Abstract
Maternal immunization holds tremendous promise to improve maternal and neonatal health for a number of infectious conditions. The unique susceptibilities of pregnant women to infectious conditions, as well as the ability of maternally-derived antibody to offer vital neonatal protection (via placental transfer), together have produced the recent increased attention on maternal immunization. The Advisory Committee on Immunization Practices (ACIP) currently recommends 2 immunizations for all pregnant women lacking contraindication, inactivated Influenza and tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap). Given ongoing research the number of vaccines recommended during pregnancy is likely to increase. Thus, achieving high vaccination coverage of pregnant women for all recommended immunizations is a key public health enterprise. This review will focus on the present state of vaccine acceptance in pregnancy, with attention to currently identified barriers and determinants of vaccine acceptance. Additionally, opportunities for improvement will be considered.
Keywords: pregnancy, influenza, pertussis, vaccine, vaccination, immunization
Abbreviations
- Tdap
tetanus toxoid, reduced diphtheria toxoid and acellular pertussis
- ACIP
Advisory Committee on Immunization Practices
- ACOG
American College of Obstetricians and Gynecologists
- AAP
American Academy of Pediatrics
- MMR
measles, mumps, and rubella
- CDC
Centers for Disease Control and Prevention
Maternal Immunization: A Historical Context
Immunizations are one of the most effective interventions in modern medicine. Numerous infectious conditions previously responsible for significant morbidity and mortality have been either completely eliminated or greatly reduced as significant contributors to global infectious disease burden. The majority of benefits have been realized in pediatric health and are also noted for numerous infectious conditions affecting adolescents and adults.
Dramatic immunization successes have also been seen in the field of reproductive health. The domestic rate of Congenital Rubella Syndrome has been radically reduced (at least in part) by the combination of the measles, mumps, and rubella (MMR) vaccine programs for children and adolescents and standardized testing during pregnancy for susceptibility to rubella along with postpartum immunization of women lacking immunity.1 Internationally, programs using a maternal immunization approach (immunizing mother for maternal and importantly neonatal benefit via trans-placental antibody transfer) have had a large impact on lowering the rates of neonatal tetanus.2 Additionally, with the advent of anti-D immune globulin use among Rh-negative mothers, the rates of Rh-alloimmunization have dramatically fallen since the 1970s.3 Given the proven track record of these interventions, as well as emerging data and related heightened attention on the benefits of maternal influenza and pertussis immunization, renewed and robust efforts are underway to promote maternal immunization as a powerful intervention against numerous maternal and neonatal pathogens.
In light of this re-emerging emphasis in public policy, academic, federal, and pharmaceutical circles about the potential impact of maternal immunization, improved understanding and mechanisms allowing for optimal vaccine uptake in pregnancy are timely and necessary.4 This review will address these very important issues with a focus on how to overcome known barriers in order to achieve meaningful progress with regard to maternal vaccine acceptance.
Current Recommendations for Vaccination During Pregnancy
Currently, the ACIP recommends 2 vaccines for all pregnant women: (1) inactivated Influenza, and (2) tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis (Tdap; Table 1).5,6 This section will review the evidence and rationale supporting the current guidelines for maternal immunization. Data in this area are evolving, and Figure 1 contains resources with regularly updated information on maternal immunization guidelines.
Table 1.
Recommendations for vaccination during pregnancy5,6
| Vaccine | General recommendation for use in pregnancy | Timing of vaccination | Vaccine formulation | Exceptions/contraindications |
|---|---|---|---|---|
| Influenza (inactivated) | Recommended during each pregnancy | Preconception or as early in pregnancy as possible during influenza season | IIV (IIV3 or IIV4) | Prior severe allergic reaction to influenza vaccine; individuals with history of severe allergic reactions to eggs should be assessed by a physician with expertise in management of allergic conditions |
| Influenza (LAIV) | Not recommended | n/a | n/a | n/a |
| Tdap | Recommended during each pregnancy | Optimal timing is between 27–36 wk gestation (but may be given at any time) | Tdap | Prior severe allergic reaction to Tdap vaccine, encephalopathy not due to other causes within 7 d of Tdap administration; neurologic reactions to Tdap should be considered on a case by case basis with a physician. |
| Td | Used if otherwise indicated (i.e., for wound management if at least 5 y since the previous Td booster) | When indicated | If Td is indicated for a pregnant woman, administer Tdap | As above for Tdap |
*Abbreviations: LAIV, live attenuated inactivate influenza vaccine; IIV, inactivated influenza vaccine; IIV3, trivalent formulation; IIV4 quadrivalent formulation; Tdap, tetanus toxoid, reduced diphtheria toxoid and acellular pertussis; Td, tetanus and diphtheria;**Other vaccines not routinely recommended for pregnant women but safe to administer during pregnancy when a clinical indication exists include: hepatitis B, hepatitis A, pneumococcal, meningococcal, yellow fever, rabies and anthrax vaccines.102-105 Of note, the live attenuated MMR vaccine is contraindicated during pregnancy, but recommended for postpartum administration to rubella nonimmune pregnant women.102 The live attenuated Typhoid vaccine is not recommended during pregnancy due to lack of available data.106
Figure 1.

Resources with regularly updated information about vaccination during pregnancy.
Influenza
Influenza exposure is common, affecting an estimated 11% of pregnant women.7,8 Twentieth century influenza pandemics, including the 2009 H1N1 pandemic, demonstrated that pregnant women are at increased risk for influenza-related morbidity and mortality.9-14 Seasonal influenza also imparts disproportionate morbidity and mortality to pregnant women,15,16 partly due to the altered immunologic and physiologic parameters of pregnancy.17,18 Numerous studies have documented increased respiratory, febrile, and cardiopulmonary morbidity among pregnant women during seasonal influenza epidemics.7,8,19-24 These maternal consequences increasingly appear to be associated with numerous adverse pregnancy outcomes (preterm delivery, fetal distress, fetal growth restriction, cesarean delivery), and the potential for detrimental neonatal outcomes.20,25,26
Influenza vaccination is the most effective strategy for influenza prevention during pregnancy. Multiple studies document the immunogenicity27-29 and safety30,31 of maternal influenza vaccination. Studies of seasonal32 and pandemic33,34 influenza vaccination in the United States and abroad suggest that exposure to the influenza vaccine during pregnancy does not appear to be associated with obstetric or fetal complications.35-38 In fact, maternal immunization may reduce rates of preterm birth and suboptimal growth among infants of vaccinated mothers.39,40 In a landmark randomized controlled trial published in 2008, Zaman and colleagues demonstrated that maternal influenza vaccination confers neonatal protection against influenza,41 presumably due to transplacental passage of maternal IgG antibody to the fetus.42 Maternal influenza vaccination has also been associated with reduced rates of influenza-related hospitalization of infants less than 6 mo of age.43 These findings are particularly important given that neonates under 6 mo are at heightened risk of severe complications from influenza, and they also are ineligible to receive any currently available influenza vaccine formulations. Maternal immunization is thus the only current mechanism to provide antibody to the newborn, and is a highly cost-effective intervention.44
Based on the well-documented benefits and safety of maternal influenza vaccination, yearly influenza vaccination is recommended by the ACIP and ACOG for all women who are pregnant or who might become pregnant during influenza season.5,45 Women who are or will be pregnant during influenza season are advised to receive the trivalent inactivated influenza vaccine (newer quadrivalent vaccines also are compatible with pregnancy). Live attenuated influenza vaccine is not recommended for use in women who are pregnant, given the theoretical risks of viremia and fetal infection from a live-attenuated vaccine. The inactivated vaccine can be administered before or during influenza season, regardless of gestational age (ideally as soon as vaccine is available).5
Tetanus, diphtheria, pertussis
Pertussis (also called whooping cough) is a highly infectious bacterial disease that causes an acute respiratory infection with 3 classic phases: catarrhal, paroxysmal, and convalescent. Adults can develop symptoms of pertussis, but the overwhelming majority of pertussis disease affects newborns ≤3 mo of age,46 who are more susceptible to pertussis infection and serious morbidity and mortality.47 Infants do not begin their own vaccination series until 2 mo of age, and, until this time, are critically dependent on the immunity of family members and other caregivers for protection against pertussis (a protective ‘cocoon’).
Starting in 2006, the ACIP recommended vaccination of postpartum women and all other family members and caregivers who had not previously received the vaccine in order to protect the newborn. This strategy, known as “cocooning,” was in part based on data that three-fourths of infected infants appear to contract pertussis from close family members.48 Cocooning proved challenging to broadly implement and inadequate as an isolated strategy to prevent newborn pertussis. In the face of persistent increases in pertussis disease, in June of 2011, the ACIP issued a new recommendation that previously unvaccinated women receive Tdap during pregnancy.49 In the setting of ongoing increases in pertussis disease in newborns, reassuring data on the safety of Tdap in pregnancy, and evolving data on the waning of immunity after initial vaccination,50,51 another revision was made by the ACIP in February of 2012. These most current recommendations advise that all pregnant women receive a dose of Tdap during their current pregnancy, regardless of prior immunization history. Vaccination is acceptable at any gestational age, but optimally timed at 27 to 36 wk to maximize antibody transfer and newborn immunity.6 These guidelines are supported by ACOG52 and the American Academy of Pediatrics (AAP).53 If Tdap is not received during pregnancy and has not previously been administered, it should be administered immediately postpartum. The current recommendations are based at least in part on rationale similar to that underlying recommendations for influenza vaccination in pregnancy, namely that maternal vaccination is safe, effective, and protects an otherwise vulnerable newborn in the first few weeks of life. Monitoring through the CDC's Vaccine Adverse Event Report System from 2005–2010 suggests the safety of Tdap immunization during pregnancy.54 These reassuring findings from passive surveillance were confirmed in a recent randomized, controlled phase 1–2 trial.55 In this study of the safety and immunogenicity of Tdap immunization of third trimester pregnant women, no Tdap vaccine-related adverse events or adverse pregnancy outcomes were observed. Adverse event monitoring related to Tdap vaccination is ongoing at a population level. A recent decision analysis suggests that antenatal vaccination with Tdap will avert more infant pertussis cases, hospital admission and deaths than prior cocooning strategies.56
Other vaccines
In addition to influenza and pertussis, the Centers for Disease Control and Prevention (CDC) vaccination schedule identifies immunizations that may be safely administered during pregnancy based on individual risk factors. These important but non-routine immunizations will not be addressed in detail here, but have been discussed elsewhere.57
Importantly, numerous industry-sponsored investigations have been undertaken or are underway for at least 2 new vaccines under development targeting maternal immunization specifically, group B streptococcus (GBS) and respiratory syncytial virus (RSV).58,59 If the vaccines are found to be safe and efficacious for prevention of neonatal infection after maternal immunization, first-ever FDA-approved vaccines for use specifically in pregnancy could be available for widespread use. Such a development would expand the total number of immunizations recommended during pregnancy in the future, emphasizing the importance of maternal vaccine acceptance in enabling achievement of the important goal of improved maternal/neonatal health.
Vaccine Acceptance: A Crucial Consideration for Implementing Maternal Immunization Recommendations
Despite the strong endorsement of recommended immunizations in pregnancy by the ACIP, ACOG, the AAP, and Healthy People 2020, vaccine uptake during pregnancy has been suboptimal. National estimates of influenza vaccine coverage of pregnant women have historically been persistently and discouragingly low, around 15%.60-64 Vaccination rates increased during the 2009 H1N1 pandemic (to approximately 45–50% in the 2009–2010 season), and these rates were seemingly sustained in the 2010–2011,65,66 2011–2012,67 and 2012–201368 seasons. Although encouraging, such estimates of vaccine coverage of pregnant women remain far below the Healthy People 2020 goal of 80% influenza vaccine coverage of pregnant women.62
There is limited available data to assess rates of Tdap vaccine uptake among pregnant women in the United States. Available estimates suggest that rates as low as 3% are the likely starting point for national data around the time of the first Tdap during pregnancy vaccination recommendation from the ACIP in 2011.6 Such low rates are not surprising given the novelty of the recommendation and the understanding that obstetric providers have not traditionally viewed pertussis as a relevant vaccine-preventable disease for their primary patient population (pregnant women), and may not have regarded themselves as vaccinators. Expectations are high for some measure of increase from this low baseline in the ensuing years.
Determinants of Maternal Vaccine Acceptance
Maternal acceptance of recommended vaccinations is a key consideration in improving maternal immunization rates. Certain segments of the obstetric population seem more at risk for non-vaccination. National and international studies have demonstrated that vaccination is less likely in pregnant women with lower socio-economic status,69 less educational attainment,70 and in women belonging to racial/ethnic minority groups.71,72 Vaccine non-receipt among certain population sub-segments is likely mediated, at least in part, by sociocultural and psychological factors influencing maternal vaccine acceptance, as has been demonstrated in non-pregnant populations.73,74
The Health Belief Model75 is a well-known conceptual framework that addresses social and psychological determinants of health behaviors. According to the Health Belief Model, belief in a personal threat coupled with a belief in the effectiveness of a behavior in reducing that threat will predict the likelihood of that behavior. This model can be used to better understand vaccine declination.76-79 In a conceptual model based on the Health Belief framework, key determinants of maternal influenza vaccination include perceived vulnerability to influenza disease, perceived benefits that outweigh costs of vaccination, vaccination-related normative beliefs and prior behaviors, and self-efficacy (Fig. 2). The effects of these determinants can be modified by perceived regret about vaccination behaviors and by cues to action regarding vaccine-related decisions in pregnancy.
Figure 2.
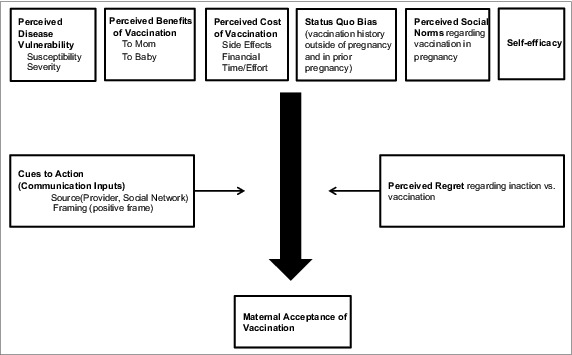
Modified health belief model as a theoretical framework for maternal acceptance of vaccination.
This conceptual framework is reflected in known barriers to maternal acceptance of vaccination in pregnancy, including: lack of knowledge about susceptibility to severe influenza disease in pregnancy and susceptibility to severe pertussis disease in newborns, misinformation about vaccine effectiveness, concerns about vaccine safety and side effects, prior vaccination behavior, lack of precedent for receiving a vaccination from an obstetric provider, fear of needles, general mistrust of the healthcare profession, and poor access to care.80 Some of these barriers will be addressed in detail here.
Lack of perceived susceptibility and misinformation about vaccine effectiveness
Some pregnant and postpartum women may not appreciate their increased susceptibility to influenza infection and potentially severe influenza disease.81,82 False beliefs about influenza susceptibility and severity and the effectiveness of vaccination have been associated with reduced maternal uptake of the vaccine.83 Similarly many pregnant women may be unaware of their risk of acquiring and then exposing their infant to pertussis, an infant's increased susceptibility to pertussis, and the consequences of neonatal pertussis.84 Perceived susceptibility to disease and accurate understanding of the disproportionate severity of influenza disease in pregnancy and pertussis in neonates appear to be necessary precedents to acceptance of vaccination in pregnancy. Indeed, in one cross-sectional survey of 173 pregnant women, worry about acquiring influenza was the strongest predictor of vaccine intention.85 This factor will continue to be an issue as more vaccines are investigated and eventually recommended for use in pregnancy to combat maternal and/or neonatal disease.
Concerns about vaccine safety and side effects
In multiple studies, a primary reason for declining influenza16,82,86 or pertussis84 immunization during pregnancy is concern that the vaccine might harm the baby. Unvaccinated women in Georgia cited a variety of reasons for declining the influenza vaccine in 2006–7, including concerns that the vaccine might harm their babies (27.4%) or themselves (25.5%).16 In a cross-sectional survey of postpartum women in Canada, 46 of 58 respondents (80%) incorrectly believed that influenza vaccination can cause birth defects.81 In another cross-sectional survey of more than 500 pregnant women at an academic, tertiary care center in Pennsylvania, over 60% were concerned about vaccine safety during pregnancy, with a worrisome 8% believing that the influenza vaccine causes influenza disease.82 In one study of the 2011–12 influenza season, only 36% of pregnant women were vaccinated, and concerns about vaccine safety were the most cited reason for not receiving the influenza vaccine.67,87 Reassuringly, patients’ focus on potential vaccine harm comes at a time of growing data about the benefits and safety of maternal vaccination for babies.32,33,35,39-41 Emphasizing the benefits of maternal immunization, including its safety and absence of fetal harm, should help decrease the perceived cost of maternal immunizations.
Prior vaccination behavior
Prior vaccination behaviors appear to influence current immunization acceptance in non-pregnant individuals, including healthcare workers.88,89 Maternal acceptance of immunization during pregnancy may also be influenced by prior vaccination behaviors. Some young women may have previously received vaccines only during childhood and may feel that adults do not need vaccines as children do.71,90 Such beliefs may contribute to vaccine declination. Conversely, prior receipt of the seasonal influenza vaccine may predict influenza vaccine receipt. In one recent cross-sectional survey of 199 pregnant women, respondents who had received influenza vaccination in the previous season were 5 times as likely to have accepted influenza immunization during pregnancy than those who had not.78
Other barriers
Pregnant women may be unaccustomed to receiving vaccines from obstetrician gynecologists; it is unclear how this may affect willingness to request or receive vaccines in an obstetrician's office. Some women may harbor general mistrust of the medical establishment, and this may affect their willingness to receive vaccines during pregnancy. Lack of perceived family and/or peer approval of vaccination during pregnancy may also be an important barrier to vaccine uptake.91 In the general public, poor access and out of pocket expense are known barriers to uptake of preventive health services. While specific data are not available in pregnancy, lack of insurance, unreliable transportation, and inconsistent or absent prenatal care may also represent barriers to maternal acceptance of immunization. Lastly, there may be significant provider-related barriers to maternal acceptance of immunization.80 Financial barriers may constrain obstetricians’ ability to administer vaccines in their offices,92,93 which may act as a logistic barrier to maternal immunization. Lack of knowledge about influenza or concerns about current vaccination guidelines may affect a provider's likelihood of making a recommendation or the strength of their recommendation for maternal vaccination.86,94 Additionally, obstetrician gynecologists may not consider themselves vaccinators.92,93,95 These attitudinal barriers are vitally important, given that compelling provider recommendation has consistently been shown to be critically important to a pregnant woman's decision to accept vaccination.16,96
Opportunities for Improving Implementation of Recommended Immunizations During Pregnancy
We support the following key tasks as evidence-based approaches to promoting maternal immunization acceptance: Educate, Recommend, Normalize, Maximize Convenience (Table 2; concepts adapted from the ACOG Committee Opinion on Integrating Immunizations into Practice45).
Table 2.
Evidence-based approaches to promoting maternal vaccine acceptance*
| Educate | Explicitly address vaccination with all obstetric patients. Counseling should focus on effectiveness and safety of vaccination for both mom and baby. Help patients weigh anticipated regret of inaction vs. regret of vaccination. Involve the patient's social network in counseling whenever possible. |
| Recommend | Talk to patient directly. Recommend indicated vaccines. Explicitly state benefits to both mom and baby. Address any misinformation about vaccines (especially the notion that a vaccine can make one sick). |
| Normalize | Make control of vaccine-preventable disease a routine part of obstetric and gynecologic care. Help patients establish a new status quo by offering indicated vaccines during non-obstetric care. Address vaccines as a standard part of anticipatory guidance at first obstetric visits. |
| Maximize Convenience | Use prompts (e.g., pop-up reminder in electronic record) to consistently and clearly identify vaccine-eligible patients and remind staff to order recommended vaccines. Consider using the electronic record to streamline documentation of vaccination |
*Concepts adapted from the American College of Obstetricians and Gynecologists’ Committee Opinion on Integrating immunizations into practice.45
Educate
Insufficient knowledge about susceptibility to and morbidity of vaccine-preventable disease and the risks and benefits of vaccination are modifiable barriers to vaccine uptake. Women's health providers should explicitly address vaccination with all obstetric patients. Counseling should focus on the effectiveness and safety of vaccination for both mom and baby. Individuals who perceive susceptibility to influenza and believe in the vaccine's effectiveness are more likely to get vaccinated.91 A positively framed message that stresses the benefits of vaccination (i.e., ‘influenza vaccination protects you and your baby from influenza’) may be more effective than a negatively framed message that stresses the risks of foregoing influenza or pertussis vaccination (i.e., ‘if you don't get the influenza vaccine, you could get really sick and need intensive care’).97 This may be particularly true if the message emphasizes the validated benefits to the infant.90 These conversations are an opportunity to ask a patient about her concerns and address any specific misinformation about vaccines (especially the notion that a vaccine can give a person an illness). Helping a patient explore her fears about vaccination is important, given the role emotions play in moderating decisions for vaccine acceptance vs. declination in non-pregnant groups.77 Providers should help patients weigh anticipated regret of inaction (i.e., foregoing vaccination) vs. regret of vaccination. Because a patient's family and friends are trusted resources in healthcare decisions, providers should try to involve the patient's influential social network in counseling and education about maternal vaccination whenever possible. Additionally, many women report receiving conflicting information about vaccination, and providers should work to ensure that everyone on their care team provides consistent messaging about the importance of vaccination during pregnancy.90
Recommend
Verbal, face to face communication from a physician appears to be an overwhelmingly powerful motivator of vaccine acceptance in pregnancy.67,98 In one recent study of postpartum women in Delaware, those who recalled a specific recommendation from a healthcare provider about influenza vaccination were 3 times more likely to be vaccinated than those who did not report a healthcare provider recommendation.70 In a similar study in Australia with overall lower influenza vaccine coverage of participants (25%), women who reported receiving provider recommendation of influenza vaccination were 41 times more likely to have received antepartum vaccination.99 Similarly, lack of recommendation from an obstetric provider appears to be a strong predictor of not receiving indicated maternal vaccines.98
These findings underscore the importance of a strong and direct provider recommendation of indicated vaccines during pregnancy. Providers should talk directly to patients about recommended vaccinations. In these conversations, obstetric practitioners should clearly state their support of recommended vaccines during pregnancy and specifically recommend indicated vaccines for the particular patient. In addition to recommending the vaccine, offering the vaccine in the office has a combined effect in terms of greatly increasing the odds of acceptance and reducing access barriers to vaccination by maximizing convenience for patients.67,68 ACOG has undertaken an impressive campaign to educate providers about recommended vaccines for women of reproductive age, and such efforts are likely to strengthen provider recommendations for indicated vaccines during pregnancy.93,94 Such educational efforts will also be critical as future vaccines are approved and recommended for use during pregnancy. In addition to her obstetric care team, a woman's social network may be a particularly trusted source of information about vaccination during pregnancy.90 Perceived interpersonal support of maternal vaccination appears to predict vaccine uptake.91 More research is needed to better understand how best to use community networks, including online social networks, to promote maternal vaccination. Novel tools for communicating the safety and effectiveness of maternal vaccination include the internet, social media, and eHealth technologies like text messaging and web-based applications. Findings from studies of mobile interventions like text messaging to promote maternal influenza vaccination have been mixed,100,101 and further study is needed to determine how best to capitalize on the promise of novel communication interventions for maternal vaccine acceptance.
Normalize
Obstetric practitioners should frame infectious disease prevention for women and infants as a routine part of obstetric care, presenting vaccines as a standard part of anticipatory guidance at first obstetric visits. All providers involved in the care of pregnant women should also accept the influenza vaccine themselves as a powerful message in support of immunizations and as a safeguard to the well-being of the patients for whom they care. This can help to contribute to a culture of immunization normalization in the office setting.
More broadly, women's health providers should strive to promote universal adult influenza vaccination as an annual behavior for all adults. Such an approach promotes vaccination as a routine part of annual health maintenance. It should increase vaccination of women pre-conception and may help vaccination behaviors become more habitual among women of reproductive age.
On a systems level, organizations can implement strategies to ensure consistent provider recommendation of vaccination, including standing orders, ongoing provider education about vaccination, and use of vaccination as a quality indicator. At a population level, the implementation of the Patient Protection and Affordable Care Act holds promise to reduce access and cost barriers to care, which may enhance uptake of routine care and utilization of preventive services, including immunization. Together, these changes may help modify the status quo and help women perceive vaccination as a more normative.
Maximize convenience
Obstetric providers see pregnant women frequently and are viewed as a trusted, reliable source of medical information. As such, obstetric providers should offer on-site administration of recommended vaccines in pregnancy, to reduce the time and effort costs for patients. Obstetric caregivers can consider using prompts (such as pop-up reminders or best-practice tabs in the electronic record) to consistently and clearly identify vaccine-eligible obstetric patients. Electronic records also afford opportunities to remind staff to order recommended vaccines and to streamline documentation of vaccination counseling. It will also be important to implement reimbursement structures that incentivize maintaining a vaccine supply and cover the cost of vaccine administration.
Conclusion
Maternal immunization against influenza and pertussis has known benefits for both mother and child. Despite safety and effectiveness of maternal immunization, vaccine uptake in pregnant populations is suboptimal. Maternal acceptance of vaccination crucially hinges on perceived vulnerability to disease, perceived effectiveness that outweighs costs of vaccination, and normative beliefs about vaccination behaviors during pregnancy. The fundamental importance of an obstetric care provider's recommendation of indicated maternal immunizations cannot be overstated. Further research is needed to develop evidence-based communication strategies for reaching under-vaccinated obstetric populations, including women with limited socioeconomic means and educational attainment and women of racial/ethnic minority descent. Optimizing maternal acceptance of indicated vaccines during pregnancy is becoming an increasingly important evidence-based practice in obstetrics, and a key way for obstetric practitioners to advocate for a family unit. Improving rates of uptake of currently recommended vaccines in pregnancy will help optimize acceptance when future vaccines are approved for use in pregnancy. Doing so can help create a cultural shift in how providers and patients view the prenatal period as a time of tremendous opportunity for infectious disease prevention for mothers and babies alike.
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
References
- 1. Centers for Disease Control and Prevention Vaccines and Immunizations, Chapter 15: Congenital Rubella Syndrome. Available at: http://www.cdc.gov/vaccines/pubs/surv-manual/chpt15-crs.html. Retrieved March 31, 2014. [Google Scholar]
- 2. Roper MH, Vandelaer JH, Gasse FL. Maternal and neonatal tetanus. Lancet 2007; 370:1947-59; PMID:17854885; http://dx.doi.org/ 10.1016/S0140-6736(07)61261-6 [DOI] [PubMed] [Google Scholar]
- 3. American College of Obstetricians and Gynecologists Practice Bulletin #4, Prevention of Rh D Alloimmunization. Washington, DC: American College of Obstetricians and Gynecologists; reaffirmed 2013 Available at http://www.acog.org/Resources_And_Publications/Practice_Bulletins/Committee_on_Practice_Bulletins_-_Obstetrics/Prevention_of_Rh_D_Alloimmunization. Accessed March 31, 2014. [Google Scholar]
- 4. Beigi RH, Goldkind SF, Jevaji I. Research on vaccines and antimicrobials during pregnancy: challenges and opportunities. Vaccine 2013; 31:4261-3; PMID:23978280; http://dx.doi.org/ 10.1016/j.vaccine.2013.05.060 [DOI] [PubMed] [Google Scholar]
- 5. Centers for Disease Control and Prevention (CDC) Prevention and control of seasonal influenza with vaccines. Recommendations of the Advisory Committee on Immunization Practices–United States, 2013-2014 MMWR Recomm Rep 2013; 62(RR-07):1-43 [PubMed] [Google Scholar]
- 6. Centers for Disease Control and Prevention (CDC) . Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccine (Tdap) in pregnant women–Advisory Committee on Immunization Practices (ACIP), 2012. [eng.]. MMWR Morb Mortal Wkly Rep 2013; 62:131-5; PMID:23425962 [PMC free article] [PubMed] [Google Scholar]
- 7. Fiore AE, Shay DK, Broder K, Iskander JK, Uyeki TM, Mootrey G, Bresee JS, Cox NJ. Centers for Disease Control and Prevention. Prevention and control of seasonal influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2009; 58(RR-8):1-52; PMID:19644442 [PubMed] [Google Scholar]
- 8. Irving WL, James DK, Stephenson T, Laing P, Jameson C, Oxford JS, Chakraverty P, Brown DW, Boon AC, Zambon MC. Influenza virus infection in the second and third trimesters of pregnancy: a clinical and seroepidemiological study. BJOG 2000; 107:1282-9; PMID:11028582; http://dx.doi.org/ 10.1111/j.1471-0528.2000.tb11621.x [DOI] [PubMed] [Google Scholar]
- 9. Harris JM. Influenza occurring in pregnat women: a statistical study of thirteen hundred and fifty cases. J Am Med Assoc 1919; 72:978-80; http://dx.doi.org/ 10.1001/jama.1919.02610140008002 [DOI] [Google Scholar]
- 10. Freeman DW, Barno A. Deaths from Asian influenza associated with pregnancy. Am J Obstet Gynecol 1959; 78:1172-5; PMID:13824729 [DOI] [PubMed] [Google Scholar]
- 11. Jamieson DJ, Honein MA, Rasmussen SA, Williams JL, Swerdlow DL, Biggerstaff MS, Lindstrom S, Louie JK, Christ CM, Bohm SR, et al.; Novel Influenza A (H1N1) Pregnancy Working Group. H1N1 2009 influenza virus infection during pregnancy in the USA. Lancet 2009; 374:451-8; PMID:19643469; http://dx.doi.org/ 10.1016/S0140-6736(09)61304-0 [DOI] [PubMed] [Google Scholar]
- 12. Louie JK, Acosta M, Jamieson DJ, Honein MA. California Pandemic (H1N1) Working Group. Severe 2009 H1N1 influenza in pregnant and postpartum women in California. N Engl J Med 2010; 362:27-35; PMID:20032319; http://dx.doi.org/ 10.1056/NEJMoa0910444 [DOI] [PubMed] [Google Scholar]
- 13. Siston AM, Rasmussen SA, Honein MA, Fry AM, Seib K, Callaghan WM, Louie J, Doyle TJ, Crockett M, Lynfield R, et al.; Pandemic H1N1 Influenza in Pregnancy Working Group. Pandemic 2009 influenza A(H1N1) virus illness among pregnant women in the United States. JAMA 2010; 303:1517-25; PMID:20407061; http://dx.doi.org/ 10.1001/jama.2010.479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Saleeby E, Chapman J, Morse J, Bryant A. H1N1 influenza in pregnancy: cause for concern. Obstet Gynecol 2009; 114:885-91; PMID:19888049; http://dx.doi.org/ 10.1097/AOG.0b013e3181bb44bb [DOI] [PubMed] [Google Scholar]
- 15. Creanga AA, Kamimoto L, Newsome K, D’Mello T, Jamieson DJ, Zotti ME, Arnold KE, Baumbach J, Bennett NM, Farley MM, et al. Seasonal and 2009 pandemic influenza A (H1N1) virus infection during pregnancy: a population-based study of hospitalized cases. Am J Obstet Gynecol 2011; 204(Suppl 1):S38-45; PMID:21507375; http://dx.doi.org/ 10.1016/j.ajog.2011.02.037 [DOI] [PubMed] [Google Scholar]
- 16. Ahluwalia IB, Jamieson DJ, Rasmussen SA, D’Angelo D, Goodman D, Kim H. Correlates of seasonal influenza vaccine coverage among pregnant women in Georgia and Rhode Island. Obstet Gynecol 2010; 116:949-55; PMID:20859160; http://dx.doi.org/ 10.1097/AOG.0b013e3181f1039f [DOI] [PubMed] [Google Scholar]
- 17. Moniz MH, Beigi RH. Influenza Infection During Pregnancy: Virology, Pathogenesis, and Clinical Challenges. Future Virology 2013; 8:11-23; http://dx.doi.org/ 10.2217/fvl.12.117 [DOI] [Google Scholar]
- 18. Goodrum LA. Pneumonia in pregnancy. Semin Perinatol 1997; 21:276-83; PMID:9298716; http://dx.doi.org/ 10.1016/S0146-0005(97)80070-5 [DOI] [PubMed] [Google Scholar]
- 19. Neuzil KM, Reed GW, Mitchel EF, Simonsen L, Griffin MR. Impact of influenza on acute cardiopulmonary hospitalizations in pregnant women. Am J Epidemiol 1998; 148:1094-102; PMID:9850132; http://dx.doi.org/ 10.1093/oxfordjournals.aje.a009587 [DOI] [PubMed] [Google Scholar]
- 20. Cox S, Posner SF, McPheeters M, Jamieson DJ, Kourtis AP, Meikle S. Hospitalizations with respiratory illness among pregnant women during influenza season. Obstet Gynecol 2006; 107:1315-22; PMID:16738158; http://dx.doi.org/ 10.1097/01.AOG.0000218702.92005.bb [DOI] [PubMed] [Google Scholar]
- 21. Cox S, Posner SF, McPheeters M, Jamieson DJ, Kourtis AP, Meikle S. Influenza and pregnant women: hospitalization burden, United States, 1998-2002. J Womens Health (Larchmt) 2006; 15:891-3; PMID:17087611; http://dx.doi.org/ 10.1089/jwh.2006.15.891 [DOI] [PubMed] [Google Scholar]
- 22. Wilkinson DJ, Buttery JP, Andersen CC. Influenza in the neonatal intensive care unit. J Perinatol 2006; 26:772-6; PMID:17122787; http://dx.doi.org/ 10.1038/sj.jp.7211625 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hartert TV, Neuzil KM, Shintani AK, Mitchel EF, Jr., Snowden MS, Wood LB, Dittus RS, Griffin MR. Maternal morbidity and perinatal outcomes among pregnant women with respiratory hospitalizations during influenza season. Am J Obstet Gynecol 2003; 189:1705-12; PMID:14710102; http://dx.doi.org/ 10.1016/S0002-9378(03)00857-3 [DOI] [PubMed] [Google Scholar]
- 24. Lindsay L, Jackson LA, Savitz DA, Weber DJ, Koch GG, Kong L, Guess HA. Community influenza activity and risk of acute influenza-like illness episodes among healthy unvaccinated pregnant and postpartum women. Am J Epidemiol 2006; 163:838-48; PMID:16554352; http://dx.doi.org/ 10.1093/aje/kwj095 [DOI] [PubMed] [Google Scholar]
- 25. Pierce M, Kurinczuk JJ, Spark P, Brocklehurst P, Knight M. UKOSS. Perinatal outcomes after maternal 2009/H1N1 infection: national cohort study. BMJ 2011; 342:d3214; PMID:21672992; http://dx.doi.org/ 10.1136/bmj.d3214 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Naresh A, Fisher BM, Hoppe KK, Catov J, Xu J, Hart J, Lynch AM, Gibbs R, Eschenbach D, Gravett M, et al. A multicenter cohort study of pregnancy outcomes among women with laboratory-confirmed H1N1 influenza. J Perinatol 2013; 33:939-43; PMID:24051575; http://dx.doi.org/ 10.1038/jp.2013.110 [DOI] [PubMed] [Google Scholar]
- 27. Sumaya CV, Gibbs RS. Immunization of pregnant women with influenza A/New Jersey/76 virus vaccine: reactogenicity and immunogenicity in mother and infant. J Infect Dis 1979; 140:141-6; PMID:479636; http://dx.doi.org/ 10.1093/infdis/140.2.141 [DOI] [PubMed] [Google Scholar]
- 28. Englund JA, Mbawuike IN, Hammill H, Holleman MC, Baxter BD, Glezen WP. Maternal immunization with influenza or tetanus toxoid vaccine for passive antibody protection in young infants. J Infect Dis 1993; 168:647-56; PMID:8354906; http://dx.doi.org/ 10.1093/infdis/168.3.647 [DOI] [PubMed] [Google Scholar]
- 29. Jackson LA, Patel SM, Swamy GK, Frey SE, Creech CB, Munoz FM, Artal R, Keitel WA, Noah DL, Petrie CR, et al. Immunogenicity of an inactivated monovalent 2009 H1N1 influenza vaccine in pregnant women. J Infect Dis 2011; 204:854-63; PMID:21849282; http://dx.doi.org/ 10.1093/infdis/jir440 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Mak TK, Mangtani P, Leese J, Watson JM, Pfeifer D. Influenza vaccination in pregnancy: current evidence and selected national policies. Lancet Infect Dis 2008; 8:44-52; PMID:18156088; http://dx.doi.org/ 10.1016/S1473-3099(07)70311-0 [DOI] [PubMed] [Google Scholar]
- 31. Tamma PD, Ault KA, del Rio C, Steinhoff MC, Halsey NA, Omer SB. Safety of influenza vaccination during pregnancy. Am J Obstet Gynecol 2009; 201:547-52; PMID:19850275; http://dx.doi.org/ 10.1016/j.ajog.2009.09.034 [DOI] [PubMed] [Google Scholar]
- 32. Moro PL, Broder K, Zheteyeva Y, Walton K, Rohan P, Sutherland A, Guh A, Haber P, Destefano F, Vellozzi C. Adverse events in pregnant women following administration of trivalent inactivated influenza vaccine and live attenuated influenza vaccine in the Vaccine Adverse Event Reporting System, 1990-2009. Am J Obstet Gynecol 2011; 204:e1-7; PMID:20965490 [DOI] [PubMed] [Google Scholar]
- 33. Håberg SE, Trogstad L, Gunnes N, Wilcox AJ, Gjessing HK, Samuelsen SO, Skrondal A, Cappelen I, Engeland A, Aavitsland P, et al. Risk of fetal death after pandemic influenza virus infection or vaccination. N Engl J Med 2013; 368:333-40; PMID:23323868; http://dx.doi.org/ 10.1056/NEJMoa1207210 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Pasternak B, Svanström H, Mølgaard-Nielsen D, Krause TG, Emborg HD, Melbye M, Hviid A. Risk of adverse fetal outcomes following administration of a pandemic influenza A(H1N1) vaccine during pregnancy. JAMA 2012; 308:165-74; PMID:22782418; http://dx.doi.org/ 10.1001/jama.2012.6131 [DOI] [PubMed] [Google Scholar]
- 35. Moro PL, Tepper NK, Grohskopf LA, Vellozzi C, Broder K. Safety of seasonal influenza and influenza A (H1N1) 2009 monovalent vaccines in pregnancy. Expert Rev Vaccines 2012; 11:911-21; PMID:23002972; http://dx.doi.org/ 10.1586/erv.12.72 [DOI] [PubMed] [Google Scholar]
- 36. Bednarczyk RA, Adjaye-Gbewonyo D, Omer SB. Safety of influenza immunization during pregnancy for the fetus and the neonate. Am J Obstet Gynecol 2012; 207(Suppl):S38-46; PMID:22920058; http://dx.doi.org/ 10.1016/j.ajog.2012.07.002 [DOI] [PubMed] [Google Scholar]
- 37. Munoz FM. Safety of influenza vaccines in pregnant women. Am J Obstet Gynecol 2012; 207(Suppl):S33-7; PMID:22920057; http://dx.doi.org/ 10.1016/j.ajog.2012.06.072 [DOI] [PubMed] [Google Scholar]
- 38. Irving SA, Kieke BA, Donahue JG, Mascola MA, Baggs J, DeStefano F, Cheetham TC, Jackson LA, Naleway AL, Glanz JM, et al.; Vaccine Safety Datalink. Trivalent inactivated influenza vaccine and spontaneous abortion. Obstet Gynecol 2013; 121:159-65; PMID:23262941 [DOI] [PubMed] [Google Scholar]
- 39. Steinhoff MC, Omer SB, Roy E, El Arifeen S, Raqib R, Dodd C, Breiman RF, Zaman K. Neonatal outcomes after influenza immunization during pregnancy: a randomized controlled trial. CMAJ 2012; 184:645-53; PMID:22353593; http://dx.doi.org/ 10.1503/cmaj.110754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Omer SB, Goodman D, Steinhoff MC, Rochat R, Klugman KP, Stoll BJ, Ramakrishnan U. Maternal influenza immunization and reduced likelihood of prematurity and small for gestational age births: a retrospective cohort study. PLoS Med 2011; 8:e1000441; PMID:21655318; http://dx.doi.org/ 10.1371/journal.pmed.1000441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Zaman K, Roy E, Arifeen SE, Rahman M, Raqib R, Wilson E, Omer SB, Shahid NS, Breiman RF, Steinhoff MC. Effectiveness of maternal influenza immunization in mothers and infants. N Engl J Med 2008; 359:1555-64; PMID:18799552; http://dx.doi.org/ 10.1056/NEJMoa0708630 [DOI] [PubMed] [Google Scholar]
- 42. Puck JM, Glezen WP, Frank AL, Six HR. Protection of infants from infection with influenza A virus by transplacentally acquired antibody. J Infect Dis 1980; 142:844-9; PMID:7462695; http://dx.doi.org/ 10.1093/infdis/142.6.844 [DOI] [PubMed] [Google Scholar]
- 43. Poehling KA, Szilagyi PG, Staat MA, Snively BM, Payne DC, Bridges CB, Chu SY, Light LS, Prill MM, Finelli L, et al.; New Vaccine Surveillance Network. Impact of maternal immunization on influenza hospitalizations in infants. Am J Obstet Gynecol 2011; 204(Suppl 1):S141-8; PMID:21492825; http://dx.doi.org/ 10.1016/j.ajog.2011.02.042 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Beigi RH, Wiringa AE, Bailey RR, Assi TM, Lee BY. Economic value of seasonal and pandemic influenza vaccination during pregnancy. Clin Infect Dis 2009; 49:1784-92; PMID:19911967; http://dx.doi.org/ 10.1086/649013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. American College of Obstetricians and Gynecologists . ACOG committee opinion no. 558: Integrating immunizations into practice. Obstet Gynecol 2013; 121:897-903; PMID:23635707; http://dx.doi.org/ 10.1097/01.AOG.0000428788.74725.90 [DOI] [PubMed] [Google Scholar]
- 46. Van Rie A, Wendelboe AM, Englund JA. Role of maternal pertussis antibodies in infants. Pediatr Infect Dis J 2005; 24(Suppl):S62-5; PMID:15876928; http://dx.doi.org/ 10.1097/01.inf.0000160915.93979.8f [DOI] [PubMed] [Google Scholar]
- 47. Pertussis report California Department of Public Health. 2010. Available at: http://www.cdph.ca.gov/programs/immunize/Documents/PertussisReport2010-12-15.pdf. Retrieved April 11, 2014. [Google Scholar]
- 48. Bisgard KM, Pascual FB, Ehresmann KR, Miller CA, Cianfrini C, Jennings CE, Rebmann CA, Gabel J, Schauer SL, Lett SM. Infant pertussis: who was the source? Pediatr Infect Dis J 2004; 23:985-9; PMID:15545851; http://dx.doi.org/ 10.1097/01.inf.0000145263.37198.2b [DOI] [PubMed] [Google Scholar]
- 49. Centers for Disease Control and Prevention (CDC) . Updated recommendations for use of tetanus toxoid, reduced diphtheria toxoid and acellular pertussis vaccine (Tdap) in pregnant women and persons who have or anticipate having close contact with an infant aged <12 months — Advisory Committee on Immunization Practices (ACIP), 2011. [eng.]. MMWR Morb Mortal Wkly Rep 2011; 60:1424-6; PMID:22012116 [PubMed] [Google Scholar]
- 50. Healy CM, Rench MA, Baker CJ. Importance of timing of maternal combined tetanus, diphtheria, and acellular pertussis (Tdap) immunization and protection of young infants. Clin Infect Dis 2013; 56:539-44; PMID:23097585; http://dx.doi.org/ 10.1093/cid/cis923 [DOI] [PubMed] [Google Scholar]
- 51. Halperin BA, Morris A, Mackinnon-Cameron D, Mutch J, Langley JM, McNeil SA, Macdougall D, Halperin SA. Kinetics of the antibody response to tetanus-diphtheria-acellular pertussis vaccine in women of childbearing age and postpartum women. Clin Infect Dis 2011; 53:885-92; PMID:21946190; http://dx.doi.org/ 10.1093/cid/cir538 [DOI] [PubMed] [Google Scholar]
- 52. ACOG Committee Opinion No . ACOG Committee Opinion No. 566: Update on immunization and pregnancy: tetanus, diphtheria, and pertussis vaccination. Obstet Gynecol 2013; 121:1411-4; PMID:23812487; http://dx.doi.org/ 10.1097/01.AOG.0000431054.33593.e3 [DOI] [PubMed] [Google Scholar]
- 53. Committee on infectious diseases . Recommendations for prevention and control of influenza in children, 2013-2014. Pediatrics 2013; 132:e1089-104; PMID:23999962; http://dx.doi.org/ 10.1542/peds.2013-2377 [DOI] [PubMed] [Google Scholar]
- 54. Zheteyeva YA, Moro PL, Tepper NK, Rasmussen SA, Barash FE, Revzina NV, Kissin D, Lewis PW, Yue X, Haber P, et al. Adverse event reports after tetanus toxoid, reduced diphtheria toxoid, and acellular pertussis vaccines in pregnant women. Am J Obstet Gynecol 2012; 207:e1-7; PMID:22727350; http://dx.doi.org/ 10.1016/j.ajog.2012.05.006 [DOI] [PubMed] [Google Scholar]
- 55. Munoz FM, Bond NH, Maccato M, Pinell P, Hammill HA, Swamy GK, Walter EB, Jackson LA, Englund JA, Edwards MS, et al. Safety and immunogenicity of tetanus diphtheria and acellular pertussis (Tdap) immunization during pregnancy in mothers and infants: a randomized clinical trial. [eng.]. JAMA 2014; 311:1760-9; PMID:24794369; http://dx.doi.org/ 10.1001/jama.2014.3633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Terranella A, Asay GR, Messonnier ML, Clark TA, Liang JL. Pregnancy dose Tdap and postpartum cocooning to prevent infant pertussis: a decision analysis. Pediatrics 2013; 131:e1748-56; PMID:23713104; http://dx.doi.org/ 10.1542/peds.2012-3144 [DOI] [PubMed] [Google Scholar]
- 57. Swamy GK, Garcia-Putnam R. Vaccine-preventable diseases in pregnancy. Am J Perinatol 2013; 30:89-97; PMID:23271378 [DOI] [PubMed] [Google Scholar]
- 58. Novartis Vaccines Safety and Immunogenicity of a Trivalent Group B Streptococcus Vaccine in Healthy Pregnant Women. ClinicalTrials.gov Identifier NCT02046148. Available at: https://clinicaltrials.gov/ct2/show/NCT02046148?term=gbs&rank=8. Accessed June 4, 2014. [Google Scholar]
- 59. ClinicalTrials.gov Ongoing clinical trials related to maternal immunization. Available at: https://clinicaltrials.gov/ct2/results?term=maternal+immunization. Accessed June 4, 2014. [Google Scholar]
- 60. Fiore AE, Uyeki TM, Broder K, Finelli L, Euler GL, Singleton JA, Iskander JK, Wortley PM, Shay DK, Bresee JS, et al.; Centers for Disease Control and Prevention (CDC). Prevention and control of influenza with vaccines: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2010. MMWR Recomm Rep 2010; 59(RR-8):1-62; PMID:20689501 [PubMed] [Google Scholar]
- 61. Naleway AL, Smith WJ, Mullooly JP. Delivering influenza vaccine to pregnant women. Epidemiol Rev 2006; 28:47-53; PMID:16731574; http://dx.doi.org/ 10.1093/epirev/mxj002 [DOI] [PubMed] [Google Scholar]
- 62. Healthy People 2020 Topics and Objectives: Immunizations and Infectious Diseases, IID-12.10. Available at http://www.healthypeople.gov/2020/topicsobjectives2020/overview.aspx?topicid=23. Accessed March 2, 2014. [Google Scholar]
- 63. American College of Obstetricians and Gynecologists Committee on Obstetric Practice . ACOG Committee Opinion No. 468: Influenza vaccination during pregnancy. Obstet Gynecol 2010; 116:1006-7; PMID:20859176; http://dx.doi.org/ 10.1097/AOG.0b013e3181fae845 [DOI] [PubMed] [Google Scholar]
- 64. Lu P, Bridges CB, Euler GL, Singleton JA. Influenza vaccination of recommended adult populations, U.S., 1989-2005. Vaccine 2008; 26:1786-93; PMID:18336965; http://dx.doi.org/ 10.1016/j.vaccine.2008.01.040 [DOI] [PubMed] [Google Scholar]
- 65. Centers for Disease Control and Prevention (CDC) . Influenza vaccination coverage among pregnant women—United States, 2010-11 influenza season. MMWR Morb Mortal Wkly Rep 2011; 60:1078-82; PMID:21849964 [PubMed] [Google Scholar]
- 66. Kennedy ED, Ahluwalia IB, Ding H, Lu PJ, Singleton JA, Bridges CB. Monitoring seasonal influenza vaccination coverage among pregnant women in the United States. Am J Obstet Gynecol 2012; 207(Suppl):S9-16; PMID:22920065; http://dx.doi.org/ 10.1016/j.ajog.2012.06.069 [DOI] [PubMed] [Google Scholar]
- 67. Centers for Disease Control and Prevention (CDC) . Influenza vaccination coverage among pregnant women: 2011-12 influenza season, United States. [eng.]. MMWR Morb Mortal Wkly Rep 2012; 61:758-63; PMID:23013721 [PubMed] [Google Scholar]
- 68. Centers for Disease Control and Prevention (CDC) . Influenza vaccination coverage among pregnant women–United States, 2012-13 influenza season. [eng.]. MMWR Morb Mortal Wkly Rep 2013; 62:787-92; PMID:24067583 [PMC free article] [PubMed] [Google Scholar]
- 69. Freund R, Le Ray C, Charlier C, Avenell C, Truster V, Tréluyer JM, Skalli D, Ville Y, Goffinet F, Launay O; Inserm COFLUPREG Study Group . Determinants of non-vaccination against pandemic 2009 H1N1 influenza in pregnant women: a prospective cohort study. PLoS One 2011; 6:e20900; PMID:21695074; http://dx.doi.org/ 10.1371/journal.pone.0020900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70. Kuehn BM. Mothers take physicians’ advice on vaccines. JAMA 2010; 304:2577-8; PMID:21156941; http://dx.doi.org/ 10.1001/jama.2010.1785 [DOI] [PubMed] [Google Scholar]
- 71. Fisher BM, Scott J, Hart J, Winn VD, Gibbs RS, Lynch AM. Behaviors and perceptions regarding seasonal and H1N1 influenza vaccination during pregnancy. Am J Obstet Gynecol 2011; 204(Suppl 1):S107-11; PMID:21419386; http://dx.doi.org/ 10.1016/j.ajog.2011.02.041 [DOI] [PubMed] [Google Scholar]
- 72. Drees M, Johnson O, Wong E, Stewart A, Ferisin S, Silverman PR, Ehrenthal DB. Acceptance of 2009 H1N1 influenza vaccine among pregnant women in Delaware. Am J Perinatol 2012; 29:289-94; PMID:22147638; http://dx.doi.org/ 10.1055/s-0031-1295660 [DOI] [PubMed] [Google Scholar]
- 73. Linn ST, Guralnik JM, Patel KV. Disparities in influenza vaccine coverage in the United States, 2008. J Am Geriatr Soc 2010; 58:1333-40; PMID:20533970; http://dx.doi.org/ 10.1111/j.1532-5415.2010.02904.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Logan JL. Disparities in influenza immunization among US adults. J Natl Med Assoc 2009; 101:161-6; PMID:19378634 [DOI] [PubMed] [Google Scholar]
- 75. Glanz K, Rimer BK, Viswanath K. Health behavior and health education: theory, research, and practice. 4th ed. San Francisco, CA: Jossey-Bass; 2008. xxxiii, 552 p. p. [Google Scholar]
- 76. Brewer NT, Chapman GB, Gibbons FX, Gerrard M, McCaul KD, Weinstein ND. Meta-analysis of the relationship between risk perception and health behavior: the example of vaccination. Health Psychol 2007; 26:136-45; PMID:17385964; http://dx.doi.org/ 10.1037/0278-6133.26.2.136 [DOI] [PubMed] [Google Scholar]
- 77. Chapman GB, Coups EJ. Emotions and preventive health behavior: worry, regret, and influenza vaccination. Health Psychol 2006; 25:82-90; PMID:16448301; http://dx.doi.org/ 10.1037/0278-6133.25.1.82 [DOI] [PubMed] [Google Scholar]
- 78. Gorman JR, Brewer NT, Wang JB, Chambers CD. Theory-based predictors of influenza vaccination among pregnant women. Vaccine 2012; 31:213-8; PMID:23123019; http://dx.doi.org/ 10.1016/j.vaccine.2012.10.064 [DOI] [PubMed] [Google Scholar]
- 79. Henninger M, Naleway A, Crane B, Donahue J, Irving S. Predictors of seasonal influenza vaccination during pregnancy. Obstet Gynecol 2013; 121:741-9; PMID:23635673; http://dx.doi.org/ 10.1097/AOG.0b013e3182878a5a [DOI] [PubMed] [Google Scholar]
- 80. Shavell VI, Moniz MH, Gonik B, Beigi RH. Influenza immunization in pregnancy: overcoming patient and health care provider barriers. Am J Obstet Gynecol 2012; 207(Suppl):S67-74; PMID:22920063; http://dx.doi.org/ 10.1016/j.ajog.2012.06.077 [DOI] [PubMed] [Google Scholar]
- 81. Yudin MH, Salaripour M, Sgro MD. Pregnant women's knowledge of influenza and the use and safety of the influenza vaccine during pregnancy. J Obstet Gynaecol Can 2009; 31:120-5; PMID:19327210 [DOI] [PubMed] [Google Scholar]
- 82. Moniz MH, Vitek WS, Akers A, Meyn LA, Beigi RH. Perceptions and acceptance of immunization during pregnancy. J Reprod Med 2013; 58:383-8; PMID:24050026 [PubMed] [Google Scholar]
- 83. Eppes C, Wu A, You W, Cameron KA, Garcia P, Grobman W. Barriers to influenza vaccination among pregnant women. Vaccine 2013; 31:2874-8; PMID:23623863; http://dx.doi.org/ 10.1016/j.vaccine.2013.04.031 [DOI] [PubMed] [Google Scholar]
- 84. Varan AK, Esteves-Jaramillo A, Richardson V, Esparza-Aguilar M, Cervantes-Powell P, Omer SB. Intention to accept Bordetella pertussis booster vaccine during pregnancy in Mexico City. Vaccine 2014; 32:785-92; PMID:24394441; http://dx.doi.org/ 10.1016/j.vaccine.2013.12.054 [DOI] [PubMed] [Google Scholar]
- 85. Tucker Edmonds BM, Coleman J, Armstrong K, Shea JA. Risk perceptions, worry, or distrust: what drives pregnant women's decisions to accept the H1N1 vaccine? Matern Child Health J 2011; 15:1203-9; PMID:20936337; http://dx.doi.org/ 10.1007/s10995-010-0693-5 [DOI] [PubMed] [Google Scholar]
- 86. Silverman NS, Greif A. Influenza vaccination during pregnancy. Patients’ and physicians’ attitudes. J Reprod Med 2001; 46:989-94; PMID:11762156 [PubMed] [Google Scholar]
- 87. Ahluwalia IB, Singleton JA, Jamieson DJ, Rasmussen SA, Harrison L. Seasonal influenza vaccine coverage among pregnant women: pregnancy risk assessment monitoring system. J Womens Health (Larchmt) 2011; 20:649-51; PMID:21438700; http://dx.doi.org/ 10.1089/jwh.2011.2794 [DOI] [PubMed] [Google Scholar]
- 88. Naleway AL, Henkle EM, Ball S, Bozeman S, Gaglani MJ, Kennedy ED, Thompson MG. Barriers and facilitators to influenza vaccination and vaccine coverage in a cohort of health care personnel. Am J Infect Control 2014; 42:371-5; PMID:24679562; http://dx.doi.org/ 10.1016/j.ajic.2013.11.003 [DOI] [PubMed] [Google Scholar]
- 89. Hollmeyer HG, Hayden F, Poland G, Buchholz U. Influenza vaccination of health care workers in hospitals–a review of studies on attitudes and predictors. Vaccine 2009; 27:3935-44; PMID:19467744; http://dx.doi.org/ 10.1016/j.vaccine.2009.03.056 [DOI] [PubMed] [Google Scholar]
- 90. Marsh HA, Malik F, Shapiro E, Omer SB, Frew PM. Message Framing Strategies to Increase Influenza Immunization Uptake Among Pregnant African American Women. Matern Child Health J 2013; (Forthcoming); PMID:24337776; http://dx.doi.org/ 10.1007/s10995-013-1404-9 [DOI] [PubMed] [Google Scholar]
- 91. Frew PM, Saint-Victor DS, Owens LE, Omer SB. Socioecological and message framing factors influencing maternal influenza immunization among minority women. Vaccine 2014; 32:1736-44; PMID:24486366; http://dx.doi.org/ 10.1016/j.vaccine.2014.01.030 [DOI] [PubMed] [Google Scholar]
- 92. Kissin DM, Power ML, Kahn EB, Williams JL, Jamieson DJ, MacFarlane K, Schulkin J, Zhang Y, Callaghan WM. Attitudes and practices of obstetrician-gynecologists regarding influenza vaccination in pregnancy. Obstet Gynecol 2011; 118:1074-80; PMID:22015875; http://dx.doi.org/ 10.1097/AOG.0b013e3182329681 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93. Power ML, Leddy MA, Anderson BL, Gall SA, Gonik B, Schulkin J. Obstetrician-gynecologists’ practices and perceived knowledge regarding immunization. Am J Prev Med 2009; 37:231-4; PMID:19596538; http://dx.doi.org/ 10.1016/j.amepre.2009.05.019 [DOI] [PubMed] [Google Scholar]
- 94. Panda B, Stiller R, Panda A. Influenza vaccination during pregnancy and factors for lacking compliance with current CDC guidelines. J Matern Fetal Neonatal Med 2011; 24:402-6; PMID:20593974; http://dx.doi.org/ 10.3109/14767058.2010.497882 [DOI] [PubMed] [Google Scholar]
- 95. Gonik B, Jones T, Contreras D, Fasano N, Roberts C. The obstetrician-gynecologist's role in vaccine-preventable diseases and immunization. Obstet Gynecol 2000; 96:81-4; PMID:10862847; http://dx.doi.org/ 10.1016/S0029-7844(00)00860-7 [DOI] [PubMed] [Google Scholar]
- 96. Tong A, Biringer A, Ofner-Agostini M, Upshur R, McGeer A. A cross-sectional study of maternity care providers’ and women's knowledge, attitudes, and behaviours towards influenza vaccination during pregnancy. J Obstet Gynaecol Can 2008; 30:404-10; PMID:18505664 [DOI] [PubMed] [Google Scholar]
- 97. Gallagher KM, Updegraff JA. Health message framing effects on attitudes, intentions, and behavior: a meta-analytic review. Ann Behav Med 2012; 43:101-16; PMID:21993844; http://dx.doi.org/ 10.1007/s12160-011-9308-7 [DOI] [PubMed] [Google Scholar]
- 98. Blanchard-Rohner G, Meier S, Ryser J, Schaller D, Combescure C, Yudin MH, Burton-Jeangros C, de Tejada BM, Siegrist CA. Acceptability of maternal immunization against influenza: the critical role of obstetricians. J Matern Fetal Neonatal Med 2012; 25:1800-9; PMID:22339083; http://dx.doi.org/ 10.3109/14767058.2012.663835 [DOI] [PubMed] [Google Scholar]
- 99. Maher L, Hope K, Torvaldsen S, Lawrence G, Dawson A, Wiley K, Thomson D, Hayen A, Conaty S. Influenza vaccination during pregnancy: coverage rates and influencing factors in two urban districts in Sydney. Vaccine 2013; 31:5557-64; PMID:24076176; http://dx.doi.org/ 10.1016/j.vaccine.2013.08.081 [DOI] [PubMed] [Google Scholar]
- 100. Stockwell MS, Westhoff C, Kharbanda EO, Vargas CY, Camargo S, Vawdrey DK, Castaño PM. Influenza vaccine text message reminders for urban, low-income pregnant women: a randomized controlled trial. Am J Public Health 2014; 104(Suppl 1):e7-12; PMID:24354839; http://dx.doi.org/ 10.2105/AJPH.2013.301620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Moniz MH, Hasley S, Meyn LA, Beigi RH. Improving influenza vaccination rates in pregnancy through text messaging: a randomized controlled trial. Obstet Gynecol 2013; 121:734-40; PMID:23635672; http://dx.doi.org/ 10.1097/AOG.0b013e31828642b1 [DOI] [PubMed] [Google Scholar]
- 102. Centers for Disease Control and Prevention . Recommended adult immunization schedule – United States, 2014. J Midwifery Womens Health 2014; 59:205-9; PMID:24618112; http://dx.doi.org/ 10.1111/jmwh.12184 [DOI] [PubMed] [Google Scholar]
- 103. Staples JE, Gershman M, Fischer M; Centers for Disease Control and Prevention (CDC) . Yellow fever vaccine: recommendations of the Advisory Committee on Immunization Practices (ACIP). MMWR Recomm Rep 2010; 59(RR-7):1-27; PMID:20671663 [PubMed] [Google Scholar]
- 104. Manning SE, Rupprecht CE, Fishbein D, Hanlon CA, Lumlertdacha B, Guerra M, Meltzer MI, Dhankhar P, Vaidya SA, Jenkins SR, et al.; Advisory Committee on Immunization Practices Centers for Disease Control and Prevention (CDC). Human rabies prevention–United States, 2008: recommendations of the Advisory Committee on Immunization Practices. MMWR Recomm Rep 2008; 57(RR-3):1-28; PMID:18496505 [PubMed] [Google Scholar]
- 105. Wright JG, Quinn CP, Shadomy S, Messonnier N; Centers for Disease Control and Prevention (CDC) . Use of anthrax vaccine in the United States: recommendations of the Advisory Committee on Immunization Practices (ACIP), 2009. MMWR Recomm Rep 2010; 59(RR-6):1-30; PMID:20651644 [PubMed] [Google Scholar]
- 106. New York, NY: CDC Oxford University Press. CDC Health Information for International Travel; 2012; 2012 [Google Scholar]


