Abstract
Physico-chemical analysis of pneumococcal polysaccharide (PS)-protein conjugate vaccine components used for two commercially licensed vaccines was performed to compare the serotype- and carrier protein-specific stabilities of these vaccines. Nineteen different monovalent pneumococcal conjugates from commercial vaccines utilizing CRM197, diphtheria toxoid (DT), Protein D (PD) or tetanus toxoid (TT) as carrier proteins were incubated at temperatures up to 56°C for up to eight weeks or were subjected to freeze-thawing (F/T). Structural stability was evaluated by monitoring their size, integrity and carrier protein conformation. The molecular size of the vaccine components was well maintained for Protein D, TT and DT conjugates at -20°C, 4°C and F/T, and for CRM197 conjugates at 4°C and F/T. It was observed that four of the eight serotypes of Protein D conjugates tended to form high molecular weight complexes at 37°C or above. The other conjugated carrier proteins also appeared to form oligomers or ‘aggregates’ at elevated temperatures, but rarely when frozen and thawed. There was evidence of degradation in some of the conjugates as evidenced by the formation of lower molecular weight materials which correlated with measured free saccharide. In conclusion, pneumococcal-Protein D/TT/DT and most CRM197 bulk conjugate vaccines were stable when stored at 2–8°C, the recommended temperature. In common between the conjugates produced by the two manufacturers, serotypes 1, 5, and 19F were relatively less stable and 6B was the most stable, with types 7F and 23F also showing good stability.
Keywords: Streptococcus pneumoniae, pneumococcal, polysaccharide, conjugate vaccine, Protein D, stability, HPLC, HPAEC-PAD, fluorescence spectroscopy
Abbreviations
- CRM197
Cross-Reacting Material- 197
- DT
diphtheria toxoid
- F/T
freeze-thawing, HPAEC-PAD, high pH anion-exchange chromatography with pulsed amperometric detection
- MW
molecular weight
- Pn
pneumococcal
- PS
polysaccharide
- PD
Protein D
- SEC
size-exclusion chromatography
- TT
tetanus toxoid
Introduction
Streptococcus pneumoniae is a major cause of morbidity and mortality worldwide due to the invasive diseases it causes: bacteremia, meningitis and septicaemia. Plain capsular polysaccharides vaccines of S. pneumoniae have been available for decades but their inability to protect infants and toddlers younger than 2 y of age has led to the development of conjugate vaccines.1 Following the licensure of a 7-valent pneumococcal polysaccharide (PnPs)-CRM197 conjugate vaccine in the European Union and the USA in 2001, and demonstration of its efficacy against invasive pneumococcal disease in young children,2,3 higher valency pneumococcal conjugate vaccines covering additional serotypes have been licensed. In addition to the serotypes covered in the 7-valent PnPs-CRM197 conjugate vaccine, namely serotypes 4, 6B, 9V, 14, 18C, 19F and 23F, a 9-valent PnPs-CRM197 conjugate vaccine also contains serotypes 1 and 5, which are important causes of invasive pneumococcal disease throughout the world.4 A 10-valent pneumococcal polysaccharide-protein conjugate vaccine containing either Protein D (PD), tetanus toxoid (TT), and diphtheria toxoid (DT) as carrier proteins (PD/TT/DT) was licensed in 2009.31 In addition to the aforementioned serotypes the 10-valent vaccine contains serotype 7F. All PS in this vaccine are conjugated to Protein D, a novel carrier protein, except for type 18C which is conjugated to TT, and type 19F, conjugated to DT.
The potency of conjugate vaccines relies on the effective conjugation of oligo- or polysaccharide to carrier protein(s) and the integrity of the vaccine molecules throughout their shelf-life. Factors adversely affecting of the stability of conjugates may reduce vaccine potency through reducing the amount, accessibility and solubility of conjugated saccharide and carrier protein epitopes, potentially reducing their protective efficacy. Monitoring the stability-indicating markers of integrity and molecular size of conjugate vaccine molecules are key steps in assuring their quality, and are useful in assessing the suitability of batch release methods used to detect changes in the quality of vaccines that could potentially affect their efficacy. Physico-chemical analysis methods are widely used in the pre- and post- licensing quality control of these vaccines to ensure compliance with manufacturing specifications and batch-to-batch consistency of the products.
Previous studies have shown that CRM197 conjugate vaccines including MenC-CRM197 and Hib-CRM197 are conformationally stable when stored at their recommended temperatures, but evidence of less well-folded conformation of the carrier protein with hydrolysis or depolymerisation of the oligosaccharide chains was observed at elevated temperature.5,6 While tetanus toxoid and its conjugated forms are more resistant to secondary and tertiary conformational changes due to formaldehyde-induced intramolecular cross-linking, changes in its self-association have been observed under some conditions.7,8
Studies reporting the stability of Protein D conjugates have not been previously published. Protein D is a highly conserved cell surface protein of H. influenzae with affinity for human IgD.9 Although Protein D was developed as a novel carrier protein capable of providing immunoprotection against non-typeable Hemophilus influenzae, in its own right, little has been published on the structure and solution behavior of native Protein D or Protein D conjugate vaccines. This study was performed to assess the thermo stability of the PnPs-Protein D bulk conjugates, as well as a DT and a TT conjugate, present in the current 10-valent vaccine using physico-chemical methods, and to compare these with PnPs-CRM197 bulk conjugates. The relationship between the molecular size and conjugated carrier protein structure was also investigated for the monovalent PnPs-Protein D/TT/DT/CRM197 bulk conjugates. Some patterns in the stability of the conjugates could be attributed to specific serotype polysaccharides or carrier proteins.
Results
Thermostability of pneumococcal conjugate molecular size
To assess the size and integrity of the pneumococcal conjugate samples under stability, HPLC-SEC was performed. Unlike globular proteins, the conjugate molecules have long branches of carbohydrate chains, often highly charged, attached to the carrier protein, and their retention times are dependent on the hydrodynamic size as well as molecular mass. Because charge interactions can also affect elution, the polymeric TSK PWXL matrix was used to reduce column interactions.
Half of the 8 monovalent bulk pneumococcal-Protein D conjugates stored at 4°C, from manufacturer A (types 1-PD, 4-PD, 7F-PD and 14-PD) had a characteristically similar elution pattern, with a peak of high molecular weight (MW) material eluting at the void volume (5.4 ml) followed by a main broad peak (Fig. 1). This elution pattern was maintained in samples stored at -20°C or subjected to repeated freeze-thawing for these serotypes conjugates, and at 37°C for the type 14-PD conjugate. Following storage for 5 wk at 37°C and above, serotypes 1, 4, and 7F-PD conjugate, and at 56°C for 14-PD, samples were found to contain an increasing amount of earlier-eluting high MW materials.
Figure 1.
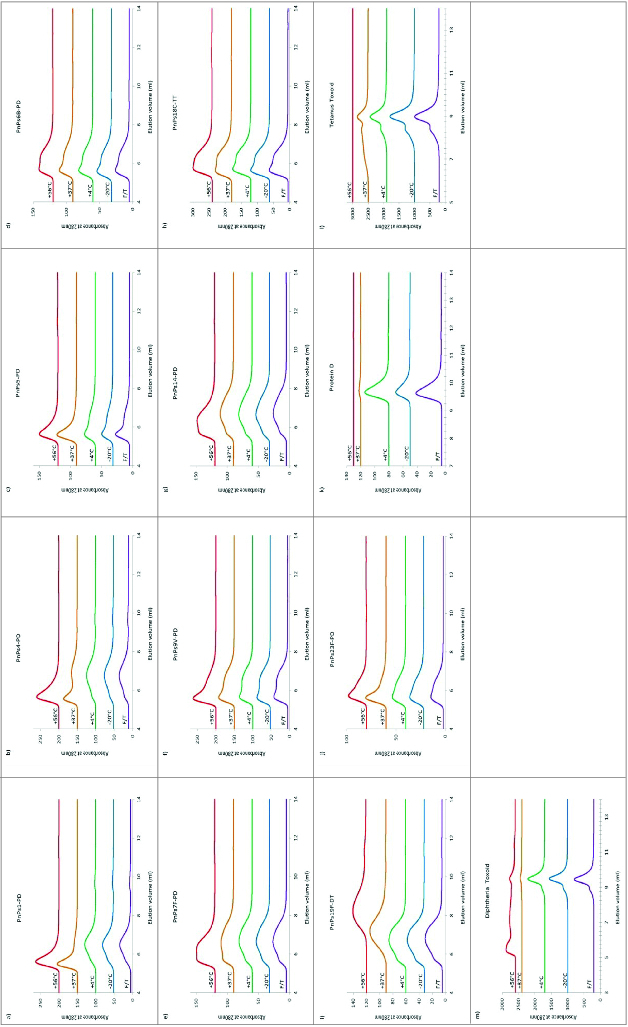
HPLC-SEC chromatograms of pneumococcal-PD/TT/DT bulk conjugates and carrier proteins stability samples. Serotypes 1, 4, 5, 6B, 7F, 9V, 14, 18C, 19F, 23F and PD, TT, DT (A to M) were stored at temperatures of -20, 4, 37, or 56°C for 5 wk, as labeled, or exposed to repeated freeze-thawing (F/T). Samples were loaded onto a TSK5000 PWXL column and eluted in PBS, pH7.4 with a flow rate 0.3 ml/min.
The other 4 monovalent bulk Protein D conjugate vaccines (5-PD, 6B-PD, 9V-PD and 23F-PD) were of higher average MW, eluting with a more prominent peak at the void volume with a second broad peak on its trailing edge. At 37°C and above, types 5-PD, 9V-PD and 23F-PD bulk conjugates also tended to form relatively higher MW forms, probably due to the association of ‘main peak’ conjugated material, as judged from the disappearance of this peak and appearance of the earlier-eluting peak. There was, however, no obvious peak profile change of 6B-PD bulk conjugate following storage at up to 37°C for 5 wk; only a small increase in the low MW conjugate shoulder was apparent at 56°C. Protein D, itself, eluted as a single broad peak, which was affected by high temperature storage, and to a lesser extent by freeze-thawing, which lead to an apparent increase in MW (Fig. 1K).
The DT and TT conjugates eluted as expected for molecules of larger molecular mass. Type 18C-TT showed stability to storage at elevated temperatures, as well as to freeze-thawing (Fig. 1H), as did tetanus toxoid, on its own (Fig. 1L). TT did, however, show a pattern of aggregation after 37°C storage, and loss at 56°C. Likewise, DT, maintained its quaternary structure upon freeze-thawing, but was aggregated at elevated temperatures (Fig. 1M). The high MW component of type 19F-DT bulk conjugate started to disappear following storage 37°C, with an accompanying decrease in the main peak size indicating degradation; further degradation of this conjugates was observed at 56°C (Fig. 1I).
The 9 monovalent pneumococcal-CRM197 conjugates eluted between 5 and 9 ml with varying distributions, typical of heterogeneous conjugates with higher oligomers. There was no obvious change in the molecular size of the CRM197 bulk conjugates stored at -20°C except for serotype 5, which showed a slight increase in high MW species. After incubation at 55°C for 8 wk, types 1, 5, 9V, 14, 18C, and 19F-CRM197 conjugates lost high MW material and there was the appearance of low MW peaks eluting between 12 to 15 ml. There were no main peak profile changes in chromatograms of types 4 or 6B-CRM197 conjugates at 55°C but an increased amount of low MW materials were detected within the elution volume range of 11.5 to 15 ml. Type 23F-CRM197 conjugate tended to form more ‘aggregates’ with low MW materials at 55°C. The only bulk conjugate sample which significantly degraded with a complete loss of all the high MW eluting materials was 19F-CRM197 conjugate (Fig. 2, A to I).
Figure 2.
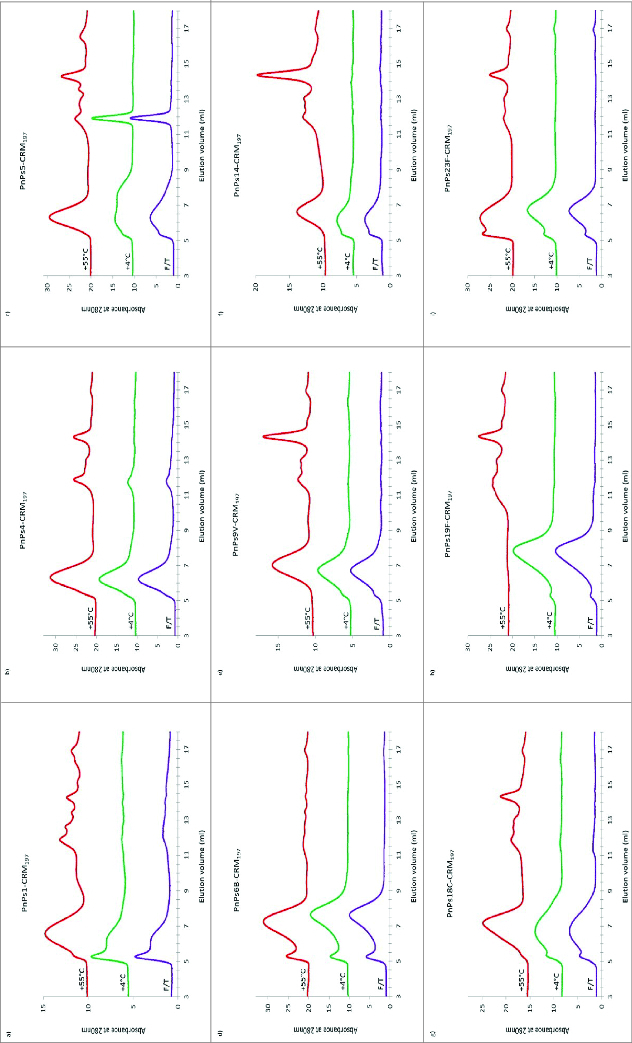
HPLC-SEC chromatograms of pneumococcal-CRM197 bulk conjugate stability samples. Serotypes 1, 4, 5, 6B, 9V, 14, 18C, 19F, and 23F (A to I) were stored at temperatures of -20, 4, 37, or 56°C for for 8 wk or exposed to repeated freeze-thawing (F/T). Conditions were as for Figure 1.
Saccharide stability of pneumococcal-Protein D, TT and DT conjugate molecules
The % free, or unconjugated, saccharide of each pneumococcal polysaccharide serotype determined for the 5 wk stability samples is shown in Figure 3. The % free saccharide values of all serotypes were less than 10% at temperatures up to 37°C. Likewise, the freeze-thawed samples did not show a significant increase in free saccharide with the exception of the type 5-PD conjugate which contained 25% free saccharide. Most serotypes of the bulk conjugates tended to release a higher amount of free saccharide during storage at 56°C for 5 wk, and types 1-PD, 5-PD, 18C-TT, and 19F-DT conjugates showed significantly increased % free saccharide at 56°C, with 60%, 56%, 18%, and 55% respectively.
Figure 3.
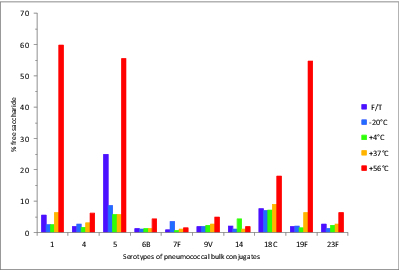
Percent free saccharide of monovalent pneumococcal-PD/TT/DT bulk conjugate stability samples. Samples were stored at -20, 4, 37, or 56°C for 5 wk or exposed to repeated freeze-thawing (F/T), with colors indicated in the legend. The free PS was separated from bulk conjugates by ultrafiltration and determined by HPAEC-PAD using PS standards.
The pH stability of a conjugate vaccine sample can be critically important; changes in pH can trigger PS depolymerization, and decreases in pH can occur following PS degradation, for example, following the cleavage of phosphodiester bonds (present in types 6B, 18C, 19F and 23F), or from de-O-acetylation, potentially in types 1, 7F, 9V, and 18C. The pH of all 10 serotypes of samples of pneumococcal-Protein D/TT/DT bulk conjugates stored in 150 mM NaCl, was stable at all temperatures up to 37°C, with the range from 5.4 to 6.1 across serotypes stored at 4°C (Fig. 4). A reduction in the pH was observed in all samples stored at 56°C (of 0.03 to 0.33 pH units) compared with samples stored at the recommended storage temperature, 2–8°C. The most significant changes were in serotypes 1, 5, 9V, 18C and 19F. Freezing did not generally cause a significant change in pH, although two freeze-thawed samples showed a significant change in pH (type 1 and type 18C). Four of the 5 serotypes showing increased acidity also had significantly enhanced free saccharide: 1, 5, 18C and 19F.
Figure 4.
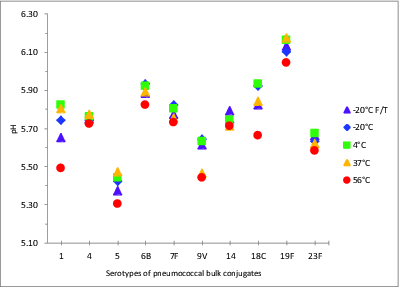
pH values of stability samples of monovalent pneumococcal-protein D/TT/DT bulk conjugates. Samples were stored at -20, 4, 37, or 56°C for 5 wk or exposed to repeated freeze-thawing (F/T), with colors indicated in the legend. pH measurements were made with samples equilibrated to room temperature.
Due to a paucity of material, it was not possible to perform direct saccharide analysis on the CRM197-conjugate polysaccharides. However, comparison of the 280 nm chromatograms (detecting protein Trp and Tyr side-chain residue signals) with the RI trace (detecting both protein and saccharide), did show extensive degradation of the polysaccharides for types 1, 4, 5, 9V, 14, and 19F-CRM197 conjugates following storage at 55°C for 8 wk, with the formation of late- eluting unique saccharide peaks, or, in the case of 19F, a loss of distinguishable peaks before the total volume of the column (Fig. S1). These can be compared with 280 nm and RI traces of types 1 and 6B-Protein D conjugates (Fig. S2) shown as examples of relatively labile and stable serotypes, respectively. The 214 nm detector signals were very similar to the 280 nm signals, when normalized, and any absorbance contributions from uronic acids or acetamido sugars at this wavelength would have been weak (not shown).
Carrier protein conformational stability of pneumococcal-Protein D, TT and DT conjugates
Intrinsic fluorescence spectroscopy has been used previously to detect changes in the protein conformation of CRM197 or TT conjugates5-7 using an excitation wavelength of 280 nm to report on the contribution of the side-chain of tryptophan and to a lesser extent tyrosine.
Protein D, the carrier protein of 8 of the 10 serotypes of the 10-valent PnPs-Protein D/TT/DT conjugate vaccine, is a highly conserved 40 kDa surface lipoprotein (Janson et al., 1991)21 found in all Hemophilus influenzae, including non-typeable H. influenzae. It contains 6 tryptophan residues and 17 tyrosine residues22 giving the protein an absorption maximum at 280 nm. The fluorescence emission maxima (Fmax) were 340 to 342 nm for all 8 Protein D conjugates (1, 4, 5, 6B, 7F, 9V, 14, and 23F) compared with 343 nm for Protein D on its own (Fig. 5). The emission maxima of the stability samples (Protein D conjugates) generally showed blue-shifts (up to 3 nm) of the emission maxima in samples stored at 37°C compared with 4°C. Significant blue-shifts were observed in all Protein D conjugates stored at 56°C, resulting in the Fmax range of 330 to 339 nm (Fig. 5), indicating an internalisation of the Trp residues, as occurs with oligomerisation or aggregation. Protein D on its own showed the most significant blue shift at 56°C (310 nm).
Figure 5.
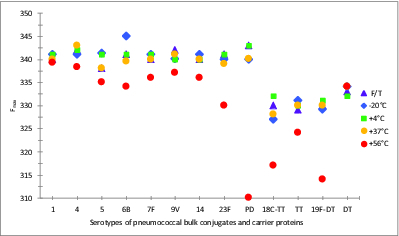
Intrinsic fluorescent emission maxima (Fmax) of stability samples of monovalent pneumococcal-PD/TT/DT bulk conjugate vaccines. Samples were stored at -20, 4, 37, or 56°C for 5 wk or exposed to repeated freeze-thawing (F/T), with colors indicated in the legend. An excitation wavelength of 280 nm was used.
The Fmax of TT, 330 nm, was slightly red-shifted (+2 nm) when conjugated to 18C, suggestive of some unfolding, while the Fmax of DT, 332 nm showed a slight blue-shift (-1 nm) when conjugated to polysaccharide 19F. Blue-shifts, up to 5 nm, can be seen in both TT and DT conjugates stored at lower (-20°C and F/T) or higher (up to 37°C) temperatures, with significant blue-shifts being observed upon storage at 56°C, from 332 to 317 nm, or 331 to 314 nm, for 18C-TT or 19F-DT bulk conjugates, respectively. Unlike the pattern with Protein D, which was stabilized following conjugation, TT and DT were more stable on their own than compared with their conjugated forms.
Discussion
Physico-chemical analysis methods have been widely used in determining the structural integrity and consistency of conjugate vaccines for controlling their quality. In the present study, in vitro physico-chemical methods have been applied to thermo-stability samples of 10 monovalent PnPs-Protein D/TT/DT and 9 monovalent PnPs-CRM197 conjugate vaccine components from two manufacturers.
Differences in the hydrodynamic size of the PS-protein conjugate molecules could be attributed in part to the carrier proteins’ quaternary structure. Within the same polysaccharide serotype, CRM197 conjugates have slightly larger hydrodynamic size than Protein D conjugates, as judged from their SEC elution. The mass of monomeric CRM197 (58.4 g/mol)23 is larger than monomeric Protein D (40 g/mol) based on amino acid composition.21 Of the carrier proteins used in these vaccines, TT and CRM197 have been previously shown to be relatively stable.5–7,24 In this study, Protein D, and to a lesser extent DT, showed a propensity to form higher sized forms; conjugation conferred some degree of stability. Some aggregation of the CRM197 carrier protein was evident after storage at 56°C for 8 wk.
The stability samples showed different patterns of higher MW association, which may have been due to non-specific aggregation of the carrier protein. A loss of highly charged PS, such as the negatively charged serotypes 1, 6, 18, 19, and 23, could conceivably result in a greater access of protein hydrophobic patches and non-specific association which could lead to aggregation. Of these, types 1, 18C, and 19F did have significantly increased free saccharide and correlative increase in acidity, as did serotype 5. Types 1 and 5 contain tri- and di-deoxysugars which are susceptible to acid hydrolysis and backbone depolymerization.11,13 While mild acid hydrolysis of types 18C and 19F can lead to the formation of free phosphate, the mechanisms are different, with the depolymerization of 18C being via the breakage of the rhamnopyranosidic bond rather than the branching glycerophosphate,18 and the degradation of 19F being through the labile in-chain phosphodiester-amino-sugar linkage.19
Repeated freeze-thawing had little effect on the size of 8 of 9 serotypes of CRM197 conjugates; only type 5-CRM197 conjugates became slightly ‘aggregated’ without a change of low MW material peak. Apart from type 6B-CRM197 conjugates, all other serotypes of CRM197 conjugates released significant amounts of low MW materials with concomitant reduction of hydrodynamic size of molecules at storage temperature of 55°C. This may be due to loss of saccharide linked to the conjugated protein when stored at elevated temperature, as well as to protein degradation itself, as supported by evidence from the variable detector signals.
The molecular size distribution of all serotypes of Protein D/TT/DT conjugates remained consistent at storage temperatures of -20°C, 4°C, or repeated freeze-thawing except serotype 5, which presented as an enhanced aggregate formation with increased free saccharide.
Pneumococcal polysaccharide serotype 19F has been previously reported to be relatively sensitive to depolymerization25,26 with the highest relative molecular size change in a 23-valent panel tested without demonstrating antigenic lability over time; its instability was attributed to its less compact structure,27 although the weak in-chain Rha-phosphodiester linkage would also play a role.19
The long-term (5°C) stability study of 23-valent pneumococcal polysaccharides by the Hennessey group26 demonstrated that epitope recognition of polysaccharides by polyvalent antibodies could change over time (types 1, 9V and 18C), without a concomitant reduction in molecular size through depolymerization, and this was attributed to the loss or migration of O-acetyl groups. That free saccharide of these serotypes was generated following one to two months at 55–56°C as found in the present study should not be surprising. Following 70°C for several hours, Type 1 was also reported by Hennessey to be unstable.28
Some serotypes may have undergone changes to saccharide substituents (such as O-acetyl, amino, pyruvyl, and phosphoryl groups), which may not have resulted in backbone degradation detectable by the HPAEC-PAD method, but these could potentially impact on immunological protection. These would also include the PneNAc on type 5, the Rha on type 7F, the Gal of type 14 and the P-Gro of types 18C and 23F. A limitation of this study is that analysis such as NMR, which could be performed on bulk conjugates, was not performed to determine the extent of these changes. Types 7F-PD and 9V-PD, which have relatively stable backbones containing uronic acid, hexoses and amino sugars, could also have had significant de-O-acetylation. Further information about the stability of type 1, is particularly important, in light of the high incidence of type 1 disease in the African meningitis belt.29
A further limitation of the study is that the high MW species were not completely resolvable from the TSK5000PWXL column void, although the column could discriminate between high and low MW populations. A column combination such as the TSK6000+5000PWXL or mixed bed polymer column, may be preferable in this regard. A separate study of TSK polymer columns from the authors’ group has demonstrated a correlation between elution volume and MALLS-derived mean molecular mass for six different conjugated polysaccharide types.
The HPAEC-PAD method of Talaga et al.,10 used here is a specific and sensitive technique for the analysis of monovalent bulk conjugates. The use of a common hydrolysis method across all serotypes meant that particularly resistant or labile serotypes may not give as highly accurate results as other serotypes, due to their lower recovery.10 The method applied in this study, however, used purified polysaccharide rather than monosaccharide quantitative standards, which would ameliorate this issue. It is recognized that serological and colorimetric-based methods do not necessarily give improved quantitation.
From the present study, all data indicated that 6B was the most stable serotype. Athough 6B contains a phosphodiester bond; it is comparatively more stable than 6A.30 Types 7F and 23F were also comparatively more stable than the other 7 serotypes of the Protein D/TT/DT conjugate vaccine. Despite their structural stability, a number of studies have pointed to 6B and 23F-Protein D conjugates being less immunogenic than their corresponding CRM197 conjugates.31-33 The correlation between molecule structure and immune response for these two serotypes in the two different vaccines is still unclear; though they induce a weaker immune response, both are efficacious.34 Future studies should address the stability of serotype conjugates in final containers, and the role that protein aggregation may play in immunogenicity. MALLS analysis of PS, carrier protein and CRM197 conjugate stability is both information and reproducible.35
This study is the first which presents a comprehensive analysis of components from different commercially licensed pneumococcal conjugate vaccines. The methodological approaches in this study which are routinely used in the pre-clinical and batch release testing of Hib and meningococcal conjugate vaccines could discriminate between high quality and degraded pneumococcal vaccines components.
All pneumococcal- Protein D/TT/DT and CRM197 monovalent bulk conjugates were stable when stored at their recommended temperature, 4°C, for the duration of the study. The % free saccharide contents of all the serotypes used in the 10-valent vaccine were maintained within 10% at temperatures up to 37°C despite a pattern of ‘aggregation’ or high MW association of conjugates. Serotypes 1, 5, and 19F were less stable than other serotypes. Because the final products of both vaccines consist of adjuvanted, liquid formulations, further studies could be performed to assess the stability of the different serotypes in the final combinations. The findings here will be useful to those involved in the development and evaluation of new pneumococcal conjugate vaccines, which are required to reduce the significant burden of S. pneumoniae cases and deaths world-wide.
Materials and methods
Materials
Pneumococcal conjugate vaccine components from two different manufacturers, A and B, were studied. Bulk conjugates from manufacturer A, manufactured as consistency lots, consisted of polysaccharide of serotype 1, 4, 5, 6B, 7F, 9V, 14, and 23F conjugated to Protein D, serotype 18C conjugated to TT and 19F conjugated to DT; bulk conjugates from manufacturer B, manufactured during clinical development, included nine serotypes: 1, 4, 5, 6B, 9V, 14, 18C, 19F, and 23F, conjugated to CRM197. These bulk conjugates did not contain any adjuvant and they were stored at 4°C in saline for conjugates from manufacturer A or saline-based buffer from manufacturer B, at concentrations of 0.15 to 1 mg protein/ml.
Stability sample treatment
The Protein D, TT, and DT individual monovalent bulk conjugates and carrier proteins, supplied sterile by the manufacturer, were incubated at -20, 4, 37, or 56°C for 5 wk, and, a separate set of sample stored at -20°C was subjected to 5 once-weekly cycles of freeze-thawing (F/T). In each F/T cycle, frozen vaccines were left at 22°C -25°C for 1 h/week and were returned to -20°C in between the thawing cycles.
The CRM197 bulk conjugates were sterile-filtered with 0.2 μm low protein binding membranes and incubated at 4 or 55°C for 8 wk or subjected to 8 once weekly cycles of freeze-thawing and stored as for pneumococcal-Protein D/TT/DT vaccine components. At the end of the incubation period, all vaccines were stored at 4°C for up to 2 mo until evaluated.
HPLC-SEC conjugate size analysis
The HPLC system used for size-exclusion chromatography (SEC) analysis was a Dionex (part of ThermoFisher Scientific) DX600 that consisted of a GP50 gradient pump, AS autosampler, TCC-100 column oven, and a variable wavelength VWD UV-Vis detector. Dionex Chromeleon software (Version 6.8) was used to program the runs and analyze the data. A TSK-GEL PWXL guard column and TSK G5000PWXL analytical column (7.8 mm x 30 cm) (Tosoh Bioscience GmbH) with a fractionation range of Mr 4000 - 1 × 106 Da was kept at 30°C in a TCC-100 column oven. The columns were calibrated using DNA (Sigma D1626), thyroglobulin (Sigma T9145), bovine serum albumin (Sigma A1900), carbonic anhydrase (Sigma C7025) and tyrosine (Sigma T3754), as described in Saydam et al.8 Protein absorbance at 280 nm and 214 nm, and refractive index were monitored.
Bulk conjugate vaccine samples or carrier protein containing 50 μg of protein content in 100 μl volume were loaded onto the SEC column and eluted isocratically in PBS ‘A’ (10.1 mM Na2HPO4, 1.8 mM KH2PO4, 171 mM NaCl, 3.4 mM KCl, pH 7.4 ± 0.1) at a flow rate of 0.3 ml/min.
HPAEC-PAD saccharide analysis
The antigenically distinct polysaccharides present in the 10-valent conjugate vaccine components contain very different repeating unit structures10-20 and require separate and specific assays for their quantitation. The HPAEC-PAD system was used to measure both the free and total saccharide content of pneumococcal-Protein D/TT/DT conjugates to determine the % free, or non-conjugated, saccharide, which is a key marker of integrity, with modifications of the method of Talaga et al., 2002.10 An ICS3000 BioLC chromatography system was equipped with a gradient pump (SP), pulsed amperometric detector (ED) and an autosampler (AS). The electrochemical detector used a gold working electrode and an Ag/AgCl reference electrode. A CarboPac PA-10 (4 × 250 mm) column preceded by an Amino Trap, then PA10 guard column (Dionex, part of ThermoFisher Scientific) was used to quantify selected saccharide content from each serotype of pneumococcal bulk conjugate vaccines after hydrolysis with 2 M TFA, at 121°C for 2 h, based on Method 1 of Talaga10 (Table 1A and 1B). Sample injections were performed by an autosampler with a 100 μl loop. Samples were eluted at a flow rate of 1 ml/min with a multistep gradient as follows: 0–15 min, 18 mM NaOH; 15–18 min, 18–100 mM NaOH; 18–35 min, 100 mM NaOH; and 0–300 mM NaOAc; followed by a re-equilibration step with 18 mM NaOH for 20 min. Monosaccharides were detected by pulsed amperometric detection using the quadruple –potential waveform with the following pulsed potentials and durations: E1 = 0.1 V, t1 = 400 ms; E2 = -2 V, t2 = 20 ms; E3 = 0.6 V, t3 = 10 ms; E4 = -0.1 V, t4 = 60 ms. The quadruple waveform confers greater reproducibility between runs due to longer-term electrode surface stability.36 Dionex Chromeleon software was used to program the runs and analyze data.
Table 1A.
Saccharide substituents of pneumococcal polysaccharide vaccine hydrolysates
| Mono- and disaccharidea | Appearance in PnPs serotypes |
|---|---|
| Gro | 18C, 23F |
| Rib-ol | 6B |
| FucN | 4, 5 |
| ManNβ3FucN | 5 |
| Rha | 6B, 7F, 18C, 19F, 23F |
| GalN | 4, 7F |
| ManN | 4, 9V, 19F |
| GlcN | 7F, 14 |
| Gal | 4, 6B, 7F, 9V, 14, 18C, 23F |
| Glc | 5, 6B, 7F, 9V, 14, 18C, 19F, 23F |
| ManNβ4Glc | 9V, 19F |
| GlcAβ3FucN | 5 |
| Gro-P | 18C, 23F |
| GalA | 1 |
| Rib-ol-P | 6B |
| GlcAα3Gal | 9V |
| GlcA | 5, 9V |
| P-ManN | 19F |
aSaccharides identified by Talaga et al.10 from HPAEC-PAD analysis
Free saccharide was separated from the pneumococcal-Protein D/TT/DT conjugate vaccines by ultrafiltration using Microcon-100 filters (Merck Millipore) with 100 kDa MWCO that were centrifuged at 6900 × g for 15 min at room temperature. The 100 kDa filters were chosen for measuring degraded polysaccharide, rather than unconjugated polysaccharide necessarily. Any conjugated-polysaccharide would not be expected to filter through. Equivalent results would be expected from the use of 30 kDa filters. The filtrates subjected to acid hydrolysis were used for measurement of free saccharide content of the sample. The filtrates (for free saccharide) or conjugate vaccines (for total saccharide) were hydrolysed using TFA in final concentration of 2 M at 121°C for 2 h.10 The hydrolysate solutions were then dried using a SpeedVac to evaporate TFA and samples were re-dissolved in water to 0.6 ml prior to chromatography.
Quantitative polysaccharide standards were prepared for each serotype from bulk purified polysaccharide from manufacturer A over the range of 0.5–27 μg/ml. Purified polysaccharides were prepared in the same diluent, 150 mM NaCl, with their unitage based on dry weight. They were hydrolysed, dried and re-dissolved in water in the same way as the vaccine samples. An identical amount of D-fucose (Sigma F8150) (20 μl of 100 μg/ml) was added to 480 μl of each sample and standard as internal standard just prior to chromatography. A volume of 50 μl was injected. The average of two injections was used for determination of free and total saccharide content. Fucose eluted at 6.0 min. For each serotype, the saccharide peaks selected for quantitation and their elution times PS are listed in Table 1B.
Table 1B.
Saccharide substituents of pneumococcal polysaccharide vaccine hydrolysates utilized for quantitation of unconjugated saccharide (B)
| Serotype | Saccharide selectedb | Elution time (min) |
|---|---|---|
| 1 | GalA | 29.9 |
| 4 | Gal | 14.2 |
| 5 | Glc | 15.2 |
| 6B | Rha | 10.5 |
| 7F | GlcN | 11.2 |
| 9V | Glc | 15.1 |
| 14 | GlcN | 11.3 |
| 18C | Rha | 9.7 |
| 19F | Rha | 9.8 |
| 23F | Rha | 9.5 |
bThe identification of the saccharide chromatographic peak selected is based on that determined by Talaga.10
pH determination
The pH values of stability samples of Protein D, TT, and DT bulk conjugates were read using a Jenway 3305 pH meter (Bibby Scientific Ltd.). The pH meter was calibrated using pH standards of 4.0, 7.0 and 10.0 (Fisher Scientific) before determination, and samples were equilibrated to room temperature for ∼15 min prior to their pH measurement, but were not shaken. The pH values were accurate to ± 0.05 pH units.
Fluorescence spectroscopy
Intrinsic fluorescence spectra of Protein D, TT, and DT conjugates and carrier proteins were obtained using a Spex Fluoromax single photon-counting spectrofluorometer (Jobin Yvon Ltd.) at 25°C, in 1 cm path-length quartz cells (Hellma GmbH) with a protein concentration of 50 μg/ml. An excitation wavelength of 280 nm was used with a band pass of 4.25 nm for the excitation monochromator and 4.50 nm for the emission monochromator. Spectra were collected between 260 and 500 nm at a data increment of 0.5 nm and an integration time of 1 s. Fluorescence spectra were corrected by subtracting the corresponding base-line spectra of PBS, pH 7.4. Fluorescence emission λmax (Fmax) values obtained were accurate to ± 0.5 nm.
Supplementary Material
Disclosure of Potential Conflicts of Interest
No potential conflicts of interest were disclosed.
Acknowledgments
Our grateful thanks to the vaccine manufacturers for their invaluable assistance in providing materials and information for the establishment of quality control methods, and to Dr. Johan Descamps and Dr. Vincent Turula, in particular, for their helpful review of this manuscript and for long-standing collaborations. Dr. Rory Care, Mrs. Claire Mattick Raine and Miss Jane Bygraves kindly assisted in setting up this study. We also appreciate the support of Dr. Ian Feavers and Dr. Mike Corbel, Heads of Division of Bacteriology at NIBSC.
Funding
The work was funded by the UK Department of Health.
Supplemental Materials
Supplemental data for this article can be accessed on the publisher's website.
References
- 1. Straetemans M, Sanders EA, Veenhoven RH, Schilder AG, Damoiseaux RA, Zielhuis GA. Review of randomized controlled trials on pneumococcal vaccination for prevention of otitis media. Pediatr Infect Dis J 2003; 22:515-24; PMID:12799508; http://dx.doi.org/ 10.1097/01.inf.0000069763.08122.1c [DOI] [PubMed] [Google Scholar]
- 2. O’Brien KL, Moulton LH, Reid R, Weatherholtz R, Oski J, Brown L, Kumar G, Parkinson A, Hu D, Hackell J, et al. Efficacy and safety of seven-valent conjugate pneumococcal vaccine in American Indian children: group randomised trial. Lancet 2003; 362:355-61; PMID:12907008; http://dx.doi.org/ 10.1016/S0140-6736(03)14022-6 [DOI] [PubMed] [Google Scholar]
- 3. van Gils EJM, Veenhoven RH, Rodenburg GD, Hak E, Sanders EAM. Effect of 7-valent pneumococcal conjugate vaccine on nasopharyngeal carriage with Haemophilus influenzae and Moraxella catarrhalis in a randomized controlled trial. Vaccine 2011; 29:7595-8; PMID:21893151; http://dx.doi.org/ 10.1016/j.vaccine.2011.08.049 [DOI] [PubMed] [Google Scholar]
- 4. Hausdorff WP, Bryant J, Paradiso PR, Siber GR. Which pneumococcal serogroups cause the most invasive disease: implications for conjugate vaccine formulation and use, part I. Clin Infect Dis 2000; 30:100-21; PMID:10619740; http://dx.doi.org/ 10.1086/313608 [DOI] [PubMed] [Google Scholar]
- 5. Ho MM, Bolgiano B, Corbel MJ. Assessment of the stability and immunogenicity of meningococcal oligosaccharide C-CRM197 conjugate vaccines. Vaccine 2000; 19:716-25; PMID:11115692; http://dx.doi.org/ 10.1016/S0264-410X(00)00261-9 [DOI] [PubMed] [Google Scholar]
- 6. Bolgiano B, Mawas F, Yost SE, Crane DT, Lemercinier X, Corbel MJ. Effect of physico-chemical modification on the immunogenicity of Haemophilus influenzae type b oligosaccharide-CRM(197) conjugate vaccines. Vaccine 2001; 19:3189-200; PMID:11312015; http://dx.doi.org/ 10.1016/S0264-410X(01)00024-X [DOI] [PubMed] [Google Scholar]
- 7. Ho MM, Mawas F, Bolgiano B, Lemercinier X, Crane DT, Huskisson R, Corbel MJ. Physico-chemical and immunological examination of the thermal stability of tetanus toxoid conjugate vaccines. Vaccine 2002; 20:3509-22; PMID:12297396; http://dx.doi.org/ 10.1016/S0264-410X(02)00342-0 [DOI] [PubMed] [Google Scholar]
- 8. Saydam M, Burkin K, Care R, Rigsby P, Bolgiano B, Mawas F. Immunogenicity and thermal stability of a combined vaccine against Haemophilus influenzae type b and Neisseria meningitidis serogroup C diseases. Vaccine 2010; 28:6228-34; PMID:20638457; http://dx.doi.org/ 10.1016/j.vaccine.2010.07.005 [DOI] [PubMed] [Google Scholar]
- 9. Munson RS, Jr., Sasaki K. Protein D, a putative immunoglobulin D-binding protein produced by Haemophilus influenzae, is glycerophosphodiester phosphodiesterase. J Bacteriol 1993; 175:4569-71; PMID:8392514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Talaga P, Vialle S, Moreau M. Development of a high-performance anion-exchange chromatography with pulsed-amperometric detection based quantification assay for pneumococcal polysaccharides and conjugates. Vaccine 2002; 20:2474-84; PMID:12057602; http://dx.doi.org/ 10.1016/S0264-410X(02)00183-4 [DOI] [PubMed] [Google Scholar]
- 11. Lindberg B, Lindqvist B, Lönngren J, Powell DA. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 1. Carbohydr Res 1980; 78:111-7; PMID:7351025; http://dx.doi.org/ 10.1016/S0008-6215(00)83664-2 [DOI] [PubMed] [Google Scholar]
- 12. Jones C, Currie F, Forster MJ. N.m.r. and conformational analysis of the capsular polysaccharide from Streptococcus pneumoniae type 4. Carbohydr Res 1991; 221:95-121; PMID:1667857; http://dx.doi.org/ 10.1016/0008-6215(91)80051-N [DOI] [PubMed] [Google Scholar]
- 13. Jansson PE, Lindberg B, Lindquist U. Structural studies of the capsular polysaccharide from Streptococcus pneumoniae type 5. Carbohydr Res 1985; 140:101-10; PMID:4053092; http://dx.doi.org/ 10.1016/0008-6215(85)85053-9 [DOI] [PubMed] [Google Scholar]
- 14. Kenne L, Lindberg B, Madden JK. Structural studies of the capsular antigen from Streptococcus pneumoniae type 26. Carbohydr Res 1979; 73:175-82; PMID:38908; http://dx.doi.org/ 10.1016/S0008-6215(00)85487-7 [DOI] [PubMed] [Google Scholar]
- 15. Moreau M, Richards JC, Perry MB, Kniskern PJ. Application of high-resolution n.m.r. spectroscopy to the elucidation of the structure of the specific capsular polysaccharide of Streptococcus pneumoniae type 7F. Carbohydr Res 1988; 182:79-99; PMID:3149544; http://dx.doi.org/ 10.1016/0008-6215(88)84093-X [DOI] [PubMed] [Google Scholar]
- 16. Perry MB, Daoust V, Carlo DJ. The specific capsular polysaccharide of Streptococcus pneumoniae type 9V. Can J Biochem 1981; 59:524-33; PMID:6271367; http://dx.doi.org/ 10.1139/o81-073 [DOI] [PubMed] [Google Scholar]
- 17. Lindberg B, Lönngren J, Powell DA. Structural studies on the specific type-14 pneumococcal polysaccharide. Carbohydr Res 1977; 58:177-86; PMID:21030; http://dx.doi.org/ 10.1016/S0008-6215(00)83413-8 [DOI] [PubMed] [Google Scholar]
- 18. Lugowski C, Jennings HJ. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type 18C (56). Carbohydr Res 1984; 131:119-29; PMID:6488199; http://dx.doi.org/ 10.1016/0008-6215(84)85409-9 [DOI] [PubMed] [Google Scholar]
- 19. Jennings HJ, Rosell K-G, Carlo DJ. Structural determination of the capsular polysaccharide of Streptococcus pneumoniae type 19 (19F). Can J Chem 1980; 58:1069-74; http://dx.doi.org/ 10.1139/v80-167 [DOI] [Google Scholar]
- 20. Richards JC, Perry MB. Structure of the specific capsular polysaccharide of Streptococcus pneumoniae type 23F (American type 23). Biochem Cell Biol 1988; 66:758-71; PMID:2846013; http://dx.doi.org/ 10.1139/o88-087 [DOI] [PubMed] [Google Scholar]
- 21. Janson H, Hedén LO, Grubb A, Ruan MR, Forsgren A. Protein D, an immunoglobulin D-binding protein of Haemophilus influenzae: cloning, nucleotide sequence, and expression in Escherichia coli. Infect Immun 1991; 59:119-25; PMID:1987023 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Song XM, Forsgren A, Janson H. The gene encoding protein D (hpd) is highly conserved among Haemophilus influenzae type b and nontypeable strains. Infect Immun 1995; 63:696-9; PMID:7822043 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Giannini G, Rappuoli R, Ratti G. The amino-acid sequence of two non-toxic mutants of diphtheria toxin: CRM45 and CRM197. Nucleic Acids Res 1984; 12:4063-9; PMID:6427753; http://dx.doi.org/ 10.1093/nar/12.10.4063 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Xing DK, Crane DT, Bolgiano B, Corbel MJ, Jones C, Sesardic D. Physicochemical and immunological studies on the stability of free and microsphere-encapsulated tetanus toxoid in vitro. Vaccine 1996; 14:1205-13; PMID:8961506; http://dx.doi.org/ 10.1016/S0264-410X(96)00032-1 [DOI] [PubMed] [Google Scholar]
- 25. Lee C-J, Lin K-T. Studies on vaccine control and immunogenicity of polysaccharides of Streptococcus pneumoniae. Rev Infect Dis 1981; 3(Suppl):S51-60; PMID:6169130; http://dx.doi.org/ 10.1093/clinids/3.Supplement_1.S51 [DOI] [PubMed] [Google Scholar]
- 26. Sweeney JA, Sumner JS, Hennessey JP, Jr. Simultaneous evaluation of molecular size and antigenic stability of PNEUMOVAX 23, a multivalent pneumococcal polysaccharide vaccine. Dev Biol (Basel) 2000; 103:11-26; PMID:11214229 [PubMed] [Google Scholar]
- 27. MacNair JE, Desai T, Teyral J, Abeygunawardana C, Hennessey JP, Jr. Alignment of absolute and relative molecular size specifications for a polyvalent pneumococcal polysaccharide vaccine (PNEUMOVAX 23). Biologicals 2005; 33:49-58; PMID:15713556; http://dx.doi.org/ 10.1016/j.biologicals.2004.11.002 [DOI] [PubMed] [Google Scholar]
- 28. Abeygunawardana C, Williams TC, Sumner JS, Hennessey JP, Jr. Development and validation of an NMR-based identity assay for bacterial polysaccharides. Anal Biochem 2000; 279:226-40; PMID:10706792; http://dx.doi.org/ 10.1006/abio.1999.4470 [DOI] [PubMed] [Google Scholar]
- 29. Gessner BD, Mueller JE, Yaro S. African meningitis belt pneumococcal disease epidemiology indicates a need for an effective serotype 1 containing vaccine, including for older children and adults. BMC Infect Dis 2010; 10:22; http://www.biomedical.com/1471-2334/10/22; PMID:20146815; http://dx.doi.org/ 10.1186/1471-2334-10-22. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Zon G, Szu SC, Egan W, Robbins JD, Robbins JB. Hydrolytic stability of pneumococcal group 6 (type 6A and 6B) capsular polysaccharides. Infect Immun 1982; 37:89-103; PMID:7107011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Vesikari T, Wysocki J, Chevallier B, Karvonen A, Czajka H, Arsène J-P, Lommel P, Dieussaert I, Schuerman L. Immunogenicity of the 10-valent pneumococcal non-typeable Haemophilus influenzae Protein D conjugate vaccine (PHiD-CV) compared to the licensed 7vCRM vaccine. Pediatr Infect Dis J 2009; 28(Suppl):S66-76 [DOI] [PubMed] [Google Scholar]
- 32. Nurkka A, Joensuu J, Henckaerts I, Peeters P, Poolman J, Kilpi T, Käyhty H. Immunogenicity and safety of the eleven valent pneumococcal polysaccharide-protein D conjugate vaccine in infants. Pediatr Infect Dis J 2004; 23:1008-14; PMID:15545855; http://dx.doi.org/ 10.1097/01.inf.0000143640.03214.18 [DOI] [PubMed] [Google Scholar]
- 33. Prymula R, Peeters P, Chrobok V, Kriz P, Novakova E, Kaliskova E, Kohl I, Lommel P, Poolman J, Prieels JP, et al. Pneumococcal capsular polysaccharides conjugated to protein D for prevention of acute otitis media caused by both Streptococcus pneumoniae and non-typable Haemophilus influenzae: a randomised double-blind efficacy study. Lancet 2006; 367:740-8; PMID:16517274; http://dx.doi.org/ 10.1016/S0140-6736(06)68304-9 [DOI] [PubMed] [Google Scholar]
- 34. De Serres G, Pilishvili T, Link-Gelles R, Reingold A, Gershman K, Petit S, Farley MM, Harrison LH, Lynfield R, Bennett NM, et al. Use of surveillance data to estimate the effectiveness of the 7-valent conjugate pneumococcal vaccine in children less than 5 years of age over a 9 year period. Vaccine 2012; 30:4067-72; PMID:22525797; http://dx.doi.org/ 10.1016/j.vaccine.2012.04.017 [DOI] [PubMed] [Google Scholar]
- 35. Turula V. Application of SEC-MALLS from vaccine development to commercial manufacturing, a paper presented at the 18th Annual Wyatt Technology International Light Scattering Colloquium, Santa Barbara, CA, 16-17 Oct 2006. [Google Scholar]
- 36. Rocklin RD, Clarke AP, Weitzhandler M. Improved long-term reproducibility for pulsed amperometric detection of carbohydrates via a new quadruple-potential waveform. Anal Chem 1998; 70:1496-501; http://dx.doi.org/ 10.1021/ac970906w [DOI] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


