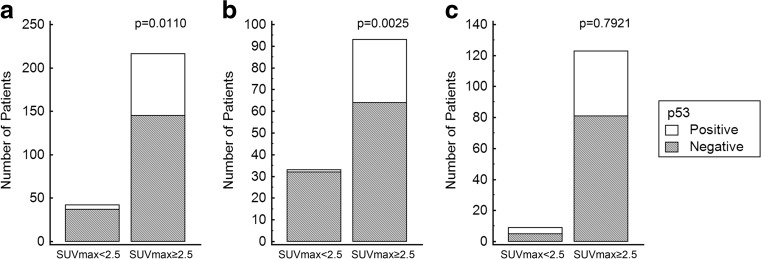
The relationships between various clinicopathologic factors and 18F-FDG uptake were summarized in Table 2. On the univariate analysis, ER (p = 0.0045), tumor size (p < 0.0001), lymphovascular invasion (p = 0.0382), p53 (p = 0.0110), NG (p < 0.0001) and pN (p = 0.0470) were significantly associated with high SUVmax in T1 and T2 breast cancer (Table 2, Fig. 1). ER (p = 0.0045), p53 (p = 0.0025) and NG (p = 0.0004) were significantly associated with high SUVmax in the T1 group on the univariate analysis (Table 2, Figs. 1 and 2). In the T2 group, only NG (p = 0.0279) was associated with high SUVmax on the univariate analysis (Table 2, Fig. 1). On the multivariate analysis, tumor size (OR 5.1925, 95 % CI 2.2384-12.0453; p = 0.0001) and NG (OR 1.5695, 95 % CI 1.2011-2.0508; p = 0.0010) remained statistically significant variables for determining high and low SUVmax. In the T1 group, p53 (OR 10.0595, 95 % CI 1.2775-79.2110; p = 0.0283) and NG (OR 1.5365, 95 % CI 1.1093-2.1281; p = 0.0098) were independent factors to determine high and low SUVmax on the multivariate analysis.
Table 2.
Univariate and multivariate analysis of clinicopathologic factors influencing 18F-FDG uptake in breast cancer
| Univariate | Multivariate | ||||
|---|---|---|---|---|---|
| All (n = 258) | SUVmax ≥ 2.5 | SUVmax < 2.5 | p value | OR (95 % CI) | p value |
| Estrogen receptor (positive) | 139 | 37 | 0.0045 | ||
| Progesterone receptor (positive) | 130 | 32 | 0.0736 | ||
| HER2 (positive) | 79 | 11 | 0.2649 | ||
| Tumor size (mm) | 2.35 ± 0.83 | 1.63 ± 0.48 | <0.0001 | 5.1925 (2.2384-12.0453) | 0.0001 |
| Surgical margin (positive) | 9 | 0 | 0.3730 | ||
| Lymphovascular invasion (positive) | 80 | 8 | 0.0382 | ||
| p53 (positive) | 71 | 5 | 0.0110 | ||
| Nottingham tumor grade | 7.17 ± 1.39 | 5.83 ± 1.32 | <0.0001 | 1.5695 (1.2011-2.0508) | 0.0010 |
| Ki67 (%) | 25.02 ± 23.49 | 23.54 ± 26.72 | 0.2818 | ||
| Pathologic N status (positive) | 106 | 13 | 0.0470 | ||
| 1 cm < T1 ≤ 2 cm (n = 126) | SUVmax ≥ 2.5 | SUVmax < 2.5 | p value | OR (95 % CI) | p value |
| Estrogen receptor (positive) | 66 | 32 | 0.0045 | ||
| Progesterone receptor (positive) | 66 | 27 | 0.3234 | ||
| HER2 (positive) | 26 | 7 | 0.5984 | ||
| Surgical margin (positive) | 3 | 0 | 0.6987 | ||
| Lymphovascular invasion (positive) | 24 | 6 | 0.5185 | ||
| p53 (positive) | 29 | 1 | 0.0025 | 10.0595 (1.2775-79.2110) | 0.0283 |
| Nottingham tumor grade | 6.63 ± 1.44 | 5.61 ± 1.30 | 0.0004 | 1.5365 (1.1093-2.1281) | 0.0098 |
| Ki67 (%) | 25.27 ± 23.81 | 20.98 ± 24.80 | 0.1946 | ||
| Pathologic N status (positive) | 33 | 8 | 0.3331 | ||
| 2 cm < T2 ≤ 5 cm (n = 132) | SUVmax ≥ 2.5 | SUVmax < 2.5 | p value | OR (95 % CI) | p value |
| Estrogen receptor (positive) | 73 | 5 | 0.8984 | ||
| Progesterone receptor (positive) | 64 | 5 | 0.8875 | ||
| HER2 (positive) | 53 | 4 | 0.7877 | ||
| Surgical margin (positive) | 6 | 0 | 0.8802 | ||
| Lymphovascular invasion (positive) | 56 | 2 | 0.3115 | ||
| p53 (positive) | 42 | 4 | 0.7921 | ||
| Nottingham tumor grade | 7.57 ± 1.21 | 6.67 ± 1.12 | 0.0279 | ||
| Ki67 (%) | 24.84 ± 23.34 | 32.89 ± 32.77 | 0.5710 | ||
| Pathologic N status (positive) | 73 | 5 | 0.8984 | ||
SUVmax maximum standardized uptake value, HER2 human epidermal growth factor receptor 2, OR odds ratio
Fig. 1.
Comparison of Nottingham tumor grade between the high SUVmax group and low SUVmax group. Nottingham tumor grade was significantly higher in the high SUVmax group than in low SUVmax group. a All-size group; b T1 group; c T2 group
Fig. 2.
Comparison of p53 between the high SUVmax group and low SUVmax group. Tumor protein p53 was a significant variable for determining high and low SUVmax groups in the T1 group. a All-size group; b T1 group; c T2 group
Discussion
Breast cancer appears to display considerably variable 18F-FDG uptake, so several studies have examined the correlation between 18F-FDG uptake and various prognostic factors [3, 9–13, 18]. Buck et al. [10] and Crippa et al. [18] examined the possible association between 18F-FDG uptake in breast cancer and several clinicopathologic factors. Both studies, and our study, indicated that there was no significant correlation between 18F-FDG uptake and the hormone receptor status. In addition, Buck et al. [10] demonstrated that there was no significant correlation between 18F-FDG uptake and HER2 overexpression, and this was consistent with our results.
In the present study, we showed that tumor size and NG could influence the intensity of 18F-FDG uptake in breast cancer. We thought that SUV values greater than 2.5 might represent relatively intense 18F-FDG uptake, so we regarded SUVmax greater than 2.5 as high SUVmax [4]. The positive relationship between SUVmax and tumor size in the present study was in accordance with previous studies. PET imaging has been reported to provide a low sensitivity to detect small breast cancers, but an increase in metabolic activity with tumor growth [9, 12, 13]. However, SUVmax of breast cancer with similar small size was also variable [4, 14–18], and small sized breast cancer might have high SUVmax. Similarly, Ueda et al. [9] reported no significant relationship between tumor size and SUVmax, especially in lesions of 2 cm or less. Also, Avril et al. [20] reported that there was no significant relationship between tumor size and 18F-FDG uptake. This discrepancy in the existing literature may be explained by immunohistochemical and histologic differences. As breast cancer is a very heterogenous disease with variable phenotypes, different histological and immunohistochemical characteristics can influence the glycolytic pathway and the variability in the glycolytic phenotype [21, 22].
In the subgroup analysis, we divided patients into two groups by tumor size, according to the pathologic T stage given by the American Joint Committee on Cancer, 7th edition [19], and presumed to have similar treatment plan and prognosis, to find additional prognostic factors in the same T stage. Tumor protein p53 and NG in the T1 group, and NG in the T2 group could make a difference of 18F-FDG uptake. These data mean that regardless of tumor size, NG was an independent factor influencing 18F-FDG uptake. A significant relationship between SUVmax and pathologic grade represented by NG has been reported, and which is due to the finding that less differentiated tumors with higher tumor growth rates exhibit higher 18F-FDG uptake [13, 20]. These results may be due to high levels of glucose transporter 1 at the membrane and increased level of hexokinase in the cytoplasm, encountered in high-grade human cancers including breast cancer [9, 23, 24]. NG is determined by evaluating morphologic features including tubule formation, nuclear pleomorphism and mitotic count [7, 25, 26]. The lowest score is given to well-differentiated tumors that all form tubules and have a low mitotic rate. Hence, the data presented here signify that a more differentiated tumor may have a lower 18F-FDG uptake in breast cancer.
In the present study, we found the relationship between p53 and SUVmax in the T1 group, but not in the T2 group. In agreement with our study, Crippa et al. [18] reported a positive correlation between 18F-FDG uptake and p53 overexpression. The p53 gene functions as a tumor suppressor, preventing cancer [27, 28]. The capability that p53 status affects biological behavior was raised in a previous study in which the presence of p53 mutations in aggressive breast cancer was demonstrated [29, 30]. Hence, the data presented here signify that T1 breast cancer, at a relatively early stage, with high 18F-FDG, may be related to aggressive breast cancer, and careful monitoring may be necessary. Also, there is evidence linking the development and progression of cancer with an accumulation of mutations at the genomic level [27, 28]. These mutations are thought to result either in the activation of proto-oncogenes or in the loss of tumor suppressor gene potential. So, their accumulation will eventually drive cancer cells further into anaplasia [29]. Compared with other clinicopathologic factors, p53 is related to the development of cancer, and it may influence the aggressive nature of tumor only in a relatively early stage.
Our study had several limitations. First, this study was a retrospective one with a relatively small number of patients. Second, we simplified the degree of 18F-FDG uptake and categorized groups according to SUVmax of 2.5. Consequently SUVmax was regarded as a discrete variable, and it led to loss of some information. Third, we did not assess the association between prognosis and clinicopathologic factors used as variables in this study. Fourth, it might have been a limitation to use two different scanners.
Conclusion
NG showed an association with 18F-FDG uptake in both T1 and T2 breast cancer independently; however, p53 in T1 breast cancer.
Acknowledgments
The manuscript has not been published before or is not under consideration for publication anywhere else and has been approved by all co-authors.
Compliance with Ethical Standards
Conflict of Interest
So Jung Kim, Seong-Jang Kim, In Joo Kim, Kyoungjune Pak, Bum Soo Kim and Seunghyeon Shin declare that they have no conflict of interest.
Ethical Statement
The study was approved by an institutional review board or equivalent and has been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. All subjects in the study gave written informed consent or the institutional review board waived the need to obtain informed consent.
References
- 1.Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127:2893–917. doi: 10.1002/ijc.25516. [DOI] [PubMed] [Google Scholar]
- 2.Buck AK, Schirrmeister H, Mattfeldt T, Reske SN. Biological characterisation of breast cancer by means of PET. Eur J Nucl Med Mol Imaging. 2004;31(Suppl 1):S80–7. doi: 10.1007/s00259-004-1529-6. [DOI] [PubMed] [Google Scholar]
- 3.Mavi A, Cermik TF, Urhan M, Puskulcu H, Basu S, Yu JQ, et al. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions. J Nucl Med. 2007;48:1266–72. doi: 10.2967/jnumed.106.037440. [DOI] [PubMed] [Google Scholar]
- 4.Avril N, Dose J, Janicke F, Bense S, Ziegler S, Laubenbacher C, et al. Metabolic characterization of breast tumors with positron emission tomography using F-18 fluorodeoxyglucose. J Clin Oncol. 1996;14:1848–57. doi: 10.1200/JCO.1996.14.6.1848. [DOI] [PubMed] [Google Scholar]
- 5.Avril N, Rose CA, Schelling M, Dose J, Kuhn W, Bense S, et al. Breast imaging with positron emission tomography and fluorine-18 fluorodeoxyglucose: use and limitations. J Clin Oncol. 2000;18:3495–502. doi: 10.1200/JCO.2000.18.20.3495. [DOI] [PubMed] [Google Scholar]
- 6.Wahl RL, Cody RL, Hutchins GD, Mudgett EE. Primary and metastatic breast carcinoma: initial clinical evaluation with PET with the radiolabeled glucose analogue 2-[F-18]-fluoro-2-deoxy-D-glucose. Radiology. 1991;179:765–70. doi: 10.1148/radiology.179.3.2027989. [DOI] [PubMed] [Google Scholar]
- 7.Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19:403–10. doi: 10.1111/j.1365-2559.1991.tb00229.x. [DOI] [PubMed] [Google Scholar]
- 8.Kwast AB, Voogd AC, Menke-Pluijmers MB, Linn SC, Sonke GS, Kiemeney LA, et al. Prognostic factors for survival in metastatic breast cancer by hormone receptor status. Breast Cancer Res Treat. 2014;145(2):503–11. doi: 10.1007/s10549-014-2964-0. [DOI] [PubMed] [Google Scholar]
- 9.Ueda S, Tsuda H, Asakawa H, Shigekawa T, Fukatsu K, Kondo N, et al. Clinicopathological and prognostic relevance of uptake level using 18F-fluorodeoxyglucose positron emission tomography/computed tomography fusion imaging (18F-FDG PET/CT) in primary breast cancer. Jpn J Clin Oncol. 2008;38:250–8. doi: 10.1093/jjco/hyn019. [DOI] [PubMed] [Google Scholar]
- 10.Buck A, Schirrmeister H, Kuhn T, Shen C, Kalker T, Kotzerke J, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29:1317–23. doi: 10.1007/s00259-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 11.Heudel P, Cimarelli S, Montella A, Bouteille C, Mognetti T. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15:588–93. doi: 10.1007/s10147-010-0120-3. [DOI] [PubMed] [Google Scholar]
- 12.Osborne JR, Port E, Gonen M, Doane A, Yeung H, Gerald W, et al. 18F-FDG PET of locally invasive breast cancer and association of estrogen receptor status with standardized uptake value: microarray and immunohistochemical analysis. J Nucl Med. 2010;51:543–50. doi: 10.2967/jnumed.108.060459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sanli Y, Kuyumcu S, Ozkan ZG, Isik G, Karanlik H, Guzelbey B, et al. Increased FDG uptake in breast cancer is associated with prognostic factors. Ann Nucl Med. 2012;26:345–50. doi: 10.1007/s12149-012-0579-2. [DOI] [PubMed] [Google Scholar]
- 14.Adler LP, Crowe JP, al-Kaisi NK, Sunshine JL. Evaluation of breast masses and axillary lymph nodes with [F-18] 2-deoxy-2-fluoro-D-glucose PET. Radiology. 1993;187:743–50. doi: 10.1148/radiology.187.3.8497624. [DOI] [PubMed] [Google Scholar]
- 15.Avril N, Bense S, Ziegler SI, Dose J, Weber W, Laubenbacher C, et al. Breast imaging with fluorine-18-FDG PET: quantitative image analysis. J Nucl Med. 1997;38:1186–91. [PubMed] [Google Scholar]
- 16.Avril N, Schelling M, Dose J, Weber WA, Schwaiger M. Utility of PET in breast cancer. Clin Positron Imaging. 1999;2:261–71. doi: 10.1016/S1095-0397(99)00032-1. [DOI] [PubMed] [Google Scholar]
- 17.Scheidhauer K, Scharl A, Pietrzyk U, Wagner R, Gohring UJ, Schomacker K, et al. Qualitative [18F]FDG positron emission tomography in primary breast cancer: clinical relevance and practicability. Eur J Nucl Med. 1996;23:618–23. doi: 10.1007/BF00834522. [DOI] [PubMed] [Google Scholar]
- 18.Crippa F, Seregni E, Agresti R, Chiesa C, Pascali C, Bogni A, et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med. 1998;25:1429–34. doi: 10.1007/s002590050319. [DOI] [PubMed] [Google Scholar]
- 19.Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York: Springer; 2010.
- 20.Avril N, Menzel M, Dose J, Schelling M, Weber W, Janicke F, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42:9–16. [PubMed] [Google Scholar]
- 21.Bertucci F, Finetti P, Birnbaum D. Basal breast cancer: a complex and deadly molecular subtype. Curr Mol Med. 2012;12:96–110. doi: 10.2174/156652412798376134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Vander Heiden MG, Plas DR, Rathmell JC, Fox CJ, Harris MH, Thompson CB. Growth factors can influence cell growth and survival through effects on glucose metabolism. Mol Cell Biol. 2001;21:5899–912. doi: 10.1128/MCB.21.17.5899-5912.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Higashi T, Tamaki N, Honda T, Torizuka T, Kimura T, Inokuma T, et al. Expression of glucose transporters in human pancreatic tumors compared with increased FDG accumulation in PET study. J Nucl Med. 1997;38:1337–44. [PubMed] [Google Scholar]
- 24.Reske SN, Grillenberger KG, Glatting G, Port M, Hildebrandt M, Gansauge F, et al. Overexpression of glucose transporter 1 and increased FDG uptake in pancreatic carcinoma. J Nucl Med. 1997;38:1344–8. [PubMed] [Google Scholar]
- 25.Bloom HJ, Richardson WW. Histological grading and prognosis in breast cancer; a study of 1409 cases of which 359 have been followed for 15 years. Br J Cancer. 1957;11:359–77. doi: 10.1038/bjc.1957.43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Genestie C, Zafrani B, Asselain B, Fourquet A, Rozan S, Validire P, et al. Comparison of the prognostic value of Scarff-Bloom-Richardson and Nottingham histological grades in a series of 825 cases of breast cancer: major importance of the mitotic count as a component of both grading systems. Anticancer Res. 1998;18:571–6. [PubMed] [Google Scholar]
- 27.Ryan KM, Phillips AC, Vousden KH. Regulation and function of the p53 tumor suppressor protein. Curr Opin Cell Biol. 2001;13:332–7. doi: 10.1016/S0955-0674(00)00216-7. [DOI] [PubMed] [Google Scholar]
- 28.Vogelstein B, Lane D, Levine AJ. Surfing the p53 network. Nature. 2000;408:307–10. doi: 10.1038/35042675. [DOI] [PubMed] [Google Scholar]
- 29.Mazars R, Spinardi L, BenCheikh M, Simony-Lafontaine J, Jeanteur P, Theillet C. P53 mutations occur in aggressive breast cancer. Cancer Res. 1992;52:3918–23. [PubMed] [Google Scholar]
- 30.Alsner J, Yilmaz M, Guldberg P, Hansen LL, Overgaard J. Heterogeneity in the clinical phenotype of TP53 mutations in breast cancer patients. Clin Cancer Res. 2000;6:3923–31. doi: 10.1186/bcr109. [DOI] [PMC free article] [PubMed] [Google Scholar]