Abstract
Objectives:
This experimental study evaluated the effect of bonding application time on the microshear bond strength of composite resin to different types of glass ionomer cements (GICs).
Materials and Methods:
One-hundred and sixty specimens (two conventional and two resin-modified GICs) were prepared and divided into 16 groups. The surface of all specimens was prepared using two different bonding systems (Frog and Stea) at three different times. After setting, the composite resin (Z100) was placed over the GICs. The specimens were then stored in distilled water for 24 hours (37°C) and exposed to microshear stresses at a crosshead speed of 1 mm/min. The results were analyzed using three-way ANOVA and Tukey’s test (P<0.05).
Results:
In conventional GICs, bond strength was affected by the type of bonding system at different times, and bond strength was significantly higher in the Fuji II group compared to Riva Self Cure group. In the Riva Self Cure group, bond strength was significantly affected by time; whereas, the type of bonding system failed to exert a significant effect on bond strength. There was no significant correlation between the type of bonding system and the two brands of resin-modified GICs. Bond strength was not affected by the type of bonding agent; however, among the two brands of resin-modified GICs, Fuji II LC yielded a significantly stronger bond.
Conclusion:
It appears that the type of bonding agent does not affect the microshear bond strength, and the bonding application time affects the microshear bond strength in Riva Self Cure GICs.
Keywords: Dentin-Bonding Agents; Acid Etching, Dental; Glass Ionomer Cements
INTRODUCTION
Application of GICs in conjunction with composite resin is a conventional restorative method known as the “sandwich technique”. This technique combines the optimal properties of composite resins (i.e. optimal esthetics and wear resistance) with those of GICs (i.e. durable bond with dentin and long term release of fluoride) [1,2].
In order to achieve a successful sandwich technique restoration, a strong bond between the composite resin and the GIC is required to overcome the internal stresses related to the setting of the composite resin and the clinical stresses imposed in long-term [1].
Numerous factors affect the quality of the bond between the composite resin and the conventional GICs which include the time elapsed between mixing the cement and etching [3,4], the etching time [5], type of cement [6], application of an intermediate unfilled resin [4,7] and the properties of the unfilled resin such as wettability and texture [8]. The bond between the conventional GIC and composite resin is micromechanical and achieved through irregularities developed on the surface of the GIC during the bonding procedure (i.e. similar to bonding composite resin to dental hard tissues).
These porosities are formed by acid etching using phosphoric acid. It was demonstrated that rapid etching of the newly set GIC may compromise its mechanical properties and bond strength; whereas delaying the etching by up to 24 hours post application of cement improved the bond strength [9]. Some have suggested delaying the etching for 15 minutes after the initiation of mixing [3]. Since long deferment is impractical, researchers have proposed the application of resin-modified GICs, to form a direct chemical bond between the methacrylate components in both materials within a shorter period of time [10].
Currently, there is an increasing trend toward the use of self-etch bonding systems. These systems contain acidic monomers, which are capable of concurrent etching and priming, eliminating the need for extra steps of etching and rinsing. This reduces technique sensitivity and the overall duration of the procedure resulting in improved clinical efficacy [11].
Although numerous studies have been carried out on different properties of sandwich restorations including bond strength, microleakage and the clinical efficacy of this technique, the majority of these studies have studied the etch and rinse bonding systems [12,13].
Ghassemi et al. conducted a study on the bonding application time in association with the microleakage of Class V sandwich restorations and revealed that time did not affect microleakage at the occlusal margins; whereas, in the gingival margins, microleakage decreased with time [14].
However, there is limited information regarding the effect of bonding application time in self-etch systems on the bond strength between GICs and composite resins. Thus, the present study sought to evaluate the effect of etching time and the application of self etch bonding systems on the microshear bond strength between composite resins and GICs.
MATERIALS AND METHODS
The present in vitro study involved the use of four types of GICs: Two resin-modified GICs namely Riva Light Cure (SDI) and Fuji II LC (GC International), and two conventional GICs namely Riva Self Cure (SDI) and Fuji II (GC International), two bonding systems namely an etch & rinse Stea (SDI) and self etch Frog (SDI) and a light cure composite resin (Z100, 3M ESPE).
Table 1 lists the materials evaluated in this study. A total of 160 GIC specimens were made using molds with dimensions of 2×4×6 mm.
Table 1.
Materials evaluated in this study
| Material | Type | Composition | Manufacturer | Working time | Setting time |
|---|---|---|---|---|---|
| Fuji II | Conventional glass ionomer | Powder: Fluoroaluminosilicate glass | GC International Corp., Tokyo, Japan | 1 min., 45 s | 5 min., 30 s |
| Liquid: Acrylic acid, maleic acid, tartaric acid, water | |||||
| Fuji II LC | Resin modified glass ionomer | Powder: Fluoroaluminosilicate glass | GC International Corp., Tokyo, Japan | 3 min, 45 s | 20 s |
| Liquid: Acrylic acid, maleic acid, HEMA, water, camphorquinone | |||||
| Riva Self Cure | Conventional glass ionomer | Powder: Fluoroaluminosilicate glass | SDI, Victoria, Australia | 1 min., 40 s | 6 min. |
| Liquid: Acrylic acid, tartaric acid, water | |||||
| Riva Light Cure | Resin modified glass ionomer | Powder: Fluoroaluminosilicate glass | SDI, Victoria, Australia | 2 min., 10 s | 20 s |
| Liquid: Acrylic acid, HEMA, water, camphorquinone | |||||
| Frog | Etch & rinse bonding | Primer: Phosphoric acid ester monomer, 2-hydroxyethyl methacrylate (HEMA), Dimethacrylate monomer, Water, Photoinitiators, Stabilizer | SDI, Victoria, Australia | - | 20 s |
| Bonding agent: Phosphoric acid ester monomer, 2-hydroxyethyl methacrylate (HEMA), Dimethacrylate monomer, Silicon dioxide filler, Photoinitiator | |||||
| Stae | Self etch bonding | Acrylic monomer, Acetone, Fluoride, Stabilizer | SDI, Victoria, Australia | - | 20 s |
| Z100 | Composite resin | BIS-GMA (Bisphenol A diglycidyl ether dimethacrylate), TEGDMA (triethylene glycol dimethacrylate), 66% (volume) silica/zirconia filler | 3M ESPE, St. Paul, MN, USA | - | 40 s |
The GICs were prepared according to the manufacturers’ instructions. After filling the molds with the cement, the surface was covered with a Mylar strip and a glass slab was placed on top of them to achieve a smooth surface. The surface of the conventional GICs was then prepared using the Stea and Frog bonding systems at three time points (after working time, immediately after setting and 15 minutes after mixing). The resin-modified GICs were also etched using the two bonding systems immediately after curing. Table 2 shows the study groups and the preparation procedures.
Table 2.
Study groups and microshear bond strength values of GICs
| Group* | Glass inomer | Bonding agent | Time | Mean± SD** |
|---|---|---|---|---|
| 1 | Riva Self Cure | Stae | After working time | 12.59±4.48 |
| 2 | Riva Self Cure | Stae | After setting | 13.78±6.08 |
| 3 | Riva Self Cure | Stae | 15 minutes after mixing | 9.72±4.38 |
| 4 | Riva Light Cure | Stae | After curing | 14.47±2.25 |
| 5 | Fuji II | Stae | After working time | 15.35±6.50 |
| 6 | Fuji II | Stae | After setting | 19.93±8.48 |
| 7 | Fuji II | Stae | 15 minutes after mixing | 16.91±3.90 |
| 8 | Fuji II LC | Stae | After curing | 27.81±8.14 |
| 9 | Riva Self Cure | Frog | After working time | 16.65±3.36 |
| 10 | Riva Self Cure | Frog | After setting | 10.96±4.96 |
| 11 | Riva Self Cure | Frog | 15 minutes after mixing | 11.69±3.20 |
| 12 | Riva Light Cure | Frog | After curing | 19.30±8.95 |
| 13 | Fuji II | Frog | After working time | 18.89±7.35 |
| 14 | Fuji II | Frog | After setting | 15.74±7.76 |
| 15 | Fuji II | Frog | 15 minutes after mixing | 13.61±3.05 |
| 16 | Fuji II LC | Frog | After curing | 25.96±9.95 |
10 specimens in each group
SD: Standard deviation
Glass ionomer surface treatment:
For the etch & rinse group (Stea), the surface of the GIC blocks was initially etched with 37% phosphoric acid for 15 seconds, rinsed (10 seconds) and moist-dried. The bonding was then applied and cured for 40 seconds using Arialux (650 mW/cm2) light curing unit (ApadanaTak, Tehran, Iran).
For the self-etch group (Frog), the surface of the GIC blocks was initially treated by the primer for 10 seconds and gently dried with air spray. The bonding agent was then applied using a fine applicator and gently dried and cured for 10 seconds. Finally, Z100 composite resin (A2) in plastic tubes (Tygon tubes) with an internal diameter of 0.7mm and height of 1mm was placed on the surface of the GIC holding the tube perpendicular to the specimens. The composite was cured for 40 seconds and subsequently stored in distilled water (37°C) for 24 hours. The tubes were then cut using a scalpel and removed from around the composite resins. To evaluate the microshear bond strength, the samples were placed in a microtensile tester (Bisco, Schaumburg, IL, USA) and subjected to microshear stress at a crosshead speed of 1 mm/min. The force needed to break the samples was recorded in Newton and the microshear bond strength was calculated by dividing the maximum force by the surface area of the specimens (MPa). The data were subjected to three-way ANOVA and Tukey’s test. Level of significance was set at P<0.05.
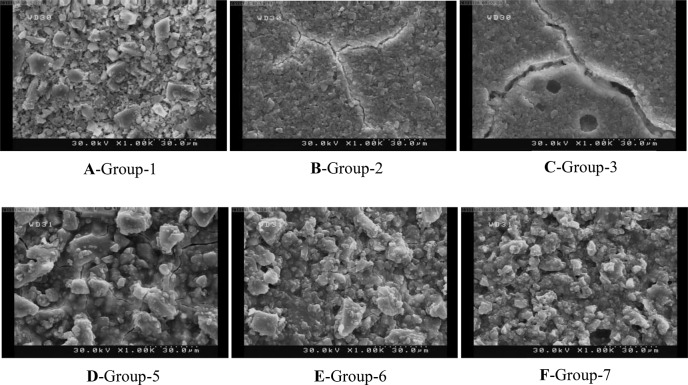
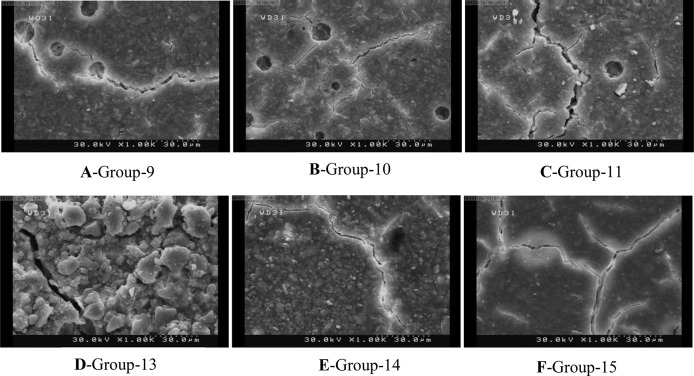
To assess the surface characteristics of glass ionomers after different treatments under scanning electron microscope (SEM), one more specimen was made in each group. Each specimen was treated the same as its corresponding group; the only difference was that on these specimens no adhesive resin was applied. Samples were then mounted on SEM stubs and sputter coated with gold. Characteristic photomicrographs were obtained at ×1000 magnification.
RESULTS
Table 2 illustrates the microshear bond strength values in conventional GICs treated with the two bonding systems at different times. The multivariate effect of the two brands of GICs, two bonding systems and the three different times on the microshear bond strength was assessed using the three-way ANOVA.
The test results failed to reveal a significant three-way combined effect of the covariates or the interaction effect of the GIC-bonding agent and the GIC-time on the bond strength. However, the cross-effect of the bonding and time was significant (P<0.05). In other words, microshear bond strength at different intervals was shown to be affected by the type of bonding agent. On the other hand, the type of GIC markedly affected the bond strength (P<0.001).
The results yielded significantly higher bond strength in Fuji II compared to Riva Self Cure, regardless of the type of bonding agent and time.
To further evaluate the correlation between the bonding material and time, the effect of the two factors on microshear bond strength was calculated using the two-way ANOVA. It was observed that in the Riva Self Cure group, the type of bonding agent failed to affect the bond strength (P=0.365), while the bonding application time significantly affected the bond strength (P<0.05). Tukey’s test for pairwise comparison of the bonding application times revealed that the maximum bond strength was achieved with immediate application of the bonding agent while the minimum bond strength was achieved when the bonding agent was applied 15 minutes after mixing. In the Fuji II group, the time and type of bonding agent had no effect on the microshear bond strength.
Table 2 summarizes the microshear bond strength values of resin-modified GICs relative to the type of bonding agent. Two-way ANOVA was applied to compare the microshear bond strength values between the two brands of resin-modified GICs (Fuji II LC and Riva Light Cure) and to assess the effect of type of bonding system on microshear bond strength. The statistical analyses revealed no significant interaction effect between the type of bonding agent and the type of resin-modified GIC.
Furthermore, applying either of the two bonding systems on the two brands of GICs failed to result in different bond strength values. This suggested that the type of bonding material had no significant effect on the microshear bond strength. However, the type of resin-modified GIC demonstrated a significant effect on the shear bond strength (Fuji II LC exhibited higher microshear bond strength compared to Riva Light Cure)(Table 1).
The SEM analysis revealed that etching in early stages produced more irregularities. This phenomenon was more obvious in Riva Self Cure. However, it was recognized that Fuji II yielded a more irregular and coarse surface than Riva self cure at all time points (Figs. 1A–1F). In addition, self etch primer made a different surface with lower roughness in comparison with phosphoric acid as seen on SEM micrographs (Figs. 2A–2F). Light cured GICs’ surface was obviously different. Fewer irregularities were seen on their surfaces after etching or priming; in other words, a smoother surface was observed in all samples (Figs. 3A–3D).
Fig. 1.
SEM micrographs of self cure GICs treated with phosphoric acid
Fig. 2.
SEM micrographs of self cure GICs treated with self etch primer
Fig. 3.
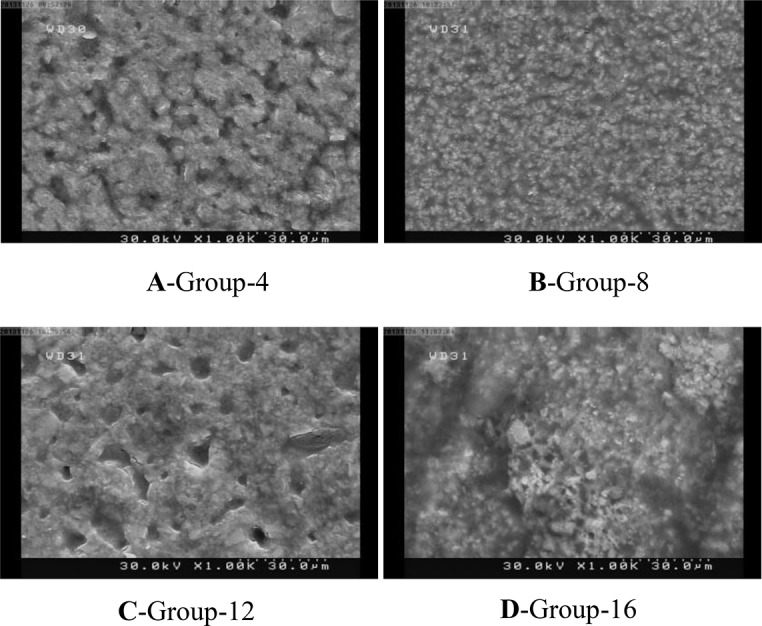
SEM micrographs of light cure GICs
DISCUSSION
Application of GIC under composite restorations (Sandwich technique) is a common restorative procedure, with the desired properties of both materials. To attain optimal results, adequate bond strength is required between the composite resin and the GIC to resist the stresses developed during the setting of the composite and over long-term clinical service. For the first time, McLean et al. attempted to etch the surface of the GIC via phosphoric acid and used resin bonding agent to bond composite to GIC [15]. Generally, etch and rinse bonding systems have widely been used in sandwich technique restorations. However, given the nature of the tooth structure and properties of dentin, this system presents problems such as inadequate etching, rinsing and drying and compromised bonding ability [16]. Thus, with the introduction of self-etch systems, researchers tried to overcome these shortcomings.
One of the major concerns of clinicians with regard to the use of sandwich technique is the time of bonding application.
A considerable amount of time may be required for complete setting of the GIC and its favorable properties. Lund et al. believed that contamination of GICs with moisture prior to complete maturation, results in increased solubility and decreased mechanical resistance of the substance [17]. This study was, therefore, conducted to evaluate the effect of bonding application time on the bond strength between GIC and the composite resin and the possibility of applying the bonding agent at the early stages of setting while achieving optimum properties.
The results revealed that Fuji II and Fuji II LC displayed higher microshear bond strength compared to Riva Self Cure and Riva Light Cure. This difference may be due to possibly higher strength of Fuji II compared to that of Riva as it has been stated that bond strength is a function of cohesive strength of a material. The other factor, which may account for the higher bond strength of Fuji II is the rougher surface of this material as revealed on SEM micrographs (Figs. 1D, 1E and 1F). Unfortunately, the available data fail to allow for direct comparison between the two brands. The results indicated that only Riva Self Cure group was affected by time. The highest bond strength was achieved immediately after working time and the lowest bond strength was recorded 15 minutes after mixing the cement. In other words, the bond between Riva Self Cure and the composite resin became weaker with time and maturation of the cement. Riva Self Cure manufacturers claim that this product has low acid erosion.
Therefore, it may be legitimate to assume that with complete setting and maturation of the material, the solubility of the cement decreases significantly resulting in reduced porosity and micromechanical retention and ultimately reduced bond strength. This may explain why in the early stages of mixing when the cement had not reached complete maturation it demonstrated greater solubility in acid and was likely to provide optimum surface roughness and porosities for a stronger bond. In fact, SEM micrographs supported these findings in which the roughest surface was seen when Riva Self Cure was etched after working time (Fig. 1A).
Regarding the differences noted between the two brands of GICs, it may be appropriate to claim that as Fuji II reaches maturation at a faster rate, its solubility is not significantly affected by time. Therefore, we tend not to see any significant differences in the bond strength at different times. Riva Self Cure, on the other hand, undergoes maturation at a slower rate; hence it demonstrates greater solubility in the early stages of maturation and gradually becomes less soluble rendering less surface roughness with time and resulting in compromised bond strength. Actually, SEM micrographs showed that etching of Riva self cure in early stages made a rougher surface (Figs. 1A, 1B and 1C) In contrast, the rough surface of Fuji II exhibited an identical pattern at all time points (Figs. 1D, 1E and 1F).
Ghassemi et al. evaluated the microleakage of class V sandwich restorations and concluded that in all self-cure GICs treated with the total-etch bonding system, microleakage significantly decreased in gingival margins over time [14]. This was in line with the findings of the current study showing that when the cement is exposed to acid at early stages of maturation, greater surface roughness would be achieved rendering a stronger bond and less microleakage.
These hypotheses are supported by previous studies on the solubility of GICs. Gemalmaz et al, [18] and Oilo [19] reported that GICs displayed minimum solubility six to seven minutes after the initiation of the setting reaction, and the solubility decreased with time and maturation of the cement. Furthermore, Hamouda showed that there was a direct correlation between solubility and surface roughness [20].
Our results also revealed that resin-modified GICs developed a stronger bond compared to the conventional ones. Numerous reasons explain this finding. The residual unpolymerized HEMA molecules on the surface of the set resin-modified GIC can facilitate wetting by the bonding agent and the composite resin during the bonding procedure. Furthermore, the presence of unreacted methacrylate groups within resin-modified GICs and presence of oxygen inhibition layer on the surface can develop a strong chemical covalent bond with the bonding resin, which enhances the bond strength of GICs [21–23]. Moreover, due to the higher cohesive strength of resin-modified GICs versus the conventional GICs, the former cements display greater cohesive strength, which may account for this difference. Resin-modified GICs set faster due to their light curing mechanism; therefore, the effect of etching or priming systems on the surface of these cements significantly differs from that of conventional GICs. The smooth surface of resin-modified GICs as seen on SEM micrographs support a faster setting and, in turn, less solubility (Fig. 3)
This study failed to show any difference in terms of the bond strength among the two bonding systems, i.e. self-etch and etch and rinse. Thus, based on a previous study by Zhang et al, reporting a stronger bond between self-etch bonding systems and conventional GICs compared to etch and rinse systems [24], it may be concluded that the new generations of bonding systems can be readily used in sandwich technique restorations.
CONCLUSION
Based on the findings of the present study, postponing the application of the new generations of bonding systems on GICs to achieve more optimal results, is unnecessary and fails to affect the bond strength of composite resin to GICs.
ACKNOWLEDGMENT
The authors wish to express their gratitude to SDI Company for providing part of glass ionomers and bonding agents used in this study.
REFERENCES
- 1-. Kerby RE, Knobloch L. The relative shear bond strength of visible light- curing and chemically curing glass ionomer cement to composite resin. Quintessence Int. 1992. September; 23 (9): 641– 4. [PubMed] [Google Scholar]
- 2-. Aboush YE, Torabzadeh H. Fluoride release from tooth-colored restorative materials: a 12-month report. J Can Dent Assoc. 1998. September; 64(8):561–4, 568. [PubMed] [Google Scholar]
- 3-. Chin YH, Tyas MJ. Adhesion of composite resin to etched glass ionomer cement. Aust Dent J. 1988. April; 33 ( 2): 87– 90. [DOI] [PubMed] [Google Scholar]
- 4-. Welbury RR, McCabe JF, Murray JJ, Rusby S. Factors affecting the bond strength of composite resin to etched glass ionomer cement. J Dent. 1988. August; 16 ( 4): 188– 93. [DOI] [PubMed] [Google Scholar]
- 5-. Taggart SE, Pearson GJ. The effect of etching on glass polyalkenoate cement. J Oral Rehabil. 1991. January; 18 ( 1): 31– 42. [DOI] [PubMed] [Google Scholar]
- 6-. Mount GJ. The tensile strength of the union between various glass ionomer cements and various composite resins. Aust Dent J. 1989. April; 34 ( 2): 136– 46. [DOI] [PubMed] [Google Scholar]
- 7-. Woolford MJ, Grieve AR. The use of intermediary resins when bonding glass polyalkenoate (ionomer) cement to composite resin. J Oral Rehabil. 1993. May; 20 ( 3): 249– 55. [DOI] [PubMed] [Google Scholar]
- 8-. Mount GJ. The wettability of bonding resins in the composite resin/glass ionomer sandwich technique. Aust Dent J. 1989. February; 34 ( 1): 32– 5. [DOI] [PubMed] [Google Scholar]
- 9-. Taggart SE, Pearson GJ. The effect of etching on glass polyalkenoate cement. J Oral Rehabil. 1991. January; 18 ( 1): 31– 42. [DOI] [PubMed] [Google Scholar]
- 10-. Mount GJ. Glass ionomer cements: past, present, and future. Oper Dent. 1994. May-Jun; 19 ( 3): 82– 90. [PubMed] [Google Scholar]
- 11-. Van Landuyt KL, Kanumilli P, De Munck J, Peumans M, Lambrechts P, Van Meerbeek B. Bond strength of a mild self-etch adhesive with and without prior acid-etching. J Dent. 2006. January; 34 ( 1): 77– 85. [DOI] [PubMed] [Google Scholar]
- 12-. Navimipour EJ, Oskoee SS, Oskoee PA, Bahari M, Rikhtegaran S, Ghojazadeh M. Effect of acid and laser etching on shear bond strength of conventional and resin-modified glass-ionomer cements to composite resin. Lasers Med Sci. 2012. March; 27 ( 2): 305– 11. [DOI] [PubMed] [Google Scholar]
- 13-. Aboush YE, Torabzadeh H. Clinical performance of Class II restorations in which resin composite is laminated over resin-modified glass-ionomer. Oper Dent. 2000. Sep-Oct; 25 ( 5): 367– 73. [PubMed] [Google Scholar]
- 14-. Ghasemi A, Torabzadeh H, Mahdian M, Afkar M, Fazeli A, Akbarzadeh Baghban A. Effect of bonding application time on the microleakage of Class V sandwich restorations. Aust Dent J. 2012. September; 57 ( 3): 334– 8. [DOI] [PubMed] [Google Scholar]
- 15-. McLean JW, Powis DR, Prosser HJ, Wilson AD. The use of glass ionomer cements in bonding composite resin to dentin. Br Dent J. 1985. June 8; 158 ( 11): 410– 4. [DOI] [PubMed] [Google Scholar]
- 16-. Burrow M. Understanding adhesive dentistry. Ann R Australas Coll Dent Surg. 2010. March; 20: 75– 9. [PubMed] [Google Scholar]
- 17-. Lund RG, da Silva AF, Demarco FF, Del-pino FA, Piva E, Michelon D. Band cementation materials: Solubility and Fluoride Release. Oral Health Prev Dent. 2008; 6 ( 4): 323– 9. [PubMed] [Google Scholar]
- 18-. Gemalmaz D, Yoruc B, Ozcan M, Alkumu HN. Effect of early water contact on solubility of glass ionomer luting cements. J Prosthet Dent. 1998. October; 80 ( 4): 474– 8. [DOI] [PubMed] [Google Scholar]
- 19-. Oilo G. Early erosion of dental cements. Scand J Dent Res. 1984. December; 92 ( 6): 539– 43. [PubMed] [Google Scholar]
- 20-. Hamouda IM. Effect of various beverages on hardness, roughness and solubility of esthetic restorative materials. J Esthet Restor Dent. 2011. October; 23 ( 5): 315– 22. [DOI] [PubMed] [Google Scholar]
- 21-. Nakanuma K, Hayakawa T, Tomita T, Yamazaki M. Effect of the application of dentin primers and a dentin bonding agent on the adhesion between the resin- modified glass ionomer cement and dentin. Dent Mater. 1998. July; 14 ( 4): 281– 6. [DOI] [PubMed] [Google Scholar]
- 22-. Pereira PN, Yamada T, Inokoshi S, Burrow MF, Sano H, Tagami J. Adhesion of resin modified glass ionomer cement using resin-bonding systems. J Dent. 1998. Jul-Aug; 26 ( 5–6): 479– 85. [DOI] [PubMed] [Google Scholar]
- 23-. Fritz UB, Finger WJ, Uno S. Resin modified glass ionomer cements: Bonding to enamel and dentin. Dent Mater. 1996. May; 12 ( 3): 161– 6. [DOI] [PubMed] [Google Scholar]
- 24-. Zhang Y, Burrow MF, Palamara JE, Thomas CD. Bonding to glass ionomer cements using resin based adhesives. Oper Dent. 2011. Nov-Dec; 36 ( 6): 618– 25. [DOI] [PubMed] [Google Scholar]