Abstract
Human immunodeficiency virus type 1 (HIV-1) proviral mutants that lack viral regulatory genes are unable to replicate unless rescued by complementation in trans. Structurally intact virus can be produced by infecting recombinant cell lines expressing the deficient genes. A HIV-1 mutant functionally defective in tat and rev (vIIIB delta Tat/Rev), which replicates only in a recombinant T-cell line expressing tat and rev (CEMTART), is described in this report. Infection of the CEMTART cell line with vIIIB delta Tat/Rev permits the complete HIV-1 life cycle, including cytopathology, decreased expression of CD4, and production of viral structural proteins, to be biologically contained. Culture supernatants from infected CEMTART contain virus that is able to replicate only in uninfected CEMTART. No reversion of vIIIB delta Tat/Rev to wild-type HIV-1 was observed as measured either by sequencing proviral vIIIB delta Tat/Rev or by detecting the ability of vIIIB delta Tat/Rev to replicate in CEM or activated CD4-bearing T lymphocytes. Defective HIV-1 mutants produced by trans complementation of essential genes permit infection and analysis of defined genotypes on cellular function and phenotype. Authentic HIV-1 structural proteins and infected cells can be prepared in mass, and agents that interfere with the HIV-1 life cycle can be studied on a large scale with minimum risk of exposing workers to virulent HIV-1.
Full text
PDF

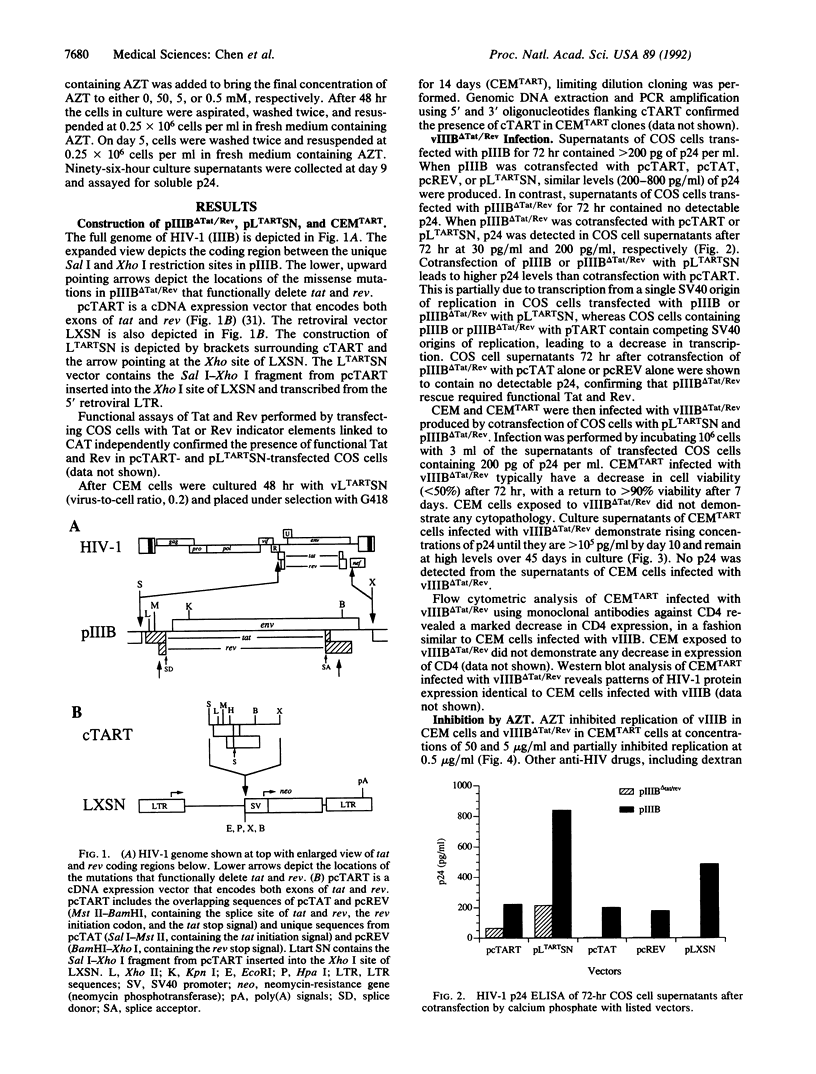
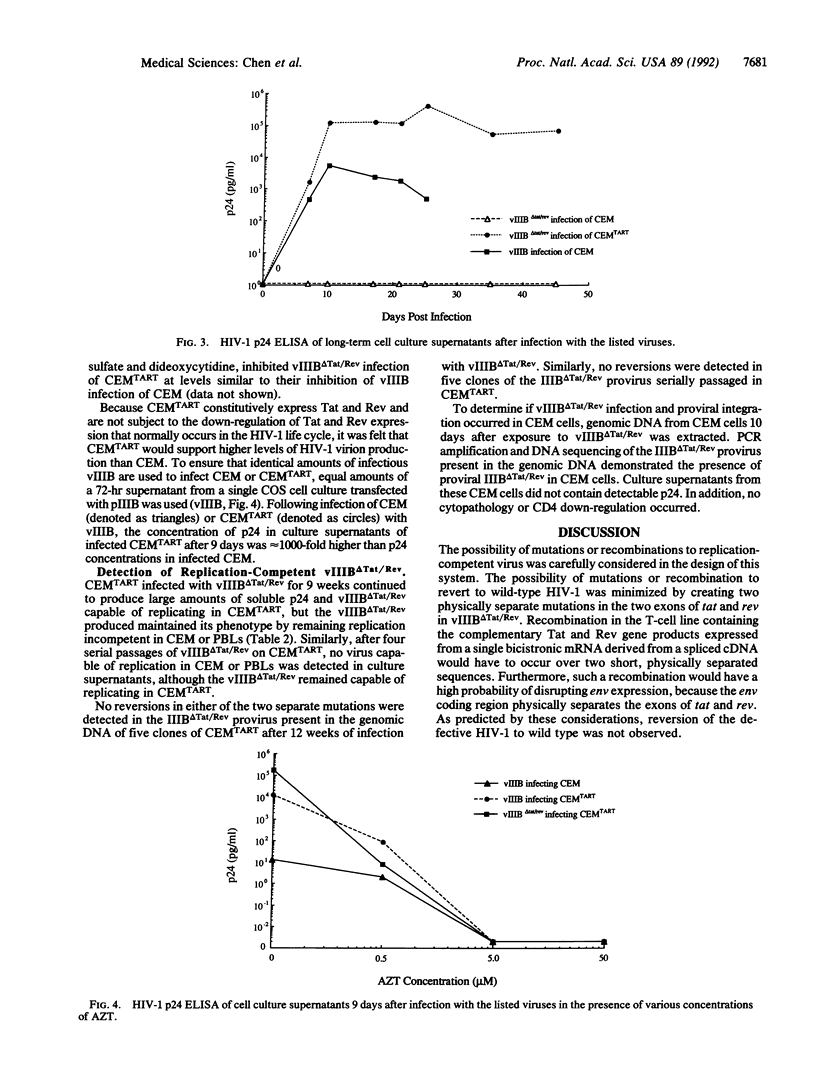

Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arya S. K., Beaver B., Jagodzinski L., Ensoli B., Kanki P. J., Albert J., Fenyo E. M., Biberfeld G., Zagury J. F., Laure F. New human and simian HIV-related retroviruses possess functional transactivator (tat) gene. Nature. 1987 Aug 6;328(6130):548–550. doi: 10.1038/328548a0. [DOI] [PubMed] [Google Scholar]
- Botstein D., Falco S. C., Stewart S. E., Brennan M., Scherer S., Stinchcomb D. T., Struhl K., Davis R. W. Sterile host yeasts (SHY): a eukaryotic system of biological containment for recombinant DNA experiments. Gene. 1979 Dec;8(1):17–24. doi: 10.1016/0378-1119(79)90004-0. [DOI] [PubMed] [Google Scholar]
- Conte J. E., Jr Infection with human immunodeficiency virus in the hospital. Epidemiology, infection control, and biosafety considerations. Ann Intern Med. 1986 Nov;105(5):730–736. doi: 10.7326/0003-4819-105-5-730. [DOI] [PubMed] [Google Scholar]
- Cornetta K., Anderson W. F. Protamine sulfate as an effective alternative to polybrene in retroviral-mediated gene-transfer: implications for human gene therapy. J Virol Methods. 1989 Feb;23(2):187–194. doi: 10.1016/0166-0934(89)90132-8. [DOI] [PubMed] [Google Scholar]
- Cullen B. R. Regulation of HIV-1 gene expression. FASEB J. 1991 Jul;5(10):2361–2368. doi: 10.1096/fasebj.5.10.1712325. [DOI] [PubMed] [Google Scholar]
- Curtiss R., 3rd Biological containment and cloning vector transmissibility. J Infect Dis. 1978 May;137(5):668–675. doi: 10.1093/infdis/137.5.668. [DOI] [PubMed] [Google Scholar]
- Desrosiers R. C., Hunter E. AIDS biosafety. Science. 1991 May 31;252(5010):1231–1231. doi: 10.1126/science.1953906. [DOI] [PubMed] [Google Scholar]
- Fisher E., Lincoln D. R. Assessing physical containment in recombinant DNA facilities. Recomb DNA Tech Bull. 1984 Mar;7(1):1–7. [PubMed] [Google Scholar]
- Greene W. C. The molecular biology of human immunodeficiency virus type 1 infection. N Engl J Med. 1991 Jan 31;324(5):308–317. doi: 10.1056/NEJM199101313240506. [DOI] [PubMed] [Google Scholar]
- Grumet F. C., MacPherson J. L., Hoppe P. A., Smallwood L. A. Application of biosafety principles in blood establishments. Transfusion. 1988 Sep-Oct;28(5):502–505. doi: 10.1046/j.1537-2995.1988.28588337348.x. [DOI] [PubMed] [Google Scholar]
- Ishak R., Linhares A. C., Ishak M. O. Biossegurança no laboratório. Rev Inst Med Trop Sao Paulo. 1989 Mar-Apr;31(2):126–131. doi: 10.1590/s0036-46651989000200011. [DOI] [PubMed] [Google Scholar]
- Kestler H. W., 3rd, Ringler D. J., Mori K., Panicali D. L., Sehgal P. K., Daniel M. D., Desrosiers R. C. Importance of the nef gene for maintenance of high virus loads and for development of AIDS. Cell. 1991 May 17;65(4):651–662. doi: 10.1016/0092-8674(91)90097-i. [DOI] [PubMed] [Google Scholar]
- Knudsen S. M., Karlström O. H. Development of efficient suicide mechanisms for biological containment of bacteria. Appl Environ Microbiol. 1991 Jan;57(1):85–92. doi: 10.1128/aem.57.1.85-92.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuehne R. W. Biological containment facility for studying infectious disease. Appl Microbiol. 1973 Sep;26(3):239–243. doi: 10.1128/am.26.3.239-243.1973. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Böhnlein S., Fenrick R., Le S. Y., Maizel J. V., Cullen B. R. Functional comparison of the Rev trans-activators encoded by different primate immunodeficiency virus species. Proc Natl Acad Sci U S A. 1989 Nov;86(21):8222–8226. doi: 10.1073/pnas.86.21.8222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Fenrick R., Ballard D. W., Hauber J., Böhnlein E., Cullen B. R. Functional characterization of a complex protein-DNA-binding domain located within the human immunodeficiency virus type 1 long terminal repeat leader region. J Virol. 1989 Aug;63(8):3213–3219. doi: 10.1128/jvi.63.8.3213-3219.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Fenrick R., Cullen B. R. Immunodeficiency virus rev trans-activator modulates the expression of the viral regulatory genes. Nature. 1988 Sep 8;335(6186):181–183. doi: 10.1038/335181a0. [DOI] [PubMed] [Google Scholar]
- Malim M. H., Hauber J., Le S. Y., Maizel J. V., Cullen B. R. The HIV-1 rev trans-activator acts through a structured target sequence to activate nuclear export of unspliced viral mRNA. Nature. 1989 Mar 16;338(6212):254–257. doi: 10.1038/338254a0. [DOI] [PubMed] [Google Scholar]
- Miller A. D. Retrovirus packaging cells. Hum Gene Ther. 1990 Spring;1(1):5–14. doi: 10.1089/hum.1990.1.1-5. [DOI] [PubMed] [Google Scholar]
- Miller A. D., Rosman G. J. Improved retroviral vectors for gene transfer and expression. Biotechniques. 1989 Oct;7(9):980-2, 984-6, 989-90. [PMC free article] [PubMed] [Google Scholar]
- Miller A. D., Trauber D. R., Buttimore C. Factors involved in production of helper virus-free retrovirus vectors. Somat Cell Mol Genet. 1986 Mar;12(2):175–183. doi: 10.1007/BF01560664. [DOI] [PubMed] [Google Scholar]
- Mitsuya H., Weinhold K. J., Furman P. A., St Clair M. H., Lehrman S. N., Gallo R. C., Bolognesi D., Barry D. W., Broder S. 3'-Azido-3'-deoxythymidine (BW A509U): an antiviral agent that inhibits the infectivity and cytopathic effect of human T-lymphotropic virus type III/lymphadenopathy-associated virus in vitro. Proc Natl Acad Sci U S A. 1985 Oct;82(20):7096–7100. doi: 10.1073/pnas.82.20.7096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newsom S. W. Class II (laminar flow) biological safety cabinet. J Clin Pathol. 1979 May;32(5):505–513. doi: 10.1136/jcp.32.5.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saiki R. K., Gelfand D. H., Stoffel S., Scharf S. J., Higuchi R., Horn G. T., Mullis K. B., Erlich H. A. Primer-directed enzymatic amplification of DNA with a thermostable DNA polymerase. Science. 1988 Jan 29;239(4839):487–491. doi: 10.1126/science.2448875. [DOI] [PubMed] [Google Scholar]
- Sanger F., Nicklen S., Coulson A. R. DNA sequencing with chain-terminating inhibitors. Proc Natl Acad Sci U S A. 1977 Dec;74(12):5463–5467. doi: 10.1073/pnas.74.12.5463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szybalski Waclaw. Summary and critique of the new NIH guidelines for recombinant DNA research. Gene. 1979;5:179–181+. [PubMed] [Google Scholar]
- Talbot B. Development of the National Institutes of Health Guidelines for Recombinant DNA Research. Public Health Rep. 1983 Jul-Aug;98(4):361–368. [PMC free article] [PubMed] [Google Scholar]
- Vaishnav Y. N., Wong-Staal F. The biochemistry of AIDS. Annu Rev Biochem. 1991;60:577–630. doi: 10.1146/annurev.bi.60.070191.003045. [DOI] [PubMed] [Google Scholar]
- Viglianti G. A., Mullins J. I. Functional comparison of transactivation by simian immunodeficiency virus from rhesus macaques and human immunodeficiency virus type 1. J Virol. 1988 Dec;62(12):4523–4532. doi: 10.1128/jvi.62.12.4523-4532.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss S. H., Goedert J. J., Gartner S., Popovic M., Waters D., Markham P., di Marzo Veronese F., Gail M. H., Barkley W. E., Gibbons J. Risk of human immunodeficiency virus (HIV-1) infection among laboratory workers. Science. 1988 Jan 1;239(4835):68–71. doi: 10.1126/science.3336776. [DOI] [PubMed] [Google Scholar]



