Abstract
Objective:
To evaluate whether the use of multiphase CT angiography (CTA) improves interrater agreement for intracranial occlusion detection between stroke neurology trainees and an expert neuroradiologist.
Methods:
A neuroradiologist and 2 stroke neurology fellows independently reviewed 100 prospectively collected single-phase and multiphase CTA scans from acute ischemic stroke patients with mild symptoms (NIH Stroke Scale score ≤5). The presence and location of a vascular occlusion(s) were documented. Interrater agreement single- and multiphase CTA was quantified using unweighted κ statistics. We assessed for any occlusions, anterior vs posterior occlusions, and pial vessel asymmetry.
Results:
Using multiphase CTA, the neuroradiologist detected 50 scans with anterior circulation occlusions and 15 scans with posterior circulation occlusions. Median reading time was 2 minutes per scan. Median reading time for the neurologists was 3 minutes per multiphase CTA scan. Interrater agreement was fair between the 2 neurologists and neuroradiologist when using single-phase CTA (κ = 0.45 and 0.32). Agreement improved minimally when stratified by anterior vs posterior circulation. When using multiphase CTA, agreement was high for detection of occlusion or asymmetry of pial vessels in the anterior circulation (κ = 0.80 and 0.84).
Conclusions:
Multiphase CTA improves diagnostic accuracy in minor ischemic stroke for detection of anterior circulation intracranial occlusion.
Classification of evidence:
This study provides Class II evidence that multiphase CTA, compared to single-phase CTA, improves the interrater agreement between stroke neurology trainees and an expert neuroradiologist for detecting anterior circulation intracranial vascular occlusion in patients with minor acute ischemic strokes.
Neurologists are critical to the treatment of acute ischemic stroke and are called on to rapidly interpret imaging, including CT angiography (CTA) for early detection of neurovascular occlusions. Accurate identification of intracranial vessel occlusions is crucial when making clinical decisions.1–5 In the minor stroke and TIA spectrum of ischemic cerebrovascular disease, intracranial occlusion has been shown to be associated with early stroke recurrence, progression, and disabled outcome.6 Current clinical trials are assessing the value of endovascular and medical thrombolytic therapy in populations defined by the presence of vessel occlusion. BASICS (NCT01717755) is a trial evaluating the efficacy of endovascular therapy in the posterior circulation occlusion, and TEMPO-2 (NCT02398656) is examining the efficacy of IV tenecteplase (TNK–tissue plasminogen activator) in minor strokes and TIAs with proven symptomatic intracranial occlusion.7,8
Detecting an intracranial occlusion can be challenging, especially when the occlusion is in the distal intracranial vasculature. Multiphase CTA is a novel but simple imaging technique that provides time-resolved cerebral angiograms of the intracranial vasculature.9–11 The objective of this study was to evaluate whether the use of multiphase CTA improves interrater reliability and detection of proximal and distal intracranial occlusions among TIA and minor stroke patients.
METHODS
Data are from 100 prospectively collected multiphase CTA scans from 2 studies: the TEMPO-1 Trial (TNK-tPA Evaluation for Minor Ischemic Stroke with Proven Occlusion) and the PRoveIT Study (Precise and Rapid Assessment of Collaterals Using Multiphase CTA in the Triage of Patients with Acute Ischemic Stroke for Intra-arterial Therapy; NCT02184936).8,9 TEMPO-1 was a phase 2, dose-escalation safety and feasibility trial that enrolled patients with a proven intracranial occlusion and clinical TIAs or minor ischemic strokes, defined as NIH Stroke Scale score ≤5; all were treated with IV tenecteplase. Because TEMPO-1 was a multicenter study, not all patients were imaged by multiphase CTA. Only patients who underwent a multiphase CTA scan were included. PRoveIT is an ongoing prospective, observational study analyzing the utility of multimodal imaging, including multiphase CTA, in the triage of patients with acute ischemic stroke for treatment decisions. To obtain a homogeneous group of patients, we selected consecutive patients enrolled in PRoveIT with an NIH Stroke Scale score ≤5. Patients in PRoveIT did not necessarily have an intracranial occlusion and were treated according to the attending physician's discretion. Scans from both studies were merged and randomly reordered by a research assistant who was not involved in reading the scans so that the readers were blinded to the clinical symptoms, the study of origin, and the total number of occlusions.
Standard protocol approvals, registrations, and patient consents.
The TEMPO-1 and PRoveIT studies received approval from the University of Calgary institutional review board for research using human subjects. Written informed consent was obtained from patients enrolled in TEMPO-1 and waiver of consent was approved for enrollment in PRoveIT.
Imaging protocol and analysis.
The full imaging protocol for multiphase CTA has been previously described.9 Briefly, the first phase of the multiphase CTA is from aortic arch to vertex using a multidetector CT scanner, acquired in the late arterial phase with scanning triggered by contrast bolus monitoring in the aortic arch with average dose length product of 700 to 800 mGy·cm. The scan time is less than 10 seconds on modern generation scanners. The second and third phases of the multiphase CTA, each taken approximately 8 seconds apart, take images from the skull base to vertex in the midvenous and late-venous phases of the initial contrast bolus. Images were acquired with 0.625-mm section thickness and automatic postprocessing of multiphase CTA maps are complete in less than 3 minutes. Radiocontrast media (80 mL) is injected at a rate of 5 mL/s and followed by a 50-mL normal saline chase at a rate of 6 mL/s. The multiphase CTA protocol does not require additional contrast medium and there is minimal extra radiation as the second and third imaging phases are acquired with lower radiation dose since imaging is from the skull base to vertex only.10,11
For our study, the first phase of the multiphase CTA acquired in the late arterial phase was defined as single-phase CTA. An expert neuroradiologist (Z.A.) and 2 stroke neurology fellows (A.Y.X.Y., C.Z.) reviewed all scans blinded to any clinical and other imaging data. When reading single-phase CTA, the readers had access only to the first-phase source images, axial thick maximum intensity projection (MIP), and the axial, sagittal, and coronal reformatted thin MIPs. When reading the multiphase CTA, the readers had access to all 3 phases, including sources images and the second and third phases of the axial thick MIPs. To ensure the interpretations of the single- and multiphase CTA were independent from each other, the 2 scan types were read 2 weeks or more apart.
Each reader documented the presence and location of an intracranial occlusion. The classification of intracranial occlusion location was as follows: left and/or right anterior cerebral artery (ACA-A1, ACA-A2), middle cerebral artery (MCA-M1, MCA-M2, MCA-M3), posterior cerebral artery (PCA), vertebral artery and posterior inferior cerebellar artery (VA-PICA), and the basilar artery. After reading the multiphase CTA, even if no occlusion was found, readers documented any asymmetry of the ACA, MCA, PCA, or VA-PICA pial vasculature. This could be seen as decrease or delay in filling or washout of contrast in pial vessels on the second and third phases representing the mid- and late-venous phases (figures 1–3). Finally, follow-up neuroimaging was reviewed by the neuroradiologist, and the presence and location of acute infarcts as a reference standard for the occlusion detection were documented.
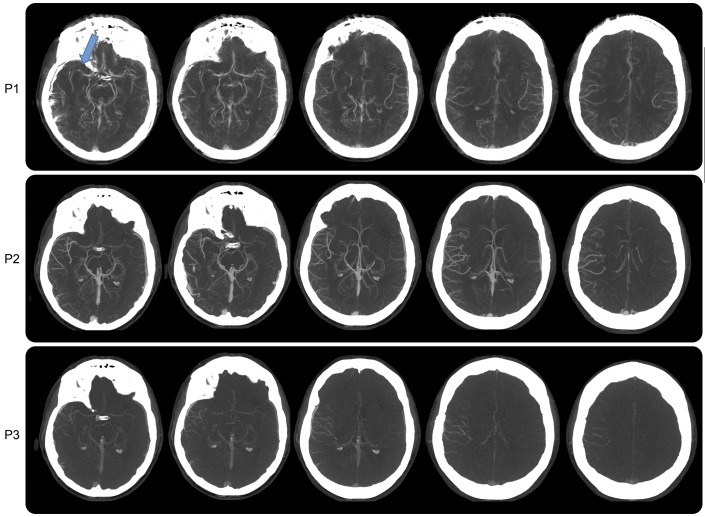
Figure 1. Case study 1.
Axial multiphase CT angiography showing 3 phases as P1 (late arterial phase), P2 (midvenous phase), and P3 (late-venous phase). P1 images are degraded by motion artifacts. There is evidence of a right MCA mid-M1 loss of contrast opacification, indicating an occlusion (blue arrow). P2 and P3 show asymmetry in pial arteries in the right MCA distribution with delayed washout of the contrast, confirming the vascular occlusion despite motion artifact. MCA = middle cerebral artery.
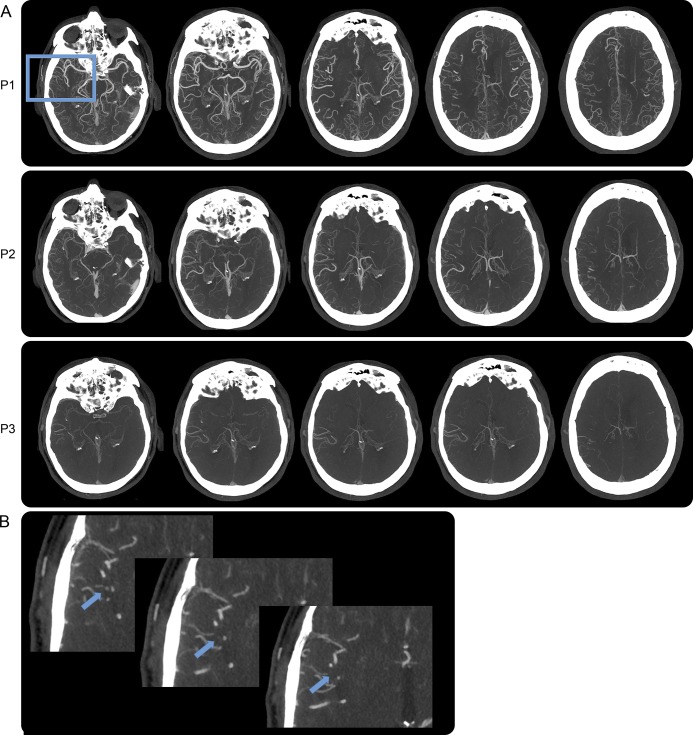
Figure 2. Case study 2.
(A) Multiphase CT angiography with right MCA occlusion in the distal branches, made evident by delayed washout of contrast in the right MCA vascular distribution in P2 and P3. (B) Magnified axial maximum intensity projection of P1 showing gradual loss of contrast opacification of a distal M2 branch of the MCA in the sylvian fissure indicating a vascular occlusion (blue arrows). MCA = middle cerebral artery.
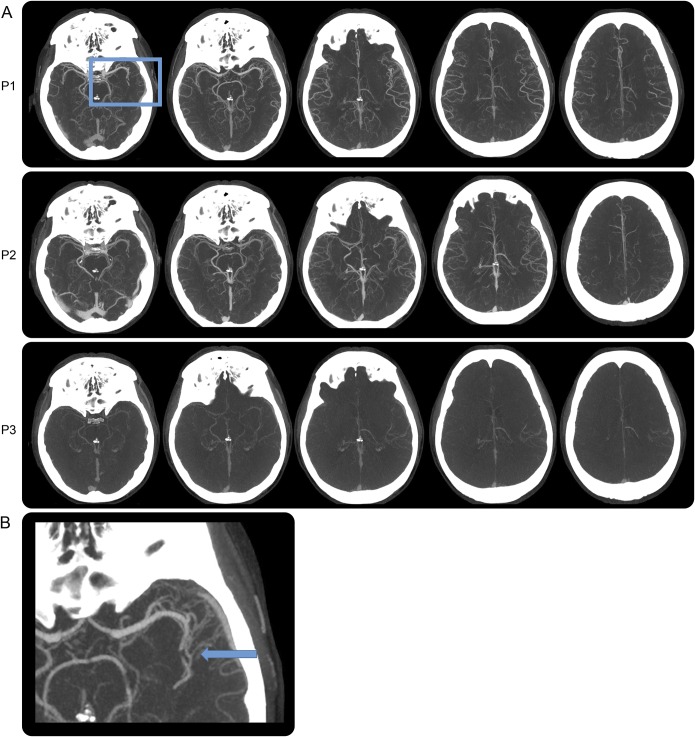
Figure 3. Case study 3.
(A) Multiphase CT angiography with left MCA-M2 branch occlusion in P1 and delayed contrast washout in the left MCA vascular distribution (P2, P3). (B) Magnified axial maximum intensity projection of P1 showing abrupt vessel cutoff with distal vessel opacification, either through slow anterograde or retrograde collateral filling (blue arrow). MCA = middle cerebral artery.
Statistical methods.
We used descriptive analyses to describe the frequency and location of intracranial occlusion detection for each scan type and the median reading time per scan. The occlusion locations were grouped into anterior (ACA, MCA) and posterior (PCA, VA-PICA, basilar) circulation territories. The anterior circulation occlusions were further classified as proximal (ACA-A1 and MCA-M1) and distal (ACA-A2, MCA-M2, and MCA-M3). We did not stratify the posterior circulation occlusions. We used unweighted κ statistics and 95% confidence intervals to evaluate interrater agreement between each neurologist and the neuroradiologist. Agreement was quantified as fair (κ = 0.21–0.40), moderate (κ = 0.41–0.60), substantial (κ = 0.61–0.80), or almost perfect (κ = 0.81–0.99).12 For the single-phase CTA, we reported agreement for any occlusion detection and also stratified by anterior vs posterior circulation territories. For the multiphase CTA, we reported agreement for detection of any occlusion, anterior vs posterior occlusion, and anterior vs posterior occlusion and/or asymmetry of pial vessels. We reported agreement for the detection of proximal and distal occlusions in the anterior circulation for both scan types. All analyses were performed using STATA 14.0 (StataCorp, College Station, TX).
RESULTS
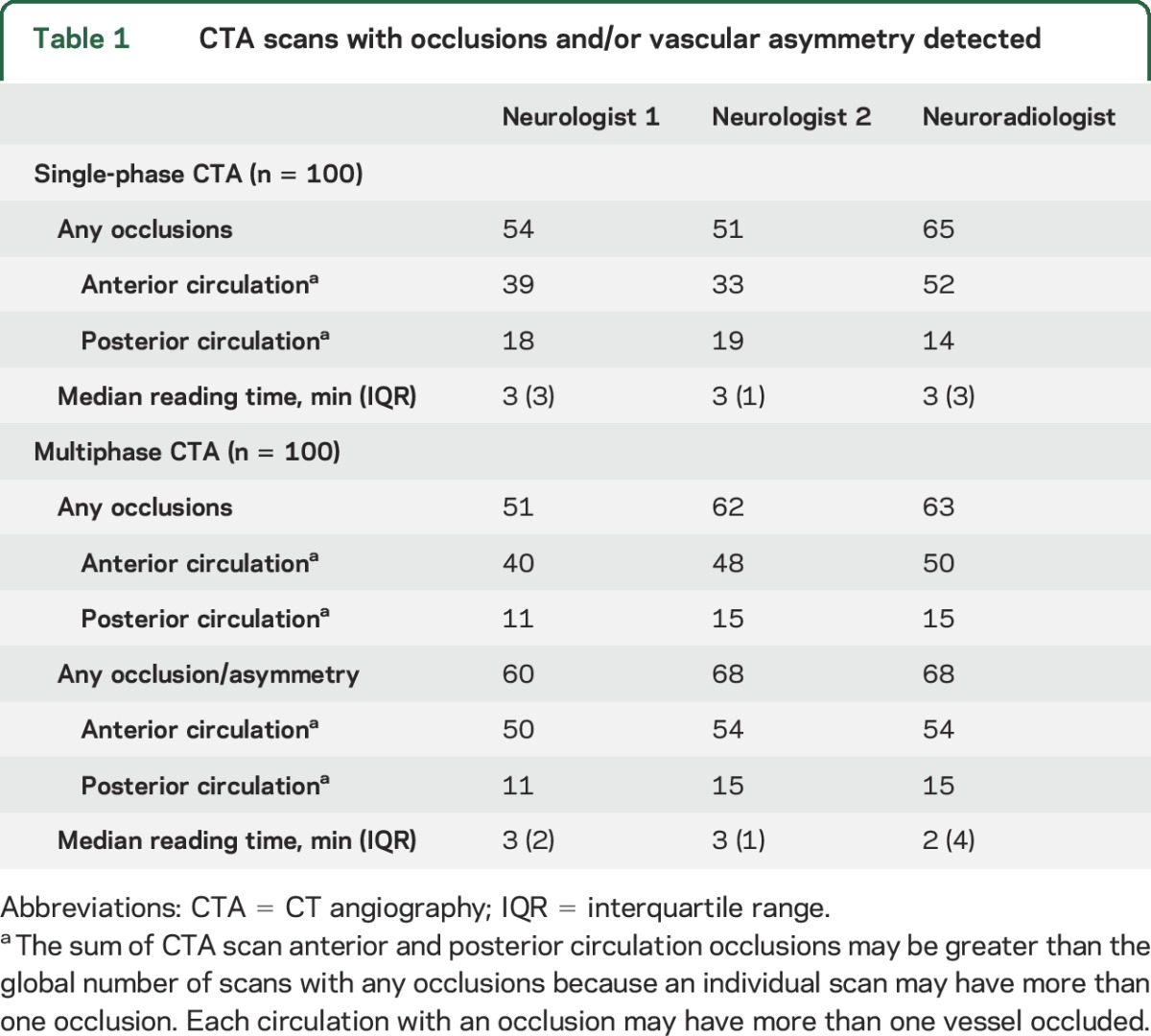
Single- and multiphase CTA scans from 100 patients were interpreted. Table 1 outlines the total number of scans with an occlusion, as identified by each reader, in any circulation and categorized into anterior and posterior circulation. The median reading time was 3 minutes per single-phase CTA scan for all readers and 3 minutes per multiphase CTA scan for the neurologists and 2 minutes for the neuroradiologist. On single-phase CTA, of the 52 anterior and 14 posterior occlusions identified by the neuroradiologist, neurologist 1 correctly identified 34 (65%) anterior and 12 (86%) posterior circulation occlusions and neurologist 2 correctly identified 28 (54%) anterior and 9 (64%) posterior circulation occlusions. Neurologist 1 identified 5 false-positive cases in the anterior circulation and 6 in the posterior circulation (occlusions detected by the neurologist that were not reported by the neuroradiologist). Neurologist 2 identified 6 and 10 false-positive cases in the anterior and posterior circulations, respectively. On multiphase CTA, when assessing both vessel occlusion and pial vessel asymmetry, of the 54 anterior and 15 posterior circulation abnormalities identified by the neuroradiologist, neurologist 1 correctly identified 47 (87%) anterior and 6 (40%) posterior circulation abnormalities and neurologist 2 correctly identified 50 (93%) anterior and 7 (47%) posterior circulation abnormalities. Neurologist 1 identified 3 and 4 false-positive cases in the anterior and posterior circulations and neurologist 2 identified 5 and 8 false-positive cases in those respective circulations.
Table 1.
CTA scans with occlusions and/or vascular asymmetry detected
Interrater agreement was fair (κ = 0.45 and 0.32) between the neurologists and neuroradiologist for occlusion detection using the single-phase CTA (table 2). The agreement remained similar when stratifying the single-phase CTA results by anterior vs posterior occlusion. However, there was substantial agreement (κ = 0.76 and 0.72) for anterior circulation occlusions using multiphase CTA and the agreement further improved for identification of an anterior circulation occlusion or asymmetry of pial vessel filling using multiphase CTA (κ = 0.80 and 0.84). Most of the anterior occlusions detected by the neuroradiologist on the multiphase CTA were distal (n = 53; some scans had more than one occlusion in the same anterior circulation territory): 8 proximal M1 branch occlusions, 26 M2, and 19 M3 and beyond. The agreement was fair to moderate for proximal vs distal occlusions on single-phase CTA (κ = 0.56 and 0.40 for proximal and κ = 0.39 and 0.33 for distal occlusions, for neurologist 1 and 2, respectively, compared to the neuroradiologist). The agreement improved with multiphase CTA (κ = 0.71 and 0.74 for proximal and κ = 0.62 and 0.59 for distal occlusions, for neurologist 1 and 2, respectively).
Table 2.
Kappa coefficient and 95% confidence intervals for agreement between readers
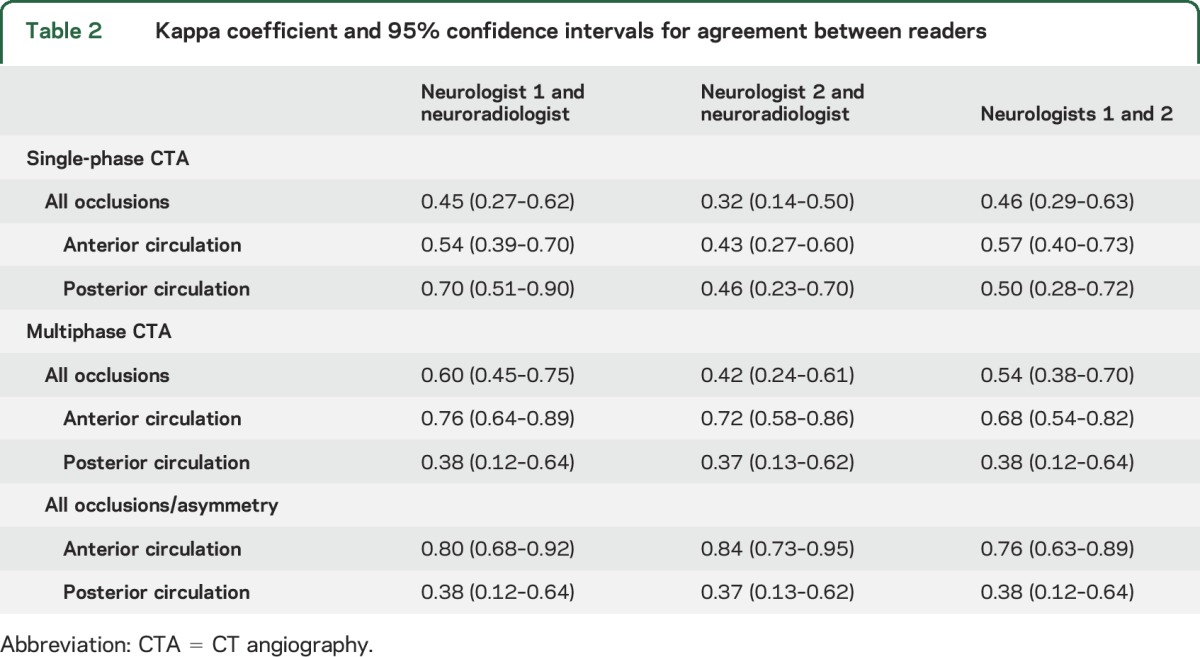
Interrater agreement appeared lower using multiphase CTA for occlusions in the posterior circulation (κ = 0.38 and 0.37, table 2). Most posterior circulation occlusions (n = 15) detected by the neuroradiologist were distal: one proximal PCA, 6 distal PCA, one anterior inferior cerebellar artery, one superior cerebellar artery, 3 VA-PICA, and 3 basilar artery, including one partially thrombosed ectatic basilar.
Ninety-three patients had a follow-up MRI, 6 had a CT, and one had no follow-up imaging. Of the 54 anterior circulation occlusions or asymmetries detected by the neuroradiologist on multiphase CTA, 45 (83%) had evidence of cerebral ischemia in the relevant anterior circulation distribution on follow-up imaging. Of the 15 posterior circulation occlusions detected by the neuroradiologist on multiphase CTA, 12 (80%) had evidence of posterior circulation cerebral ischemia.
DISCUSSION
We show that the interrater agreement for detection of intracranial anterior circulation occlusion between nonexpert stroke neurology trainees and an expert neuroradiologist is high when using multiphase CTA. In contrast, the agreement is only fair with the standard single-phase CTA.
Because acute ischemic stroke treatment is time-sensitive, front-line physicians, including stroke neurologists, general neurologists, emergency room physicians, and trainees have important roles in image interpretation. Neurovascular imaging interpretation in patients with acute ischemic stroke is analogous to ECG interpretation by emergency room physicians or internists for acute myocardial infarction. We showed that evaluation of pial vessel asymmetry allowed for detection of additional potential ischemic vascular territory in the anterior circulation. In the context of suspected acute stroke, asymmetric pial vessel filling suggests asymmetric blood flow, due to either intra- or extracranial vascular high-grade stenosis or occlusion. Identifying the acute ischemic stroke patients with symptomatic vascular occlusions helps distinguish the most high-risk patients and has the potential to lead to more rapid institution of treatment.7,8,13 Another explanation for pial vessel asymmetry without detection of occlusion is early recanalization without reperfusion. Identifying these patients is relevant because reperfusion has been shown to be a stronger predictor of good clinical and imaging outcome compared to recanalization.14,15 Furthermore, multiphase CTA has been shown to have higher interrater agreement for grading collateral status, which is an independent predictor for clinical outcome after acute ischemic stroke.16,17 Despite increased information with this advanced imaging technique, patient management is not expected to be delayed as reading times are similar between the 2 imaging modalities and between neurologists and neuroradiologist. Moreover, our results are consistent between the 2 stroke neurology trainees who read the images independently, suggesting that our findings can be replicated by other neurologists.
It may be more difficult for trainees to detect occlusions in the posterior circulation; we showed low interrater agreement. The additional phases on the multiphase CTA did not seem to confer more information for posterior circulation occlusions. Plausible reasons for this observation include that (1) most of the posterior circulation findings were in the distal vasculature where the vessels are smaller and the anatomy is more variable, (2) the deep cerebral veins on phases 2 and 3 of the multiphase CTA make imaging interpretation more challenging, and (3) blinding to clinical symptoms may have also made these interpretations challenging for nonradiologist clinicians.
A limitation of our study is that the number of posterior circulation occlusions was small (20% of the cohort) and our confidence in the results in this stratum is lower. However, this is representative of the proportion reported in the acute ischemic stroke literature.7 We did not capture any patients with anterior cerebral artery occlusions. There may be challenges in the generalization of our findings to other centers. Imaging interpretation is the last step of a multistep process, including proper installation of advanced imaging equipment, training technologists, as well as troubleshooting patient factors, such as poor cardiac output and motion artifact. Addressing these issues to yield a high-quality image is as important as imaging interpretation. Finally, while the current study did not evaluate other acute neuroimaging tools for suspected cerebral ischemia, such as the CT perfusion or acute MRI, a future study comparing these tools with multiphase CTA may be of interest. CT perfusion has shown potential to provide additional information, but it is currently limited by the lack of standardization of the imaging protocol, the absence of uniform definition for the ischemic core and penumbra, as well as its vulnerability to motion artifact and image processing delays.13
Multiphase CTA is an innovative, user-friendly imaging tool that can improve diagnostic accuracy for anterior circulation intracranial occlusions, even when used by relatively inexperienced readers and even when occlusions are distal. The inclusion of the multiphase CTA in acute ischemic stroke imaging protocols has the potential to allow non–radiology trained physicians and trainees to rapidly and accurately determine anterior circulation occlusions, a crucial point in the clinical management of the patient for revascularization procedures.
Glossary
- ACA
anterior cerebral artery
- CTA
CT angiography
- MCA
middle cerebral artery
- MIP
maximum intensity projection
- PCA
posterior cerebral artery
- PRoveIT
Precise and Rapid Assessment of Collaterals Using Multiphase CTA in the Triage of Patients with Acute Ischemic Stroke for Intra-arterial Therapy
- TEMPO-1
TNK-tPA Evaluation for Minor Ischemic Stroke with Proven Occlusion
- VA-PICA
vertebral artery–posterior inferior cerebellar artery
AUTHOR CONTRIBUTIONS
A.Y.X. Yu contributed to the study concept and design, acquisition, analysis, and interpretation of the data, as well as drafting and revising the manuscript. C. Zerna contributed to the study concept and design, acquisition, analysis, and interpretation of the data, and revising the manuscript. Z. Assis contributed to the study concept and design, acquisition, and revising the manuscript. J.K. Holodinsky contributed to the data analysis and interpretation and revising the manuscript. P.A. Randhawa contributed to acquisition of data and revising the manuscript. M. Najm contributed to acquisition of data and revising the manuscript. M. Goyal contributed to study concept and design, data interpretation, contribution of vital tools, and revising the manuscript. B.K. Menon contributed to study concept and design, data interpretation, contribution of vital tools, and revising the manuscript. A.M. Demchuk contributed to study concept and design, data interpretation, contribution of vital tools, and revising the manuscript. S.B. Coutts contributed to study concept and design, data interpretation, and revising the manuscript. M.D. Hill contributed to the study concept and design, acquisition, analysis, and interpretation of the data, as well as drafting and revising the manuscript and overall study supervision.
STUDY FUNDING
The PRoveIT Study (NCT02184936) is funded by the Canadian Institute of Health Research. Data and material presented in this study are also supported by the Heart and Stroke Foundation/University of Calgary Professorship that B.K.M. currently holds. The TEMPO-1 Study (NCT02398656) was funded by the Heart and Stroke Foundation of Alberta and Nunavut and Alberta Innovates: Health Solutions.
DISCLOSURE
A. Yu, C. Zerna, Z. Assis, J. Holodinsky, P. Randhawa, and M. Najm report no disclosures relevant to the manuscript. M. Goyal is a consultant for Covidien for teaching engagements and for design and conduct of SWIFT PRIME trial; University of Calgary received partial funding for ESCAPE trial provided by Covidien through an unrestricted grant to the institution. Dr. Goyal has a patent systems and methods for diagnosing strokes (PCT/CA2013/000761) licensed to GE Healthcare. B.K. Menon reports membership of the steering and executive committee, ESCAPE trial that received support from Covidien Inc., site principal investigator, SOCRATES Trial, sponsored by AstraZeneca, honoraria from Penumbra Inc., a provisional patent 62/086077 for triaging systems in ischemic stroke, research funding from CIHR, HSFC, AIHS, HBI, and the Faculty of Medicine, University of Calgary, and reports board membership of QuikFlo Health Inc. A. Demchuk received honoraria for CME and an unrestricted grant to support the ESCAPE trial from Covidien. S. Coutts received salary support from an Alberta Innovates Clinical Investigator award. M. Hill reports consulting fees from Vernalis Group; grant support from Covidien and Hoffmann–La Roche Canada; lecture fees from Hoffmann–La Roche Canada, Servier Canada, Bristol-Myers Squibb Canada; stock ownership in Calgary Scientific; financial support from Heart and Stroke Foundation of Alberta, Northwest Territories, and Nunavut and Alberta Innovates–Health Solutions, and board membership of QuikFlo Health Inc. Go to Neurology.org for full disclosures.
REFERENCES
- 1.Berkhemer OA, Fransen PS, Beumer D, et al. A randomized trial of intraarterial treatment for acute ischemic stroke. N Engl J Med 2015;372:11–20. [DOI] [PubMed] [Google Scholar]
- 2.Goyal M, Demchuk AM, Menon BK, et al. Randomized assessment of rapid endovascular treatment of ischemic stroke. N Engl J Med 2015;372:1019–1030. [DOI] [PubMed] [Google Scholar]
- 3.Campbell BC, Mitchell PJ, Kleinig TJ, et al. Endovascular therapy for ischemic stroke with perfusion-imaging selection. N Engl J Med 2015;372:1009–1018. [DOI] [PubMed] [Google Scholar]
- 4.Jovin TG, Chamorro A, Cobo E, et al. Thrombectomy within 8 hours after symptom onset in ischemic stroke. N Engl J Med 2015;372:2296–2306. [DOI] [PubMed] [Google Scholar]
- 5.Saver JL, Goyal M, Bonafe A, et al. Stent-retriever thrombectomy after intravenous t-PA vs. t-PA alone in stroke. N Engl J Med 2015;372:2285–2295. [DOI] [PubMed] [Google Scholar]
- 6.Coutts SB, Modi J, Patel SK, et al. What causes disability after transient ischemic attack and minor stroke? Results from the CT and MRI in the Triage of TIA and Minor Cerebrovascular Events to Identify High Risk Patients (CATCH) Study. Stroke 2012;43:3018–3022. [DOI] [PubMed] [Google Scholar]
- 7.van der Hoeven EJ, Schonewille WJ, Vos JA, et al. The Basilar Artery International Cooperation Study (BASICS): study protocol for a randomised controlled trial. Trials 2013;14:200–208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Coutts SB, Dubuc V, Mandzia J, et al. Tenecteplase-tissue-type plasminogen activator evaluation for minor ischemic stroke with proven occlusion. Stroke 2015;46:769–774. [DOI] [PubMed] [Google Scholar]
- 9.Menon BK, d'Esterre CD, Qazi EM, et al. Multiphase CT angiography: a new tool for the imaging triage of patients with acute ischemic stroke. Radiology 2015;275:510–520. [DOI] [PubMed] [Google Scholar]
- 10.Yang CY, Chen YF, Lee CW, et al. Multiphase CT angiography versus single-phase CT angiography: comparison of image quality and radiation dose. AJNR Am J Neuroradiol 2008;29:1288–1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Demchuk AM, Menon BK, Goyal M. Comparing vessel imaging: noncontrast computed tomography/computed tomographic angiography should be the new minimum standard in acute disabling stroke. Stroke 2015;47:273–281. [DOI] [PubMed] [Google Scholar]
- 12.Landis JR, Koch GG. The measurement of observer agreement for categorical data. Biometrics 1977;33:159–174. [PubMed] [Google Scholar]
- 13.Zerna C, Assis Z, d'Esterre CD, Menon BK, Goyal M. Imaging, intervention, and workflow in acute ischemic stroke: the Calgary approach. AJNR Am J Neuroradiol 2016;37:978–984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cho TH, Nighoghossian N, Mikkelsen IK, et al. Reperfusion within 6 hours outperforms recanalization in predicting penumbra salvage, lesion growth, final infarct, and clinical outcome. Stroke 2015;46:1582–1589. [DOI] [PubMed] [Google Scholar]
- 15.Tsai JP, Albers GW. Reperfusion versus recanalization: the winner is…. Stroke 2015;46:1433–1434. [DOI] [PubMed] [Google Scholar]
- 16.Flores A, Rubiera M, Ribo M, et al. Poor collateral circulation assessed by multiphase computed tomographic angiography predicts malignant middle cerebral artery evolution after reperfusion therapies. Stroke 2015;46:3149–3153. [DOI] [PubMed] [Google Scholar]
- 17.Shin NY, Kim KE, Park M, et al. Dual-phase CT collateral score: a predictor of clinical outcome in patients with acute ischemic stroke. PLoS One 2014;9:e107379. [DOI] [PMC free article] [PubMed] [Google Scholar]