Abstract
Objective:
We investigated type 2 diabetes mellitus (T2DM) as a risk factor for brain atrophy and glucose hypometabolism in older adults with or at risk of cognitive impairment.
Methods:
Participants with the T2DM were identified from the Alzheimer's Disease Neuroimaging Initiative (ADNI-1/GO/2 cohorts). Analysis of covariance models were used to compare participants with and without T2DM, controlling for potential confounding factors.
Results:
Whole brain volume and whole brain [18F]-fluorodeoxyglucose (FDG) uptake were significantly different as a function of T2DM status, independent of baseline clinical diagnosis. On post hoc analysis, a lower whole brain volume was seen in participants with both mild cognitive impairment (MCI) and T2DM (n = 76) compared with participants who had MCI but not T2DM (n = 747; p = 0.009). Similarly, mean FDG uptake in gray matter and white matter was lower in participants with both MCI and T2DM (n = 72) than in participants with MCI without T2DM (n = 719; p = 0.04). Subsequent regional analysis revealed that the decreased FDG uptake in participants with both MCI and T2DM was mainly manifested in 3 brain regions: frontal lobe, sensory motor cortex, and striatum.
Conclusions:
T2DM may accelerate cognition deterioration in patients with MCI by affecting glucose metabolism and brain volume.
Type 2 diabetes mellitus (T2DM) is a risk factor for cognitive impairment,1 as more than 30% of patients with T2DM have mild cognitive impairment (MCI).2,3 T2DM not only is associated with a high MCI prevalence but may also shorten the conversion time from normal cognition to MCI.4,5 In 1997, a population-based cohort study reported that T2DM could significantly increase Alzheimer disease (AD) risk.6 In 1999, the Rotterdam Study reported that T2DM could almost double the AD risk.7 However, no direct link has been found between T2DM and AD from the pathophysiologic perspective.8 In addition, it is not clear how T2DM interacts with existing cognitive impairment in the form of MCI or AD. Thus, the goal of this study was to evaluate how T2DM affects brain structure and glucose metabolism in participants with normal cognition, MCI, or AD. Specifically, the effects of T2DM were examined in a secondary data analysis of the structural brain atrophy and glucose metabolism measures from older adults with normal cognition, MCI, or AD, who were enrolled in the Alzheimer's Disease Neuroimaging Initiative (ADNI). The findings may provide important insight into the biological mechanisms by which T2DM increases the risk of cognitive impairment.
METHODS
Alzheimer's Disease Neuroimaging Initiative.
Demographic and imaging data were downloaded from the ADNI database (adni.loni.usc.edu). As an ongoing project, ADNI was launched in 2003 and has been sponsored by the following agencies: National Institute on Aging, National Institute of Biomedical Imaging and Bioengineering, Food and Drug Administration, private pharmaceutical companies, and nonprofit organizations. The primary goal of ADNI has been to test whether serial MRI, PET, biological markers, and clinical and neuropsychological assessment can be combined to measure the progression of MCI and early AD. In 3 phases (1, GO, and 2) and from more than 50 sites across the United States and Canada, ADNI has recruited more than 1,600 adult participants. The participants are older people (aged 55–90 years) with normal cognition, MCI, or mild AD. Further information can be found at http://www.adni-info.org/ and in previous reports.9–14
Selection of T2DM participants.
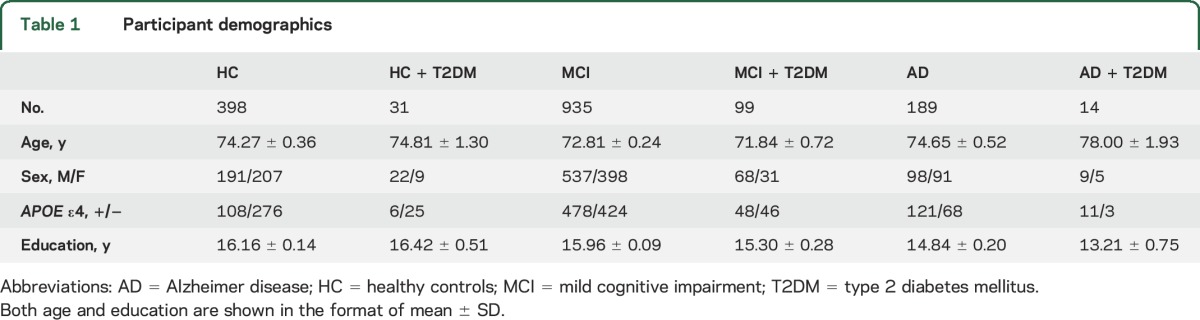
The following search terms were used to screen ADNI participants' medical history database: diabetes, diabetic, insulin, insulin-dependent diabetes mellitus (IDDM), and non-insulin dependent diabetes mellitus (NIDDM). Demographic information from 1,666 included participants is shown in table 1. Based on the medical history information (age at onset of diabetes, clinical diagnosis and/or use of diabetic medications), 159 participants from the ADNI were found to have T2DM at the screening visit of whom 76.73% (122/159) were being treated with antidiabetic medications. These diabetic participants had an average fasting glucose level between 110 and 120 mg/dL at the baseline and 12-, 24-, and 36-month follow-ups, indicating generally good control of their glucose levels. However, the average baseline fasting glucose level was 118.74 ± 35.69 mg/dL (n = 91) in those participants using antidiabetic medications; this level was significantly higher than the same measure of 106.79 ± 18.70 mg/dL (n = 24) in those who did not use any medications for control of their diabetes. One explanation is that those who received antidiabetic medications had more severe hyperglycemic conditions than those who did not have antidiabetic treatments. However, some participants with T2DM did not have their baseline fasting glucose data available for comparison.
Table 1.
Participant demographics
Magnetic resonance imaging.
Preprocessed MRI scans are available from the LONI ADNI site (http://adni.loni.usc.edu). Before being downloaded, the scans were corrected as previously described.15,16 FreeSurfer (version 5.1) was then used to extract MRI data for volumetric and cortical thickness measurements. Since MRI scanner types and acquisition protocols varied for the 3 ADNI phases (ADNI-1 = 1.5 tesla; ADNI-GO/2 = 3 tesla), MRI field strength was included as a covariate for the MRI data analyses along with age, sex, intracranial volume, and APOE ε4 carrier status.
[18F]-Fluorodeoxyglucose–PET.
Preprocessed [18F]-fluorodeoxyglucose (FDG)-PET scans were downloaded from the LONI ADNI site (http://adni.loni.usc.edu). Images were preprocessed by the ADNI PET core and at the Indiana University School of Medicine, as previously described.10,17 Briefly, before the download, the raw dynamic frames (6 × 5 minute frames from 30 to 60 minutes postinjection) were averaged to create a single average image, which was then aligned with a standard space, resampled to a standard image and voxel size, normalized in terms of intensity, and smoothed to a uniform resolution. Downloaded scans were normalized to MNI (Montreal Neurologic Institute) space using the same time point MRI, and the image was intensity normalized to the mean pons signal to create FDG standardized uptake value ratio (SUVR) images. Mean SUVR values were extracted from the following brain regions: whole brain, frontal lobe, parietal lobe, temporal lobe, limbic lobe, and occipital lobe using regions of interest from the automated anatomical labeling atlas.18
Statistical analysis.
SPSS (version 22.0; IBM Corp., Armonk, NY) was used for all statistical analyses. Two-way analysis of covariance models were used to evaluate the effect of T2DM on whole brain volume and cerebral glucose metabolism in the context of diagnostic group (cognitively healthy control [HC], MCI, AD). Based on this 2-way analysis of covariance model including both baseline cognition diagnosis and T2DM status, participants were divided into 6 groups: HC with no T2DM (HC; n = 398), MCI with no T2DM (MCI; n = 935), AD with no T2DM (AD; n = 189), HC with T2DM (HC + T2DM; n = 31), MCI with T2DM (MCI + T2DM; n = 99), and AD with T2DM (AD + T2DM; n = 14) (table 1). Then the neuroimaging data were compared among the 6 groups by controlling appropriate confounding factors. Specifically, for whole brain volume, data were analyzed by controlling age, sex, APOE ε4 carrier status, intracranial volume, and MRI field strength. FDG-PET data were assessed controlling for age, sex, and APOE ε4 carrier status. Data are shown in the form of mean ± SD, and p < 0.05 was considered as significant for all statistical analyses. Figures were created using SigmaPlot (version 10.0).
Standard protocol approvals, registrations, and patient consents.
Written informed consent was obtained from all individuals (or guardians of participants) participating in the study according to the Declaration of Helsinki (consent for research).
RESULTS
A significant effect of T2DM status on whole brain volume (p = 0.009) and whole brain FDG SUVR (p = 0.004) was observed, with participants with T2DM showing lower volume and metabolism than nondiabetic participants. An expected significant main effect of diagnosis was also observed, but no interaction between baseline diagnosis and T2DM status was seen for either outcome.
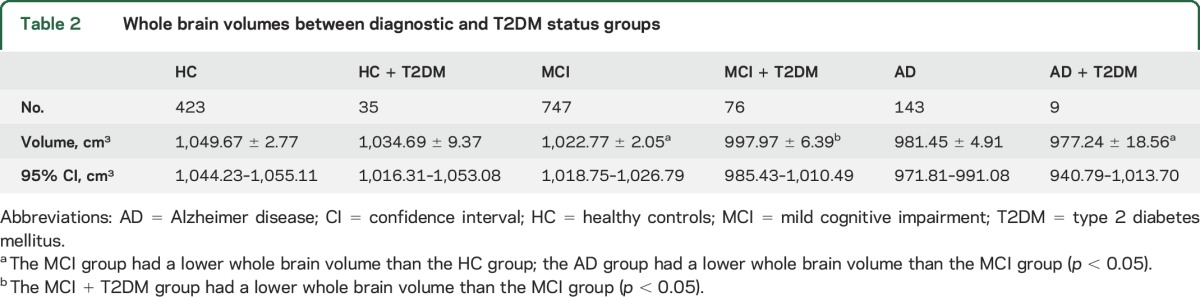
Whole brain volume and glucose metabolism were then compared among the 6 groups (HC, HC + T2DM, MCI, MCI + T2DM, AD, and AD + T2DM). A significant difference between the MCI + T2DM group and the MCI group was observed, with the MCI + T2DM group showing a whole brain volume of 997.96 ± 6.39 cm3 (95% confidence interval [CI]: 985.43–1,010.49 cm3; n = 76), which was lower than the whole brain volume for the MCI group (mean whole brain volume: 1,022.77 ± 2.05 cm3 [95% CI: 1,018.75–1,026.79 cm3; n = 747; p = 0.003]) (figure 1, table 2).
Figure 1. Whole brain volumes were compared among the 6 groups.
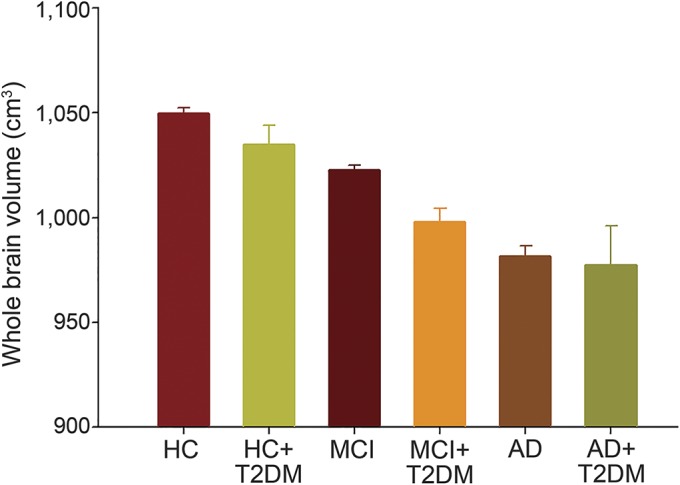
Whole brain volumes were different among the 6 groups based on T2DM status (p = 0.009), but the effects of T2DM were only seen in participants with MCI. The whole brain volume in the MCI + T2DM group was lower than that in the MCI group (p = 0.003). AD = Alzheimer disease; HC = healthy controls; MCI = mild cognitive impairment; T2DM = type 2 diabetes mellitus.
Table 2.
Whole brain volumes between diagnostic and T2DM status groups
Mean whole brain FDG SUVR was also significantly different between the MCI + T2DM group and the MCI group. Specifically, the MCI + T2DM group showed a mean whole brain FDG of 1.237 ± 0.014 (95% CI: 1.209–1.264; n = 72), which was lower than the MCI group (1.281 ± 0.004 [95% CI: 1.272–1.289; n = 719; p = 0.04]) (figure 2).
Figure 2. Whole brain FDG SUVR was compared among the 6 groups.
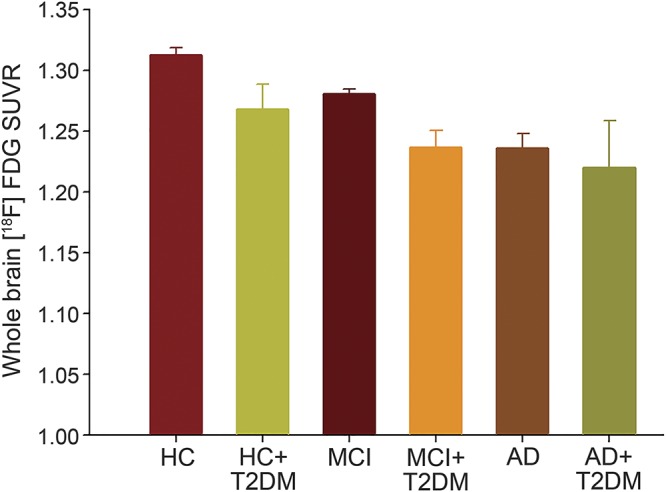
Whole brain FDG SUVR was different among the 6 groups when T2DM status was used as the independent variable (p = 0.001). However, the effects of T2DM on the whole brain FDG SUVR were only seen in participants with MCI. The MCI + T2DM group had a lower whole brain FDG SUVR than the MCI group (p = 0.04). AD = Alzheimer disease; FDG = [18F]-fluorodeoxyglucose; HC = healthy controls; MCI = mild cognitive impairment; SUVR = standardized uptake value ratio; T2DM = type 2 diabetes mellitus.
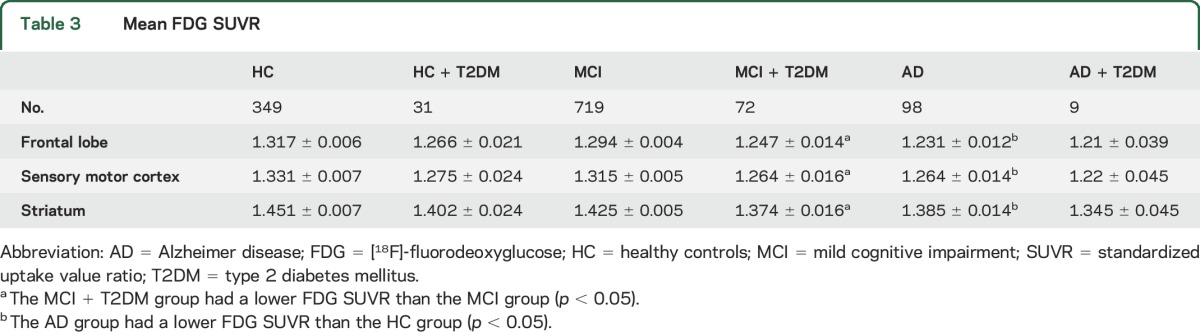
Subsequently, glucose metabolism from multiple brain regions was examined. The MCI + T2DM group had a lower FDG SUVR than the MCI group in the frontal lobe, sensory motor cortex, and striatum (p < 0.05; table 3). The effects of T2DM did not reach the level of significance within the other 2 baseline cognition diagnosis groups for any comparison (HC or AD).
Table 3.
Mean FDG SUVR
DISCUSSION
A lower whole brain volume, as well as whole brain hypometabolism, was seen in participants with both MCI and T2DM than in those with MCI only. In addition, participants with MCI + T2DM showed reduced glucose metabolism in the frontal lobe, sensory motor cortex, and striatum relative to MCI without T2DM. T2DM showed no effects within either HC or AD groups. The findings suggest that deterioration caused by T2DM affected patients with MCI more than patients with AD or healthy older adults.
The majority of studies have found that patients with T2DM have a lower whole brain volume than patients with normoglycemia19–22 except one.23 Findings from the current study showed that T2DM had effects on the whole brain volume primarily in patients with MCI. In addition, no volume change was detected in any subregion of the brain, so the effects of T2DM were only observed when considering the whole brain volume. The MCI-specific effects of T2DM suggest that the atrophic changes seen in the prodromal stages of dementia may be accelerated by T2DM. In addition, the MCI-specific effects of T2DM may explain the inconsistent reports on the whole brain volume measures in patients with T2DM.
In a previous study, hippocampal atrophy was associated with cerebral small vessel disease (SVD) in patients with T2DM, but no association was found between vascular risk factors and cortical atrophy.24 T2DM was associated with a higher count of cerebral infarcts and a lower total brain volume.25 Although SVD was reported to be associated with cognitive decline in patients with T2DM,26 the presence of silent brain infarct and white matter lesions was not assessed in this study. Thus, the role of SVD in brain atrophy in patients with T2DM deserves further investigations.27,28
A previous study found that the odds ratio for hypometabolism in AD signature regions was doubled in individuals with T2DM in comparison to nondiabetic individuals.29 Several frontotemporal brain regions in patients with T2DM were shown to have a reduced cerebral glucose metabolism, even after various vascular risk factors were controlled.30 In another report, a reduced glucose metabolism was found in the frontal lobe in adults with T2DM.31 In this study, we demonstrated an association between T2DM and a lower hypometabolism in the whole brain in participants with MCI. On regional analysis, reduced glucose metabolism was mainly manifested in the frontal lobe, the sensory motor cortex, and the striatum in participants with T2DM and MCI relative to those with MCI only.
We studied the effects of antidiabetic treatments or other features of diabetes on the outcomes of either whole brain volume or glucose metabolism in the brain as well. The concurrent antidiabetic treatment showed no effects on either outcome. By contrast, other characteristics of diabetes, such as fasting glucose level and duration of T2DM, have previously been shown to be associated either with brain structure or glucose metabolism changes in the brain.27 In this study, participants with T2DM showed stable fasting glucose levels and, thus, relatively controlled disease.
Previous studies have shown an association between T2DM and accelerated cognitive decline.5,19 For example, T2DM at baseline was found to accelerate the conversion from normal cognition to MCI by 7 years.5 The decreased total brain volume and the lowered glucose metabolism in regions of frontal lobe, sensory motor cortex, and striatum in the present study may provide some biological evidence for supporting T2DM's role in impairing cognition functions.25,26,32
This study was limited by its cross-sectional design. A longitudinal follow-up study is warranted to investigate how T2DM correlates with an accelerated cognitive deterioration over time. Furthermore, future fluid biomarker studies may shed more light on the underlying mechanism of T2DM-associated cognitive decline. Another limitation is that the participants with T2DM were ascertained based on their self-reported medical history, which may not be complete or accurate. Future studies using digital medical history would be warranted to confirm the findings in the present study.
In summary, the findings from this study provide biological evidence regarding the influence of the T2DM that may help explain previously reported associations of T2DM with cognition decline, especially in patients with MCI. T2DM appears to have additive effects with AD pathology in patients with MCI to reduce the whole brain volume and glucose metabolism in multiple brain regions. Although it is difficult to delineate the specific contributions of atrophic brain structural changes relative to changes in function represented by impaired glucose metabolism, both processes may be involved in cognitive decline in patients with T2DM and MCI.
Supplementary Material
GLOSSARY
- AD
Alzheimer disease
- ADNI
Alzheimer's Disease Neuroimaging Initiative
- CI
confidence interval
- FDG
[18F]-fluorodeoxyglucose
- HC
healthy control
- MCI
mild cognitive impairment
- SUVR
standardized uptake value ratio
- SVD
small vessel disease
- T2DM
type 2 diabetes mellitus
Footnotes
Supplemental data at Neurology.org
AUTHOR CONTRIBUTIONS
Wei Li conceived the study design and drafted the manuscript. All authors contributed to acquisition of data, data analysis and interpretation, and critical revision of the manuscript for important intellectual content.
STUDY FUNDING
Data collection and sharing for this project were funded by the Alzheimer's Disease Neuroimaging Initiative (ADNI) (NIH grant U01 AG024904) and Department of Defense (DOD) ADNI (W81XWH-12-2-0012). ADNI was also supported by NIH grants P30 AG010129, K01 AG030514, and the Dana Foundation. In addition to the above referenced support for collection of the ADNI data, the analyses reported here were supported in part by the following sources: P30 AG10133 (A.J.S.), National Institute on Aging (NIA) R01 AG19771 (A.J.S.), NIA K01 AG049050 (S.L.R.), an Alzheimer's Association New Investigator Research Grant (S.L.R.), the Indiana University Health–Indiana University School of Medicine Strategic Research Initiative (S.L.R., A.J.S.), and the Indiana Clinical and Translational Science Institute (S.L.R., A.J.S.).
DISCLOSURE
The authors report no disclosures relevant to the manuscript. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Luchsinger JA, Reitz C, Patel B, Tang MX, Manly JJ, Mayeux R. Relation of diabetes to mild cognitive impairment. Arch Neurol 2007;64:570–575. [DOI] [PubMed] [Google Scholar]
- 2.Lee YJ, Kang HM, Kim NK, et al. Factors associated for mild cognitive impairment in older Korean adults with type 2 diabetes mellitus. Diabetes Metab J 2014;38:150–157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gorska-Ciebiada M, Saryusz-Wolska M, Ciebiada M, Loba J. Mild cognitive impairment and depressive symptoms in elderly patients with diabetes: prevalence, risk factors, and comorbidity. J Diabetes Res 2014;2014:179648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gao Y, Xiao Y, Miao R, et al. The prevalence of mild cognitive impairment with type 2 diabetes mellitus among elderly people in China: a cross-sectional study. Arch Gerontol Geriatr 2016;62:138–142. [DOI] [PubMed] [Google Scholar]
- 5.Kryscio RJ, Abner EL, Lin Y, et al. Adjusting for mortality when identifying risk factors for transitions to mild cognitive impairment and dementia. J Alzheimers Dis 2013;35:823–832. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Leibson CL, Rocca WA, Hanson VA, et al. Risk of dementia among persons with diabetes mellitus: a population-based cohort study. Am J Epidemiol 1997;145:301–308. [DOI] [PubMed] [Google Scholar]
- 7.Ott A, Stolk RP, van Harskamp F, Pols HA, Hofman A, Breteler MM. Diabetes mellitus and the risk of dementia: the Rotterdam Study. Neurology 1999;53:1937–1942. [DOI] [PubMed] [Google Scholar]
- 8.Heitner J, Dickson D. Diabetics do not have increased Alzheimer-type pathology compared with age-matched control subjects: a retrospective postmortem immunocytochemical and histofluorescent study. Neurology 1997;49:1306–1311. [DOI] [PubMed] [Google Scholar]
- 9.Jack CR Jr, Bernstein MA, Borowski BJ, et al. Update on the magnetic resonance imaging core of the Alzheimer's Disease Neuroimaging Initiative. Alzheimers Dement 2010;6:212–220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Jagust WJ, Bandy D, Chen K, et al. The Alzheimer's Disease Neuroimaging Initiative positron emission tomography core. Alzheimers Dement 2010;6:221–229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Petersen RC, Aisen PS, Beckett LA, et al. Alzheimer's Disease Neuroimaging Initiative (ADNI): clinical characterization. Neurology 2010;74:201–209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Saykin AJ, Shen L, Foroud TM, et al. Alzheimer's Disease Neuroimaging Initiative biomarkers as quantitative phenotypes: genetics core aims, progress, and plans. Alzheimers Dement 2010;6:265–273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Trojanowski JQ, Vandeerstichele H, Korecka M, et al. Update on the biomarker core of the Alzheimer's Disease Neuroimaging Initiative subjects. Alzheimers Dement 2010;6:230–238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Weiner MW, Aisen PS, Jack CR Jr, et al. The Alzheimer's Disease Neuroimaging Initiative: progress report and future plans. Alzheimers Dement 2010;6:202–211.e7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Domínguez RO, Marschoff ER, González SE, Repetto MG, Serra JA. Type 2 diabetes and/or its treatment leads to less cognitive impairment in Alzheimer's disease patients. Diabetes Res Clin Pract 2012;98:68–74. [DOI] [PubMed] [Google Scholar]
- 16.Jack CR Jr, Bernstein MA, Fox NC, et al. The Alzheimer's Disease Neuroimaging Initiative (ADNI): MRI methods. J Magn Reson Imaging 2008;27:685–691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Risacher SL, Kim S, Shen L, et al. The role of apolipoprotein E (APOE) genotype in early mild cognitive impairment (E-MCI). Front Aging Neurosci 2013;5:1–12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Brett M, Anton JL, Valabregue R, Poline JB. Region of interest analysis using an SPM toolbox. Presented at the 8th International Conference on Functional Mapping of the Human Brain; June 2–6, 2002; Sendai, Japan.
- 19.Morris JK, Vidoni ED, Honea RA, Burns JM. Impaired glycemia increases disease progression in mild cognitive impairment. Neurobiol Aging 2014;35:585–589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.de Bresser J, Tiehuis AM, van den Berg E, et al. Progression of cerebral atrophy and white matter hyperintensities in patients with type 2 diabetes. Diabetes Care 2010;33:1309–1314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Moulton CD, Costafreda SG, Horton P, Ismail K, Fu CH. Meta-analyses of structural regional cerebral effects in type 1 and type 2 diabetes. Brain Imaging Behav 2015;9:651–662. [DOI] [PubMed] [Google Scholar]
- 22.Biessels GJ, Reijmer YD. Brain changes underlying cognitive dysfunction in diabetes: what can we learn from MRI? Diabetes 2014;63:2244–2252. [DOI] [PubMed] [Google Scholar]
- 23.Wang C, Fu K, Liu H, Xing F, Zhang S. Brain structural changes and their correlation with vascular disease in type 2 diabetes mellitus patients: a voxel-based morphometric study. Neural Regen Res 2014;9:1548–1556. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brundel M, van den Heuvel M, de Bresser J, Kappelle LJ, Biessels GJ. Cerebral cortical thickness in patients with type 2 diabetes. J Neurol Sci 2010;299:126–130. [DOI] [PubMed] [Google Scholar]
- 25.Moran C, Phan TG, Chen J, et al. Brain atrophy in type 2 diabetes: regional distribution and influence on cognition. Diabetes Care 2013;36:4036–4042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Imamine R, Kawamura T, Umemura T, et al. Does cerebral small vessel disease predict future decline of cognitive function in elderly people with type 2 diabetes? Diabetes Res Clin Pract 2011;94:91–99. [DOI] [PubMed] [Google Scholar]
- 27.Bryan RN, Bilello M, Davatzikos C, et al. Effect of diabetes on brain structure: the action to control cardiovascular risk in diabetes MR imaging baseline data. Radiology 2014;272:210–216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Abner EL, Nelson PT, Kryscio RJ, et al. Diabetes is associated with cerebrovascular but not Alzheimer neuropathology. Alzheimers Dement Epub 2016 Jan 23. [DOI] [PMC free article] [PubMed]
- 29.Roberts RO, Knopman DS, Cha RH, et al. Diabetes and elevated hemoglobin A1c levels are associated with brain hypometabolism but not amyloid accumulation. J Nucl Med 2014;55:759–764. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.García-Casares N, Berthier ML, Jorge RE, et al. Structural and functional brain changes in middle-aged type 2 diabetic patients: a cross-sectional study. J Alzheimers Dis 2014;40:375–386. [DOI] [PubMed] [Google Scholar]
- 31.Baker LD, Cross DJ, Minoshima S, Belongia D, Watson GS, Craft S. Insulin resistance and Alzheimer-like reductions in regional cerebral glucose metabolism for cognitively normal adults with prediabetes or early type 2 diabetes. Arch Neurol 2011;68:51–57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Szémán B, Nagy G, Varga T, et al. Changes in cognitive function in patients with diabetes mellitus [in Hungarian]. Orv Hetil 2012;153:323–329. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


