Abstract
We have recently shown that nitric oxide or authentic endothelium-derived relaxing factor generated in a biologic system reacts in the presence of specific protein thiols to form S-nitrosoprotein derivatives that have endothelium-derived relaxing factor-like properties. The single free cysteine of serum albumin, Cys-34, is particularly reactive toward nitrogen oxides (most likely nitrosonium ion) under physiologic conditions, primarily because of its anomalously low pK; given its abundance in plasma, where it accounts for approximately 0.5 mM thiol, we hypothesized that this plasma protein serves as a reservoir for nitric oxide produced by the endothelial cell. To test this hypothesis, we developed a methodology, which involves UV photolytic cleavage of the S--NO bond before reaction with ozone for chemiluminescence detection, with which to measure free nitric oxide, S-nitrosothiols, and S-nitrosoproteins in biologic systems. We found that human plasma contains approximately 7 microM S-nitrosothiols, of which 96% are S-nitrosoproteins, 82% of which is accounted for by S-nitroso-serum albumin. By contrast, plasma levels of free nitric oxide are only in the 3-nM range. In rabbits, plasma S-nitrosothiols are present at approximately 1 microM; 60 min after administration of NG-monomethyl-L-arginine at 50 mg/ml, a selective and potent inhibitor of nitric oxide synthetases, S-nitrosothiols decreased by approximately 40% (greater than 95% of which were accounted for by S-nitrosoproteins, and approximately 80% of which was S-nitroso-serum albumin); this decrease was accompanied by a concomitant increase in mean arterial blood pressure of 22%. These data suggest that naturally produced nitric oxide circulates in plasma primarily complexed in S-nitrosothiol species, principal among which is S-nitroso-serum albumin. This abundant, relatively long-lived adduct likely serves as a reservoir with which plasma levels of highly reactive, short-lived free nitric oxide can be regulated for the maintenance of vascular tone.
Full text
PDF
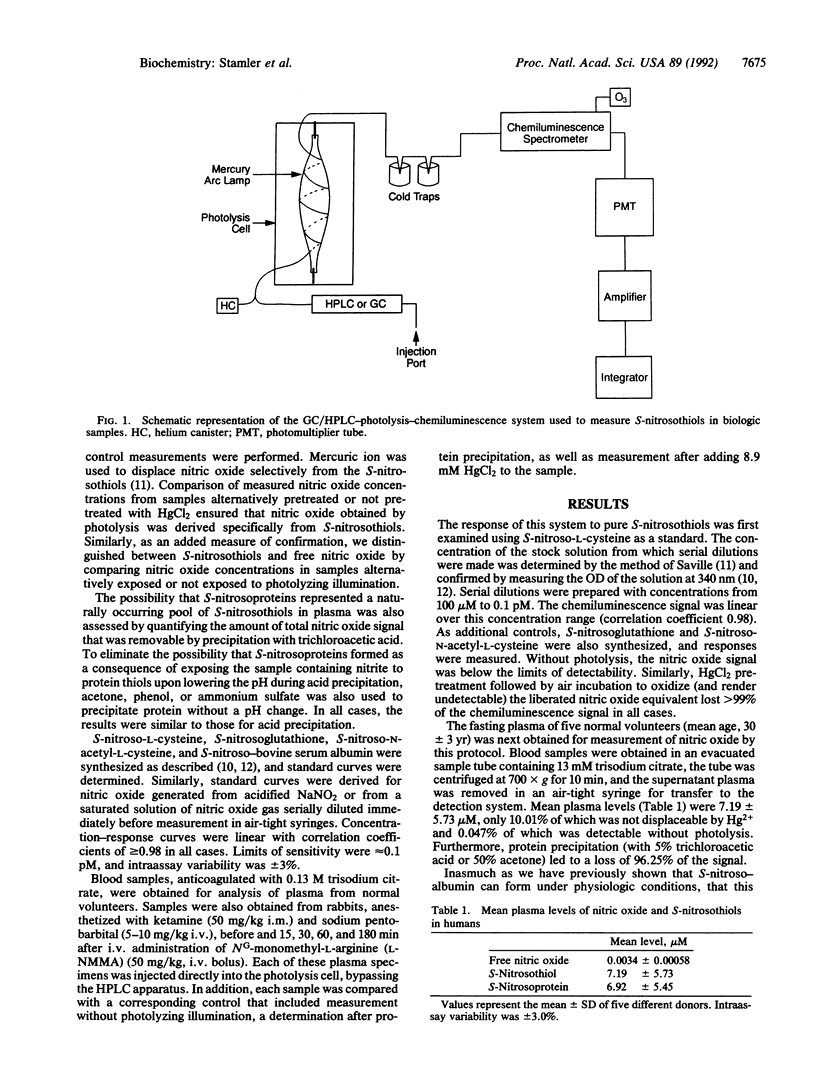
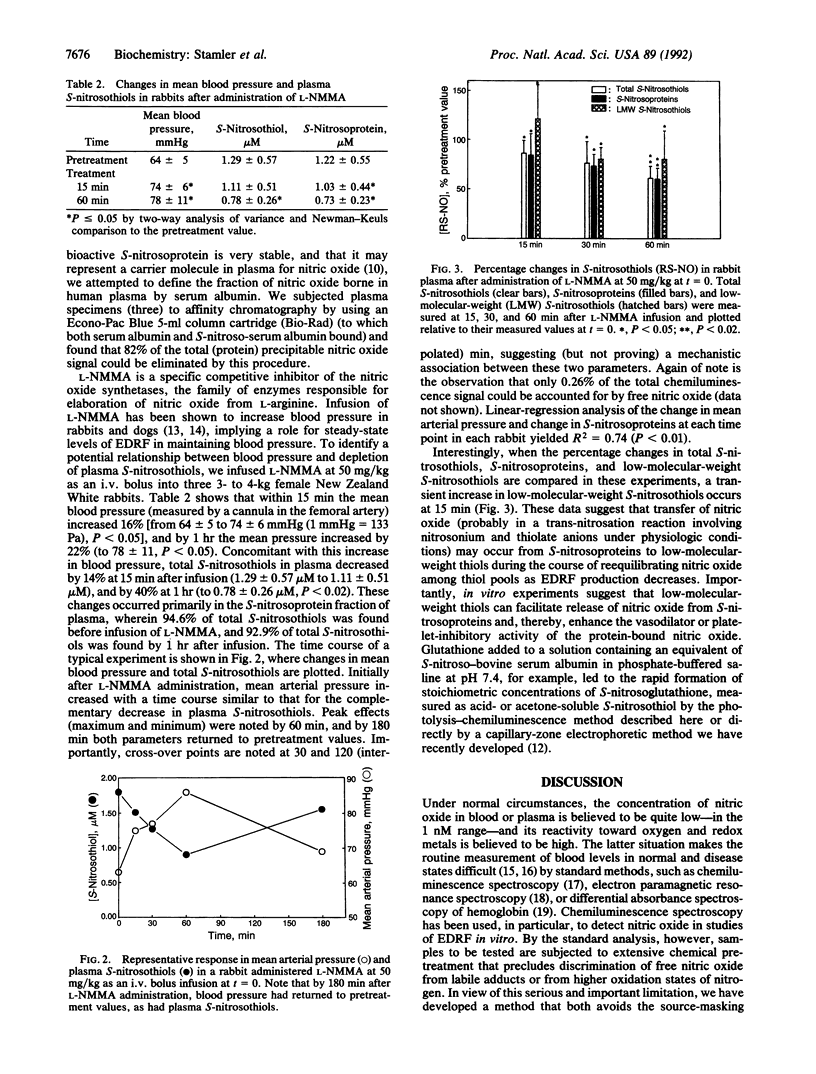

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arroyo C. M., Kohno M. Difficulties encountered in the detection of nitric oxide (NO) by spin trapping techniques. A cautionary note. Free Radic Res Commun. 1991;14(2):145–155. doi: 10.3109/10715769109094127. [DOI] [PubMed] [Google Scholar]
- Azuma H., Ishikawa M., Sekizaki S. Endothelium-dependent inhibition of platelet aggregation. Br J Pharmacol. 1986 Jun;88(2):411–415. doi: 10.1111/j.1476-5381.1986.tb10218.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu A., Chambers D. E., Lin C. C., Kuehl W. D., Cobb F. R. Nitric oxide modulates epicardial coronary basal vasomotor tone in awake dogs. Am J Physiol. 1990 Apr;258(4 Pt 2):H1250–H1254. doi: 10.1152/ajpheart.1990.258.4.H1250. [DOI] [PubMed] [Google Scholar]
- Dinh-Xuan A. T., Higenbottam T. W., Clelland C. A., Pepke-Zaba J., Cremona G., Butt A. Y., Large S. R., Wells F. C., Wallwork J. Impairment of endothelium-dependent pulmonary-artery relaxation in chronic obstructive lung disease. N Engl J Med. 1991 May 30;324(22):1539–1547. doi: 10.1056/NEJM199105303242203. [DOI] [PubMed] [Google Scholar]
- Downes M. J., Edwards M. W., Elsey T. S., Walters C. L. Determination of a non-volatile nitrosamine by using denitrosation and a chemiluminescence analyser. Analyst. 1976 Sep;101(1206):742–748. doi: 10.1039/an9760100742. [DOI] [PubMed] [Google Scholar]
- Furchgott R. F., Zawadzki J. V. The obligatory role of endothelial cells in the relaxation of arterial smooth muscle by acetylcholine. Nature. 1980 Nov 27;288(5789):373–376. doi: 10.1038/288373a0. [DOI] [PubMed] [Google Scholar]
- Goretski J., Hollocher T. C. Trapping of nitric oxide produced during denitrification by extracellular hemoglobin. J Biol Chem. 1988 Feb 15;263(5):2316–2323. [PubMed] [Google Scholar]
- Ignarro L. J., Buga G. M., Wood K. S., Byrns R. E., Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987 Dec;84(24):9265–9269. doi: 10.1073/pnas.84.24.9265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelm M., Schrader J. Control of coronary vascular tone by nitric oxide. Circ Res. 1990 Jun;66(6):1561–1575. doi: 10.1161/01.res.66.6.1561. [DOI] [PubMed] [Google Scholar]
- Martin W., Furchgott R. F., Villani G. M., Jothianandan D. Depression of contractile responses in rat aorta by spontaneously released endothelium-derived relaxing factor. J Pharmacol Exp Ther. 1986 May;237(2):529–538. [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Biosynthesis of nitric oxide from L-arginine. A pathway for the regulation of cell function and communication. Biochem Pharmacol. 1989 Jun 1;38(11):1709–1715. doi: 10.1016/0006-2952(89)90403-6. [DOI] [PubMed] [Google Scholar]
- Palmer R. M., Ferrige A. G., Moncada S. Nitric oxide release accounts for the biological activity of endothelium-derived relaxing factor. Nature. 1987 Jun 11;327(6122):524–526. doi: 10.1038/327524a0. [DOI] [PubMed] [Google Scholar]
- Radomski M. W., Palmer R. M., Moncada S. The anti-aggregating properties of vascular endothelium: interactions between prostacyclin and nitric oxide. Br J Pharmacol. 1987 Nov;92(3):639–646. doi: 10.1111/j.1476-5381.1987.tb11367.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rees D. D., Palmer R. M., Moncada S. Role of endothelium-derived nitric oxide in the regulation of blood pressure. Proc Natl Acad Sci U S A. 1989 May;86(9):3375–3378. doi: 10.1073/pnas.86.9.3375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stamler J. S., Loscalzo J. Capillary zone electrophoretic detection of biological thiols and their S-nitrosated derivatives. Anal Chem. 1992 Apr 1;64(7):779–785. doi: 10.1021/ac00031a014. [DOI] [PubMed] [Google Scholar]
- Stamler J. S., Simon D. I., Osborne J. A., Mullins M. E., Jaraki O., Michel T., Singel D. J., Loscalzo J. S-nitrosylation of proteins with nitric oxide: synthesis and characterization of biologically active compounds. Proc Natl Acad Sci U S A. 1992 Jan 1;89(1):444–448. doi: 10.1073/pnas.89.1.444. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westenberger U., Thanner S., Ruf H. H., Gersonde K., Sutter G., Trentz O. Formation of free radicals and nitric oxide derivative of hemoglobin in rats during shock syndrome. Free Radic Res Commun. 1990;11(1-3):167–178. doi: 10.3109/10715769009109680. [DOI] [PubMed] [Google Scholar]
- Yamamoto H., Bossaller C., Cartwright J., Jr, Henry P. D. Videomicroscopic demonstration of defective cholinergic arteriolar vasodilation in atherosclerotic rabbit. J Clin Invest. 1988 Jun;81(6):1752–1758. doi: 10.1172/JCI113516. [DOI] [PMC free article] [PubMed] [Google Scholar]



