Abstract
Objective:
Epileptic seizures, syncope, and psychogenic nonepileptic seizures (PNES) account for over 90% of presentations with transient loss of consciousness (TLOC). The patient's history is crucial for the diagnosis, but the diagnostic value of individual semiologic features is limited. This study explores the diagnostic potential of a comprehensive questionnaire focusing on TLOC-associated symptoms.
Methods:
A total of 386 patients with proven epilepsy, 308 patients with proven PNES, and 371 patients with proven syncope were approached by post to recruit 100 patients in each diagnostic group. Symptoms were self-reported on an 86-item questionnaire (the Paroxysmal Event Profile [PEP]) using a 5-point Likert scale (always to never). Data were subjected to exploratory factor analysis (EFA) followed by confirmatory factor analysis (CFA). Factors were used to differentiate between diagnoses by pairwise and multinomial regression.
Results:
Patients with PNES reported more and more frequent TLOC-associated symptoms than those with epilepsy or syncope (p < 0.001). EFA/CFA identified a 5-factor structure based on 74/86 questionnaire items with loadings ≥0.4. Pairwise logistic regression analysis correctly classified 91% of patients with epilepsy vs those with syncope, 94% of those with PNES vs those with syncope, and 77% of those with epilepsy vs those with PNES. Multinomial logistic regression analysis yielded a similar pattern.
Conclusions:
Clusters of self-reported TLOC symptoms can be used to direct patients to appropriate investigation and treatment pathways for syncope on the one hand and seizures on the other, although additional information is required for a reliable distinction, especially between epilepsy and PNES.
Transient loss of consciousness (TLOC) is the second most common neurologic emergency.1 Three conditions account for over 90% of presentations: epilepsy and psychogenic nonepileptic seizures (PNES) and syncope.2,3 Accurate distinction among these conditions is crucial because treatment choice depends on it. However, misdiagnosis rates of over 25% have been reported in different primary and secondary care settings.4–6 The gold standard test in this situation would be the synchronous recording of a typical event by video, heart rhythm by ECG, and electrical brain activity by EEG. However, in many patients, the observation of spontaneous episodes of TLOC is impractical or impossible.
Previous research suggests that there is no single demographic, clinical, or semiologic feature that distinguishes clearly among epilepsy, syncope, and PNES.4,7 In routine clinical practice, the diagnosis is usually based on a combination of facts derived from the patient's history and witness accounts (if available). The diagnosis also takes account of interictal investigations like blood pressure recordings, ECG, EEG, and brain CT or MRI, although these investigations are of limited utility.8–11 However, in the absence of a clear pretest probability of one specific cause of TLOC, interictal test abnormalities may be misinterpreted, especially by nonexperts.5,6,12
The misdiagnosis of PNES is particularly common. In fact, almost all patients with this disorder initially receive a diagnosis of epilepsy or syncope.13,14 The mean latency between manifestation and diagnosis of PNES is 4–7 years, putting patients at risk of inappropriate emergency treatments and even death.13,15,16
For these reasons, recent epilepsy management guidelines emphasize the need for an early expert assessment.17 However, access to experts is limited, and even experts currently lack evidence-based tools that would allow them to express the level of certainty with which they have made their initial clinical diagnosis: an accurate determination of a posttest probability of a particular diagnosis would require a clear understanding of its pretest probability.18
The present study determines whether a comprehensive profile of patients' TLOC experiences can contribute to the differentiation among the 3 common causes of this clinical phenomenon and explores whether such a questionnaire can provide a pretest probability adding value to the interpretation of interictal investigations.
METHODS
Patients: Epilepsy and PNES groups.
Patients with epilepsy or PNES supported by video-EEG recordings of typical seizures involving TLOC were identified from clinical databases of the Department of Clinical Neurophysiology at the Royal Hallamshire Hospital in Sheffield and the National Hospital of Neurology and Neurosurgery in London, United Kingdom.
Syncope group.
Patients with a diagnosis of recurrent syncope supported by pathophysiologic evidence (typical warning symptoms or complete TLOC associated with typical heart rate or blood pressure changes during a tilt-table test/presyncopal or syncopal symptoms associated with explanatory heart rate or rhythm changes during ECG monitoring) were identified from the centers above and the database of the Falls and Syncope Service based at Newcastle upon Tyne, United Kingdom.
Patients with one of the causes of TLOC were approached by post until 100 in each diagnostic group had returned completed questionnaires. The clinical diagnoses of all patients were confirmed by each patients' consultant neurologist/physician. All patients were ≥16 years of age at the time of the study.
Sample size.
We recruited a total sample of n = 300 (100 per group) to allow us to conduct factor analyses across all participants as well as to test for moderate differences (effect size ≥0.40) between groups with at least 80% power.19
Paroxysmal event profile (PEP).
The 86-item PEP included questions previously shown to differentiate between tonic-clonic seizures and syncope.20,21 It also included questions thought by experts, among whom earlier versions of this questionnaire were circulated, potentially to differentiate between epilepsy and PNES. Additional items were based on ideas derived from metaphor analytic studies suggesting that patients with epilepsy perceive their episodes of TLOC as opponents with independent agency whereas those with PNES experience their TLOC as a space or place they themselves go through.22,23 The PEP also contained questions about dissociation (based on the Dissociative Experience Scale Taxon)24 and about symptoms of panic listed in the DSM-IV-TR.25 Responses were invited on a 5-point Likert scale (always/frequently/sometimes/rarely/never). In addition to the 86 items focusing on TLOC manifestations, respondents were asked to answer 7 questions about demographic and clinical features (see PEP questionnaire, appendix e-1 on the Neurology® Web site at Neurology.org, for details).
Study procedure.
PEP questionnaires were sent out by post with a free return envelope and an information sheet stating that the return of the completed questionnaire would be interpreted as consent. Patients were incentivized to return the questionnaires by the offer of participation in a prize draw for a digital radio (value £60, $80).
Statistical analysis.
Overall mean symptom scores for each participant were calculated for each diagnostic group and compared using analysis of variance (ANOVA). Frequencies of reporting extremes (never or always responses) were tabulated and compared between diagnostic groups using χ2 statistics.
An exploratory factor analysis (EFA) of the PEP items was conducted using geomin oblique rotation. Items with factor loadings ≥0.4 were retained. In a confirmatory factor analysis (CFA), the factor structure suggested by the EFA was subjected to Goodness of Fit statistics, including root mean square error approximation (RMSEA), Comparative Fit Index (CFI), and Tucker Lewis Index (TLI). RMSEA <0.06, CFI >0.90, and TLI >0.90 were predetermined as standards for good model fitting.
Mean factor scores were compared across the 3 diagnostic groups and differences tested using 1-way ANOVA. To assess the ability of all of the factors simultaneously to discriminate participants by diagnostic groups, pairwise and multinomial logistic regression analyses were used with all 5 factor scores as continuous predictors. Separate models including models using only one factor score at a time were also produced. In addition to regression models based entirely on self-reported TLOC symptom factors, we report regression models combining TLOC symptom factors with additional patient demographic/clinical information (self-reported data on sex, number of episodes of TLOC in the last year, lifetime number of hospitalizations, and whether or not there had been any admissions to intensive care for the treatment of TLOC or whether there was a family history of TLOC). All clinical variables were included as categorical predictors (as in table 1). Age and age at onset were not included in models including patients with syncope because syncope patients were predominantly recruited in a health care setting attracting older adults.
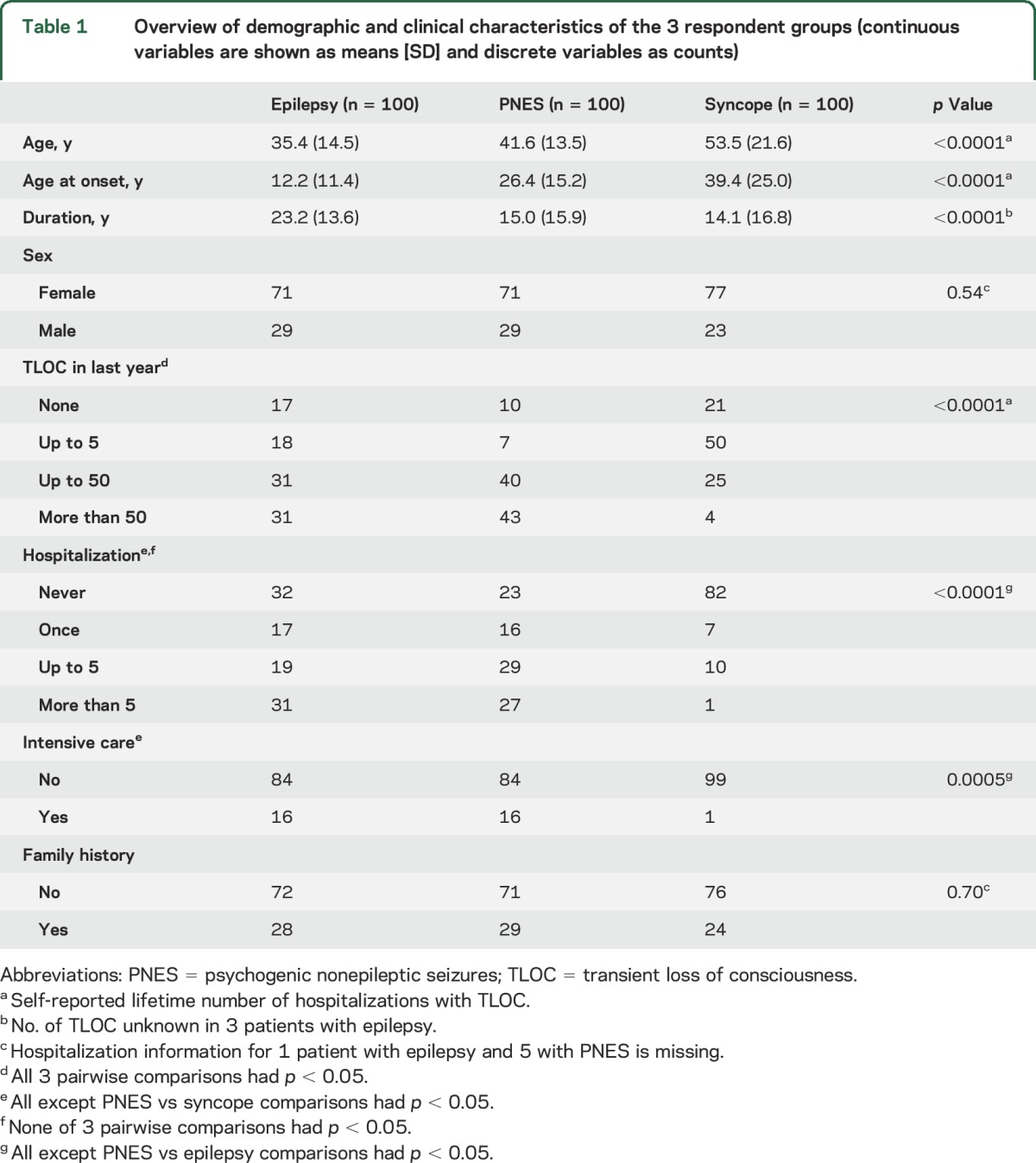
Table 1.
Overview of demographic and clinical characteristics of the 3 respondent groups (continuous variables are shown as means [SD] and discrete variables as counts)
All factor analyses were conducted in Mplus version 7.0. Logistic regression was performed in SAS (SAS Institute, Cary, NC) version 9.3 for Windows. Two-sided p values ≤0.05 were considered statistically significant. The ANOVA on 86 items was controlled for multiple testing (i.e., significance was determined at 0.05/86 = 0.00058).
Standard protocol approvals, registrations, and patient consents.
Ethical approval for this study was granted by the Northern and Yorkshire Multi-Centre Research Ethics Committee. Patients were informed that their return of the completed PEP questionnaire would be considered as consent to the analysis and publication of the data provided.
RESULTS
Respondents.
A total of 386 patients with epilepsy, 308 with PNES, and 371 with syncope were approached until 100 patients had been recruited in each group (see table 1).
Descriptive findings.
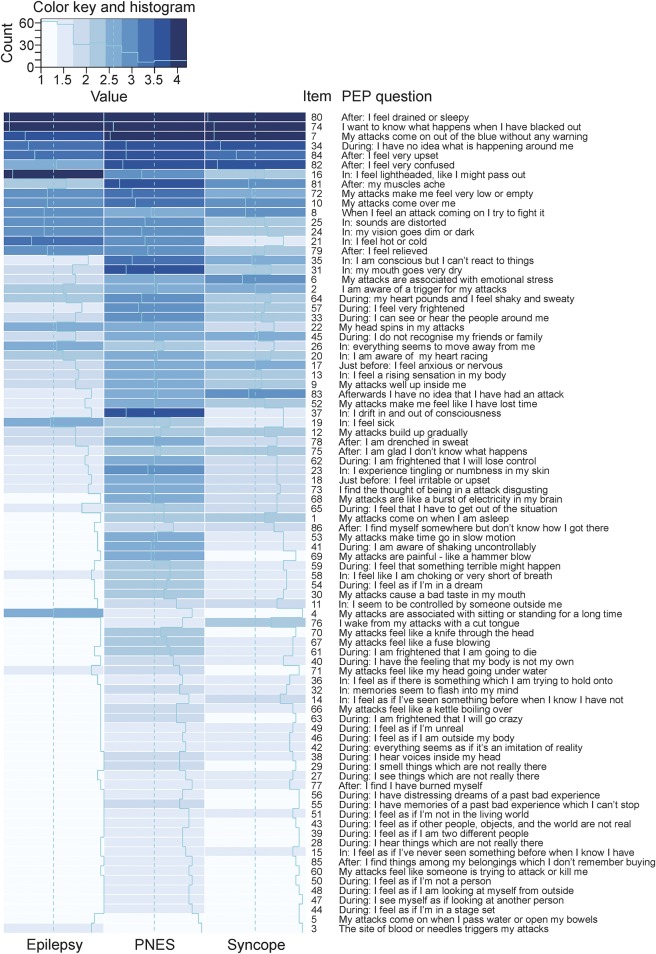
The mean item scores for each PEP question by the 3 diagnostic groups are presented in figure 1. As indicated by the darker shading of the PNES column in figure 1, patients in this group reported more frequent symptoms overall (mean score 2.4, where 1 is never and 5 is always) than those with epilepsy (mean score 2.0) or syncope (mean score 1.8, differences: PNES vs epilepsy p < 0.0001, epilepsy vs syncope p = 0.007). PNES were also associated with a wider range of symptoms, reflected by the lower number of white squares in the PNES column in figure 1 and by the lower mean percentage of never replies in the PNES group (46.1%) than the epilepsy (60.3%) or syncope (73.4%) groups (differences PNES vs epilepsy p < 0.0001, epilepsy vs syncope p < 0.0001). As indicated by the significantly lower percentage of extremes (i.e., never plus always), PNES (59.4%) emerged as a less stereotyped phenomenon than epilepsy (70.4%) or syncope (82.9%, differences PNES vs epilepsy p < 0.0001, epilepsy vs syncope p < 0.0001).
Figure 1. Range and frequency of self-reported transient loss of consciousness symptoms in patients with epilepsy, syncope, and psychogenic nonepileptic seizures (PNES).
Graphic representation of the respondents' mean answers to the 86 questions posed in the paroxysmal event profile (PEP) illustrates the relative diagnostic value of individual items. Mean answers are indicated for each group by the light blue line. The shade of the background color also indicates the mean response (with dark shades reflecting more frequent experiences and light shades less frequent experiences).
Responses to 57 out of 76 items differed significantly among the 3 diagnostic groups in an ANOVA (p ≤ 0.0005). Only 10 items (P2, P5, P8, P10, P20, P26, P34, P74, P80, and P84) did not differentiate among the 3 groups, even at the more liberal 0.05 level.
Latent factor analysis.
EFA models with 1–6 factors were tested due to there being 6 eigenvalues greater than 1. Models with 4–6 factors all had a good model fit (RMSEA ≤0.05 and CFI/TLI ≥0.90), but the 5-factor model was favored for its better interpretability. Of the 86 items, 74 had factor loadings ≥0.4. Seven loaded on 2, the remaining items on 1 latent factor. The 5-factor structure, with the selected 74 items, was tested by CFA. The fit indices of CFA model were CFI = 0.93, TLI = 0.92, and REMSA = 0.04.
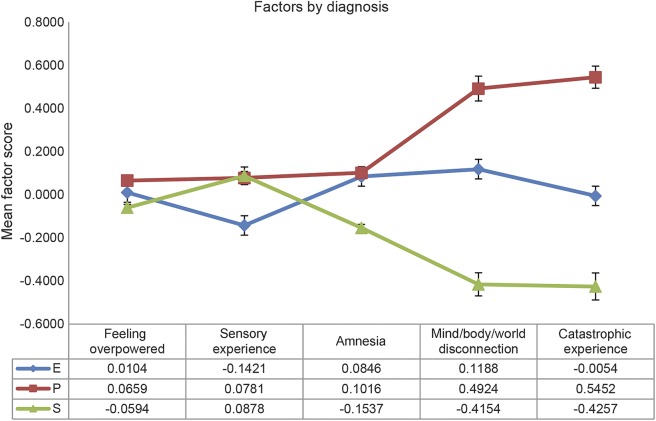
Based on our semantic interpretation, the 5 latent factors were called feeling overpowered, sensory experience, mind/body/world disconnection, catastrophic experience, and amnesia (see table 2 for more details). The mean factor scores from the CFA analysis across the epilepsy (E), PNES (P), and syncope (S) groups are shown in figure 2. The factors feeling overpowered, mind/body/world disconnection, and catastrophic experience were significantly different among all 3 groups (p < 0.001), while amnesia only differentiated syncope and sensory experience only epilepsy from the other 2 diagnostic groups (p < 0.001) (see table 3 for more details).
Table 2.
Five latent factors: Sample questions contributing to the different factors
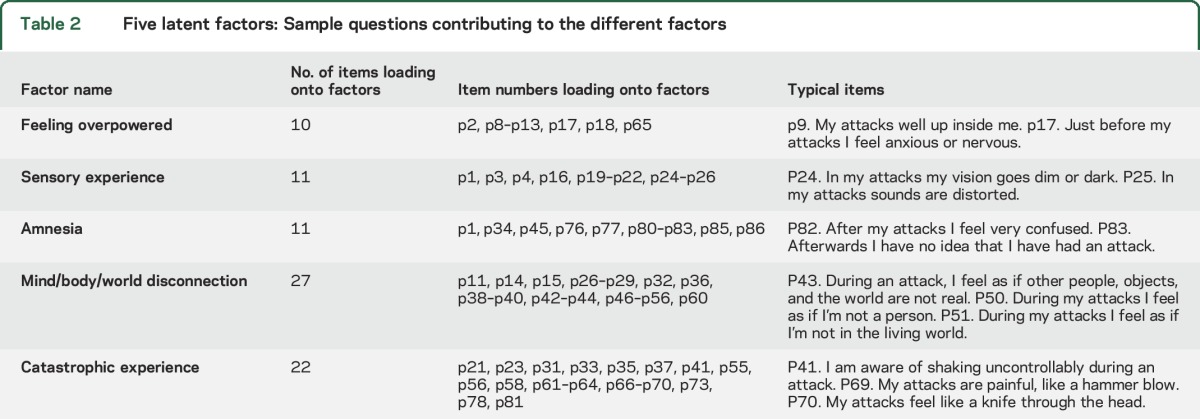
Figure 2. Transient loss of consciousness (TLOC) symptom profiles in epilepsy, syncope, and psychogenic nonepileptic seizures.
Symptom profiles based on 5 latent factors characterizing the subjective patient experience of the 3 common causes of TLOC (means and SE bars). E = epilepsy; P = psychogenic nonepileptic seizures; S = syncope.
Table 3.
ANOVA demonstrating the differentiating potential of individual factor scores among the 3 possible causes of TLOC
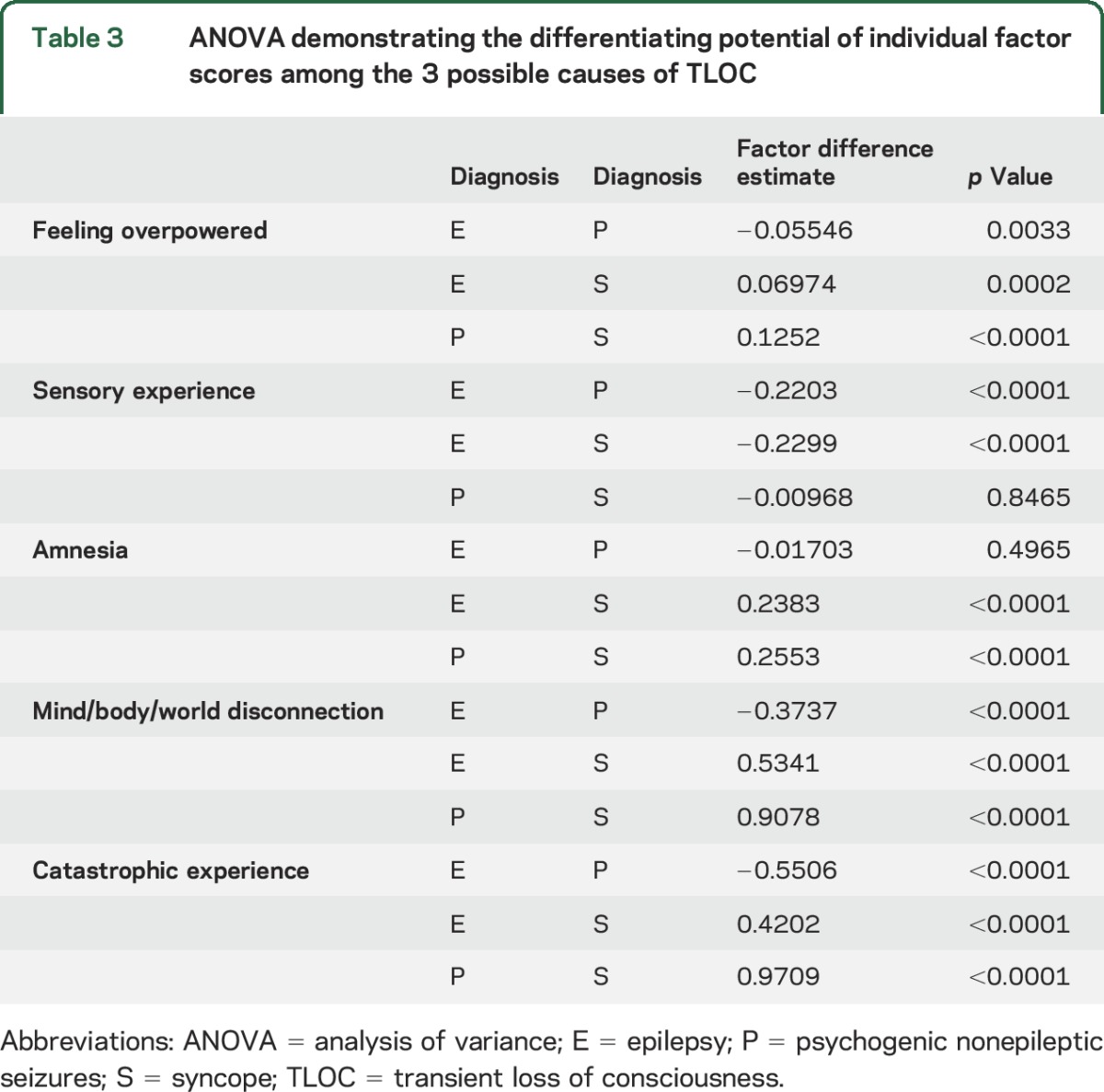
Differential diagnostic value of latent factors.
Initial pairwise logistic regression established the discriminating power of the factors between each pair of the 3 possible clinical diagnoses (table 4). The combination of symptom-based factor scores and basic patient demographic/clinical information (excluding age and age at onset) correctly classified more patients than the symptom-based data alone. In each regression, the factor scores (based on patients' self-reported seizure experiences) contributed more to the differential diagnosis than patient demographic/clinical information.
Table 4.
Binary logistic regression demonstrating differentiating potential of factor scores or demographic/clinical patient information or both combined
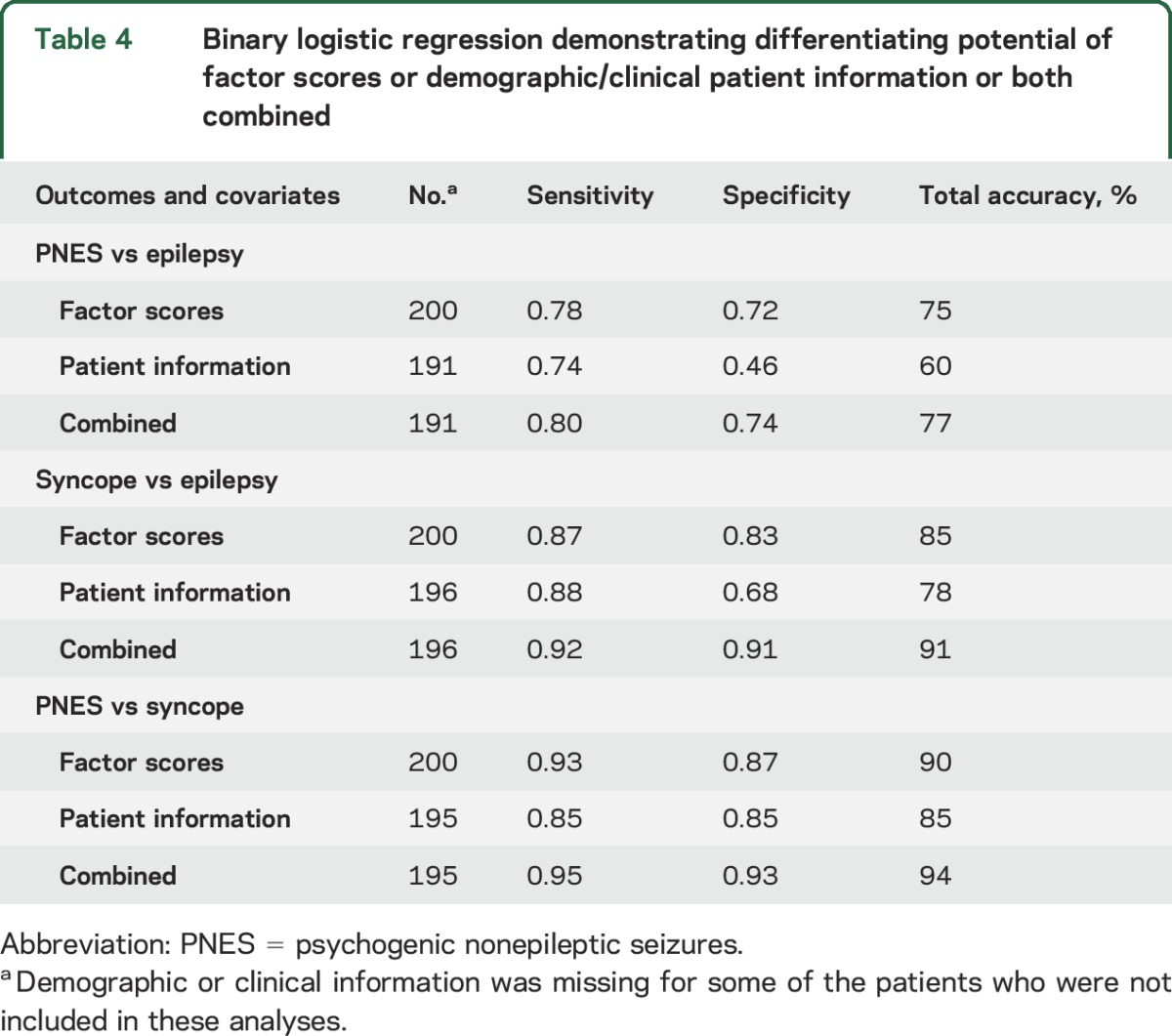
Multinomial logistic regression analysis revealed a similar pattern to the analyses described above: 91 out of 100 (91%) syncope diagnoses could be predicted correctly. Correct classification rates were lower for epilepsy and PNES: 63 out of 96 (66%) epilepsy patients and 74 out of 95 (78%) PNES patients were classified correctly, and most of the confounding was between these 2 diagnostic categories as well (the PNES and epilepsy groups contained fewer than 100 patients because some demographic/clinical details had not been provided by a small number of participants).
DISCUSSION
Our study demonstrates that a comprehensive self-report tool focusing on TLOC-associated symptoms can differentiate with high accuracy between syncope and the other 2 common causes of TLOC and slightly less well between PNES and epilepsy. This finding is of considerable clinical importance because it shows that self-reported symptom profiles can help direct patients to the most appropriate specialist services and provide a numeric pretest probability enhancing the diagnostic value of interictal investigations.
While to date, no diagnostic self-report tool designed to aid the differentiation among the 3 common causes of TLOC has been tested, a 118-item questionnaire used in 671 patients achieved a correct differentiation between recurrent generalized tonic clonic seizures or syncope in 94% of cases.21 However, patients with loss of awareness without collapse or those with PNES were excluded from this study.
In another study, a 29-item patient questionnaire was used in combination with a 6-item witness questionnaire to identify patients who ultimately received an expert diagnosis of epileptic seizure from a group of 94 patients with TLOC. A logistic regression model based on 4 items (age, sweating before the event, tongue biting, and witnessed rapid orientation after the event) worked least well in uncertain cases.20
Another project (excluding those with probable syncope) used a 209-item questionnaire in patients referred for video-EEG with possible diagnoses of epilepsy or PNES. This self-report tool categorized patients correctly with a sensitivity and specificity of 85%.26 While a small proportion of questions used in this study asked about subjective seizure symptoms, other studies suggest that the subjective experiences and patients' accounts of PNES differ from those of epileptic seizures.23,27–32
It is a particular strength of our study that the clinical diagnoses of all participants had been proven by the recording of typical TLOC episodes during appropriate physiologic monitoring. Previous studies aiming to validate diagnostic questionnaires have often used much lower diagnostic standards, for instance the working diagnosis after a certain period of follow-up,20,21 the opinion of an experienced clinician,33 or the diagnosis recorded in medical registers.34,35 Given how important the patient's history is for the diagnosis, validation studies not involving objective confirmation by the recording of a typical episode of TLOC are at risk of demonstrating a match between a clinician's history-taking efforts and a history taken by questionnaire rather than a match between the actual diagnosis and the diagnostic questionnaire result. Having said this, the fact that we deliberately chose patients with medical gold standard diagnoses means that our findings were based on a relatively chronic patient population. Now that there is clear proof-of-concept that a symptom-based questionnaire can contribute to the differential diagnosis of TLOC, we can use the results of our study to identify the most highly discriminating questions and develop a shorter questionnaire specifically for use in clinical settings where patients initially present with TLOC.
In our study, patients with PNES reported more different and frequent TLOC-associated symptoms than those with epilepsy or syncope. These observations are consistent with a previous study that demonstrated a greater range of subjective ictal experiences in PNES than epilepsy.28 They could simply reflect the tendency of patients with PNES to report higher rates of physical symptoms generally—as demonstrated by studies using general somatization measures.36,37 However, impairment of consciousness associated with PNES may also be less profound than that associated with the other 2 disorders.38,39 Third, TLOC caused by PNES may be a less stereotyped experience. This interpretation would be supported by the lower ratio of extreme vs middling responses seen in this group.
Our study demonstrates that self-reported symptom profiles made a greater contribution to the diagnostic models than the available demographic and clinical facts, although the addition of these facts improved the accuracy of the diagnostic models. The models did not differentiate equally well among all 3 causes of TLOC: our self-report tool accurately categorized over 90% of patients with either epilepsy or syncope. Similar levels of diagnostic separation were achieved in the distinction of patients with PNES from those with syncope. However, even when information derived from symptom reports was combined with demographic/clinical information, our detailed questionnaire only classified 77% of patients accurately when we attempted to distinguish between epilepsy and PNES (82% with the additional consideration of age at onset). The high levels of correct differentiation between syncope and epilepsy are in keeping with the results of previous studies.20,21 However, in contrast to the previous studies, our study includes a well-characterized group of patients with PNES, a condition as common in neurology clinics as syncope.2,3 This means that our findings are applicable to a wider range of patients, including those with partial or absence seizures involving TLOC, not only those with generalized tonic-clonic seizures, on which the largest similar previous study focused.21
While responses to 57 of the 86 items in the PEP questionnaire differed among the diagnostic groups, questions asking about symptoms of disconnection and patients' tendency to catastrophize made the greatest contribution to the 3-way differentiation. Responses to these questions suggested that PNES more than epileptic seizures and both of these types of TLOC more than syncope involve ictal experiences similar to those that characterize dissociative or anxiety disorders.
Our study has a number of limitations. Given that we wanted to base our study on patients with gold standard diagnoses, our findings are derived from patients who presented to specialist centers. The fact that the participants in all diagnostic groups studied were predominantly female meant that the groups were well-matched for sex but also suggests that male patients may have been underrepresented, at least in the epilepsy group. Our findings may also have been affected by the response rate: while a rate of around 30% may be all that is achievable in a postal study of this kind, there may have been significant differences between patients who participated in this study and those who did not. The relatively high number of episodes of TLOC and long duration of TLOC disorders is likely to have enabled respondents to answer questions about their symptoms particularly well. However, the preferential inclusion of patients with relatively chronic disorders means that our findings should be replicated in other settings before they are generalized more widely. We also cannot rule out that patients' symptom reporting was influenced by them being aware of their diagnosis. For instance, patients with PNES may have been encouraged by doctors to reflect on the presence of dissociative or anxiety symptoms. Finally, the differential diagnostic value of self-reported subjective symptoms could be greater if they had been combined with information only obtainable from other sources, for instance from witnesses. For instance, it may have been helpful to know whether collapses were atonic or involved shaking.
Despite these limitations, our study clearly demonstrates that the different pathophysiologic mechanisms causing TLOC in epilepsy, PNES, and syncope are associated with different TLOC experiences. Self-reported TLOC manifestations differentiate well between patients with syncope and those with seizures. However, the distinction of epileptic from nonepileptic seizures was less secure. Self-report tools based on TLOC manifestations can therefore be used to guide patients to relevant medical investigation and treatment pathways (e.g., cardiology for syncope, neurology for seizures) and can help to quantify the posttest probability of particular diagnoses.
Supplementary Material
GLOSSARY
- ANOVA
analysis of variance
- CFA
confirmatory factor analysis
- CFI
Comparative Fit Index
- DSM-IV-TR
Diagnostic and Statistical Manual of Mental Disorders, 4th edition, text revision
- EFA
exploratory factor analysis
- PEP
paroxysmal event profile
- PNES
psychogenic nonepileptic seizures
- RMSEA
root mean square error approximation
- TLI
Tucker Lewis Index
- TLOC
transient loss of consciousness
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
M. Reuber: conceptualization of research project, development of Paroxysmal Event Profile Questionnaire, obtaining regulatory approval, identifying participants, confirming participants' diagnoses by chart review, data collection, statistical analysis, drafting of manuscript. M. Chen: statistical analysis, drafting of manuscript. J. Jamnadas-Khoda: obtaining regulatory approval, identifying participants, data collection, data entry, drafting of manuscript. M. Broadhurst: conceptualization of research project, development of Paroxysmal Event Profile Questionnaire, obtaining regulatory approval, drafting of manuscript. M. Wall: analytic strategy development, statistical analysis, drafting of manuscript. R.A. Grünewald: development of Paroxysmal Event Profile Questionnaire, identifying participants, confirming participants' diagnoses by chart review, drafting of manuscript. S.J. Howell: development of Paroxysmal Event Profile Questionnaire, identifying participants, confirming participants' diagnoses by chart review, drafting of manuscript. M. Koepp: identifying participants, confirming participants' diagnoses by chart review, drafting of manuscript. S. Parry: development of Paroxysmal Event Profile Questionnaire, obtaining regulatory approval, identifying participants, confirming participants' diagnoses by chart review, data collection, drafting of manuscript. S. Sisodiya: identifying participants, confirming participants' diagnoses by chart review, data collection, drafting of manuscript. M. Walker: identifying participants, confirming participants' diagnoses by chart review, data collection, drafting of manuscript. D. Hesdorffer: analytic strategy development, statistical analysis, drafting of manuscript.
STUDY FUNDING
This study was supported by the Sheffield Hospitals Charitable Trust.
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Dickson JM, Taylor LH, Shewan J, Baldwin T, Gruenewald RA, Reuber M. A cross-sectional study of the pre-hospital management of patients with a suspected seizure (EPIC1). BMJ Open 2016;6:1–10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Angus-Leppan H. Diagnosing epilepsy in neurology clinics: a prospective study. Seizure 2008;17:431–436. [DOI] [PubMed] [Google Scholar]
- 3.Kotsopoulos IA, de Krom MC, Kessels FG, et al. The diagnosis of epileptic and non-epileptic seizures. Epilepsy Res 2003;57:59–67. [DOI] [PubMed] [Google Scholar]
- 4.Malmgren K, Reuber M, Appleton R. Differential diagnosis of epilepsy. In: Shorvon S, ed. Oxford Textbook of Epilepsy and Epileptic Seizures. Oxford: Oxford University Press; 2012:81–94. [Google Scholar]
- 5.Leach JP, Lauder R, Nicolson A, Smith DF. Epilepsy in the UK: misdiagnosis, mistreatment and undertreatment. Seizure 2005;14:514–520. [DOI] [PubMed] [Google Scholar]
- 6.Smith D, Defalla BA, Chadwick DW. The misdiagnosis of epilepsy and the management of refractory epilepsy in a specialist clinic. Q J Med 1999;92:15–23. [DOI] [PubMed] [Google Scholar]
- 7.Reuber M, Grunewald R, Panayiotopoulos CP. Newly Identified Seizures in Adults: Is it Epilepsy? The Educational Kit On Epilepsies. Oxford: Medicinae, 2007:66–71. [Google Scholar]
- 8.Hakami T, McIntosh A, Todaro M, et al. MRI-identified pathology in adults with new-onset seizures. Neurology 2013;81:920–927. [DOI] [PubMed] [Google Scholar]
- 9.Reuber M, Fernández G, Bauer J, Singh DD, Elger CE. Interictal EEG abnormalities in patients with psychogenic non-epileptic seizures. Epilepsia 2002;43:1013–1020. [DOI] [PubMed] [Google Scholar]
- 10.Reuber M, Fernández G, Helmstaedter C, Qurishi A, Elger CE. Evidence of brain abnormality in patients with psychogenic nonepileptic seizures. Epilepsy Behav 2002;3:246–248. [DOI] [PubMed] [Google Scholar]
- 11.Hoefnagels WAJ, Padberg GW, Overweg J, Roos RAC, van Dijk JG, Kamphuisen HA. Syncope or seizure? The diagnostic value of the EEG and hyperventilation test in transient loss of consciousness. J Neurol Neurosurg Psychiatry 1991;54:953–956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Benbadis SR, Tatum WO. Overinterpretation of EEGs and misdiagnosis of epilepsy. J Clin Neurophysiol 2003;20:42–44. [DOI] [PubMed] [Google Scholar]
- 13.Reuber M, Fernández G, Bauer J, Helmstaedter C, Elger CE. Diagnostic delay in psychogenic nonepileptic seizures. Neurology 2002;58:493–495. [DOI] [PubMed] [Google Scholar]
- 14.Benbadis SR, Chichkova R. Psychogenic pseudosyncope: an underestimated and provable diagnosis. Epilepsy Behav 2006;9:106–110. [DOI] [PubMed] [Google Scholar]
- 15.Hall-Patch L, Brown R, House A, et al. Acceptability and effectiveness of a communication strategy for the diagnosis of non-epileptic attacks. Epilepsia 2010;51:70–78. [DOI] [PubMed] [Google Scholar]
- 16.Reuber M, Baker GA, Gill R, Smith DF, Chadwick D. Failure to recognize psychogenic nonepileptic seizures may cause death. Neurology 2004;62:834–835. [DOI] [PubMed] [Google Scholar]
- 17.National Institute of Clinical Excellence. The epilepsies: the diagnosis and management of the epilepsies in adults and children in primary and secondary care: NICE clinical guideline 137 [online]. Available at: https://www.nice.org.uk/guidance/cg137?unlid=23807307520166920405. Accessed September 19, 2015.
- 18.Sackett DL, Haynes RB, Tugwell P. The interpretation of diagnostic data. In: Sackett DL, Haynes RB, eds. Clinical Epidemiology. Boston: Little, Brown; 1985:59–138. [Google Scholar]
- 19.Costello A, Osborne J. Best practices in exploratory factor analysis: four recommendations for getting the most from your analysis. Pract Assess Res Eval 2005;10:1–9. [Google Scholar]
- 20.Hoefnagels WAJ, Padberg GW, Overweg J, von der Velde EA, Roos RAC. Transient loss of consciousness: the value of the history for distinguishing seizure from syncope. J Neurol 1991;238:39–43. [DOI] [PubMed] [Google Scholar]
- 21.Sheldon R, Rose S, Ritchie D, et al. Historical criteria that distinguish syncope from seizures. J Am Coll Cardiol 2002;40:142–148. [DOI] [PubMed] [Google Scholar]
- 22.Surmann V. Anfallsbilder: Metaphorische Konzepte im Sprechen anfallskranker Menschen. Wuerzburg: Koenigshausen & Neumann; 2005. [Google Scholar]
- 23.Plug L, Sharrack B, Reuber M. Seizure metaphors differ in patients' accounts of epileptic and psychogenic non-epileptic seizures. Epilepsia 2009;50:994–1000. [DOI] [PubMed] [Google Scholar]
- 24.Waller NG, Ross CA. The prevalence and biometric structure of pathological dissociation in the general population: taxometric and behavior genetic findings. J Abnormpsychol 1997;106:499–510. [DOI] [PubMed] [Google Scholar]
- 25.American Psychiatric Association. Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition, Text Revision. Washington, DC: American Psychiatric Association; 2000. [Google Scholar]
- 26.Syed TU, Arozullah AM, Loparo KL, et al. A self-administered screening instrument for psychogenic nonepileptic seizures. Neurology 2009;72:1646–1652. [DOI] [PubMed] [Google Scholar]
- 27.Hendrickson R, Popescu A, Dixit R, Ghearing G, Bagic A. Panic attack symptoms differentiate patients with epilepsy from those with psychogenic nonepileptic spells (PNES). Epilepsy Behav 2014;37:210–214. [DOI] [PubMed] [Google Scholar]
- 28.Ali F, Rickards H, Bagary M, Greenhill L, McCorry D, Cavanna AE. Ictal consciousness in epilepsy and nonepileptic attack disorder. Epilepsy Behav 2010;19:522–525. [DOI] [PubMed] [Google Scholar]
- 29.Reuber M, Monzoni C, Sharrack B, Plug L. Using conversation analysis to distinguish between epilepsy and non-epileptic seizures: a prospective blinded multirater study. Epilepsy Behav 2009;16:139–144. [DOI] [PubMed] [Google Scholar]
- 30.Plug L, Sharrack B, Reuber M. Seizure, fit or attack? The use of diagnostic labels by patients with epileptic and non-epileptic seizures. Appl Linguistics 2009;31:94–114. [Google Scholar]
- 31.Goldstein LH, Mellers JD. Ictal symptoms of anxiety, avoidance behaviour, and dissociation in patients with dissociative seizures. J Neurol Neurosurg Psychiatry 2006;77:616–621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Deacon C, Wiebe S, Blume WT, McLachlan RS, Young GB, Matijevic S. Seizure identification by clinical description in temporal lobe epilepsy. Neurology 2003;61:1686–1689. [DOI] [PubMed] [Google Scholar]
- 33.Reutens DC, Howell RA, Gebert KE, Berkovic SF. Validation of a questionnaire for clinical seizure diagnosis. Epilepsia 1992;33:1065–1071. [DOI] [PubMed] [Google Scholar]
- 34.Placencia M, Sander JW, Shorvon SD, Ellison RH, Cascante SM. Validation of a screening questionnaire for the detection of epileptic seizures in epidemiological studies. Brain 1992;115:783–794. [DOI] [PubMed] [Google Scholar]
- 35.Ottman R, Barker-Cummings C, Leibson CL, Vasoli VM, Hauser WA, Buchhalter JR. Validation of a brief screening instrument for the ascertainment of epilepsy. Epilepsia 2010;51:191–197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Reuber M, House AO, Pukrop R, Bauer J, Elger CE. Somatization, dissociation and psychopathology in patients with psychogenic nonepileptic seizures. Epilepsy Res 2003;57:159–167. [DOI] [PubMed] [Google Scholar]
- 37.Owczarek K. Somatisation indexes as differential factors in psychogenic pseudoepileptic and epileptic seizures. Seizure 2003;12:178–181. [DOI] [PubMed] [Google Scholar]
- 38.Bell WL, Park YD, Thompson EA, Radtke RA. Ictal cognitive assessment of partial seizures and pseudoseizures. Arch Neurol 1998;55:1456–1459. [DOI] [PubMed] [Google Scholar]
- 39.Kuyk J, Spinhoven P, van Dyck R. Hypnotic recall: a positive criterion in the differential diagnosis between epileptic and pseudoepileptic seizures. Epilepsia 1999;40:485–491. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.