Abstract
Hemipteran insects are devastating pests of crops due to their wide host range, rapid reproduction, and ability to transmit numerous plant-infecting pathogens as vectors. While the field of plant–virus–vector interactions has flourished in recent years, plant–bacteria–vector interactions remain poorly understood. Leafhoppers and psyllids are by far the most important vectors of bacterial pathogens, yet there are still significant gaps in our understanding of their feeding behavior, salivary secretions, and plant responses as compared to important viral vectors, such as whiteflies and aphids. Even with an incomplete understanding of plant–bacteria–vector interactions, some common themes have emerged: (1) all known vector-borne bacteria share the ability to propagate in the plant and insect host; (2) particular hemipteran families appear to be incapable of transmitting vector-borne bacteria; (3) all known vector-borne bacteria have highly reduced genomes and coding capacity, resulting in host-dependence; and (4) vector-borne bacteria encode proteins that are essential for colonization of specific hosts, though only a few types of proteins have been investigated. Here, we review the current knowledge on important vector-borne bacterial pathogens, including Xylella fastidiosa, Spiroplasma spp., Liberibacter spp., and ‘Candidatus Phytoplasma spp.’. We then highlight recent approaches used in the study of vector-borne bacteria. Finally, we discuss the application of this knowledge for control and future directions that will need to be addressed in the field of vector–plant–bacteria interactions.
Keywords: vector-borne bacteria, vascular bacteria, phloem, xylem, plant–insect interactions, plant–microbe interactions, leafhoppers, psyllids
Introduction
The plant vascular system is a rich source of nutrients and represents a transport pathway for colonizers. It consists of phloem and xylem tissues, two different host environments for plant pathogens. Phloem tissue consists of companion cells, providing metabolic and regulatory components to the phloem sap, and sieve elements, forming a long distance transport system throughout the plant (Lucas, 2006; Will et al., 2013). Because this specialized transport system offers access to a rich source of carbohydrates, proteins, and amino acids, numerous viral and bacterial microbes colonize the phloem specifically (Bove and Garnier, 2003; Lough and Lucas, 2006). In contrast, the xylem vessels mainly transport water and contain lower nutrient levels in comparison to the phloem (Bae et al., 2015). Despite the low nutrient content of xylem, plant pathogens have also been identified that can colonize the xylem (Purcell and Hopkins, 1996; Bae et al., 2015).
In addition to viral and bacterial microorganisms, macro organisms also rely on the plant vascular system for their primary nutrient source. These include hemipteran pests, such as whiteflies, aphids, psyllids, and leafhoppers. Specialized mouthparts, known as stylets, allow hemipterans to penetrate the plant’s epidermal tissues and reach their preferred tissue. Some hemipterans feed from the mesophyll and vascular system, while others only probe the mesophyll and feed exclusively from the phloem or xylem. As a result of this specialized feeding, hemipterans interact with microbes colonizing the plant vascular system and can serve as vectors. A vector is the specific organism that transmits a pathogen (Purcell, 1982) and hemipteran insects are by far the most important vectors of plant-infecting pathogens (Nault and Ammar, 1989; Orlovskis et al., 2015).
While the interactions between plant–pathogenic viruses and their hemipteran vectors have been studied in depth, far less is known about the interactions between plant–infecting bacteria and their hemipteran vectors (Ng and Falk, 2006; Hogenhout et al., 2008a; Walling, 2008; Ammar et al., 2011; Blanc et al., 2011, 2014; Gray et al., 2014; Gilbertson et al., 2015; Whitfield et al., 2015). In recent decades, vector-borne bacteria have caused some of the most devastating plant diseases in perennial and annual crops. For example, in North America ‘Candidatus Liberibacter asiaticus,’ the causative agent of citrus greening, has rapidly spread across several regions of the world. Citrus greening continues to cost growers over $4 billion each year and has resulted in the loss of 1000s of jobs (Gottwald, 2010). Here, we review the current mechanistic knowledge of interactions shared among vector-borne bacteria, hemipteran vectors, and host plants. As most vector-borne bacteria cannot be cultured and are difficult to study in the lab, we then highlight current approaches used to study these tri-partite systems. Finally, we discuss application of recent knowledge for control and propose future directions for research on vector-borne bacteria and their hemipteran vectors.
Redefining the Relationships Vector-Borne Bacteria Share With Hemipteran Insects
Early studies of plant pathogens used microscopy, serological testing, and host inoculation to determine the etiological agents of diseases. While insect transmission of plant viruses was first described in 1920, insect transmission of plant bacteria was not reported until 1967 (Purcell, 1982). Because of the historic precedence of research on vector-borne viruses, concepts and terminology from virus research were applied to the study of vector-borne bacteria. In spite of this methodological connection, the actual similarities between viruses and bacteria as vector-transmitted plant pathogens may be quite limited. Here, we briefly define the common terminology found in the literature for describing pathogen–vector interactions and highlight terms that are useful for vector-borne bacteria specifically.
Persistence: Non-Persistent, Semi-persistent, or Persistent
The transmission process of vector-borne viruses is categorized by two features: (1) the time period required by the vector for acquisition of the virus and inoculation of the virus, and (2) the retention time of viral particles in the vector (Ng and Falk, 2006). Based on these features, virus-vector relationships can be categorized as non-persistent, semi-persistent, or persistent. For non-persistent viruses, transmission can occur within minutes of acquiring the viral particles (virions) and particles are retained in the stylet or in the alimentary canal of the insect (Ng and Falk, 2006; Uzest et al., 2007; Whitfield et al., 2015). Viral particles can be lost quickly in this transmission mode and multiple encounters with infected plants are required for vectors to remain viruliferous (Ng and Falk, 2006). Semi-persistent retention of virions can last for days and retention sites are found in the alimentary canal or gut lumen of the insect for the majority of these viruses (Chen et al., 2011; Ng and Zhou, 2015). For semi-persistent relationships, feeding for hours to days is required to acquire the virus and if acquisition occurs during vector immature stages, infectivity is lost after each molt. Finally for persistent associations, vectors remain infective until death after a single encounter with an infected plant. Long feeding periods (hours to days) are required for acquisition of persistent viruses by vectors.
Persistence of vector-borne bacteria varies according to plant-tissue specialization. Xylella fastidiosa, the only known vector-borne xylem specialist, has a semi-persistent association with its vectors (Table 1). Dozens of crops and native plants are hosts for X. fastidiosa and a diverse array of vectors transmits the pathogen compared to other species of vector-borne bacteria (Redak et al., 2004). The ability to utilize diverse plant and vector species may be due to X. fastidiosa’s semi-persistent relationship with insects. For example, semi-persistent bacteria may be more easily acquired and transmitted by vectors to diverse host species during pre-feeding and host finding behavior. In contrast, all known phloem-limited bacteria appear to establish persistent associations with their respective vectors (Table 1). Persistence of phloem specialists may be due to the intracellular relationship they share with plant and insect hosts. However, conclusion about tissue trophisms may be premature, as only one vector-borne xylem specialists is known so far.
Table 1.
Vector-borne phloem limited plant pathogenic bacteria.
| Class Family | Pathogen | Genome size (Mb) | Plant tissue tropism | Plant host | Vectors | Location/ Insect organs | Reference |
|---|---|---|---|---|---|---|---|
| Gammaproteobacteria Xanthomonadaceae |
Xylella fastidiosa | 2.7 | Xylem (a) | Wide host range | Homalodisca vitripennis, Graphocephala atropunctata (+ Others) | Non-Circulative/ Cybarium, foregut |
Backus and Morgan, 2011 |
| Mollicutes Spiroplasmataceae | Spiroplasma citri | 1.8 | Phloem (a) | Citrus | Circulifer tenellus | Circulative/ | Fletcher et al., 1998 |
| Spiroplasma kunkelii | Corn | Dalbulus maidis | Hemolymph bacteriocyte, salivary glands | ||||
| Mollicutes Acholeplasmataceae |
“Candidatus Phytoplasma spp.” | 0.8 | Phloem (b) | Wide host range: Asteraceae horticulture crops |
Macrosteles quadrilineatus (+ Others) |
Circulative/ Hemolymph bacteriocyte, salivary glands |
Beanland et al., 2000 |
| Alphaproteobacteria Rhizobiaceae |
“Candidatus Liberibacter spp.” | 1.2 | Phloem (b) | Citrus Solanaceae Apiaceae | Diaphorina citri, Bactericera cockerelli, Bactericera trigonica, Trioza apicalis | Circulative/ Hemolymph bacteriocyte, salivary glands |
Ammar et al., 2011 |
The bacterial group is routinely culturable (a) or non-culturable (b).
Location: Circulative or Non-circulative
The interactions plant viruses share with their insect vectors can either be “non-circulative” or “circulative.” In non-circulative interactions, the virus does not enter the insect body as part of the transmission process and the virus particles are retained in the stylet or the foregut region (Ng and Zhou, 2015). Viruses that are transmitted in a circulative manner in contrast, pass beyond the foregut into the insect intestine and enter the body as part of the transmission process. Circulative viruses can be retained for the life of the insect vector (Gray et al., 2014). For vector-borne bacteria both non-circulative and circulative relationships exist among pathogen–vector interactions (Table 1); and like persistence, relationships correlate with plant-tissue specialization of the pathogen. For example, the xylem colonizer, X. fastidiosa, is non-circulative, while all known phloem colonizers interact in a circulative manner with vectors (Table 1).
Differences in pathogen location within vectors may be explained by ancestral origins (Nadarasah and Stavrinides, 2011). In one scenario, bacteria pre-adapted to plant environments may have evolved to use insects as alternative hosts. Alternatively, insect pathogens or symbionts, pre-adapted to thrive in hemipterans, may have found an additional niche in plants (Nadarasah and Stavrinides, 2011). X. fastidiosa is most closely related to the genus Xanthomonas (Table 1). Members of Xanthomonas are exclusively plant-associated and commonly plant pathogens. Inability to cross insect membranes may be due to the fact that X. fastidiosa has evolved to be restricted to dead cells of the plant (xylem). The ability to cross plant cellular membranes may have been lost from its genetic arsenal over time. Liberibacters also are related to plant pathogens as a member of the family Rhizobiaceae, yet liberibacters have circulative relationships with insect vectors. A more striking phylogenetic observation for liberibacters is that many members of the Rhizobiaceae, have intracellular associations with hosts as pathogens and symbionts (insect and plant hosts; genera Bradyrhizobium, Bartonella, Brucella, and Afipia; Jagoueix et al., 1994). This trend may explain the origin of the circulative associations of liberibacters with their vectors. As microbiome projects for hemipterans expand, the relationship among these bacteria and the traits responsible for interactions inside the insect will likely be revealed.
Replication: Propagative or Non-propagative
Circulative viral pathogens can either circulate through the insect vector’s body without reproducing, in which case they are described as “non-propagative,” or they can circulate and multiply within the insect vector, in which case they are described as “propagative.” In the latter case, the vector serves as an alternative host for the plant pathogen (Nadarasah and Stavrinides, 2011). Typically, the vector acquires the plant pathogen by feeding on infected plants. Once inside the insect body, the virus crosses intestinal barriers, internal organs, and visceral muscles, and can be found throughout the hemolymph (Hogenhout et al., 2008a; Orlovskis et al., 2015). From the hemolymph the virus must spread to the salivary glands before the vector can subsequently transmit the pathogen to a new plant host. Only a few families of vector-borne plant viruses have propagative relationships with vectors. These families include Rhabdoviridae, Reoviridae, and Bunyaviridae (Hogenhout et al., 2008a; Ammar et al., 2009; Whitfield et al., 2015).
All described vector-borne bacteria utilize their insect vectors as alternative hosts, and are thus considered propagative (Bove and Garnier, 2003; Orlovskis et al., 2015). Vector-borne bacteria can propagate extracellularly (between host cells) or intracellularly (within host cells; Table 1). For example, the xylem colonizer, X. fastidiosa, propagates extracellularly within the vector and is non-circulative. This is in contrast to all vector-borne viruses, which can only be propagative and circulative, as they are all parasites of the cellular replication machinery. It is assumed all phloem colonizers propagate intracellularly within their vectors as they are found in diverse tissues and hemolymph (Table 1). However, detailed intracellular propagation of bacteria is not easily studied and the current knowledge may reflect methodological limitations for evaluating bacterial replication in different insect organs and cavities. Specific mechanisms mediating insect recognition, attachment, and multiplication in organs are also not yet clear, and seem to be unique for each bacteria–vector interaction.
Hemipterans and Their Role as Vectors of Bacterial Plant Pathogens
The ability to serve as a viral and/or bacterial vector appears to vary across hemipteran lineages (Figure 1; Supplementary Table S1). Vector-borne bacteria most commonly rely on members of the suborder Auchenorrhyncha for transmission, including leafhoppers (Membracoidea), froghoppers/spittlebugs (Cercopoidea), and planthoppers (Fulgoroidea; Figure 1) (Bove and Garnier, 2003). However, several psyllids (Psylloidea) from the subgroup Sternorrhyncha are also important vectors of bacterial plant pathogens (Figure 1; Supplementary Table S1). In these groups, transmission has been demonstrated for mesophyll, xylem, and phloem-feeding hemipterans (Figure 1; Supplementary Table S1). The efficiency of pathogen transmission, however, depends on the specific insect–plant interaction and on pathogen biology.
FIGURE 1.
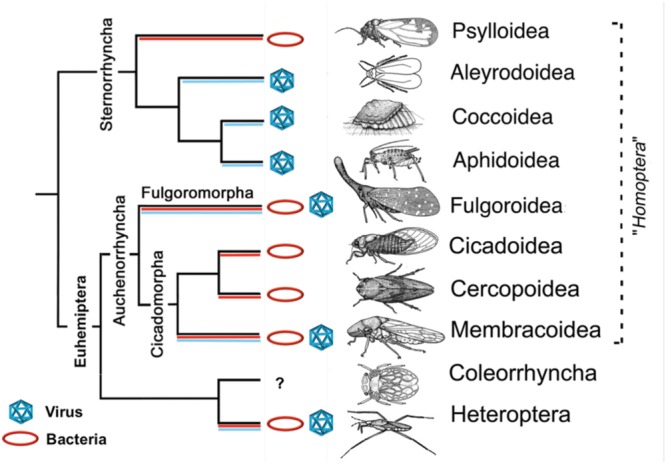
Hemiptera taxa: reported vectors and groups of plant pathogens. Specific plant pathogens are listed in Supplementary Table S1. Branches where species have been reported as transmitting virus are labeled in blue, while those transmitting bacteria are labeled in red. Figure modified with permission from Gullan and Cranston (2014).
Vectors of bacterial plant pathogens and vectors of viral plant pathogens have been reported in multiple superfamilies of the Euhemiptera lineage (Auchenorrhyncha, Coleorrhyncha, and Heteroptera; Figure 1). Surprisingly, only a few individual species within these superfamilies have been reported to serve as efficient vectors for both bacterial and viral plant pathogens (Weintraub and Beanland, 2006). One such example is the Beet Leafhopper (Circulifer tenellus, Baker), which is a vector for the Beet curly top virus ([BCTV], Geminiviridae), as well as two different bacterial pathogens (“Candidatus Phytoplasma trifolii” and Spiroplasma citri; Weintraub and Beanland, 2006).
The suborder Sternorrhyncha contains psyllids, aphids (Aphidoidea), whiteflies (Aleyrodoidea), and scales (Coccoidea), though the latter three are more closely related to one another phylogenetically (Gullan and Cranston, 2014). This is interesting because aphids, whiteflies, and scales have only been reported as vectors of viruses, while psyllids have only been reported as vectors of bacteria (Figure 1; Supplementary Table S1). In fact, aphids and whiteflies are the most important vectors of plant viruses, transmitting 46% of all described plant-infecting viruses (Hogenhout et al., 2008a; Gilbertson et al., 2015). Recent work on the Asian citrus psyllid’s (Diaphorina citri) viral metagenome found viral sequences from diverse groups of animal viruses but no sequences related to any known viral plant pathogens were reported (Nouri et al., 2015). These results provide further evidence that psyllids may lack the ability to transmit plant viruses, however, additional work in this area is needed.
Our literature review suggests that some hemipteran groups are capable of transmitting bacterial pathogens while other groups are not (Figure 1). Despite extensive research into various vector systems, the mechanisms that mediate vector specificity remain largely unknown for all but a few vector-borne phytopathogens (Uzest et al., 2007; Chen et al., 2011; Blanc et al., 2014). Variations in vector specificity among lineages suggest physical, physiological, or temporal constraints on vector-pathogen relationships. Vectors that transmit viruses and bacteria may have fewer constraints, or constraints that are easier for the pathogens to overcome. Differences in insect physiology, immunity, or feeding behavior among groups may mediate some aspects of vector specificity. However, conclusions on differences in insect biology are difficult to make at this point, as the basic biology of many hemipterans remains poorly understood and complete genomes are available for only a few vectors of plant pathogens (Leshkowitz et al., 2006; Ramsey et al., 2007; Legeai et al., 2010; Chen et al., 2015; Upadhyay et al., 2015). Variations in vector specificity may also depend on location and timing of vectors and pathogens. Geographic factors, environmental conditions, and agricultural economics are all dynamic forces that may limit host distribution, insect populations, and plant-pathogen-vector associations. Alternatively, these observations may be the result of a lack of information on the full extent of vector-borne pathogens and their hemipteran vectors, as the current inventory is still likely underrepresented (Malmstrom et al., 2011).
Vector-Borne Bacteria: Dual Host Interactions
All known vector-borne bacteria share certain biological features, including plant vascular tissue specialization, propagative relationships with vectors, and complete dependence on their hosts. Host dependence is likely a result of genome degradation, where essential biosynthetic pathways from the bacterial ancestor have been lost where the same resources can be obtained from the host environment (Nadarasah and Stavrinides, 2011). The xylem colonizer, X. fastidiosa, has the largest genome of the group (Table 1). This may be due to the fact that xylem represent an inferior nutrient sources as compared to the phloem. Therefore, more essential biosynthetic pathways in the genome may be required for the bacteria to survive in the nutrient limited xylem. Despite these similarities, the bacterial groups that depend on hemipteran vectors for transmission occur in several different phyla and orders (Table 1). Accordingly, many differences exist, including diverse mechanisms for promoting host colonization and dispersal (Orlovskis et al., 2015).
Xylem-Limited Vector-Borne Bacteria
The only known xylem-limited bacterial pathogen that is also transmitted by hemipteran vectors is X. fastidiosa. X. fastidiosa (class Gammaproteobacteria) has a very wide host range, colonizing and causing disease in grapes (Pierce’s disease), citrus (citrus variegated chlorosis), olives (leaf scorch), almonds (leaf scorch), and several other plant species (Chatterjee et al., 2008a; Saponari et al., 2014; Almeida and Nuney, 2015). Because of its economic importance, X. fastidiosa was the first bacterial plant pathogen genome to be completely sequenced (Simpson et al., 2000). The genome of X. fastidiosa is ~2.7 Mb, half the size of its closest relatives (Xanthomonas group; Table 1). X. fastidiosa is transmitted in a non-circulative manner by a diverse set of xylem-feeding hemipterans including members from the superfamilies Membracoidea, Cercopoidea, and Cicadoidea (PaiãO et al., 1996; Purcell and Hopkins, 1996; Saponari et al., 2014). Experimental evidence suggests that a wide range of additional xylem feeding hemipterans are potential vectors, however, efficiency of transmission may vary depending on the vector species (Redak et al., 2004). The planthoppers Homalodisca vitripennis and Graphocephala atropunctata are the two most well studied vectors of X. fastidiosa. Knowledge of these two vectors has been used to model the relationship X. fastidiosa share with vectors in general (Chatterjee et al., 2008b; Backus and Morgan, 2011; Rapicavoli et al., 2015).
Numerous research tools have been developed to study X. fastidiosa, including in vitro culture techniques, transformation, and bacterial strain mutants (Killiny et al., 2012; Purcell, 2013; Webster et al., 2014; Rapicavoli et al., 2015). These tools have facilitated the dissection of many genetic components involved in pathogenicity. X. fastidiosa colonize and propagate extracellularly in the plant and insect (Chatterjee et al., 2008a), with some overlapping mechanisms. X. fastidiosa uses a cell-to-cell signaling sensor (RpfC), which acts as a negative regulator. This signaling system is mediated by diffusible signaling factors (DSF) in order to modulate different aspects of behavior in a population dependent manner (i.e., quorum sensing). DSF is secreted into the extracellular environment, activating motility, biofilm formation, and virulence mechanisms when a threshold concentration is reached outside the cell (Chatterjee et al., 2008a). Aspects of X. fastidiosa colonization that are dependent on quorum sensing include the production of toxins, extracellular polysaccharides (EPS), adhesins, and hemaglutinins (Table 2) (Chatterjee et al., 2008b; Nascimento et al., 2016). Inside the insect, the bacteria do not invade the epithelial gut, hemolymph, or salivary glands, and are retained in the alimentary canal. Transmission can occur within minutes of acquisition (Killiny et al., 2012). Recently it was determined that lipopolysaccharide (LPS), the outermost layer of sugar polymers surrounding Gram-negative bacteria, is critical for attachment to the vector and subsequent transmission (Table 2) (Rapicavoli et al., 2015).
Table 2.
Reported gene product (or structure) associated with host interaction for vector-borne bacteria.
| Vector-borne bacteria | Gene product | Descriptions | Mechanisms | Phenotype | Reference |
|---|---|---|---|---|---|
| Xylella fastidiosa | RpfC | Signaling sensor | Negative regulator for DSF | Mutants show hyper attachment phenotype in xylem vessels and cybarium of insect vector | Chatterjee et al., 2008b |
| FimA, FimF | Type I fimbrial adhesins | Facilitates cell–cell aggregation | – | Chatterjee et al., 2008b | |
| HxfA, HxfB | Hemaglutinins | Facilitates cell–cell aggregation and cell–surface interactions | HxfA mutants slightly reduced attachment | Chatterjee et al., 2008b | |
| PglA | Polygalacturonase | – | Mutants lack pathogenicity and systemic movement in plants | Roper et al., 2007 | |
| β-1,4 endoglucanases Xylanases Xylosidases | Cell degrading enzymes | Degradation of plant cell wall components | – | Chatterjee et al., 2008b | |
| LesA | Lipase/esterase Type II toxin | Phytotoxicity | Phytotoxicity in early plant infection | Nascimento et al., 2016 | |
| O-antigen in LPS | O-Lipopolysaccharide | Mutants lack full pathogenicity | Rapicavoli et al., 2015 | ||
| Spiroplasma kunkelii | – | Pili, extracellular structure | Attachment to insects | – | Ammar et al., 2004 |
| Spiroplasma citri | P58 | Membrane protein | Attachment to insects | – | Ye et al., 1997 |
| SARP1 | Membrane protein | Attachment to insects | – | Berg et al., 2001 | |
| Spiralin | Membrane protein | Attachment to insects | Mutants have reduced transmission by insects | Gasparich, 2010 | |
| P32 | Membrane protein encoded in plasmid (pSci6) | Attachment to insects | Mutants have reduced attachment to insects | Berho et al., 2006 | |
| “Candidatus Phytoplasma spp.” | SAP11 | Sec-exported, NLS signal | Block JA biosynthesis in plants | Sugio et al., 2011 | |
| Amp | Sec-exported, Transmembrane domain | Interacts with insect proteins | Increase vector fecundity | Rashidi et al., 2015 | |
| SAP54/PHYL | Sec-exported | Interaction floral transcription factors | Floral abnormalities as phyllody | MacLean et al., 2014 | |
| NLS signal | Degrades MADS-box proteins | Maejima et al., 2014 | |||
| TENGU | Sec-exported | Inhibits auxin-related pathway | Dwarf plants | Minato et al., 2014 | |
| P38 | Adhesin domain | Interacts with insect proteins | – | Neriya et al., 2014 | |
| HflB | Protease | Virulence factor | – | Seemüller et al., 2013 | |
| VmpA | Membrane protein | Interaction with insects | – | Renaudin et al., 2015 | |
| “Candidatus Liberibacter asiaticus” | LasAI | Autotransporter | Unknown | – | Hao et al., 2013 |
| SC2_gp095 | Glutathione peroxidase | Detoxify ROS | – | Jain et al., 2015 |
In plants, X. fastidiosa relies on bacterial multiplication, attachment, and dispersion into neighboring vessels to colonize the xylem (Chatterjee et al., 2008b; Nascimento et al., 2016). Phytotoxicity during early stages of infection is associated with the lipase/esterase effector LesA, a type II secreted enzyme produced abundantly in culture (Nascimento et al., 2016). By degrading plant cell walls, nutrients are acquired and the bacteria are able to disperse throughout the plant (Purcell, 2013; Fatima and Senthil-Kumar, 2015). Degradation of vascular plant tissue requires the combined action of multiple enzymes, such as β-1,4 endoglucanases, xylanases, xylosidases, and polygalacturonases (Purcell, 2013; Fatima and Senthil-Kumar, 2015). X. fastidiosa mutants impaired in polygalacturonases enzyme (PglA) production, lack pathogenicity, and systemic movement in the plant (Table 2) (Roper et al., 2007). Given the reduced nutritional content of the xylem and the extracellular location of X. fastidiosa, many differences may exist for pathogenicity strategies among xylem and phloem colonizers.
Phloem-Limited Vector-Borne Bacteria
Diverse phylogenetic groups converge in phloem specialization and hemipteran transmission and it is hypothesized that those traits have been acquired independently multiple times over the course of bacteria evolution (Orlovskis et al., 2015). The majority of phytoplasmas (class Mollicutes) and liberibacters (class Alphaproteobacteria) are vector-borne phytopathogens (Bressan, 2014; Fagen et al., 2014a). For other groups, such as spiroplasmas (class Mollicutes), only some species are phytopathogens. Despite this diversity, all known phloem-limited vector-borne bacteria appear to colonize both the insect vector and the plant host intracellularly (Orlovskis et al., 2015). The bacteria cross the gut barrier and circulate in the vector body, eventually reaching the hemolymph, and salivary glands (Table 1) (Gasparich, 2010). The journey to the salivary glands requires a latent period, ranging from days to months, before transmission can occur (Thebaud et al., 2009). Here, we will discuss three examples of phylogenetic groups containing vector-borne bacteria: spiroplasmas, phytoplasmas, and liberibacters.
Spiroplasmas
Spiroplasma spp. have a distinctive helical morphology and use pili–like structures to move in a corkscrew-like motion (Ammar et al., 2004). They are classified as Mollicutes as they lack a cell wall. Spiroplasmas share diverse relationships with plant and insect hosts spanning pathogenic, commensal, and mutualistic interactions (Ammar et al., 2004). Most are associated with diverse insect orders, such as Hymenoptera, Coleoptera, Diptera, Lepidoptera, and Hemiptera (Gasparich, 2010). However, there are three phytopathogenic spiroplasmas that are also transmitted by leafhoppers (Cicadellidae): Spiroplasma citri, S. kunkelii, and S. phoeniceum (Table 1) (Gasparich, 2010). S. citri, the causal agent of citrus stubborn, was the first vector-borne bacteria to be cultured. It was first discovered in 1970 and culture methods were developed shortly after this (Bove and Garnier, 2003). Cultivation of spiroplasmas is not trivial, as it requires complex media enriched with cholesterol and fatty acids. Tools and information derived for spiroplasmas culture methods have served as references for attempts to culture phytoplasmas and liberibacter.
After acquisition, spiroplasmas adhere to receptors in the lumen of the insect midgut, where endocytosis occurs (Fletcher et al., 1998; Gasparich, 2010). Intracellular vesicular transport mediates migration to the hemolymph and exocytosis (Fletcher et al., 1998). Once inside the hemolymph, the bacteria are transported throughout the insect body, eventually reaching the salivary glands after additional intracellular crossings (Fletcher et al., 1998). Currently, the specific insect receptors/factors mediating the journey inside the vector remain unknown for spiroplasmas. However, several potential proteins required for insect attachment have been identified using S. citri mutants impaired in insect transmission and with S. citri strains that have lost insect attachment properties after multiple in vitro cultivations (Ye et al., 1997; Fletcher et al., 1998; Berho et al., 2006; Mutaqin et al., 2011) (Table 2).
One of the first approaches developed to study bacterial protein–insect interactions was the use of leafhopper (C. tenellus) monolayer cell culture assays with spiroplasmas. In this technique, researchers exposed insect cells (CT1) in vitro to S. citri. After exposure, electron microscopy (Wayadande and Fletcher, 1998) or immunofluorescence assays (Labroussaa et al., 2010) were used to evaluate bacterial phenotypes. Numerous candidate attachment proteins have been identified in this way, including P58, SARP, and the plasmid-borne protein P32 (Table 2) (Wayadande and Fletcher, 1998; Berg et al., 2001). Another very abundant membrane protein of S. citri that has been implicated in transmission is spiralin. S. citri mutants compromised in spiralin production exhibit reduced transmission by the vector, Circulifer haematoceps (Table 2) (Gasparich, 2010). This suggests that spiralin may mediate pathogen interactions within the insect vector, though specific mechanisms remain unknown.
Phytoplasmas
Phytoplasmas are another category of the Mollicutes that depend on insect vectors for transmission (Table 1), but unlike Spiroplasma, they have pleomorphic shapes and are very difficult to culture (Contaldo et al., 2012). Phytoplasmas are a diverse monophyletic group, with more than 30 “Candidatus Phytoplasma” species described and 100s of subgroups (Hogenhout et al., 2008b). As a taxon they have a wide host range, infecting more than 800 different plant species (Hogenhout et al., 2008b), but individual strains have highly restricted insect and plant hosts. Collectively, more than 1000 plant diseases are caused by phytoplasmas that are transmitted by leafhoppers and, to a lesser extent, a few other hemipterans (Mitchel, 2004; Weintraub and Beanland, 2006).
Phytoplasmas have the smallest genomes of all described phytopathogenic bacteria, averaging ~0.7 Mb with a low G+C content, high number of repetitive regions, and interesting variability in genome features across the taxon (Table 2) (Kube et al., 2012, 2014). At least six types of ATP-binding cassette (ABC) transporters are conserved in the evaluated genomes. ABC transporters shuttle metabolites across bacterial membranes, and are predicted to allow nutrient and metabolite uptake from the host. Other common features include a superoxide dismutase enzyme (SOD), possibly used to counteract reactive oxygen species produced by hosts, and a protease (HflB), which is a virulence factor for ‘Candidatus Phytoplasma mali (Wang et al., 2014). Recently a conserved Mollicutes adhesion motif (MAM) was identified in the Onion Yellow Phytoplasma genome. This candidate protein (P38) interacts with crude insect extracts and weakly with plants extracts (Table 2) (Neriya et al., 2014), however, specific host targets are unknown.
Phytoplasmas also encode translocase SecA, part of the Type II secretion system for bacteria. This secretion system allows the delivery of functionally distinct proteins with a characteristic signal peptide at the n-terminal to the bacterial membrane. Because phytoplasmas have a single membrane, after the signal peptide is cleaved the proteins are released into the host environment (secreted). Secreted phytoplasma proteins can alter host functions and act as effectors (Bai et al., 2009). A single phytoplasma genome can encodes over 50 secreted proteins (SAP’s), however, the function of each one during host colonization and propagation is only known for a few (Bai et al., 2009). SAP effectors often alter host function by manipulating plant hormone homeostasis. For example, the effector TENGU inhibits auxin-related pathways leading to a dwarf plant phenotype and floral sterility (Table 2) (Minato et al., 2014). Further, Arabidopsis transgenic lines expressing SAP11, produce less jasmonic acid (JA) compared to controls (Table 2) (Sugio et al., 2014). This leads to abnormal vegetative growth and increased fecundity for leafhopper vectors on infected plants (Lu et al., 2014b). SAP effectors can also modulate pathogenicity through changes in development. SAP54/PHYL interacts with floral transcription factors and promotes degradation of the MADS-box proteins. MADS-box proteins are critical for floral meristem development and plants expressing SAP54/PHYL flower abnormally (Table 1) (MacLean et al., 2014; Maejima et al., 2014).
A second group of proteins delivered by the Sec-secretion system are the immunodominant membrane proteins (IMPs), which remain anchored and decorate the external membrane of phytoplasmas. IMPs are unique for phytoplasmas and are categorized into three subgroups depending on whether the n- or c- terminal side of the protein is exposed extracellularly (Amp, IdpA, or Imp; Kakizawa et al., 2006). When a monoclonal anti-AMP from “Candidatus Phytoplasma asteris” Chrysanthemums Yellows strain (CPY) was fed to the leafhopper vector, internalization of the phytoplasma and transmission efficiency was reduced. These results imply that anti-Amp impedes attachment of the bacteria in the vector gut (Table 2) (Rashidi et al., 2015).
Liberibacter
The genus Liberibacter spp. contains six species of phloem-limited bacteria (Haapalainen, 2014): “Ca. Liberibacter africanus,” “Ca. Liberibacter americanus,” “Ca. Liberibacter asiaticus,” “Ca. Liberibacter solanacearum,” “Ca. Liberibacter europaeus,” and Liberibacter crescens. “Ca. Liberibacter africanus,” “Ca. Liberibacter americanus,” and “Ca. Liberibacter asiaticus” are associated with citrus greening disease, also referred as Huanglongbing (HLB) in different regions around the globe (Gottwald, 2010). “Ca. Liberibacter solanacearum” (=“Ca. Liberibacter psyllaurous”) is phytopathogenic to members of the Apiaceae and Solanaceae plant families. These four species all depend on psyllid vectors for transmission and as alternative hosts (Fagen et al., 2014b; Haapalainen, 2014). “Ca. Liberibacter europaeus” has also been associated with psyllids, but its role as a plant pathogen has not been demonstrated. To date, only Liberibacter crescens has been cultured in vitro, but it is not considered phytopathogenic and it is not vector-borne (Fagen et al., 2014a). L. crescens was first isolated as a bacterial endophyte from papaya and has not been re-isolated in nature. Non-psyllid hemipterans may also be able to pick up the bacteria during feeding as bacterial DNA has been found in mealybugs (Pitino et al., 2014), however, liberibacter transmission by other hemipterans is currently not clear.
Liberibacters have a small genome of ~1.2 Mb. Comparative genomics have shown a similar gene organization across the genus and evidence of horizontal gene transfers as prophages integrated into the genomes (Table 1) (Thompson et al., 2015). Similar to phytoplasmas, liberibacters lack biosynthesis genes for amino acids, sugars, and nitrogenated bases, which imply they obtain those metabolic products from their host (Thompson et al., 2015). Accordingly, many ABC transporters are encoded in liberibacter genomes (Lin et al., 2011; Mafra et al., 2013; Yan et al., 2013). Active importation of nutrients from phloem and insect vectors may lead to nutrient imbalances, partially explaining the foliar symptoms observed in liberibacter-infected plants (Rashed et al., 2013).
Potential pathogenicity mechanisms of liberibacters have recently been suggested based on comparative bioinformatics with other phloem-limited bacteria. Liberibacters encode the basic proteins for Sec-dependent translocation, similar to phytoplasmas (Lin et al., 2011; Yan et al., 2013). However, as liberibacters have two membranes of different composition in contrast to phytoplasmas, it is not known whether putative liberibacter Sec-transported proteins cross the outer membrane and interact with the plant or insect host. Recently, two unusual autotransporters were identified in the liberibacter genome (LasAI and LasAII) and these may serve as an alternative secretion system to the Sec-system (Table 2) (Hao et al., 2013). Evidence suggests that plant transcripts and metabolites related to salicylic acid (SA) production are altered during ‘Candidatus Liberibacter solanacerum’ infection (Casteel et al., 2012; Chin et al., 2014). SA is an important signaling molecule involved in plant defense to pathogens and insects (Glazebrook, 2005; Walling, 2009; Erb et al., 2012). Recently, a NahG-like salicylate hydroxylase gene was found in the liberibacter genome. NahG is predicted to cleave salicylates derived from SA (Lin et al., 2013) and may be used to modify the plant defense system. Although comparative bioinformatics has revealed many potential proteins used by liberibacter to alter plant and vector metabolism and vector–plants interactions, exact mechanisms for host colonization and transmission remain largely unknown.
Approaches to Study Vector-Borne Bacteria
The current understanding of pathogenicity mechanisms in vector-borne bacteria is largely influenced by the ability to culture those bacteria. To date only X. fastidiosa and Spiroplasma spp. have been cultured in vitro and both require very specific conditions (Dourado et al., 2015; Renaudin et al., 2015). Because of this limitation, much of the biology and mechanisms of host colonization for phytoplasmas and liberibacters are still poorly understood (Bove and Garnier, 2003). Another challenge of working with phloem-limited vector-borne bacteria in particular is the non-homogenous distribution in the phloem tissue. This makes choosing sampling locations difficult and can result in false negatives during detection. Further, symptoms vary significantly across plant hosts and do not necessarily correlate with pathogen titer. Despite these difficulties, approaches combining genomics, bioinformatics, transcriptomics, and genetic manipulation have contributed to recent advances in the understanding of how these bacterial pathogens colonize their host environments.
Whole Genome Sequencing and Bioinformatics of Vector-Borne Bacteria
The complete genome sequences for many strains of vector-borne bacteria have recently become available (Table 1). This has allowed scientists to study bacterial gene function within these systems without the need to culture the organism. Bioinformatics can be used to compare genome sequences with the annotated genomes of close relatives or analyze sequences using server-based algorithms to assign predicted functions to each coding region (Rutherford et al., 2000). Amino acid sequences can be further explored to identify conserved patterns and domains. In this way, proteins with low average similarity can be assigned to a predicted function (Yu et al., 2010; Caccia et al., 2013; Cortazar et al., 2015). Finally, functions of unknown proteins can even be predicted using dedicated algorithms that identify patterns associated with signal peptides, localization, cleavage sites, phosphorylation, and transmembrane domains (Yu et al., 2010).
A limitation of these various bioinformatics approaches is that all programs are trained using cultured organisms. For unculturable bacteria, many unique sequences with no homologs in cultured species exist, making comparisons and inferences difficult (Kube et al., 2008). Despite these limitations, bioinformatics have been used successfully to study gene function for many phytoplasma effectors. Bai et al. (2009) identified 56 Secreted Aster Yellows Proteins (SAPs) in the genome of ‘Candidatus Phytoplasma asteris’ strain Aster Yellows Witches’-broom (AY-WB). In this study, they utilized a pipeline to predict prokaryotic signal peptides recognized by Sec-translocases (SignalP v. 3.0) and then predicted transmembrane domains within this list to predict secretion (TMHMM v. 2.0; Bai et al., 2009). Finally, the list of 56 predicted effectors was examined for eukaryotic nuclear localization signals (predictNLS and pSORT) to select SAPs targeting plant nuclei for further investigation (Bai et al., 2009; Lu et al., 2014b).
Transcriptomics of Vector-Borne Bacteria in Their Hosts
After potential pathogenicity factors are identified, functional validation is required. For unculturable bacteria, transcription and translation of targets can only be evaluated within their hosts (plant or insect). This means RNA and protein isolations must be done from infected host tissue. By some estimates, only 0.1% of total RNA extracted from infected herbaceous hosts represents the phytoplasma RNA. Others report only 0.02% of the mRNA from the woody host was associated with the phytoplasma genome (Abba et al., 2014). However, high throughput sequencing technologies have expanded the possibilities for studying pathogens inside their hosts. Now RNAseq can be used to quantify the complete RNA population in a sample (Westermann et al., 2012). This technique has advantages over microarrays and qRT-PCR because it affords higher sensitivity for monitoring gene expression levels, independence from examining only known sequences, and wider detection ranges (Westermann et al., 2012). However, for vector-borne bacterial pathogens, RNAseq approaches have thus far had low levels of success.
RNAseq has been used to examine “Candidatus Phytoplasma mali” transcription in tobacco (Nicotiana occidentalis; Siewert et al., 2014). Prior to preparation for sequencing, total RNA was treated with a plant ribosomal depletion kit to enrich the samples for bacterial RNA. Only 0.003% of the total reads (17,046,418 reads averaging 115 b) were mapped against the protein coding regions of the predicted “Candidatus Phytoplasma mali” genome. Mapped reads corresponded to 132 genes out of the 497 predicted genes. In another RNAseq study, RNA was enriched for bacterial transcripts using a ribosomal depletion kit to remove plant cytoplasmic, mitochondrial, and chloroplast ribosomal RNA (Abba et al., 2014). Despite the enrichment and relatively deep sequencing, only 0.01% of the total reads (125,813,174 and 129,412,231, for each library) were mapped to the draft genome of the phytoplasma flavescence dore (Abba et al., 2014). In two slightly more successful studies, total RNA from psyllid vectors was used to detect transcripts from “Ca. Liberibacter solanacearum” (Ibanez et al., 2014; Yao et al., 2016). However, only 0.3% of the total (70,869,948) reads were mapped to the bacteria genome after ribosomal depletion (Ibanez et al., 2014). Transcriptomics offer a unique opportunity to overcome the many difficulties posed by these difficult pathosystems, but as evident in the above examples, many technical challenges remain.
Genetic Manipulation of Vector-Borne Bacterial Phytopathogens
The first approach that permitted gene function discovery for vector-borne bacterial plant pathogens was the use of transposon mutagenesis with spiroplasmas in the early 1990s (Fletcher et al., 1998; Mutaqin et al., 2011). In this approach, a transposon with a selective marker was integrated randomly into the chromosome of S. citri, and recombinant colonies were selected in media with antibiotics. When transformed colonies were tested in the host, the transposon was retained for a few days without antibiotic pressure. This technique was used to determine that disruption of a solute binding protein (gene sc76) reduced transmission in the leafhopper vector (Boutareaud et al., 2004). Since this first study, numerous research groups have generated collections of S. citri mutants using this technique (Foissac et al., 1997; Boutareaud et al., 2004; Mutaqin et al., 2011). Currently, X. fastidiosa and Spiroplasma spp. are the only vascular plant pathogens transmitted by hemipterans for which genetic transformation protocols and mutant libraries are currently available.
Genetic manipulation using surrogate culturable bacteria and heterologous gene expression in plants has been used to test gene function for other vector-borne bacteria (Jain et al., 2015; Renaudin et al., 2015). In a study using the flavescence dore phytoplasma, the surface protein, variable membrane protein A (VmpA), was expressed under the control of the S. citri tuf promoter in a recombinant S. citri (Table 2) (Renaudin et al., 2015). The tuf promoter was chosen because the tuf gene is expressed at high levels in most bacteria (Kim et al., 2009). In this system the leafhopper, Euscelidius variegatus, serves as a vector for both the phytoplasma and S. citri. Thus gain of function studies could be conducted with the recombinant S. citri in both hosts. In the case of phytopathogenic “Candidatus Liberibacter asiaticus,” a peroxidase protein (SC2_gp095) has been expressed in the cultivable L. crescens as a surrogate (Table 2). However, biological inferences from this system may be restricted by the lack of host infection of L. crescens after culturing.
An alternative method for studying gene function is to overexpress bacterial candidate proteins in the plant host. Model plants such as Arabidopsis thaliana and Nicotiana spp. are routinely used to evaluate gene function for plant pathogens. Once the candidate gene is selected, the coding sequence is cloned into a suitable expression vector and transgenic plants can be generated. Several authors have utilized plant heterologous expression systems to investigate the function of phytoplasma SAPs (MacLean et al., 2011; Lu et al., 2014a; Yang et al., 2015). In these studies transgenic A. thaliana expressing individual phytoplasma SAPs were screened for symptom development and plant abnormalities (MacLean et al., 2011; Lu et al., 2014a; Yang et al., 2015). After a relevant phenotype was identified, plant gene expression changes and plant proteins interacting with the phytoplasma proteins were examined (Table 2). A limitation of this approach is that only profound disturbances caused by a single bacterial gene can be identified. In addition, model plants may not serve as natural hosts for all vectored-borne bacteria and relevance of findings may be limited to an artificial system.
Application of Knowledge for ‘Next Generation’ Control Strategies
Controlling vector-borne pathogens is difficult. Chemical control of insect vectors is the most widely used method, but in most cases insecticidal applications are not sufficient to contain the spread of these pathogens and associated diseases. Furthermore, insect resistance and environmental regulations have limited the viability of long-term application of insecticides. Host plant resistance has been successful for several high value crops (Bisognin et al., 2008; Riaz et al., 2008), including grapevine tolerance to Pierce’s disease. In these plants, X. fastidiosa infection occurs, but titer remains low in the plant (Riaz et al., 2008). Due to the long time periods required to identify resistance and produce new varieties, this method may not always be a practical choice for the more aggressive and devastating outbreaks. Overall, as research on vector-borne bacteria continues to flourish, a focus on the ‘next generation’ of control strategies is needed.
One recent approach to block transmission of vector-borne bacteria used chemicals intended to saturate the pathogen-binding site in the insect or on the bacteria surface, so the insect picks up fewer pathogen cells (Killiny et al., 2012). In this study, vectors were fed artificial diet supplemented with X. fastidiosa cells and different potential transmission-blocking chemicals. Multiple lectins, carbohydrates, and antibodies were evaluated for potential transmission blocking characteristics. After feeding on the diet-bacteria mixture, insects were transferred to healthy plants to determine transmission efficiency with and without the different chemicals (Killiny et al., 2012). Diets containing certain lectins (wheat germ agglutinin and concanavalin A), N-acetyl glucosamine carbohydrates, and certain antibodies reduced the transmission efficiency under greenhouse conditions. The authors suggest that lectins probably compete with the bacteria for the binding sites inside the insect, while carbohydrate saturate X. fastidiosa adhesions on the cell surface (Killiny et al., 2012). The interference approach has also been explored in phytoplasmas using antibodies against the extracellular membrane protein Amp, with some success in the lab (Rashidi et al., 2015). Recently, phage-display libraries have been used to evaluate antibodies and protein–protein interactions inside the insect vector. In this approach, each phage contains a known peptide and the binding capacity of the peptide to an extracellular bacterial epitope is evaluated (Huang et al., 2012). The exact mechanisms mediating the ability of specific chemicals to block transmission is still unknown, and it is not clear how this technology could be used in large-scale application. How, for example, might a natural population of insects be exposed to the transmission-blocking chemical?
Some of the most extensive research efforts on ‘next generation’ control technologies for vector-borne bacteria have focused on the use of nucleic acids in gene drive systems (Sinkins and Gould, 2006), and with RNA interference strategies (Gordon and Waterhouse, 2007; Nandety et al., 2015). The first concept was explored initially in the field of medicine, and is based on the concept of ‘selfish DNA’. Selfish DNA is a naturally occurring phenomenon where certain genetic elements, such as transposable elements and others, spread in the genome of an organism and in the population by making additional copies of themselves. It has been suggested that selfish genetic elements could be used for control as a gene drive system that carries additional genes with anti-pathogen effects (Sinkins and Gould, 2006; Gantz and Jasinskiene, 2015). Populations of insects transformed with transposable elements or with a transgenic Wolbachia strain could be released into the environment, permitting the gene drive system and ‘gene of interest’ to spread in the population and block plant pathogen associations (Sinkins and Gould, 2006). Obvious concerns with this method are public acceptance of transgenic organisms, non-target impacts, and the costs of implantation.
Nucleic acids can also be utilized as a control method by inducing RNA interference (RNAi). RNAi has already been successfully exploited in plants to control viruses in commercial production (Tricoll et al., 1995; Gonsalves, 2006; Fuchs and Gonsalves, 2007; Scorza et al., 2013) and successful control of bacteria has been demonstrated (Escobar et al., 2001). RNAi can also be used to control insect species, altering insect reproduction, physiology, or survival (Gordon and Waterhouse, 2007; Wuriyanghan et al., 2011; Nandety et al., 2015). Direct injection, bait feeding, or transgenic host plants can be used to induce RNAi in insects. As direct injection is not practical for large scale control, and bait feeding is not effective in field studies for hemipteran insects, transgenic plants are the best options for using RNAi to control vectors of bacterial pathogens. While there is much excitement about the use of RNAi as an alternative control strategy (Gordon and Waterhouse, 2007; Donald et al., 2012; Li et al., 2013; Yu et al., 2016), additional research on delivery, safety, and non-target effects needs to be explored. Despite these unknowns, RNAi studies still represent an excellent attempt at next-generation control for these important plant pathogens.
Conclusion and Future Directions
Devastating outbreaks of citrus greening disease, Pierce’s disease, and zebra chip disease in recent years have contributed to a rapid growth in the literature on bacterial plant pathogens and their hemipteran vectors (Haapalainen, 2014; Almeida and Nuney, 2015; Orlovskis et al., 2015). Whereas most plant-infecting viruses depend on hemipterans for transmission, most plant-infecting bacteria do not. The small subset of known bacteria that are vector-borne are able to propagate in both the plant host and the insect vector, organisms from diverse phylogenetic kingdoms (Table 1). This is in contrast to the non-propagative relationships most vector-borne plant viruses share with hemipteran vectors. The ability to transition between divergent hosts is remarkable considering that most vector-borne bacteria have highly reduced genomes compared their free-living ancestors, yet, we still do not understand the mechanisms which make this sort of transitioning possible. Variation in vector-pathogen specificity also exists across hemipteran groups (Figure 1; Supplementary Table S1), suggesting there are still unknown constraints on these relationships. Clear differences occur between the relationships that phloem and xylem colonizers share with insect vectors (Table 1). However, conclusions based on tissue tropisms should be made with caution, as only one xylem-limited vector-borne species has been identified so far.
The analysis of the genetic mechanisms mediating interactions between vector-borne bacteria and their hosts has focused largely on membrane-bound proteins and Sec-dependent peptides in gram-positive bacteria (Table 2). For gram-negative bacteria, the primary focus has been on toxins, enzymes, and aggregation factors (Table 2). Clearly, the role of membrane-associated proteins and extracellular structures represents the first target for investigating physical recognition inside the vector and initiation of host processes. However, the methodological bias toward these functional categories may limit our understanding of other important mechanisms mediating interactions with hosts. For example, in ‘Candidatus Phytoplasma mali’ 32% of the genome has no homology to any other sequences, and for ‘Candidatus Phytoplasma asteris’ strain Onion Yellow’s almost 50% of the genome is classified as unknown (Kube et al., 2008). Considering the highly reduced genomes and host-dependence of these bacteria, genes without an assigned function likely still play a significant role in the biology of the organism and will need to be investigated. The continued expansion of “omics” and other next-generation technologies in molecular biology will likely shed new light on the role of unknown coding sequences in host colonization, pathogenesis, and how host specificity may have evolved independently in different bacterial lineages.
Despite these advances, research on vector-borne pathogens is still in its infancy. Some of the most significant gaps in our understanding concern interactions with insect vectors. In particular, our understanding of leafhopper and psyllid feeding behavior, immunity, and plant responses to these insects needs to be improved. Genetic resources for these important vectors also need to be expanded. Promisingly, the genomes for the psyllid Diaphorina citri1 and at least one planthopper have been sequenced (Noda et al., 2008) and several other genome projects for important vectors of bacterial pathogens are underway (Evans et al., 2013; Poelchau et al., 2015). However, accessibility and quality control of insect genomic data remains an ongoing concern for the entomological community. In response to this, several projects attempting to address these issues have been initiated (Legeai et al., 2010; Poelchau et al., 2015; Yin et al., 2016), though at the time of publication most of these online resources remain works-in-progress. This area of research is likely to progress rapidly in the coming years. While climate change and the global food economy will continue to drive emergence of additional vector-borne bacterial pathosystems, the advent of genome editing, single-cell–omics, and interference RNA techniques will contribute to the identification of vector-borne bacterial phytopathogens and advances in our knowledge.
Author Contributions
CC conceived the project. CC and LP-H wrote the article.
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Acknowledgments
We thank Aurelie Bak and MacKenzie Patton for helpful discussion and comments on earlier versions of this manuscript.
Funding. This work was supported in part by a grant from the NIFA (award 2013-2013-03265) to CC and University of California start-up funds to CC; and by a Fulbright-COLCIENCIAS (Colombian Administrative Department of Science, Technology and Innovation) fellowship to LP-H.
Supplementary Material
The Supplementary Material for this article can be found online at: http://journal.frontiersin.org/article/10.3389/fpls.2016.01163
References
- Abba S., Galetto L., Carle P., Carrère S., Delledonne M., Foissac X., et al. (2014). RNA-Seq profile of flavescence dorée phytoplasma in grapevine. BMC Genomics 15:1088 10.1186/1471-2164-15-1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almeida R. P., Nuney L. L. (2015). How do plant diseases caused by Xylella fastidiosa emerge? Plant Dis. 99:1457–1467. 10.1094/PDIS-02-15-0159-FE [DOI] [PubMed] [Google Scholar]
- Ammar El-D., Fulton D., Bai X., Meulia T., Hogenhout S. A. (2004). An attachment tip and pili-like structures in insect- and plant-pathogenic spiroplasmas of the class Mollicutes. Arch. Microbiol. 181 97–105. 10.1007/s00203-003-0630-8 [DOI] [PubMed] [Google Scholar]
- Ammar El-D., Shatters R. G., Hall D. G. (2011). Localization of Candidatus Liberibacter asiaticus, associated with Citrus Huanglongbing disease, in its psyllid vector using fluorescence in situ hybridization. J. Phytopathol. 159 726–734. 10.1111/j.1439-0434.2011.01836.x [DOI] [Google Scholar]
- Ammar El-D., Tsai C. W., Whitfield A. E., Redinbaugh M. G., Hogenhout S. A. (2009). Cellular and molecular aspects of Rhabdovirus interactions with insect and plant hosts. Annu. Rev. Entomol 54 447–468. 10.1146/annurev.ento.54.110807.090454 [DOI] [PubMed] [Google Scholar]
- Backus E. A., Morgan D. J. (2011). Spatiotemporal colonization of Xylella fastidiosa in its vector supports the role of egestion in the inoculation mechanism of foregut-borne plant pathogens. Phytopathology 101 912–922. 10.1094/phyto-09-10-0231 [DOI] [PubMed] [Google Scholar]
- Bae C., Han S. W., Song Y. R., Kim B. Y., Lee H. J., Lee J. M., et al. (2015). Infection processes of xylem-colonizing pathogenic bacteria: possible explanations for the scarcity of qualitative disease resistance genes against them in crops. Theor. Appl. Genet. 128 1219–1229. 10.1007/s00122-015-2521-1 [DOI] [PubMed] [Google Scholar]
- Bai X., Correa V. R., Toruno T. Y., Ammar El-D., Kamoun S., Hogenhout S. A. (2009). AY-WB phytoplasma secretes a protein that targets plant cell nuclei. Mol. Plant Microbe Interact. 22 18–30. 10.1104/pp.111.181586 [DOI] [PubMed] [Google Scholar]
- Beanland L., Hoy C. W., Miller S. A., Nault L. R. (2000). Influence of aster yellows phytoplasma on the fitness of aster leafhopper (Homoptera: Cicadellidae). Ann. Entomol. Soc. Am. 93:271–276. 10.1603/0013-8746(2000)093[0271:IOAYPO]2.0.CO;2 [DOI] [Google Scholar]
- Berg M., Melcher U., Fletcher J. (2001). Characterization of Spiroplasma citri adhesion related protein SARP1, which contains a domain of a novel family designated sarpin. Gene 257:8. [DOI] [PubMed] [Google Scholar]
- Berho N., Duret S., Danet J. L., Renaudin J. (2006). Plasmid pSci6 from Spiroplasma citri GII-3 confers insect transmissibility to the non-transmissible strain S. citri 44. Microbiology 152 2703–2716. 10.1099/mic.0.29085-0 [DOI] [PubMed] [Google Scholar]
- Bisognin C., Schneider B., Salm H., Grando M. S., Jarausch W., Moll E., et al. (2008). Apple proliferation resistance in apomictic rootstocks and its relationship to phytoplasma concentration and simple sequence repeat genotypes. Phytopathology 98 153–158. 10.1094/PHYTO-98-2-0153 [DOI] [PubMed] [Google Scholar]
- Blanc S., Drucker M., Uzest M. (2014). Localizing viruses in their insect vectors. Annu. Rev. Phytopathol. 52:403–425. 10.1146/annurev-phyto-102313-045920 [DOI] [PubMed] [Google Scholar]
- Blanc S., Uzest M., Drucker M. (2011). New research horizons in vector-transmission of plant viruses. Curr. Opin. Microbiol. 14 483–491. 10.1016/j.mib.2011.07.008 [DOI] [PubMed] [Google Scholar]
- Boutareaud A., Danet J. L., Garnier M., Saillard C. (2004). Disruption of a gene predicted to encode a solute binding protein of an ABC transporter reduces transmission of Spiroplasma citri by the leafhopper Circulifer haematoceps. Appl. Environ. Microbiol. 70 3960–3967. 10.1128/aem.70.7.3960-3967.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bove J. M., Garnier M. (2003). Phloem-and xylem-restricted plant pathogenic bacteria. Plant Sci. 164 423–438. 10.1016/S0168-9452(03)00032-3 [DOI] [Google Scholar]
- Bressan A. (2014). Emergence and evolution of Arsenophonus bacteria as insect-vectored plant pathogens. Infect. Genet. Evol. 22 81–90. 10.1016/j.meegid.2014.01.004 [DOI] [PubMed] [Google Scholar]
- Bruton B. D., Mitchell F., Fletcher J., Pair S. D., Wayadande A., Melcher U., et al. (2003). Serratia marcescens, a phloem-colonizing, squash bug-transmitted bacterium: causal agent of cucurbit yellow vine disease. Plant Dis. 87:937–944. 10.1094/PDIS.2003.87.8.937 [DOI] [PubMed] [Google Scholar]
- Caccia D., Dugo M., Callari M., Bongarzone I. (2013). Bioinformatics tools for secretome analysis. Biochim. Biophys. Acta 1834:2442–2453. 10.1016/j.bbapap.2013.01.039 [DOI] [PubMed] [Google Scholar]
- Casteel C. L., Hansen A. K., Walling L. L., Paine T. D. (2012). Manipulation of plant defense responses by the tomato psyllid (Bactericera cockerelli) and its associated endosymbiont Candidatus Liberibacter psyllaurous. PLoS ONE 7:e35191 10.1371/journal.pone.0035191 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chatterjee S., Almeida R. P., Lindow S. (2008a). Living in two worlds: the plant and insect lifestyles of Xylella fastidiosa. Annu. Rev. Phytopathol. 46 243–271. 10.1146/annurev.phyto.45.062806.094342 [DOI] [PubMed] [Google Scholar]
- Chatterjee S., Wistrom C., Lindow S. E. (2008b). A cell-cell signaling sensor is required for virulence and insect transmission of Xylella fastidiosa. Proc. Natl. Acad. Sci. U.S.A. 105 2670–2675. 10.1073/pnas.0712236105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen A. Y. S., Walker G. P., Carter D., Ng J. C. K. (2011). A virus capsid component mediates virion retention and transmission by its insect vector. Proc. Natl. Acad. Sci. U.S.A. 108 16777–16782. 10.1073/pnas.1109384108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen W., Hasegawa D. K., Arumuganathan K., Simmons A. M., Wintermantel W. M., Fei Z., et al. (2015). Estimation of the whitefly Bemisia tabaci genome size based on k-mer and flow cytometric analyses. Insects 6 704–715. 10.3390/insects6030704 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chin E. L., Mishchuk D. O., Breksa A. P., Slupsky C. M. (2014). Metabolite signature of Candidatus Liberibacter asiaticus infection in two citrus varieties. J. Agric. Food Chem. 62:6585–6591. 10.1021/jf5017434 [DOI] [PubMed] [Google Scholar]
- Contaldo N., Bertaccini A., Paltrinieri S., Windsor H. M., Windsor G. D. (2012). Axenic culture of plant pathogenic phytoplasmas. Phytopathol. Mediterr. 51:607–617. [Google Scholar]
- Cortazar A. R., Ogiza J. A., Aranzay A. M., Lavin J. L. (2015). PECAS: prokaryotic and eukaryotic classical analysis of secretome. Amino Acids 47:2659–2663. 10.1007/s00726-015-2058-2 [DOI] [PubMed] [Google Scholar]
- Danet J. L., Foissac X., Zreik L., Salar P., Verdin E., Nourrisseau J. G., et al. (2003). “Candidatus Phlomobacter fragariae” is the prevalent agent of marginal chlorosis of strawberry in french production fields and is transmitted by the planthopper Cixius wagneri (China). Phytopathology 93 644–649. 10.1094/phyto.2003.93.6.644 [DOI] [PubMed] [Google Scholar]
- Donald C. L., Kohl A., Schnettler E. (2012). New insights into control of arbovirus peplication and spread by insect RNA interference pathways. Insects 3 511–531. 10.3390/insects3020511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dourado M. N., Santos D. S., Nunes L. R., Costa De Oliveira R. L., De Oliveira M. V., Araujo W. L. (2015). Differential gene expression in Xylella fastidiosa 9a5c during co-cultivation with the endophytic bacterium Methylobacterium mesophilicum SR1.6/6. J. Basic Microbiol. 55 1357–1366. 10.1002/jobm.201400916 [DOI] [PubMed] [Google Scholar]
- Erb M., Meldau S., Howe G. A. (2012). Role of phytohormones in insect-specific plant reactions. Trends Plant Sci. 17 250–259. 10.1016/j.tplants.2012.01.003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Escobar M. A., Civerolo E. L., Summerfelt K. R., Dandekar A. M. (2001). RNAi-mediated oncogene silencing confers resistance to crown gall tumorigenesis. Proc. Natl. Acad. Sci. U.S.A. 98 13437–13442. 10.1073/pnas.241276898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans J. D., Brown S. J., Hackett K. J., Robinson G., Richards S., Lawson D., et al. (2013). The i5K Initiative: advancing arthropod genomics for knowledge, human health, agriculture, and the environment. J. Hered. 104 595–600. 10.1093/jhered/est050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fagen J. R., Leonard M. T., Coyle J. F., Mccullough C. M., Davis-Richardson A. G., Davis M. J., et al. (2014a). Liberibacter crescens gen. nov., sp. nov., the first cultured member of the genus Liberibacter. Int. J. Syst. Evol. Microbiol. 64 2461–2466. 10.1099/ijs.0.063255-0 [DOI] [PubMed] [Google Scholar]
- Fagen J. R., Leonard M. T., Mccullough C. M., Edirisinghe J. N., Henry C. S., Davis M. J., et al. (2014b). Comparative genomics of cultured and uncultured strains suggests genes essential for free-living growth of Liberibacter. PLoS ONE 9:11 10.1371/journal.pone.0084469.t001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatima U., Senthil-Kumar M. (2015). Plant and pathogen nutrient acquisition strategies. Front. Plant Sci. 6:750 10.3389/fpls.2015.00750 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher J., Wayadande A., Melcher U., Ye F. (1998). The phytopathogenic mollicute-insect vector interface: a closer look. Phytopathology 88 1351–1358. 10.1094/PHYTO.1998.88.12.1351 [DOI] [PubMed] [Google Scholar]
- Foissac X., Danet J. L., Saillard C., Gaurivaud P., Laigret F., Paré C., et al. (1997). Mutagenesis by insertion of Tn4001 into the genome of Spiroplasma citri: characterization of mutants affected in plant pathogenicity and transmission to the plant by the leafhopper vector Circulifer haematoceps. Mol. Plant Microbe Interact. 10 454–461. 10.1094/MPMI.1997.10.4.454 [DOI] [Google Scholar]
- Fuchs M., Gonsalves D. (2007). Safety of virus-resistant transgenic plants two decades after their introduction: lessons from realistic field risk assessment studies. Annu. Rev. Phytopathol. 45 173–202. 10.1146/annurev.phyto.45.062806.094434 [DOI] [PubMed] [Google Scholar]
- Gantz V. M., Jasinskiene N. (2015). Highly efficient Cas9-mediated gene drive for population modification of the malaria vector mosquito Anopheles stephensi. Proc. Natl. Acad. Sci. U.S.A. 112 E6736–E6743. 10.1073/pnas.1521077112 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gasparich G. E. (2010). Spiroplasmas and phytoplasmas: microbes associated with plant hosts. Biologicals 38 193–203. 10.1016/j.biologicals.2009.11.007 [DOI] [PubMed] [Google Scholar]
- Gilbertson R. L., Batuman O., Webster C. G., Adkins S. (2015). Role of the insect supervectors Bemisia tabaci and Frankliniella occidentalis in the emergence and global spread of plant viruses. Ann. Rev. Virol. 2 67–93. 10.1146/annurev-virology-031413-085410 [DOI] [PubMed] [Google Scholar]
- Glazebrook J. (2005). Contrasting mechanisms of defense against biotrophic and necrotrophic pathogens. Annu. Rev. Phytopathol. 43 205–227. 10.1146/annurev.phyto.43.040204.135923 [DOI] [PubMed] [Google Scholar]
- Gonsalves D. (2006). Transgenic papaya: development, release, impact and challenges. Adv. Virus Res. 67 317–354. 10.1016/S0065-3527(06)67009-7 [DOI] [PubMed] [Google Scholar]
- Gordon K. H., Waterhouse P. M. (2007). RNAi for insect-proof plants. Nat. Biotechnol. 25 1231–1232. 10.1038/nbt1107-1231 [DOI] [PubMed] [Google Scholar]
- Gottwald T. R. (2010). Current epidemiological understanding of citrus huanglongbing. Annu. Rev. Phytopathol. 48 119–139. 10.1146/annurev-phyto-073009-114418 [DOI] [PubMed] [Google Scholar]
- Gray S., Cilia M., Ghanim M. (2014). Circulative, “non-propagative” virus transmission: an orchestra of virus-, insect-, and plant-derived instruments. Adv. Virus Res. 89 141–199. 10.1016/B978-0-12-800172-1.00004-5 [DOI] [PubMed] [Google Scholar]
- Gullan P. J., Cranston P. S. (2014). The Insects: An Outline of Entomology 5th Edn Hoboken, NJ: Wiley-Blackwell; 595. [Google Scholar]
- Haapalainen M. (2014). Biology and epidemics of Candidatus Liberibacter species, psyllid-transmitted plant-pathogenic bacteria. Ann. Appl. Biol. 165 172–198. 10.1111/aab.12149 [DOI] [Google Scholar]
- Hao G., Boyle M., Zhou L., Duan Y. (2013). The intracellular citrus huanglongbing bacterium, ‘Candidatus Liberibacter asiaticus’ encodes two novel autotransporters. PLoS ONE 8:e68921 10.1371/journal.pone.0068921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiruki C. (1999). Pawlonia witches’-broom disease important in East Asia. Acta Hortic. 495:6. [Google Scholar]
- Hogenhout S. A., Ammar El-D., Whitfield A. E., Redinbaugh M. G. (2008a). Insect vector interactions with persistently transmitted viruses. Annu. Rev. Phytopathol. 46 327–359. 10.1146/annurev.phyto.022508.092135 [DOI] [PubMed] [Google Scholar]
- Hogenhout S. A., Oshima K., Ammar El-D., Kakizawa S., Kingdom H. N., Namba S. (2008b). Phytoplasmas: bacteria that manipulate plants and insects. Mol Plant Pathol 9 403–423. 10.1111/j.1364-3703.2008.00472.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang J. X., Bishop-Hurley S. L., Cooper M. A. (2012). Development of anti-infectives using phage display: biological agents against bacteria, viruses, and parasites. Antimicrob. Agents Chemother. 56 4569–4582. 10.1128/aac.00567-12 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibanez F., Levy J., Tamborindeguy C. (2014). Transcriptome analysis of “Candidatus Liberibacter solanacearum” in its psyllid vector, Bactericera cockerelli. PLoS ONE 9:e100955 10.1371/journal.pone.0100955 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jagoueix S., Bove J. M., Garnier M. (1994). The phloem-limited bacterium of greening disease of citrus is a member of the alpha subdivision of the Proteobacteria. Int. J. Syst. Bacteriol. 44 379–386. 10.1099/00207713-44-3-379 [DOI] [PubMed] [Google Scholar]
- Jain M., Fleites L. A., Gabriel D. W. (2015). Prophage-encoded peroxidase in ‘Candidatus Liberibacter asiaticus’ is a secreted effector that suppresses plant defenses. Mol. Plant Microbe Interact. 28 1330–1337. 10.1094/MPMI-07-15-0145-R [DOI] [PubMed] [Google Scholar]
- Kakizawa S., Oshima K., Namba S. (2006). Diversity and functional importance of phytoplasma membrane proteins. Trends Microbiol. 14 254–256. 10.1016/j.tim.2006.04.008 [DOI] [PubMed] [Google Scholar]
- Killiny N., Rashed A., Almeida R. P. (2012). Disrupting the transmission of a vector-borne plant pathogen. Appl. Environ. Microbiol. 78 638–643. 10.1128/aem.06996-11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim E. B., Piao da C., Son J. S., Choi Y. J. (2009). Cloning and characterization of a novel tuf promoter from Lactococcus lactis subsp. lactis IL1403. Curr. Microbiol. 59 425–431. 10.1007/s00284-009-9455-2 [DOI] [PubMed] [Google Scholar]
- Kube M., Mitrovic J., Duduk B., Rabus R., Seemuller E. (2012). Current view on phytoplasma genomes and encoded metabolism. Sci. World J. 2012:185942 10.1100/2012/185942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kube M., Schneider B., Kuhl H., Dandekar T., Heitmann K., Migdoll A. M., et al. (2008). The linear chromosome of the plant-pathogenic mycoplasma ‘Candidatus Phytoplasma mali’. BMC Genomics 9:306 10.1186/1471-2164-9-306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kube M., Siewert C., Migdoll A. M., Duduk B., Holz S., Rabus R., et al. (2014). Analysis of the complete genomes of Acholeplasma brassicae, A. palmae and A. laidlawii and their comparison to the obligate parasites from ‘Candidatus Phytoplasma’. J. Mol. Microbiol. Biotechnol. 24 19–36. 10.1159/000354322 [DOI] [PubMed] [Google Scholar]
- Labroussaa F., Arricau-Bouvery N., Dubrana M. P., Saillard C. (2010). Entry of Spiroplasma citri into Circulifer haematoceps cells involves interaction between spiroplasma phosphoglycerate kinase and leafhopper actin. Appl. Environ. Microbiol. 76 1879–1886. 10.1128/aem.02384-09 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legeai F., Shigenobu S., Gauthier J. P., Colbourne J., Rispe C., Collin O., et al. (2010). AphidBase: a centralized bioinformatic resource for annotation of the pea aphid genome. Insect Mol. Biol. 19(Suppl. 2) 5–12. 10.1111/j.1365-2583.2009.00930.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leshkowitz D., Gazit S., Reuveni E., Ghanim M., Czosnek H., Mckenzie C., et al. (2006). Whitefly (Bemisia tabaci) genome project: analysis of sequenced clones from egg, instar, and adult (viruliferous and non-viruliferous) cDNA libraries. BMC Genomics 7:79 10.1186/1471-2164-7-79 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J., Wang X. P., Wang M. Q., Ma W. H., Hua H. X. (2013). Advances in the use of the RNA interference technique in Hemiptera. Insect Sci. 20 31–39. 10.1111/j.1744-7917.2012.01550.x [DOI] [PubMed] [Google Scholar]
- Lin H., Coletta-Filho H. D., Han C. S., Lou B., Civerolo E. L., Machado M. A., et al. (2013). Draft genome sequence of “Candidatus Liberibacter americanus” bacterium associated with citrus huanglongbing in Brazil. Genome Announc. 1:e275-13. 10.1128/genomeA.00275-13 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin H., Lou B., Glynn J. M., Doddapaneni H., Civerolo E. L., Chen C., et al. (2011). The complete genome sequence of ‘Candidatus Liberibacter solanacearum’, the bacterium associated with potato zebra chip disease. PLoS ONE 6:e19135 10.1371/journal.pone.0019135 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lough T. J., Lucas W. J. (2006). Integrative plant biology: role of phloem long-distance macromolecular trafficking. Annu. Rev. Plant Biol. 57:203–232. 10.1146/annurev.arplant.56.032604.144145 [DOI] [PubMed] [Google Scholar]
- Lu Y. T., Cheng K. T., Jiang S. Y., Yang J. Y. (2014a). Post-translational cleavage and self-interaction of the phytoplasma effector SAP11. Plant Signal. Behav. 9 10.1371/journal.pone.0094391 [Epub ahead of print]. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu Y. T., Li M. Y., Cheng K. T., Tan C. M., Su L. W., Lin W. Y., et al. (2014b). Transgenic plants that express the phytoplasma effector SAP11 show altered phosphate starvation and defense responses. Plant Physiol. 164 1456–1469. 10.1111/nph.12721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas W. J. (2006). Plant viral movement proteins: agents for cell-to-cell trafficking of viral genomes. Virology 344 169–184. 10.1016/j.virol.2005.09.026 [DOI] [PubMed] [Google Scholar]
- MacLean A. M., Orlovskis Z., Kowitwanich K., Zdziarska A. M., Angenent G. C., Immink R. G., et al. (2014). Phytoplasma effector SAP54 hijacks plant reproduction by degrading MADS-box proteins and promotes insect colonization in a RAD23-dependent manner. PLoS Biol. 12:e1001835 10.1371/journal.pbio.1001835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacLean A. M., Sugio A., Makarova O. V., Findlay K. C., Grieve V. M., Toth R., et al. (2011). Phytoplasma effector SAP54 induces indeterminate leaf-like flower development in Arabidopsis plants. Plant Physiol. 157 831–841. 10.4161/mge.26145 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maejima K., Iwai R., Himeno M., Komatsu K., Kitazawa Y., Fujita N., et al. (2014). Recognition of floral homeotic MADS domain transcription factors by a phytoplasmal effector, phyllogen, induces phyllody. Plant J. 78 541–554. 10.1111/tpj.12495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mafra V., Martins P. K., Francisco C. S., Ribeiro-Alves M., Freitas-Astua J., Machado M. A. (2013). Candidatus Liberibacter americanus induces significant reprogramming of the transcriptome of the susceptible citrus genotype. BMC Genomics 14:247 10.1186/1471-2164-14-247 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Malmstrom C. M., Melcher U., Bosque-Perez N. A. (2011). The expanding field of plant virus ecology: historical foundations, knowledge gaps, and research directions. Virus Res. 159 84–94. 10.1016/j.virusres.2011.05.010 [DOI] [PubMed] [Google Scholar]
- Minato N., Himeno M., Hoshi A., Maejima K., Komatsu K., Takebayashi Y., et al. (2014). The phytoplasma virulence factor TENGU causes plant sterility by downregulating of the jasmonic acid and auxin pathways. Sci. Rep. 4:7399 10.1038/srep07399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mitchel P. L. (2004). Heteroptera as vectors of plant pathogens. Neotrop. Entomol. 33 519–545. [Google Scholar]
- Mutaqin K., Comer J. L., Wayadande A. C., Melcher U., Fletcher J. (2011). Selection and characterization of Spiroplasma citri mutants by random transposome mutagenesis. Can. J. Microbiol. 57 525–532. 10.1139/w11-026 [DOI] [PubMed] [Google Scholar]
- Nadarasah G., Stavrinides J. (2011). Insects as alternative hosts for phytopathogenic bacteria. FEMS Microbiol. Rev. 35 555–575. 10.1111/j.1574-6976.2011.00264.x [DOI] [PubMed] [Google Scholar]
- Nandety R. S., Kuo Y. W., Nouri S., Falk B. W. (2015). Emerging strategies for RNA interference (RNAi) applications in insects. Bioengineered 6 8–19. 10.4161/21655979.2014.979701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nascimento R., Gouran H., Chakraborty S., Gillespie H. W., Almeida-Souza H. O., Tu A., et al. (2016). The type II secreted lipase/esterase LesA is a key virulence factor required for Xylella fastidiosa pathogenesis in grapevines. Sci. Rep. 6:18598 10.1038/srep18598 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nault L. R., Ammar El-D. (1989). Leafhopper and planthopper transmission of plant viruses. Annu. Rev. Entomol. 34 503–529. 10.1146/annurev.en.34.010189.002443 [DOI] [Google Scholar]
- Neriya Y., Maejima K., Nijo T., Tomomitsu T., Yusa A., Himeno M., et al. (2014). Onion yellow phytoplasma P38 protein plays a role in adhesion to the hosts. FEMS Microbiol. Lett. 361:115–122. 10.1111/1574-6968.12620 [DOI] [PubMed] [Google Scholar]
- Ng J. C., Zhou J. S. (2015). Insect vector-plant virus interactions associated with non-circulative, semi-persistent transmission: current perspectives and future challenges. Curr. Opin. Virol. 15 48–55. 10.1016/j.coviro.2015.07.006 [DOI] [PubMed] [Google Scholar]
- Ng J. C. K., Falk B. W. (2006). Virus-vector interactions mediating nonpersistent and semipersistent transmission of plant viruses. Annu. Rev. Phytopathol. 44 183–212. 10.1146/annurev.phyto.44.070505.143325 [DOI] [PubMed] [Google Scholar]
- Noda H., Kawai S., Koizumi Y., Matsui K., Zhang Q., Furukawa S., et al. (2008). Annotated ESTs from various tissues of the brown planthopper Nilaparvata lugens: a genomic resource for studying agricultural pests. BMC Genomics 9:117 10.1186/1471-2164-9-117 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouri S., Salem N., Nigg J. C., Falk B. W. (2015). Diverse array of new viral sequences identified in worldwide populations of the Asian citrus psyllid (Diaphorina citri) using viral metagenomics. J. Virol. 90 2434–2445. 10.1128/jvi.02793-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Orlovskis Z., Canale M. C., Thole V., Pecher P., Lopes J. R. S., Hogenhout S. A. (2015). Insect-borne plant pathogenic bacteria: getting a ride goes beyond physical contact. Curr. Opin. Insect Sci. 9 16–23. 10.1016/j.cois.2015.04.007 [DOI] [PubMed] [Google Scholar]
- PaiãO F. G., Meneguim A. M., Casagrande E. C., Leite Junior R. P. (1996). Envolvimento de cigarras (Homoptera, Cicadidae) na transmissaão de Xylella fastidiosa em cafeeiro. Fitopatol. Bras. 27:67. [Google Scholar]
- Pitino M., Hoffman M. T., Zhou L., Hall D. G., Stocks I. C., Duan Y. (2014). The phloem-sap feeding mealybug (Ferrisia virgata) carries ‘Candidatus Liberibacter asiaticus’ populaions that do not cause disease in host plants. PLoS ONE 9:e85503 10.1371/journal.pone.0085503 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poelchau M., Childers C., Moore G., Tsavatapalli V., Evans J., Lee C. Y., et al. (2015). The i5k Workspace@NAL–enabling genomic data access, visualization and curation of arthropod genomes. Nucleic Acids Res. 43 D714–D719. 10.1093/nar/gku983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell A. (2013). Paradigms: examples from the bacterium Xylella fastidiosa. Annu. Rev. Phytopathol. 51 339–356. 10.1146/annurev-phyto-082712-102325 [DOI] [PubMed] [Google Scholar]
- Purcell A. H. (1982). Insect vector relationships with prokaryotic plant pathogens. Annu. Rev. Phytopathol. 20 397–417. 10.1146/annurev.py.20.090182.002145 [DOI] [Google Scholar]
- Purcell A. H., Hopkins D. L. (1996). Fastidious xylem-limited bacterial plant pathogens. Annu. Rev. Phytopathol. 34 131–151. 10.1146/annurev.phyto.34.1.131 [DOI] [PubMed] [Google Scholar]
- Ramsey J. S., Wilson A. C., De Vos M., Sun Q., Tamborindeguy C., Winfield A., et al. (2007). Genomic resources for Myzus persicae: EST sequencing, SNP identification, and microarray design. BMC Genomics 8:423 10.1186/1471-2164-8-423 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapicavoli J. N., Kinsinger N., Perring T. M., Backus E. A., Shugart H. J., Walker S., et al. (2015). O-antigen modulates insect vector acquisition of the bacterial plant pathogen Xylella fastidiosa. Appl. Environ. Microbiol. 81 8145–8154. 10.1128/aem.02383-15 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rashed A., Wallis C. M., Paetzold L., Workneh F., Rush C. M. (2013). Zebra chip disease and potato biochemistry: tuber physiological changes in response to ‘Candidatus Liberibacter solanacearum’ infection over time. Phytopathology 103 419–426. 10.1094/PHYTO-09-12-0244-R [DOI] [PubMed] [Google Scholar]
- Rashidi M., Galetto L., Bosco D., Bulgarelli A., Vallino M., Veratti F., et al. (2015). Role of the major antigenic membrane protein in phytoplasma transmission by two insect vector species. BMC Microbiol. 15:193 10.1186/s12866-015-0522-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redak R. A., Purcell A. H., Lopes J. R., Blua M. J., Mizell R. F., III, Andersen P. C. (2004). The biology of xylem fluid-feeding insect vectors of Xylella fastidiosa and their relation to disease epidemiology. Annu. Rev. Entomol. 49 243–270. 10.1146/annurev.ento.49.061802.123403 [DOI] [PubMed] [Google Scholar]
- Renaudin J., Beven L., Batailler B., Duret S., Desque D., Arricau-Bouvery N., et al. (2015). Heterologous expression and processing of the flavescence doree phytoplasma variable membrane protein VmpA in Spiroplasma citri. BMC Microbiol. 15:82 10.1186/s12866-015-0417-5 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riaz S., Tenscher A. C., Rubin J., Graziani R., Pao S. S., Walker M. A. (2008). Fine-scale genetic mapping of two Pierce’s disease resistance loci and a major segregation distortion region on chromosome 14 of grape. Theor. Appl. Genet. 117 671–681. 10.1007/s00122-008-0802-7 [DOI] [PubMed] [Google Scholar]
- Roper M. C., Greve C., Warren J. G., Labavitch J. M., Kirkpatrick B. C. (2007). Xylella fastidiosa requires polygalacturonase for colonization and pathogenicity in Vitis vinifera grapevines. Mol. Plant Microbe Interact. 20 411–419. 10.1094/MPMI-20-4-0411 [DOI] [PubMed] [Google Scholar]
- Rutherford K., Parkhill J., Crook J., Horsnell T., Rice P., Radjandream M. A., et al. (2000). Artemis: sequence visualization and annotation. Bioinformatics 16 944–945. 10.1093/bioinformatics/16.10.944 [DOI] [PubMed] [Google Scholar]
- Saponari M., Loconsole G., Cornara D., Yokomi R. K., De Stradis A., Boscia D., et al. (2014). Infectivity and transmission of Xylella fastidiosa by Philaenus spumarius (Hemiptera: Aphrophoridae) in Apulia, Italy. J. Econ. Entomol. 107 1316–1319. 10.1603/EC14142 [DOI] [PubMed] [Google Scholar]
- Scorza R., Callahan A., Dardick C., Ravelonandro M., Polak J., Malinowski T., et al. (2013). Genetic engineering of Plum pox virus resistance: HoneySweet plum - from concept to product. Plant Cell Tissue Organ Cult. 115 1–12. 10.1007/s11240-013-0339-6 [DOI] [Google Scholar]
- Seemuller E., Schneider B. (2004). ‘Candidatus Phytoplasma mali’, ‘Candidatus Phytoplasma pyri’ and ‘Candidatus Phytoplasma prunorum’, the causal agents of apple proliferation, pear decline and European stone fruit yellows, respectively. Int. J. Syst. Evol. Microbiol. 54(pt 4) 1217–1226. 10.1099/ijs.0.02823-0 [DOI] [PubMed] [Google Scholar]
- Seemüller E., Sule S., Kube M., Jelkmann W., Schneider B. (2013). The AAA+ ATPases and HflB/FtsH proteases of ‘Candidatus phytoplasma mali’: phylogenetic diversity, membrane topology, and relationship to strain virulence. Mol. Plant Microbe Interact. 26 367–376. 10.1094/MPMI-09-12-0221-R [DOI] [PubMed] [Google Scholar]
- Shaw M. E., Kirkpatrick B. C., Golino D. (1993). The beet leafhopper-transmitted virescence agent causes tomato big bud disease in California. Plant Dis. 77:6 10.1094/PD-77-0290 [DOI] [Google Scholar]
- Siewert C., Luge T., Duduk B., Seemüller E., Büttner C., Sauer S., et al. (2014). Analysis of expressed genes of the bacterium ‘Candidatus Phytoplasma mali’ highlights key features of virulence and metabolism. PLoS ONE 9:e94391 10.1371/journal.pone.0094391 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simpson A. J. G., Reinach F. C., Arruda P., Abreu F. A., Acencio M., Alvarenga R., et al. (2000). The Genome sequence of the plant pathogen Xylella fastidiosa. Nature 406 151–157. [DOI] [PubMed] [Google Scholar]
- Sinkins S. P., Gould F. (2006). Gene drive systems for insect disease vectors. Nat. Rev. Genet. 7 427–435. 10.1038/nrg1870 [DOI] [PubMed] [Google Scholar]
- Soto M. J., Gilbertson R. L. (2003). Distribution and rate of movement of the Curtovirus Beet mild curly top virus (Family Geminiviridae) in the beet leafhopper. Phytopathology 93:7 10.1094/PHYTO.2003.93.4.478 [DOI] [PubMed] [Google Scholar]
- Sugio A., Kingdom H. N., Maclean A. M., Grieve V. M., Hogenhout S. A. (2011). Phytoplasma protein effector SAP11 enhances insect vector reproduction by manipulating plant development and defense hormone biosynthesis. Proc. Natl. Acad. Sci. U.S.A. 108 E1254–E1263. 10.1073/pnas.1105664108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugio A., Maclean A. M., Hogenhout S. A. (2014). The small phytoplasma virulence effector SAP11 contains distinct domains required for nuclear targeting and CIN-TCP binding and destabilization. New Phytol. 202 838–848. 10.1111/nph.12721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teresani G. R., Bertolini E., Alfaro-Fernandez A., Martinez C., Tanaka F. A., Kitajima E. W., et al. (2014). Association of ‘Candidatus Liberibacter solanacearum’ with a vegetative disorder of celery in Spain and development of a real-time PCR method for its detection. Phytopathology 104 804–811. 10.1094/PHYTO-07-13-0182-R [DOI] [PubMed] [Google Scholar]
- Thebaud G., Yvon M., Alary R., Sauvion N., Labonne G. (2009). Efficient transmission of ‘Candidatus Phytoplasma prunorum’ is delayed by eight months due to a long latency in its host-alternating vector. Phytopathology 99 265–273. 10.1094/phyto-99-3-0265 [DOI] [PubMed] [Google Scholar]
- Thompson S. M., Johnson C. P., Lu A. Y., Frampton R. A., Sullivan K. L., Fiers M. W., et al. (2015). Genomes of ‘Candidatus Liberibacter solanacearum’ Haplotype A from New Zealand and the United States suggest significant genome plasticity in the species. Phytopathology 105 863–871. 10.1094/PHYTO-12-14-0363-FI [DOI] [PubMed] [Google Scholar]
- Tricoll D., Carney K. J., Russel P. F., Mcmaster J. R., Groff D. W., Hadden K. C., et al. (1995). Field evaluation of transgenic squash containing single or multiple virus coat protein gene constructs for resistance to cucumber mosaic virus, watermelon mosaic virus 2, and zucchini yellow mosaic virus. Nat. Biotech. 13 1458–1465. 10.1038/nbt1295-1458 [DOI] [Google Scholar]
- Tsai C.-W., Rowhani A., Golino D., Daane K. M., Almeida R. P. (2010). Mealybug transmission of Grapevine Leafroll Viruses: an analysis of virus–vector specificity. Phytopathology 100:830–834. 10.1094/PHYTO-100-8-0830 [DOI] [PubMed] [Google Scholar]
- Upadhyay S. K., Sharma S., Singh H., Dixit S., Kumar J., Verma P. C., et al. (2015). Whitefly genome expression reveals host-symbiont interaction in amino acid biosynthesis. PLoS ONE 10:e0126751 10.1371/journal.pone.0126751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uzest M., Gargani D., Drucker M., Hebrard E., Garzo E., Candresse T., et al. (2007). A protein key to plant virus transmission at the tip of the insect vector stylet. Proc. Natl. Acad. Sci. U.S.A. 104 17959–17964. 10.1073/pnas.0706608104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling L. L. (2008). Avoiding effective defenses: strategies employed by phloem-feeding insects. Plant Physiol. 146 859–866. 10.1104/pp.107.113142 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walling L. L. (2009). Adaptive defense responses to pathogens and pests. Adv. Bot. Res. 51 551–612. 10.1371/journal.pone.0024867 [DOI] [Google Scholar]
- Wang Q., Guo Y., Wang N., Li Y., Chen W., Chen W., et al. (2014). Identification of a conserved core genome with group-specific genes from comparative genomics of ten different Candidatus Phytoplasma strains. J. Phytopathol. 162 650–659. 10.1111/jph.12239 [DOI] [Google Scholar]
- Wayadande A. C., Fletcher J. (1998). Development and use of an established cell line of the leafhopper Circulifer tenellus to characterize Spiroplasma citri-vector interactions. J. Invertebr. Pathol. 72 126–131. 10.1006/jipa.1998.4753 [DOI] [PubMed] [Google Scholar]
- Webster R. K., Esser T., Kirkpatrick B. C., Bruening G. (2014). Cooperative efforts contained spread of Pierce’s disease and found genetic resistance. Calif. Agric. 68 134–141. 10.3733/ca.v068n04p134 [DOI] [Google Scholar]
- Weintraub P. G., Beanland L. (2006). Insect vectors of phytoplasmas. Annu. Rev. Entomol. 51 91–111. 10.1146/annurev.ento.51.110104.151039 [DOI] [PubMed] [Google Scholar]
- Westermann A. J., Gorski S. A., Vogel J. (2012). Dual RNA-seq of pathogen and host. Nat. Rev. Microbiol. 10 618–630. 10.1038/nrmicro2852 [DOI] [PubMed] [Google Scholar]
- Whitfield A. E., Falk B. W., Rotenberg D. (2015). Insect vector-mediated transmission of plant viruses. Virology 47 278–289. 10.1016/j.virol.2015.03.026 [DOI] [PubMed] [Google Scholar]
- Will T., Furch A. C., Zimmermann M. R. (2013). How phloem-feeding insects face the challenge of phloem-located defenses. Front. Plant Sci. 4:336 10.3389/fpls.2013.00336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wuriyanghan H., Rosa C., Falk B. W. (2011). Oral delivery of double-stranded RNAs and siRNAs induces RNAi effects in the potato/tomato psyllid, Bactericera cockerelli. PLoS ONE 6:e27736 10.1371/journal.pone.0027736 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan Q., Sreedharan A., Wei S., Wang J., Pelz-Stelinski K., Folimonova S., et al. (2013). Global gene expression changes in Candidatus Liberibacter asiaticus during the transmission in distinct hosts between plant and insect. Mol. Plant Pathol. 14 391–404. 10.1111/mpp.12015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang C. Y., Huang Y. H., Lin C. P. (2015). MicroRNA396-Targeted SHORT VEGETATIVE PHASE is required to repress flowering and is related to the development of abnormal flower symptoms by the phyllody symptoms1 effector. Plant Physiol. 168 1702–1716. 10.1104/pp.15.00307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yao J., Saenkham P., Levy J., Ibanez F., Noroy C., Mendoza A., et al. (2016). Interactions “Candidatus Liberibacter solanacearum” – Bactericera cockerelli: haplotype effect of vector fitness and gene expression analyses. Front. Cell. Infect. Microbiol. 6:62 10.3389/fcimb.2016.00062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye F., Melcher U., Fletcher J. (1997). Molecular characterization of a gene encoding a membrane protein of Spiroplasma citri. Gene 189 95–100. 10.1016/S0378-1119(96)00840-2 [DOI] [PubMed] [Google Scholar]
- Yin C., Shen G., Guo D., Wang S., Ma X., Xiao H., et al. (2016). InsectBase: a resource for insect genomes and transcriptomes. Nucleic Acids Res. 44 D801–D807. 10.1093/nar/gkv1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu N. Y., Wagner J. R., Laird M. R., Melli G., Rey S., Lo R., et al. (2010). PSORTb 3.0: improved protein subcellular localization prediction with refined localization subcategories and predictive capabilities for all prokaryotes. Bioinformatics 26:1608–1615. 10.1093/bioinformatics/btq249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu X. D., Liu Z. C., Huang S. L., Chen Z. Q., Sun Y. W., Duan P. F., et al. (2016). RNAi-mediated plant protection against aphids. Pest. Manag. Sci. 72 1090–1098. 10.1002/ps.4258 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


