Abstract
Years ago, intramuscular influenza vaccines were the only option for those who wanted to arm themselves against the flu. Today there are alternatives, including intradermal injections and intranasal sprays. In order to select the right influenza vaccine for their patients, pharmacists, and other healthcare professionals must have a basic understanding of the immune system. Influenza vaccines elicit different levels of immune response involving innate and adaptive immunity, which are critical to fighting infection. For the 2013–2014 flu season, there were 13 different formulations of influenza vaccines on the market with vast differences in indications, contraindications, and effectiveness. The CDC does not recommend one vaccine over another, but recommends that all patients be vaccinated against the flu. Preventing the spread of influenza is no simple task; however, the most recent evidence on influenza vaccines and sufficient knowledge of the immune system will allow pharmacists and other healthcare providers to better advocate for vaccines, determine which are most appropriate, and ensure their proper administration.
Keywords: Influenza, vaccine, pharmacists, immunity, intradermal, intranasal
Abbreviations
- CDC
Centers for Disease Control and Prevention
Background
Years ago, intramuscular influenza vaccines were the only option for those who wanted to arm themselves against the flu. Today there are alternatives, including intradermal injections and intranasal sprays. The variety of options has led to new questions such as: which vaccine is best? And, is one superior to another? The answers to these questions are particularly important as pharmacists and other healthcare providers attempt to increase immunization rates and advocate for early vaccination. Over the past several years, vaccines have become available in August, long before the first flu epidemic makes the news. Nonetheless, many wait until the virus is rampant before taking action. Pharmacists and other healthcare providers can prevent the spread of influenza by advocating for immunization as soon as vaccines are available. This article aims to guide pharmacists and other health care professionals in making evidence-based selections of influenza vaccines for their patients.
Innate vs. Adaptive Immunity
First, it is important to review how the immune system works. Skin provides the largest physical barrier, while cilia and mucous line the airways and respiratory tract to defend against inhaled organisms. When invaders make it past these physical barricades, the body deploys the innate immune system, the body's first line of defense against foreign invaders once inside the body.
Innate immunity consists of various leukocytes, including monocytes/macrophages, neutrophils, basophils, eosinophils, and mast cells. Some of these cells secrete inflammatory chemicals to trigger a greater immune response. Others, such as macrophages, act as phagocytes and destroy the invading pathogens on their own. As this innate immunity is hard at work, the chemicals released trigger the adaptive immune system to join the fight. While innate immunity is fast at recognizing and fighting pathogens that have entered the body, the adaptive immune system is more effective due to memory and specificity. This system ‘adapts’ to fight specific pathogens, becoming more efficient with subsequent infections.1,2
One very important type of cell involved in the immune response is the dendritic cell, found primarily in the skin. For years, little was known about this type of cell. However, according to recent research, dendritic cells have numerous receptors and are able to rapidly recognize and process invading organisms.3 This means that cells in the skin can begin to activate the adaptive immune system before a pathogen ever reaches the bloodstream.
For immunizations to be effective, both the innate and adaptive immune systems must be involved. When administered, flu vaccines elicit the creation of antibodies by the adaptive immune system. During the 2 to 3 weeks following immunization, these antibody levels increase and prepare for a subsequent invasion by the real flu virus. Consequently, the body's immune system rapidly mobilizes to fight the infection effectively.4
Difference in Vaccine Delivery
Does the route of vaccine administration make a difference? A closer look at the immune system reveals that it does. Most cells of the immune system are found in the bloodstream, but they are also able to migrate into tissues to fight infection.5 Because dendritic cells are predominant in the skin, vaccines that are administered intramuscularly bypass these cells.3 Other cells involved with innate immunity will migrate to the muscle and activate the adaptive immunity, but the memory of the specific pathogen (via antibody development) occurs in the bloodstream. Interestingly, influenza infection does not begin in the bloodstream, but rather in the respiratory tract.
Research is not conclusive, but this could explain why flu vaccines are not always effective—even against matched strains. The CDC estimates that general flu vaccine effectiveness is between 45–55% annually.4,6 This means that 45–55 out of every 100 individuals who receive a flu vaccine are still susceptible to infection even when the strains are properly matched to the vaccine. So how can flu vaccine effectiveness be improved?
One solution is to strengthen the immune response to a vaccine. This may be accomplished by utilizing alternative routes of vaccine delivery (Table 1). Since the intradermal and intranasal vaccines were released, there have been multiple studies comparing their efficacy to the traditional intramuscular injection. In a head-to-head comparison, the intranasal vaccine produced an 85% effective rate compared with 71% with the intramuscular vaccine.8 Such discrepancies were even greater in children.9,10
Table 1.
Routes of influenza vaccine delivery7
| Vaccine Name | Route of Administration |
|---|---|
| Flumist (LAIV) | Intranasal |
| Fluzone (TIV) | Intradermal |
| All other flu vaccines (QIV/TIV) | Intramuscular |
Similar studies have demonstrated superiority with intradermal vaccine delivery. One study used an intradermal dose 1/5th that of the intramuscular influenza vaccine, and found that even a significantly smaller dose of vaccine was able to produce a stronger immune response.11,12 These researchers also hypothesized that this response may be due in part to the dense population of dendritic cells in the skin (Table 2 for a summary of delivery comparisons).
Table 2.
Comparative vaccine efficacy based on delivery mechanism
Differences in Vaccine Composition
Vaccine delivery is not the only piece to this puzzle. The composition of the vaccine should also be considered. For the 2013–2014 flu season, there were 13 different flu vaccine formulations on the market.7 While many of these vaccines may look the same, there are vast differences in their indications, contraindications, and effectiveness. For example, some of these vaccines protect against 4 strains of influenza (quadrivalent vaccines), and provide greater protection than vaccines that protect against 3 (trivalent vaccines). One vaccine contains 4 times the amount of other intramuscular agents.13 As previously discussed, research continues to show that intradermal vaccines and live intranasal vaccines are more effective in soliciting an immune response than traditional trivalent inactivated intramuscular vaccines. The CDC does not recommend one vaccine over another, but recommends that all patients be vaccinated against the flu. Their lack of opinion is most likely an effort to avoid endorsing a certain brand or company. Table 3 summarizes the current vaccines on the market. Figure 1 provides an algorithm for deciding which flu vaccine to use.
Table 3.
Flu vaccines available for 2013–2014 season7
| Vaccine | Composition | Delivery Route | Restrictions | Notes |
|---|---|---|---|---|
| FluMist | Quadrivalent -LAIV | Intranasal | Ages 2–49 only | Not for persons with chronic disease |
| Fluarix | QIV | Intramuscular (IM) | Ages 3+ | |
| FluLaval | QIV | IM | 6 mo + | |
| Afluria | TIV | IM | Ages 9 + | Linked to fever in children under 9 |
| Fluarix | TIV | IM | Ages 3+ | |
| Flucelvax | TIV | IM | Ages 18+, contraindicated in severe egg allergy | Cell culture-based vaccine |
| FluLaval | TIV | IM | Ages 3+ | |
| Fluvirin | TIV | IM | Ages 4+ | |
| Fluzone | TIV | IM | 6 mo + | |
| Fluzone ID | TIV | Intradermal (ID) | Ages 18–64 | More common reactions at injection site |
| Fluzone HD | TIV | IM | Ages 65+ | Contains 4X as much inactivated vaccine as standard injections |
| Flublok | Trivalent recombinant | IM | Ages 18–49 | Completely egg-free |
Figure 1.
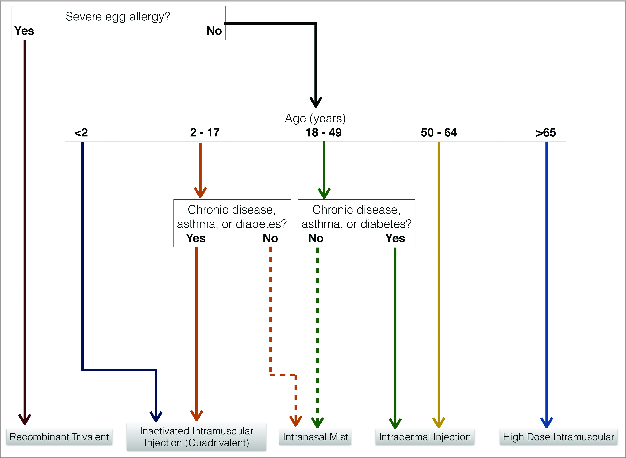
Selecting the Appropriate Flu Vaccine
Conclusion
Research is forthcoming, but most evidence shows that vaccine development is progressing to provide better solutions in preventing influenza epidemics. Examples include the development of a universal flu vaccine, influenza vaccines based on recombinant virus proteins, non-infectious virus-like particles, and harmless vectors or influenza DNA.14 Preventing the spread of influenza is no simple task; however, the most recent evidence on influenza vaccines and sufficient knowledge of the immune system will allow pharmacists and other healthcare providers to better advocate for vaccines, determine which are most appropriate, and ensure their proper administration.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1.Hall PD, Pilch N, Atchley DH. Chapter 95. Function and Evaluation of the Immune System. In: DiPiro JT, Talbert RL, Yee GC, Matzke GR, Wells BG, Posey L. eds. Pharmacotherapy: A Pathophysiologic Approach, 8e. New York: McGraw-Hill; 2011. http://accesspharmacy.mhmedical.com/content.aspx?bookid=462&Sectionid=41100874. Accessed January 10, 2014. [Google Scholar]
- 2.Delves PJ, Roitt IM. The immune system. First of two parts. N Engl J Med 2000; 343:37-49; PMID:10882768; http://dx.doi.org/ 10.1056/NEJM200007063430107 [DOI] [PubMed] [Google Scholar]
- 3.Banchereau J, Steinman RM. Dendritic cells and the control of immunity. Nature 1998; 392:245-52; PMID:9521319; http://dx.doi.org/ 10.1038/32588 [DOI] [PubMed] [Google Scholar]
- 4.Flu vaccine effectiveness: questions and answers for health professionals. Centers for Disease Control and Prevention. Available at: www.cdc.gov/flu/professionals/vaccination/effectivenessqa.htm. Accessed on: January 9, 2014. [Google Scholar]
- 5.Chaplin DD. 1. Overview of the human immune response. J Allergy Clin Immunol 2006; 117(Suppl Mini-Primer):S430-5; PMID:16455341; http://dx.doi.org/ 10.1016/j.jaci.2005.09.034 [DOI] [PubMed] [Google Scholar]
- 6.Key facts about seasonal flu vaccine. Centers for Disease Control and Prevention. Available at: http://www.cdc.gov/flu/protect/keyfacts.htm. Accessed on January 9, 2014. [Google Scholar]
- 7.Flu vaccines 2013-2014. Pharmacist's Letter/Prescriber's Letter Detail Document #291001. October 2013. Available at: www.pharmacistsletter.com. Accessed on January 9, 2014. [Google Scholar]
- 8.Treanor JJ, Kotloff K, Betts RF, Belshe R, Newman F, Iacuzio D, Wittes J, Bryant M. Evaluation of trivalent, live, cold-adapted (CAIV-T) and inactivated (TIV) influenza vaccines in prevention of virus infection and illness following challenge of adults with wild-type influenza A (H1N1), A (H3N2), and B viruses. Vaccine 1999; 18:899-906; PMID:10580204; http://dx.doi.org/ 10.1016/S0264-410X(99)00334-5 [DOI] [PubMed] [Google Scholar]
- 9.Jain VK, Rivera L, Zaman K, Espos RA, Jr., Sirivichayakul C, Quiambao BP, Rivera-Medina DM, Kerdpanich P, Ceyhan M, Dinleyici EC, et al. Vaccine for prevention of mild and moderate-to-severe influenza in children. N Engl J Med 2013; 369:2481-91; PMID:24328444; http://dx.doi.org/ 10.1056/NEJMoa1215817 [DOI] [PubMed] [Google Scholar]
- 10.Belshe RB, Edwards KM, Vesikari T, Black SV, Walker RE, Hultquist M, Kemble G, Connor EM; CAIV-T Comparative Efficacy Study Group. Live attenuated versus inactivated influenza vaccine in infants and young children. N Engl J Med 2007; 356:685-96; PMID:17301299; http://dx.doi.org/ 10.1056/NEJMoa065368 [DOI] [PubMed] [Google Scholar]
- 11.Kenney RT, Frech SA, Muenz LR, Villar CP, Glenn GM. Dose sparing with intradermal injection of influenza vaccine. N Engl J Med 2004; 351:2295-301; PMID:15525714; http://dx.doi.org/ 10.1056/NEJMoa043540 [DOI] [PubMed] [Google Scholar]
- 12.Canadian National Advisory Committee on Immunization. Recommendations on the use of intradermal trivalent inactivated influenza vaccine (TIV-ID). Canada Communicable Disease Report 2011; 37(ACS-4): 1481-8531. Available at: www.phac-aspc.gc.ca/publicat/ccdr-rmtc/11vol37/acs-dcc-4/index-eng.php. Accessed on: January 9, 2014. [Google Scholar]
- 13.Lowes R. Fluzone high-dose foils flu better in seniors, says maker. Medscape Medical News 2013. Available at: www.medscape.com/viewarticle/813203. Accessed on: January 9, 2014. [Google Scholar]
- 14.Lambert LC, Fauci AS. Influenza vaccines for the future. N Engl J Med 2010; 363:2036-44; PMID:21083388; http://dx.doi.org/ 10.1056/NEJMra1002842 [DOI] [PubMed] [Google Scholar]


