Abstract
Purpose
This study investigates the correlation of retention index (RI) using the dual phase FDG PET/CT scan with the breast cancer biomarkers.
Methods
A total of 55 patients with breast cancer underwent dual phase FDG PET/CT scans (60 and 120 min after FDG injection) before treatment. SUVmax and SUVmean of the primary breast tumors were measured, then the percent change of SUVmax and SUVmean between the two scans were calculated, and denoted as RImax and RImean, respectively. After the surgical resection of the breast tumor, the status of biomarkers (ER, PR, and HER-2) was evaluated in the postsurgical specimen.
Results
RImean was significantly higher in ER (−) (median, 16.2; IQR, 10.8–21.0) or HER-2 (+) (median, 16.1; IQR, 10.7–21.6) tumors than in ER (+) tumors (median, 9.9; IQR, 5.5–15.3) or HER-2 (−) tumors (median, 10.5; IQR, 5.5–16.1). However, there were no significant differences of SUVmax or RImax according to the ER or HER-2 status. There were no significant differences of any PET parameters between PR (+) and PR (−) tumors. Based off ROC curve analyses, RImean predicted the ER (+) tumors (AUC, 0.699; p = 0.006), and HER-2 (+) tumors (AUC, 0.674; p = 0.022), but not the PR (+) tumors. However, neither SUVmax nor RImax predicted ER (+), PR (+), or HER-2 (+) tumors.
Conclusions
Retention index of SUVmean can reflect the ER and HER-2 status of breast cancers. Higher retention index of SUVmean might associate with lower ER expression and higher HER-2 expression.
Keywords: Breast cancer, Dual phase, Estrogen receptor, Human epidermal growth factor receptor 2, Positron emission tomography, Retention index
Introduction
Breast cancer is the second most common cancer of women in Korea [1], and its incidence of rates are rising rapidly [2]. There are a variety of factors associated with breast cancer including environmental, hormonal, and genetic factors [3, 4]. Among these factors, molecular biomarkers, such as the estrogen receptor (ER), progesterone receptor (PR), and human epidermal growth factor receptor 2 (HER-2) have been reported to be associated with the patients’ prognosis and influence the treatment planning [5].
18F-fluorodeoxyglucose positron emission tomography-computed tomography (FDG PET/CT) has been important in the management of breast cancer patients [6]. It is used for screening for the extra-axillary lymph node metastasis [7] and distant metastasis [8, 9], assessing for the treatment responses [10, 11], and predicting prognosis [12], but the benefit of FDG PET/CT scanning for staging remains a matter of debate [13]. Several studies have suggested that the characteristics of FDG uptake in breast cancer are relevant to biological or histological attributes of primary tumor, including its biomarker status [14–19]. Among these previous studies using diverse PET parameters, such as the maximum standardized uptake value (SUVmax), metabolic tumor volume (MTV), or total lesion glycolysis (TLG), the correlations between the biomarker status and PET parameters were weak or modest in many studies [17, 20, 21].
The dual phase PET/CT scan is an evolving diagnostic tool in breast cancer [22, 23], but is not regarded as the standard tool. The retention index (RI) is as an alternative PET parameter which is useful not only for distinguishing malignancy from benignancy of FDG-avid lesions [24], but also for predicting survival of the patients with several malignancies such as lung [25, 26], pancreatic [27, 28], and head and neck cancer [29, 30]. Recently, the association between the RI using SUVmax and biomarker status of breast cancer was reported [21]. However, there is no data on the role of the RI using the mean standardized uptake value (SUVmean) with the biomarker status of breast cancer. Therefore, the aim of this study is to evaluate whether the calculation of the RI using both the SUVmax and the SUVmean of FDG PET/CT would be useful to predict the biomarker status of breast cancer.
Materials and Methods
Patient Population
A total of 59 consecutive patients with histologically proven breast cancer who underwent dual phase FDG PET/CT scans before treatment between September 2009 and May 2011 were retrospectively analyzed. All patients had newly diagnosed AJCC stage II or III breast cancer. To avoid the partial volume effects, patients whose tumors were less than 1.5 cm based on the MRI were excluded from the study. Other eligibility requirements included no evidence of distant metastasis confirmed by other methods previous to the PET/CT scan, no history of other malignancies except the breast cancer, and primary breast tumor showing higher FDG uptake (SUVmax of the tumor > 2.0). The Institutional Review Board of the institute approved the current study, and informed consent was waived due to its retrospective design.
FDG PET/CT Imaging
PET/CT data were acquired using a Biograph6 PET/CT scanner (Siemens Medical Solutions; Knoxville, TN, USA). All patients fasted for at least 6 h before the intravenous administration of FDG (7.4 MBq per kg of body weight), and all patients’ blood glucose levels were less than 7.2 mmol/L before the FDG injection. PET/CT imaging from the skull base to the upper thigh (5 to 6 bed positions) was performed beginning 60 min after FDG injection (first PET image). During the PET/CT scans, CT images without intravenous iodinated contrast were obtained using a 6-slice helical CT scanner, and the imaging parameters used for CT scanning were as follows: 130 kVp, 30 mA, 0.6-s/CT rotation, and a pitch of 6. Then, PET emission data were acquired over the corresponding area with a 16.2-cm axial field of view at 3.5 min per bed position. The CT data were used for attenuation correction, and the images were reconstructed using a conventional iterative algorithm (ordered-subsets expectation-maximization, two iterations, and eight subsets). The second PET imaging from the T1 to T12 level was performed beginning 120 min after FDG injection, and CT images without intravenous iodinated contrast were also obtained. The same protocols were used for the first and second PET imaging procedures.
Imaging Analysis
All PET/CT images were reviewed on e-soft workstations (Siemens Medical Systems, Iselin, NJ). An ellipsoid volume of interest (VOI) was drawn to include the entire primary tumor of the breast, the SUVmax corrected for body weight and the dose of FDG injected was measured for each PET/CT dataset.
We chose 2.0 as cut-off SUV for determining the tumor VOI. From the VOI at the cut-off SUV of 2.0, SUVmean was measured. Then the percent change of SUVmax and SUVmean between the first and second PET images, denoted as RImax and RImean, were calculated as follows:
MTV was measured by using a semi-automated contouring program with VOI at the cut-off SUV of 2.0.
Biomarker-Based Subgroup
According to the criteria suggested by Cheang et al. [31], the cases were classified into four biologically distinct subgroups based on the expression of ER, PR, HER-2, and the Ki-67 index. This classification system has been reported to be useful for both treatment planning and prediction of prognosis. Because the Ki-67 index was not evaluated, all subtypes were determined only by biomarker status as follows: a) hormone receptor-positive and HER-2-negative, b) hormone receptor-positive and HER-2-positive; c) hormone receptor-negative and HER-2-positive; and d) hormone receptor- and HER-2-negative.
Statistical Analysis
All parameters were expressed as mean ± standard deviation (SD) or median and interquartilie range (IQR). Differences of PET parameters (SUVmax, RImax, RImean, and MTV) according to the biomarker status were assessed by using a Mann–Whitney U test. The receiver-operating-characteristic (ROC) curve was analyzed to determine the ability of each PET parameter to predict the biomarker status. Area under the ROC curve (AUC), 95 % confidence interval (95 % CI), sensitivity, specificity, positive predictive value (PPV), negative predictive value (NPV), and accuracy for predicting biomarker status were reported. A Pearson correlation coefficient test was used to check for association between MTV and other PET parameters. All tests were 2-sided, and p values of 0.05 or less were considered significant. All statistical tests were performed using MedCalc (MedCalc Software 12.5.0.0, MedCalc Software bvba).
Result
Patient Characteristics
Three of 59 patients were excluded because the SUVmax of their respective tumors was less than 2.0. One patient was excluded because of the small size of tumor. Therefore, a total of 55 patients were retrospectively analyzed in the current study. The median age of the 55 patients was 46 y (IQR, 42–51), and all patients were women. On the postsurgical specimen, ER (+), PR (+), and HER-2 (+) tumors were identified in 30 (55 %), 43 (78 %), and 21 patients (38 %), respectively. About half of patients (53 %) had T3 or T4 tumors, and most patients (95 %) had regional lymph node metastasis (N1–N3). Pathologic subtypes of the primary tumor were intraductal carcinoma in 51 patients (93 %), and other in four patients (7 %) (Table 1).
Table 1.
Patient characteristics (n = 55)
| Characteristics | Number of patients (%) |
|---|---|
| Age | |
| < 35 y | 6 (10.9 %) |
| ≥ 35 y | 49 (89.1 %) |
| Gender | |
| Male | 0 (0 %) |
| Female | 55 (100 %) |
| Estrogen receptor status | |
| Positive | 30 (54.5 %) |
| Negative | 25 (46.5 %) |
| Progesterone receptor status | |
| Positive | 43 (78.2 %) |
| Negative | 12 (21.8 %) |
| HER-2/neu status | |
| Positive | 21 (38.2 %) |
| Negative | 34 (61.8 %) |
| Menopausal | |
| Premenopausal | 21 (38.2 %) |
| Menopausal | 34 (61.8 %) |
| T stage | |
| T1 | 4 (7.2 %) |
| T2 | 22 (40.0 %) |
| T3 | 18 (32.7 %) |
| T4 | 11 (20.0 %) |
| N stage | |
| N0 | 3 (5.5 %) |
| N1 | 14 (25.5 %) |
| N2 | 33 (60.0 %) |
| N3 | 5 (9.1 %) |
| M stage | |
| M0 | 55 (100 %) |
| M1 | 0 (0 %) |
| Pathologic subtypes | |
| Invasive ductal carcinoma | 51 (92.7 %) |
| Invasive lobular carcinoma | 2 (3.6 %) |
| Mucinous carcinoma | 1 (1.8 %) |
| Signet ring cell carcinoma | 1 (1.8 %) |
PET Parameters According to the Biomarker Status
Comparisons of PET parameters according to the biomarker status are detailed in Fig. 1 and Table 2. RImean was significantly higher in ER (−) tumors (median, 16.2; IQR, 10.8–21.0; Range, 0.4–42.6) than in ER (+) tumors (median, 9.9; IQR, 5.5–15.3; range, −3.2–27.9). SUVmax (median, 9.0; IQR, 6.5–12.3; range, 3.2–17.6) and RImax (median 16.2; IQR, 8.7–21.7; range, −15.1–35.6) of ER (−) tumors were also higher than those of ER (+) tumors (median 7.2; IQR, 3.5–11.1; range, 2.3–17.0 for SUVmax, and median, 15.5; IQR, 8.0–25.4; range, −10.3–47.3 for RImax). However, there were no significant differences of SUVmax (p = 0.089) or RImax (p = 0.839) between ER (+) tumors and ER (−) tumors.
Fig. 1.
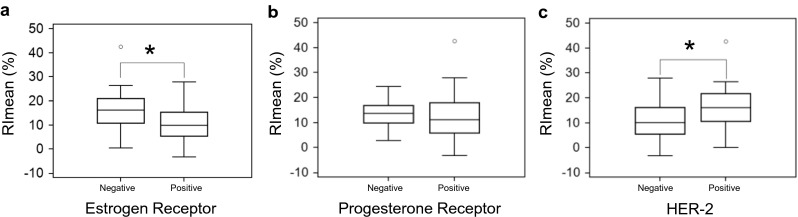
RImean according to the biomarker status. Data are presented as box plots indicating median and interquartile range; whiskers extend to most extreme data points up to 1.5 times the interquartile range. Asterisk indicates p < 0.05
Table 2.
Comparisons of PET parameters according to the biomarker status
| Number of patients | SUVmax | SUVmean | PET parameters* | |||
|---|---|---|---|---|---|---|
| RImax (%) | RImean (%) | MTV (mL) | ||||
| All patients | 55 | 7.9 (5.7–11.5) | 3.4 (2.8–4.7) | 16.0 (8.3–24.1) | 11.7 (7.0–18.0) | 20.1 (9.2–44.0) |
| ER | ||||||
| Positive | 30 | 7.2 (3.5–11.1) | 3.1 (2.5–4.5) | 15.5 (8.0–25.4) | 9.9 (5.5–15.3) | 17.4 (6.3–36.3) |
| Negative | 25 | 9.0 (6.5–12.3) | 3.6 (3.1–4.8) | 16.2 (8.7–21.7) | 16.2 (10.8–21.0) | 33.8 (16.3–51.8) |
| p value | 0.090 | 0.099 | 0.839 | 0.012 | 0.031 | |
| PR | ||||||
| Positive | 43 | 7.9 (5.3–12.8) | 3.4 (2.6–5.2) | 15.5 (4.8–25.3) | 11.3 (5.9–18.0) | 20.2 (10.9–47.7) |
| Negative | 12 | 8.2 (6.4–9.4) | 3.7 (3.2–4.4) | 16.4 (14.5–20.8) | 14.5 (10.6–17.5) | 19.0 (8.8–35.9) |
| p value | 0.744 | 0.863 | 0.313 | 0.343 | 0.501 | |
| HER-2 | ||||||
| Positive | 21 | 9.5 (6.4–11.9) | 4.1 (3.1–4.7) | 16.3 (11.1–20.8) | 16.1 (10.7–21.6) | 24.9 (8.8–47.9) |
| Negative | 34 | 7.2 (5.1–11.1) | 3.3 (2.6–4.7) | 15.5 (4.3–25.0) | 10.5 (5.5–16.1) | 19.0 (10.6–40.9) |
| p value | 0.279 | 0.396 | 0.456 | 0.031 | 0.795 | |
*PET parameters are presented as median values (interquartile range)
With respect to the HER-2 status of the tumors, RImean was higher in HER-2 (+) tumors (median, 16.1; IQR, 10.7–21.6; range, 0–42.6) than in HER-2 (−) tumors (median, 10.5; IQR, 5.5–16.1; range, −32.–27.9). SUVmax (median, 9.5; IQR, 6.4–11.9; range, 2.3–16.3) and RImax (median, 16.3; IQR, 11.1–20.8; range, 1.9–30.9) of HER-2 (+) tumors were also higher than those of HER-2 (−) tumors (median, 7.2; IQR, 5.1–11.1; range, 2.5–17.6 for SUVmax, and median, 15.5; IQR 4.3–25.0; range, −15.1–47.3 for RImax), but there were no significant differences of SUVmax (p = 0.279) or RImax (p = 0.456) between HER-2 (+) and HER-2 (−) tumors.
The SUVmax of PR (+) tumors (median, 7.9; IQR, 5.3–12.8; range, 2.3–17.6) was similar to the SUVmax of PR (−) tumors (median, 8.2; IQR, 6.4–9.4; range, 3.5–11.6). RImax (median, 16.4; IQR, 14.5–20.8; range, 8.8–30.9) and RImean (median, 14.5; IQR, 10.6–17.5; range, 2.8–24.3) of PR (−) tumors were slightly higher than those of PR (+) tumors (median, 15.5; IQR, 4.8–25.3; range, −15.1–47.3 for RImax, and median, 11.3; IQR, 5.9–18.0; range, −3.2–42.6 for RImean). However, there were no significant differences of any PET parameters between PR (+) and PR (−) tumors.
MTV showed statistically significant correlation with SUVmax (p < 0.001), RImean (p = 0.015), and the ER status (p = 0.031), but did not with the PR status (p = 0.501), HER-2 status (p = 0.795), or RImax (p = 0.139). SUVmean did not show correlation with any biomarker status.
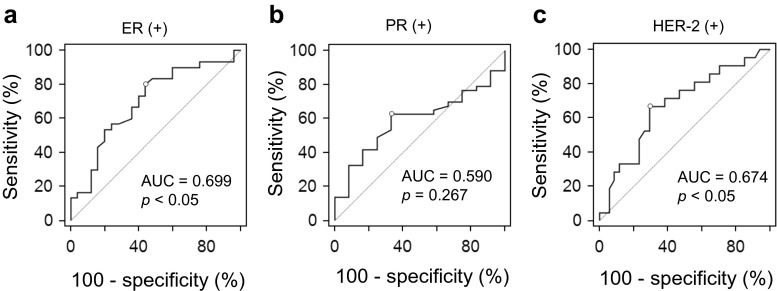
PET Parameters to Predict the Biomarker Status
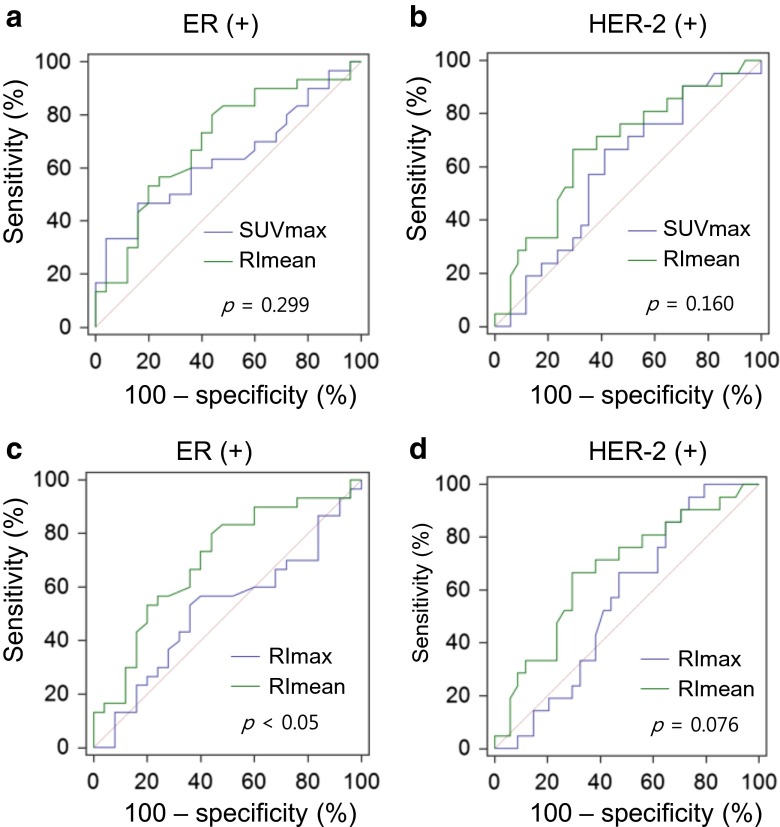
Based on ROC curve analyses, RImean predicted the ER (+) tumors (AUC, 0.699; 95 % CI, 0.560–0.815; p = 0.006), and HER-2 (+) tumors (AUC, 0.674; 95 % CI, 0.535–0.795; p = 0.022), but not the PR (+) tumors (AUC, 0.590; 95 % CI, 0.449–0.721; p = 0.276). However, neither SUVmax nor RImax predicted ER (+) tumors (AUC, 0.634; 95 % CI, 0.493–0.760; p = 0.076 for SUVmax, and AUC, 0.516; 95 % CI, 0.377–0.653; p = 0.841 for RImax, respectively), PR (+) tumors (AUC, 0.531; 95 % CI, 0.392–0.667; p = 0.691 for SUVmax, and AUC, 0.596; 95 % CI, 0.455–0.726; p = 0.215 for RImax, respectively), and HER-2 (+) tumors (AUC, 0.588; 95 % CI, 0.447–0.719; p = 0.268 for SUVmax, and AUC, 0.560; 95 % CI, 0.420–0.694; p = 0.437 for RImax, respectively) (Fig. 2). The AUC of RImean was significantly higher than that of RImax for predicting the ER status (p = 0.002). However, there were no significant differences between the AUC of SUVmax and RImean for predicting the ER status (p = 0.299) and HER-2 status (p = 0.160). There was also no significant difference between the AUC of RImax and RImean for predicting the HER-2 status (p = 0.076) (Fig. 3).
Fig. 2.
ROC curves of RImean for predicting the status of ER (a), PR (b), and HER-2 (c). RImean predicted the status of ER and HER-2, but not that of PR
Fig. 3.
Comparisons of ROC curves between RImean and other PET parameters for predicting the status of ER and HER-2. Although there are no significant differences between SUVmax and RImean for predicting the status of ER (a) or HER-2 (b), there are significant differences between RImax and RImean for predicting the status of ER (c) or HER-2 (d)
The optimal criteria, sensitivity, specificity, PPV, NPV, and accuracy of RImean were < 16.0 %, 76.7 %, 56.0 %, 67.7 %, 66.7 %, and 67.2 % for predicting ER(+) tumors, and > 12.1 %, 66.7 %, 67.7 %, 56.0 %, 76.7 %, and 67.2 %, for HER-2(+) tumors, respectively (Table 3).
Table 3.
RImean to predict the biomarker status of the breast cancer
| Optimal criteria | Sensitivity (%) | Specificity (%) | PPV (%) | NPV (%) | Accuracy (%) | |
|---|---|---|---|---|---|---|
| For prediction of ER (+) | <16.0 % | 77.7 | 56.0 | 67.7 | 66.7 | 67.2 |
| For prediction of HER-2 (+) | > 12.1 % | 66.7 | 67.7 | 56.0 | 76.7 | 67.2 |
PPV positive predictive value, NPV negative predictive value
PET Parameters Among Biomarker-Based Subgroups
The median values of RImean of subgroup a (n = 33), b (n = 15), c (n = 6), and d (n = 1) were 10.2 % (IQR, 5.2–16.0; range, −3.2–27.9), 15.3 % (IQR, 10.1–21.7; range, 0–42.6), 16.3 % (IQR, 13.7–19.6; range, 2.8–24.3), and 18.5 %, respectively. However, there were no significant differences between PET parameters among these subgroups (p = 0.107 for RImean; p = 0.444 for SUVmax; p = 0.559 for RImax) (Fig. 4).
Fig. 4.
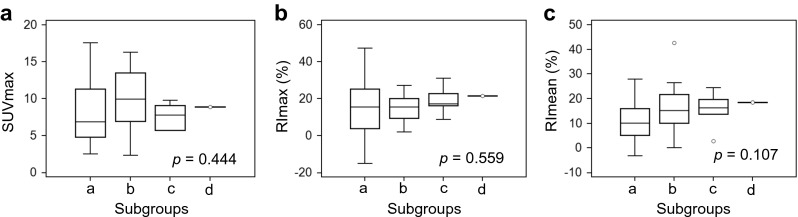
PET parameters according to the four subgroups based on the biomarker status of breast cancer. Each subgroup is defined as a) hormone receptor-positive and HER-2-negative, b) hormone receptor-positive and HER-2-positive; c) hormone receptor-negative and HER-2-positive; and d) hormone receptor- and HER-2-negative. There were no significant differences of PET parameters among these subgroups. Data are presented as box plots indicating median and interquartile range; whiskers extend to most extreme data points up to 1.5 times the interquartile range
Discussion
There are two major findings in the current study: First, among SUVmax, RImax and RImean, only RImean correlated with the biomarker status of breast cancer. Second, pretreatment RImean was useful for predicting biomarker status of breast cancer.
In the current study, SUVmax tended to be higher in ER (−) and HER-2 (+) tumors than ER (+) and HER-2 (−) tumors; however, the trend did not reach statistical significance. Other previous studies are consistent with our result [14, 16, 21, 32]. It has been reported that SUVmax of breast cancer shows a linear relationship in T1–T3 tumors and correlates with tumor size. Due to the small population in the present study, we could not evaluate the correlation between SUVmax and biomarker status in the controlled T-stage groups. Further large-scale study may be warranted.
Like SUVmax, SUVmean also failed to show the correlation with any biomarker status. It was a predictable consequence, because SUVmax would be more expected to reflect the metabolic status of the tumor than SUVmean [33].
Unlike SUVmax, RImean was significantly higher in ER (−) and HER-2 (+) tumors. It is reported that patients with ER (−) and HER-2 (+) breast cancer have poorer prognoses than patients with ER (+) and HER-2 (−) breast cancer [5, 34, 35]. Higashi et al. [36] reported that RI can predict the hexokinase-II expression and suggested possible prognostic values of RI. Because hexokinase-II is attributed with maintaining the malignant state of a tumor due to the closing of voltage-dependent anion channels [37] and prevention of mitochondria-mediated apoptosis [38], a higher RI might be relevant to the poor prognosis and negative prognostic factors such as ER (−) and HER-2 (+).
In this study, SUVmax, RImax, or RImean did not correlate with the PR status of breast cancer. Conflicting results have been reported as the correlation between FDG uptake and PR status. Some studies have claimed higher SUVmax in the PR (−) breast cancer [39, 40], but other reports showed no association between PR status and FDG uptake [41, 42]. Ekmekcioglu et al. [42] suggested that the cut-off level of the PR limit of positivity might be relevant to the result.
Although SUVmax has been widely used as a practical method, there is a limit to the complete analysis of tumor characteristics. Because SUVmax is determined by a single pixel with the highest SUV [43], it could also be effected by its vulnerability to statistical noise. Lodge et al. [33] reported that SUVmax was much more biased by noise properties than multi-pixel summarized SUV. In the current study, the PET images were acquired for 3.5 min for each bed, and therefore, the average positive biases of SUVmax and SUVmean are expected to be about 10 and 5 %, respectively. Based on this previous study, RImean would be less influenced by the SUV bias. Nonetheless, there is a limitation in the use of RImean, especially for determining the VOI of PET. Because SUVmean is strongly dependent on the region of interest [44], further studies are warranted to determine the VOI of PET from enhanced MRI of recently introduced PET-MRI [45].
We performed the dual phase PET/CT scan at 60 and 120 min after FDG injection. The dual phase PET/CT scan appears to improve the diagnostic value of PET/CT in breast cancer [22, 46]. Previous studies using dual phase PET/CT scans were also performed at 60 and 120 min after FDG injection [47–49], the delayed time of 120 min after FDG injection was decided as the scan time of the second PET in the present study. Boerner et al. [50] reported that tumor contrast in breast cancer is stronger in images 3 h after FDG injection than 1.5 h. Another study of Hamberg et al. [51] indicated that the tumor concentration of FDG in lung cancer did not reach the peak point of uptake even 120 min after FDG injection, a finding probably related to the low glucose-6-phosphatase activity and increased cellular glucose uptake [52]. These studies suggested the delayed PET/CT scan at the time of more than 120 min after FDG injection could improve the diagnostic value more than the conventional protocol of the scan at the time of 60 min after FDG injection or 120 min. In this study, we did not acquire the delayed PET/CT scan at the time of more than 120 min after FDG injection. Further studies are needed to evaluate the optimal scan time for dual phase PET/CT in breast cancer.
In this study, 2.0 was chosen as the cut-off SUV for analyzing the malignant tissue. By using this cut-off SUV, three of 59 patients were excluded because these patients had tumors showing a SUVmax < 2.0. Although a SUV of 2.5 has usually been considered as the threshold for distinguishing malignant from benign lesion [24, 53], it may not be applicable as the optimal threshold for diagnosing breast cancer [49] because phosphorylation of FDG in the breast cancer cells might be less complete, consequently it is expected that SUV of the breast cancer would be lower. In this study, five of the 59 patients had tumors showing SUVmax < 2.5. These five patients had relatively small-sized tumors or low-grade tumors. RImean using by cut-off SUV of 2.5 failed to show the correlation with the ER (p = 0.052), PR (p = 0.214), or HER-2 status (p = 0.065) in our study. On the contrary, breast tumors were not easily distinguished from surrounding breast tissue by using a cut-off SUV of 1.5. Therefore, adopted 2.0 was determined as the optimal cut-off SUV in the current study.
Biological subtypes of breast cancer determined by ER, PR, HER-2, and Ki-67 are the most common prognostic and therapeutic markers [54], and are widely used for disease stratification. These subtypes are relevant to the expression of genes to specify the tumor characteristics [55–58], and strongly supported by The 12th St Gallen International Breast Cancer Conference (2011) as the definition of therapy indication, as the subtypes incorporate many of the risk and predictive factors of breast cancer [59]. In our study, SUVmax, RImax, or RImean did not show correlation with the biological subtypes of breast cancer. The small population of the present study seemed to be not enough to show a significant difference among the subtypes. Further large-scale study may be warranted.
The present study has several limitations. First, a small number of patients were included. Second, Ki-67, which is considered to be the marker of proliferation, was not available. Finally, because of the short follow-up duration, the relationship between the PET parameters and prognosis was not evaluated.
Conclusions
Retention index of SUVmean can reflect the ER and HER-2 status of breast cancers. Higher retention index of SUVmean might associate with lower ER expression and higher HER-2 expression.
Acknowledgments
This study was supported by the Establishment Center for PET Application Technology Development of the Korea Institute of Radiological and Medical Sciences (KIRAMS) and by a grant from the Ministry of Education, Science, and Technology (50441-2014).
Compliance with Ethical Standards
Conflict of Interest
Hansol Moon, Woo Chul Noh, Hyun-Ah Kim, Eun-Kyu Kim, Ko Woon Park, Seung Sook Lee, Joon Ho Choi, Kyung Woo Han, Byung Hyun Byun, Ilhan Lim, Byung Il Kim, Chang Woon Choi, and Sang Moo Lim declare that they have no conflict of interest.
Ethical Statement
This study was approved by the Institutional Review Board at Korea Cancer Center Hospital (IRB No.K-1412-002-009), and was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. Acquisition of informed consent was exempted by the board because of the retrospective nature of the study. Details that might disclose the identity of the subjects was omitted. All authors declare that the submitted work and its essential substance have not previously been published and are not being considered for publication elsewhere.
References
- 1.Jung KW, Won YJ, Kong HJ, Oh CM, et al. Cancer statistics in Korea: incidence, mortality, survival and prevalence in 2010. Cancer Res Treat. 2013;45(1):1–14. doi: 10.4143/crt.2013.45.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ahn SH. Korean Breast Cancer S. Clinical characteristics of breast cancer patients in Korea in 2000. Arch Surg. 2004;139(1):27–30. doi: 10.1001/archsurg.139.1.31-a. [DOI] [PubMed] [Google Scholar]
- 3.Key TJ, Verkasalo PK, Banks E. Epidemiology of breast cancer. Lancet Oncol. 2001;2(3):133–140. doi: 10.1016/S1470-2045(00)00254-0. [DOI] [PubMed] [Google Scholar]
- 4.Sariego J, Zrada S, Byrd M, Matsumoto T. Breast cancer in young patients. Am J Surg. 1995;170(3):243–245. doi: 10.1016/S0002-9610(05)80007-8. [DOI] [PubMed] [Google Scholar]
- 5.Harris L, Fritsche H, Mennel R, Norton L, et al. American society of clinical oncology 2007 update of recommendations for the use of tumor markers in breast cancer. J Clin Oncol. 2007;25(33):5287–5312. doi: 10.1200/JCO.2007.14.2364. [DOI] [PubMed] [Google Scholar]
- 6.Groheux D, Giacchetti S, Rubello D, Al-Nahhas A, et al. The evolving role of PET/CT in breast cancer. Nucl Med Commun. 2010;31(4):271–273. doi: 10.1097/MNM.0b013e3283354cb4. [DOI] [PubMed] [Google Scholar]
- 7.Danforth DN, Jr, Aloj L, Carrasquillo JA, Bacharach SL, et al. The role of 18F-FDG-PET in the local/regional evaluation of women with breast cancer. Breast Cancer Res Treat. 2002;75(2):135–146. doi: 10.1023/A:1019664126220. [DOI] [PubMed] [Google Scholar]
- 8.Groheux D, Moretti JL, Baillet G, Espie M, et al. Effect of (18)F-FDG PET/CT imaging in patients with clinical Stage II and III breast cancer. Int J Radiat Oncol Biol Phys. 2008;71(3):695–704. doi: 10.1016/j.ijrobp.2008.02.056. [DOI] [PubMed] [Google Scholar]
- 9.Fuster D, Duch J, Paredes P, Velasco M, et al. Preoperative staging of large primary breast cancer with [18F]fluorodeoxyglucose positron emission tomography/computed tomography compared with conventional imaging procedures. J Clin Oncol. 2008;26(29):4746–4751. doi: 10.1200/JCO.2008.17.1496. [DOI] [PubMed] [Google Scholar]
- 10.Schwarz-Dose J, Untch M, Tiling R, Sassen S, et al. Monitoring primary systemic therapy of large and locally advanced breast cancer by using sequential positron emission tomography imaging with [18F]fluorodeoxyglucose. J Clin Oncol. 2009;27(4):535–541. doi: 10.1200/JCO.2008.17.2650. [DOI] [PubMed] [Google Scholar]
- 11.Couturier O, Jerusalem G, N’Guyen JM, Hustinx R. Sequential positron emission tomography using [18F]fluorodeoxyglucose for monitoring response to chemotherapy in metastatic breast cancer. Clin Cancer Res. 2006;12(21):6437–6443. doi: 10.1158/1078-0432.CCR-06-0383. [DOI] [PubMed] [Google Scholar]
- 12.Baba S, Isoda T, Maruoka Y, Kitamura Y, et al. Diagnostic and prognostic value of pretreatment SUV in 18F-FDG/PET in breast cancer: comparison with apparent diffusion coefficient from diffusion-weighted MR imaging. J Nucl Med. 2014;55(5):736–742. doi: 10.2967/jnumed.113.129395. [DOI] [PubMed] [Google Scholar]
- 13.Riedl CC, Slobod E, Jochelson M, Morrow M, et al. Retrospective analysis of 18F-FDG PET/CT for staging asymptomatic breast cancer patients younger than 40 years. J Nucl Med. 2014;55(10):1578–1583. doi: 10.2967/jnumed.114.143297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dehdashti F, Mortimer JE, Siegel BA, Griffeth LK, et al. Positron tomographic assessment of estrogen receptors in breast cancer: comparison with FDG-PET and in vitro receptor assays. J Nucl Med. 1995;36(10):1766–1774. [PubMed] [Google Scholar]
- 15.Crippa F, Seregni E, Agresti R, Chiesa C, et al. Association between [18F]fluorodeoxyglucose uptake and postoperative histopathology, hormone receptor status, thymidine labelling index and p53 in primary breast cancer: a preliminary observation. Eur J Nucl Med. 1998;25(10):1429–1434. doi: 10.1007/s002590050319. [DOI] [PubMed] [Google Scholar]
- 16.Avril N, Menzel M, Dose J, Schelling M, et al. Glucose metabolism of breast cancer assessed by 18F-FDG PET: histologic and immunohistochemical tissue analysis. J Nucl Med. 2001;42(1):9–16. [PubMed] [Google Scholar]
- 17.Buck A, Schirrmeister H, Kuhn T, Shen C, et al. FDG uptake in breast cancer: correlation with biological and clinical prognostic parameters. Eur J Nucl Med Mol Imaging. 2002;29(10):1317–1323. doi: 10.1007/s00259-002-0880-8. [DOI] [PubMed] [Google Scholar]
- 18.Bos R, van Der Hoeven JJ, van Der Wall E, van Der Groep P, et al. Biologic correlates of (18)fluorodeoxyglucose uptake in human breast cancer measured by positron emission tomography. J Clin Oncol. 2002;20(2):379–387. doi: 10.1200/JCO.20.2.379. [DOI] [PubMed] [Google Scholar]
- 19.Kumar R, Chauhan A, Zhuang H, Chandra P, et al. Clinicopathologic factors associated with false negative FDG-PET in primary breast cancer. Breast Cancer Res Treat. 2006;98(3):267–274. doi: 10.1007/s10549-006-9159-2. [DOI] [PubMed] [Google Scholar]
- 20.Gil-Rendo A, Martinez-Regueira F, Zornoza G, Garcia-Velloso MJ, et al. Association between [18F]fluorodeoxyglucose uptake and prognostic parameters in breast cancer. Br J Surg. 2009;96(2):166–170. doi: 10.1002/bjs.6459. [DOI] [PubMed] [Google Scholar]
- 21.Garcia Vicente AM, Castrejon AS, Relea Calatayud F, Munoz AP, et al. 18F-FDG retention index and biologic prognostic parameters in breast cancer. Clin Nucl Med. 2012;37(5):460–466. doi: 10.1097/RLU.0b013e31823926c9. [DOI] [PubMed] [Google Scholar]
- 22.Zytoon AA, Murakami K, El-Kholy MR, El-Shorbagy E. Dual time point FDG-PET/CT imaging… Potential tool for diagnosis of breast cancer. Clin Radiol. 2008;63(11):1213–1227. doi: 10.1016/j.crad.2008.03.014. [DOI] [PubMed] [Google Scholar]
- 23.Mavi A, Urhan M, Yu JQ, Zhuang H, et al. Dual time point 18F-FDG PET imaging detects breast cancer with high sensitivity and correlates well with histologic subtypes. J Nucl Med. 2006;47(9):1440–1446. [PubMed] [Google Scholar]
- 24.Zhuang H, Pourdehnad M, Lambright ES, Yamamoto AJ, et al. Dual time point 18F-FDG PET imaging for differentiating malignant from inflammatory processes. J Nucl Med. 2001;42(9):1412–1417. [PubMed] [Google Scholar]
- 25.Houseni M, Chamroonrat W, Zhuang J, Gopal R, et al. Prognostic implication of dual-phase PET in adenocarcinoma of the lung. J Nucl Med. 2010;51(4):535–542. doi: 10.2967/jnumed.109.068643. [DOI] [PubMed] [Google Scholar]
- 26.Chen HH, Lee BF, Su WC, Lai YH, et al. The increment in standardized uptake value determined using dual-phase 18F-FDG PET is a promising prognostic factor in non-small-cell lung cancer. Eur J Nucl Med Mol Imaging. 2013;40(10):1478–1485. doi: 10.1007/s00259-013-2452-5. [DOI] [PubMed] [Google Scholar]
- 27.Lyshchik A, Higashi T, Nakamoto Y, Fujimoto K, et al. Dual-phase 18F-fluoro-2-deoxy-D-glucose positron emission tomography as a prognostic parameter in patients with pancreatic cancer. Eur J Nucl Med Mol Imaging. 2005;32(4):389–397. doi: 10.1007/s00259-004-1656-0. [DOI] [PubMed] [Google Scholar]
- 28.Xi Y, Guo R, Hu J, Zhang M, et al. 18F-fluoro-2-deoxy-D-glucose retention index as a prognostic parameter in patients with pancreatic cancer. Nucl Med Commun. 2014;35(11):1112–1118. doi: 10.1097/MNM.0000000000000178. [DOI] [PubMed] [Google Scholar]
- 29.Sanghera B, Wong WL, Lodge MA, Hain S, et al. Potential novel application of dual time point SUV measurements as a predictor of survival in head and neck cancer. Nucl Med Commun. 2005;26(10):861–867. doi: 10.1097/00006231-200510000-00003. [DOI] [PubMed] [Google Scholar]
- 30.Abgral R, Le Roux PY, Rousset J, Querellou S, et al. Prognostic value of dual-time-point 18F-FDG PET-CT imaging in patients with head and neck squamous cell carcinoma. Nucl Med Commun. 2013;34(6):551–556. doi: 10.1097/MNM.0b013e32836089ab. [DOI] [PubMed] [Google Scholar]
- 31.Cheang MC, Chia SK, Voduc D, Gao D, et al. Ki67 index, HER2 status, and prognosis of patients with luminal B breast cancer. J Natl Cancer Inst. 2009;101(10):736–750. doi: 10.1093/jnci/djp082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Shimoda W, Hayashi M, Murakami K, Oyama T, et al. The relationship between FDG uptake in PET scans and biological behavior in breast cancer. Breast Cancer. 2007;14(3):260–268. doi: 10.2325/jbcs.14.260. [DOI] [PubMed] [Google Scholar]
- 33.Lodge MA, Chaudhry MA, Wahl RL. Noise considerations for PET quantification using maximum and peak standardized uptake value. J Nucl Med. 2012;53(7):1041–1047. doi: 10.2967/jnumed.111.101733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Lindstrom LS, Karlsson E, Wilking UM, Johansson U, et al. Clinically used breast cancer markers such as estrogen receptor, progesterone receptor, and human epidermal growth factor receptor 2 are unstable throughout tumor progression. J Clin Oncol. 2012;30(21):2601–2608. doi: 10.1200/JCO.2011.37.2482. [DOI] [PubMed] [Google Scholar]
- 35.Slamon DJ, Clark GM, Wong SG, Levin WJ, et al. Human breast cancer: correlation of relapse and survival with amplification of the HER-2/neu oncogene. Science. 1987;235(4785):177–182. doi: 10.1126/science.3798106. [DOI] [PubMed] [Google Scholar]
- 36.Higashi T, Saga T, Nakamoto Y, Ishimori T, et al. Relationship between retention index in dual-phase (18)F-FDG PET, and hexokinase-II and glucose transporter-1 expression in pancreatic cancer. J Nucl Med. 2002;43(2):173–180. [PubMed] [Google Scholar]
- 37.Azoulay-Zohar H, Israelson A, Abu-Hamad S, Shoshan-Barmatz V. In self-defence: hexokinase promotes voltage-dependent anion channel closure and prevents mitochondria-mediated apoptotic cell death. Biochem J. 2004;377(Pt 2):347–355. doi: 10.1042/bj20031465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Mathupala SP, Ko YH, Pedersen PL. Hexokinase II: cancer’s double-edged sword acting as both facilitator and gatekeeper of malignancy when bound to mitochondria. Oncogene. 2006;25(34):4777–4786. doi: 10.1038/sj.onc.1209603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Groheux D, Giacchetti S, Moretti JL, Porcher R, et al. Correlation of high 18F-FDG uptake to clinical, pathological and biological prognostic factors in breast cancer. Eur J Nucl Med Mol Imaging. 2011;38(3):426–435. doi: 10.1007/s00259-010-1640-9. [DOI] [PubMed] [Google Scholar]
- 40.Heudel P, Cimarelli S, Montella A, Bouteille C, et al. Value of PET-FDG in primary breast cancer based on histopathological and immunohistochemical prognostic factors. Int J Clin Oncol. 2010;15(6):588–593. doi: 10.1007/s10147-010-0120-3. [DOI] [PubMed] [Google Scholar]
- 41.Mavi A, Cermik TF, Urhan M, Puskulcu H, et al. The effects of estrogen, progesterone, and C-erbB-2 receptor states on 18F-FDG uptake of primary breast cancer lesions. J Nucl Med. 2007;48(8):1266–1272. doi: 10.2967/jnumed.106.037440. [DOI] [PubMed] [Google Scholar]
- 42.Ekmekcioglu O, Aliyev A, Yilmaz S, Arslan E, et al. Correlation of 18F-fluorodeoxyglucose uptake with histopathological prognostic factors in breast carcinoma. Nucl Med Commun. 2013;34(11):1055–1067. doi: 10.1097/MNM.0b013e3283658369. [DOI] [PubMed] [Google Scholar]
- 43.Borst GR, Belderbos JS, Boellaard R, Comans EF, et al. Standardised FDG uptake: a prognostic factor for inoperable non-small cell lung cancer. Eur J Cancer. 2005;41(11):1533–1541. doi: 10.1016/j.ejca.2005.03.026. [DOI] [PubMed] [Google Scholar]
- 44.Lee JR, Madsen MT, Bushnel D, Menda Y. A threshold method to improve standardized uptake value reproducibility. Nucl Med Commun. 2000;21(7):685–690. doi: 10.1097/00006231-200007000-00013. [DOI] [PubMed] [Google Scholar]
- 45.Lim I, Noh WC, Park J, Park JA, et al. The combination of FDG PET and dynamic contrast-enhanced MRI improves the prediction of disease-free survival in patients with advanced breast cancer after the first cycle of neoadjuvant chemotherapy. Eur J Nucl Med Mol Imaging. 2014;41(10):1852–1860. doi: 10.1007/s00259-014-2797-4. [DOI] [PubMed] [Google Scholar]
- 46.Basu S, Chen W, Tchou J, Mavi A, et al. Comparison of triple-negative and estrogen receptor-positive/progesterone receptor-positive/HER2-negative breast carcinoma using quantitative fluorine-18 fluorodeoxyglucose/positron emission tomography imaging parameters: a potentially useful method for disease characterization. Cancer. 2008;112(5):995–1000. doi: 10.1002/cncr.23226. [DOI] [PubMed] [Google Scholar]
- 47.Chen YM, Huang G, Sun XG, Liu JJ, et al. Optimizing delayed scan time for FDG PET: comparison of the early and late delayed scan. Nucl Med Commun. 2008;29(5):425–430. doi: 10.1097/MNM.0b013e3282f4d389. [DOI] [PubMed] [Google Scholar]
- 48.Chen CJ, Lee BF, Yao WJ, Cheng L, et al. Dual-phase 18F-FDG PET in the diagnosis of pulmonary nodules with an initial standard uptake value less than 2.5. AJR Am J Roentgenol. 2008;191(2):475–479. doi: 10.2214/AJR.07.3457. [DOI] [PubMed] [Google Scholar]
- 49.Zytoon AA, Murakami K, El-Kholy MR, El-Shorbagy E, et al. Breast cancer with low FDG uptake: characterization by means of dual-time point FDG-PET/CT. Eur J Radiol. 2009;70(3):530–538. doi: 10.1016/j.ejrad.2008.01.045. [DOI] [PubMed] [Google Scholar]
- 50.Boerner AR, Weckesser M, Herzog H, Schmitz T, et al. Optimal scan time for fluorine-18 fluorodeoxyglucose positron emission tomography in breast cancer. Eur J Nucl Med. 1999;26(3):226–230. doi: 10.1007/s002590050381. [DOI] [PubMed] [Google Scholar]
- 51.Hamberg LM, Hunter GJ, Alpert NM, Choi NC, et al. The dose uptake ratio as an index of glucose metabolism: useful parameter or oversimplification? J Nucl Med. 1994;35(8):1308–1312. [PubMed] [Google Scholar]
- 52.Kumar R, Loving VA, Chauhan A, Zhuang H, et al. Potential of dual-time-point imaging to improve breast cancer diagnosis with (18)F-FDG PET. J Nucl Med. 2005;46(11):1819–1824. [PubMed] [Google Scholar]
- 53.Lowe VJ, Duhaylongsod FG, Patz EF, Delong DM, et al. Pulmonary abnormalities and PET data analysis: a retrospective study. Radiology. 1997;202(2):435–439. doi: 10.1148/radiology.202.2.9015070. [DOI] [PubMed] [Google Scholar]
- 54.Zaha DC. Significance of immunohistochemistry in breast cancer. World J Clin Oncol. 2014;5(3):382–392. doi: 10.5306/wjco.v5.i3.382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Perou CM, Sorlie T, Eisen MB, van de Rijn M, et al. Molecular portraits of human breast tumours. Nature. 2000;406(6797):747–752. doi: 10.1038/35021093. [DOI] [PubMed] [Google Scholar]
- 56.Sorlie T, Perou CM, Tibshirani R, Aas T, et al. Gene expression patterns of breast carcinomas distinguish tumor subclasses with clinical implications. Proc Natl Acad Sci U S A. 2001;98(19):10869–10874. doi: 10.1073/pnas.191367098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Chung CH, Bernard PS, Perou CM. Molecular portraits and the family tree of cancer. Nat Genet. 2002;32(Suppl):533–540. doi: 10.1038/ng1038. [DOI] [PubMed] [Google Scholar]
- 58.Sorlie T, Tibshirani R, Parker J, Hastie T, et al. Repeated observation of breast tumor subtypes in independent gene expression data sets. Proc Natl Acad Sci U S A. 2003;100(14):8418–8423. doi: 10.1073/pnas.0932692100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Goldhirsch A, Wood WC, Coates AS, Gelber RD, et al. Strategies for subtypes--dealing with the diversity of breast cancer: highlights of the St. Gallen international expert consensus on the primary therapy of early breast cancer 2011. Ann Oncol. 2011;22(8):1736–1747. doi: 10.1093/annonc/mdr304. [DOI] [PMC free article] [PubMed] [Google Scholar]