Abstract
Cowden syndrome (CS) is a rare autosomal dominant disorder characterized by multiple hamartomas in various tissues and cancers (breast, thyroid, and endometrium). We report CS of the esophagus and gastrointestinal tract that was incidentally detected by positron emission tomography/computed tomography (PET/CT) at postoperative surveillance in an endometrial cancer patient. PET/CT showed mildly increased FDG uptake along the entire esophagus and stomach. Upper GI endoscopy and histologic examination revealed glycogenic acanthosis of the esophagus and several hundred gastric polyps. In our case, increased FDG uptake of the esophageal wall contributed to the diagnosis of CS.
Keywords: Cowden syndrome, Hamartoma syndrome, Multiple, Fluorodeoxyglucose F18, Positron emission tomography
Introduction
Cowden syndrome (CS) is a rare autosomal dominant disorder characterized by multiple hamartomas in various tissues (skin, colon, thyroid, etc.) and cancers (breast, thyroid, and endometrium) [1]. The prevalence of CS is between 1 in 200,000 and 1 in 250,000. This syndrome is associated with mutations in the phosphatase and tensin homolog (PTEN) gene, a tumor suppressor gene [2].
Here we report CS of the esophagus and gastrointestinal tract that was incidentally detected by postoperative positron emission tomography/computed tomography (PET/CT) in an endometrial cancer patient.
Case Report
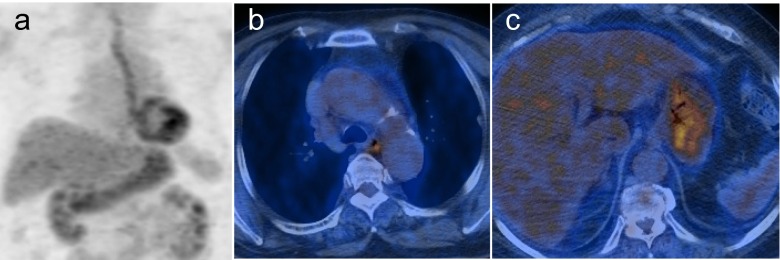
A 60-year-old female with uterine endometrial cancer who underwent total hysterectomy with bilateral salpingo-oophorectomy 7 months ago visited our hospital for tumor surveillance. Additionally, uterine fibroid and endometrial polyps were found in the hysterectomy specimens. The patient’s history included a thyroid lobectomy and total mastectomy 13 and 4 years ago, respectively, due to follicular adenomas and breast cancer. She underwent F-18 fluorodeoxyglucose (FDG) PET/CT for a systemic evaluation of endometrial cancer. PET/CT showed mildly increased FDG uptake along the entire esophagus and stomach, but definite abnormality was not detected in other organs including the large colon (Fig. 1).
Fig. 1.
FDG PET/CT images after total hysterectomy of uterine endometrial cancer. a Maximum intensity projection PET image; b and c transaxial PET/CT images showing a diffuse and increased FDG uptake of the esophagus and stomach
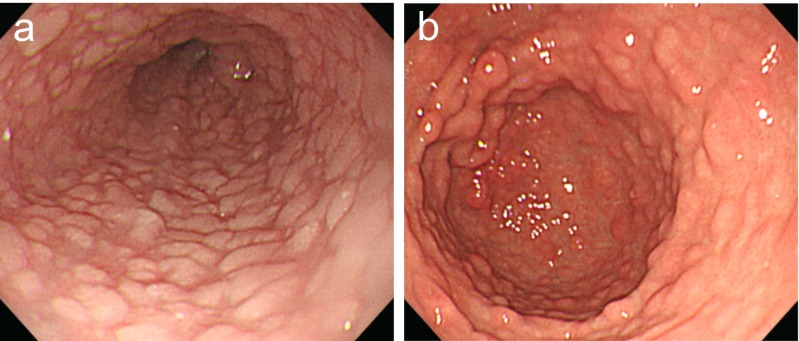
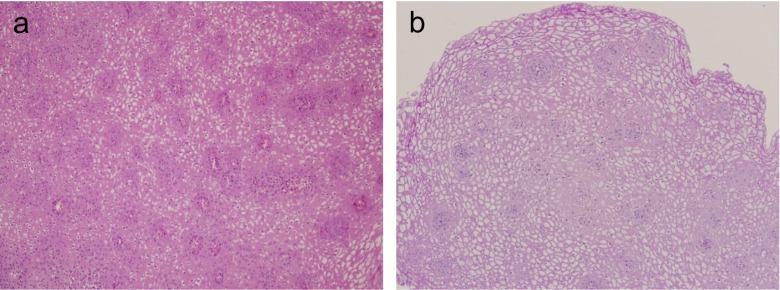
On presentation, she complained of no symptom other than dyspepsia. Laboratory results were normal. Under the impression of gastritis and esophagitis, upper GI endoscopy was performed to further evaluate the lesions. It revealed diffuse whitish papules of the entire esophagus (Fig. 2a), several hundred gastric polyps on the entire stomach (Fig. 2b), several polyps on the duodenal bulb, and a focal erosion of the gastric antrum. Histologic examination showed glycogenic acanthosis of the esophagus (Fig. 3), chronic duodenitis, and chronic active gastritis with focal lymphoid hyperplasia.
Fig. 2.
Upper GI endoscopic findings. a Diffuse whitish papules are noted throughout the entire esophagus; b several hundred gastric polyps are noted throughout the entire stomach
Fig. 3.
Histopathological analysis of the diffuse esophageal papules reveals glycogenic acanthosis (a; ×100, hematoxylin and eosin stain, b; ×100, diastase periodic acid Schiff stain)
Based on the clinical and endoscopic features, she was diagnosed with Cowden syndrome in accordance with the criteria of the International Cowden Consortium. Colonoscopy was performed to examine the large bowel. It revealed multiple polyps scattered throughout the entire colon and terminal ileum. Histology of these colonic polyps showed a variety of cell types, including tubular adenomas, xanthoma, and hyperplastic polyps. Follow-up PET/CT, upper GI endoscopy, and colonoscopy were performed to check the lesions every year. On follow-up PET/CT, mildly increased FDG uptake along the entire esophagus and stomach was not changed, and there were no new lesions during this 3-year follow-up. At serial follow-up upper GI endoscopy and colonoscopy, there were no significant interval changes in polypoid lesions, and no erosion was detected during 3 years.
Discussion
Cowden syndrome (CS), or multiple hamartoma syndrome, was first described in 1963 by Lloyd and Denis in a case report on a patient named Rachel Cowden [3]. It is regarded as part of PTEN hamartoma tumor syndrome (PHTS), which includes CS, Bannayan-Riley-Ruvalcaba syndrome, adult Lhermitte-Duclos disease, and autism spectrum disorders associated with macrocephaly [4]. PTEN mutation frequency in individuals meeting the consortium diagnostic criteria has been recently reported to be about 30–35 %. CS is only associated with cancer predisposition in PHTS disorder. Approximately 30 % of CS patients are reportedly to be associated with specific cancer types, including breast cancer, thyroid cancer (follicular), endometrial cancer, renal cell carcinoma, and colon cancer [4, 5].
CS is now well known as a highly variable multiorgan disease with cancer susceptibility as well as benign hamartomatous overgrowth. These hamartomas of CS patients can occur in any of three embryonic germ cell layers, and its origin may be ectodermal, mesodermal, or endodermal [2]. These lesions include trichilemmomas in 80–100 % of CS cases, breast fibroadenomas in 70–76 % of affected women, thyroid abnormalities such as adenomas and multinodular goiters in 40–60 % of CS cases, and gastrointestinal polyps in 35–65 % [4, 6].
Diagnosis of CS is mainly based on clinical criteria. The diagnostic criteria for CS were first proposed by Salem and Steck in 1983. They were subsequently revised and modified by the International Cowden Consortium in 2000 [7].
The pathognomonic manifestations of CS are mucocutaneous lesions such as trichilemmomas, palmoplantar keratosis, and oral mucosal papillomatosis. Its diagnosis was originally made based on characteristic cutaneous lesions and familial history [8]. Esophageal glycogenic acanthosis or GI hamartomas are considered minor criteria. However, in our case, FDG PET findings of the esophagus and stomach led to a further evaluation such as endoscopy, which showed esophageal glycogenic acanthosis as an endoscopic clue suggesting CS.
The characteristic finding of the esophagus in CS patients is diffuse glycogenic acanthosis. This has been found in 80 % of those undergoing evaluation. This lesion combined with colonic polyposis should be considered as pathognomonic for CS [4].
To the best of our knowledge, there are very few reports on F-18 FDG PET/CT of CS. In 2012, Makis et al. reported a case of FDG-avid gastric cancer on the background of extensive benign gastric polyposis in a CS patient that showed no increase in FDG uptake in diffuse esophageal glycogenic acanthosis on PET/CT [9]. Kamel et al. reported one patient with glycogenic acanthosis of the esophagus incidentally detected on PET/CT that showed focally increased moderate FDG uptake in the esophagus [10].
Increased FDG uptake on PET/CT is frequently noted in various benign lesions such as inflammation. The mechanism of FDG uptake in esophageal glycogenic acanthosis is unclear. It might be related to the proliferative activity in this lesion [10].
The main differential diagnosis for hypermetabolic lesions of the esophagus are reflux esophagitis when interpreting PET/CT or PET studies. Other potential causes of abnormal PET findings may include Barrett’s esophagus, eosinophilic esophagitis, and esophageal cancer [10–12].
Our case indicates that esophageal glycogenic acanthosis should be included as a differential diagnosis of abnormal FDG uptake in the esophageal wall.
In conclusion, we report a rare case of esophageal glycogenic acanthosis showing diffuse increased FDG uptake on PET/CT imaging. In this case, increased FDG uptake of the esophageal wall contributed to the diagnosis of CS.
Compliance with Ethical Standards
Conflict of Interest
Yun Hee Kang, Hye Kyung Lee, and Geon Park declare that they have no conflict of interest.
Ethical Statement
The study was approved by an institutional review board.
References
- 1.Lee EJ, Jung WS, Ko JM, Park HJ. Multiorgan involvements of Cowden disease in a 50-year-old woman: a case report and literature overview. J Korean So Radiol. 2013;69:251–255. doi: 10.3348/jksr.2013.69.3.251. [DOI] [Google Scholar]
- 2.Ha M, Chung JW, Hahm KB, Kim YJ, Lee W, An J, et al. A case of Cowden syndrome diagnosed from multiple gastric polyposis. World J Gastroenterol: WJG. 2012;18:861–864. doi: 10.3748/wjg.v18.i8.861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd KM, Dennis M. Cowden’s disease: a possible new symptom complex with multiple system involvement. Ann Intern Med. 1963;58:136–142. doi: 10.7326/0003-4819-58-1-136. [DOI] [PubMed] [Google Scholar]
- 4.Pilarski R, Burt R, Kohlman W, Pho L, Shannon KM, Swisher E. Cowden syndrome and the PTEN hamartoma tumor syndrome: systematic review and revised diagnostic criteria. J Natl Cancer Inst. 2013;105:1607–1616. doi: 10.1093/jnci/djt277. [DOI] [PubMed] [Google Scholar]
- 5.Saito K, Nomura E, Sasaki Y, Abe Y, Kanno N, Mizumoto N, et al. Characteristics of small bowel polyps detected in cowden syndrome by capsule endoscopy. Case Rep Gastroint Med. 2015 doi: 10.1155/2015/475705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Campos FG, Habr-Gama A, Kiss DR, Atuí FC, Rawet V, Goldstein PJ, et al. Cowden syndrome: report of two cases and review of clinical presentation and management of a rare colorectal polyposis. Curr Surg. 2006;63:15–19. doi: 10.1016/j.cursur.2005.04.015. [DOI] [PubMed] [Google Scholar]
- 7.Eng C. Will the real Cowden syndrome please stand up: revised diagnostic criteria. J Med Genet. 2000;37:828–830. doi: 10.1136/jmg.37.11.828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.McGarrity TJ, Baker MJW, Ruggiero FM, Thiboutot DM, Hampel H, Zhou X-P, et al. GI polyposis and glycogenic acanthosis of the esophagus associated with PTEN mutation positive Cowden syndrome in the absence of cutaneous manifestations. Am J Gastroenterol. 2003;98:1429–1434. doi: 10.1111/j.1572-0241.2003.07496.x. [DOI] [PubMed] [Google Scholar]
- 9.Makis W, Ciarallo A, Hickeson M. The use of 18F-FDG PET/CT in Cowden syndrome to differentiate multifocal gastric carcinoma from extensive benign gastric polyposis. Clin Nucl Med. 2012;37:311–314. doi: 10.1097/RLU.0b013e31823ea44d. [DOI] [PubMed] [Google Scholar]
- 10.Kamel EM, Thumshirn M, Truninger K, Schiesser M, Fried M, Padberg B, et al. Significance of incidental 18F-FDG accumulations in the gastrointestinal tract in PET/CT: correlation with endoscopic and histopathologic results. J Nucl Med. 2004;45:1804–1810. [PubMed] [Google Scholar]
- 11.Dong A, Wang Y, Zuo C. FDG PET/CT in eosinophilic esophagitis. Clin Nuclear Med. 2014;39:540–543. doi: 10.1097/RLU.0b013e318286c025. [DOI] [PubMed] [Google Scholar]
- 12.Roedl JB, Colen RR, King K, Fischman AJ, Mueller PR, Blake MA. Visual PET/CT scoring for nonspecific 18F-FDG uptake in the differentiation of early malignant and benign esophageal lesions. Am J Roentgenol. 2008;191:515–521. doi: 10.2214/AJR.07.3320. [DOI] [PubMed] [Google Scholar]