Abstract
A better understanding of the metabolic and diffusional limitations of photosynthesis in fluctuating irradiance can help identify targets for improving crop yields. We used different genotypes of Arabidopsis thaliana to characterise the importance of Rubisco activase (Rca), stomatal conductance (gs), non-photochemical quenching of chlorophyll fluorescence (NPQ) and sucrose phosphate synthase (SPS) on photosynthesis in fluctuating irradiance. Leaf gas exchange and chlorophyll fluorescence were measured in leaves exposed to stepwise increases and decreases in irradiance. rwt43, which has a constitutively active Rubisco enzyme in different irradiance intensities (except in darkness), showed faster increases than the wildtype, Colombia-0, in photosynthesis rates after step increases in irradiance. rca-2, having decreased Rca concentration, showed lower rates of increase. In aba2-1, high gs increased the rate of change after stepwise irradiance increases, while in C24, low gs tended to decrease it. Differences in rates of change between Colombia-0 and plants with low levels of NPQ (npq1-2, npq4-1) or SPS (spsa1) were negligible. In Colombia-0, the regulation of Rubisco activation and of gs were therefore limiting for photosynthesis in fluctuating irradiance, while levels of NPQ or SPS were not. This suggests Rca and gs as targets for improvement of photosynthesis of plants in fluctuating irradiance.
In physiological research, plants are often studied under constant environmental conditions. However, plants grow in a variable environment, with changes occurring in the time range of seconds or less1. Of the factors important for net photosynthesis (An), irradiance changes most quickly2, causing a lag between changes in irradiance and changes in An, due to the slower regulation of photosynthesis3. This lag decreases light-use efficiency relative to the steady state and transiently increases excess irradiance, possibly harming the photosynthetic apparatus4. Leaves engage various mechanisms in response to fluctuating irradiance. Among the best known mechanisms are the regulation of enzymes of carbon fixation and sucrose metabolism, non-photochemical energy dissipation and stomatal conductance (gs3,5). Although difficult to measure, cyclic electron transport may be another important mechanism (recently reviewed by Yamori and Shikanai6), due to a potential regulatory role and the balance of ATP versus NADPH production. During induction of photosynthesis in leaves adapted to darkness or low irradiance, the slow regeneration of ribulose-1,5-bisphosphate (RuBP) is typically most limiting until 60 seconds after illumination7. Thereafter, both the slow carboxylation due to partially inactive Rubisco (time to full activation: ~10 minutes) and slow stomatal opening (10–60 minutes) can limit the rate at which photosynthesis increases8. Thus, the slow rate of change of these mechanisms results in the lag between changes in irradiance and An and the resulting reduction of plant productivity9. Reductions in assimilation due to these physiological limitations can be up to 35% per day (subject to light environment and genotype10), and understanding them better may pave the road towards higher yields11,12.
Our understanding of the metabolic constraints of photosynthesis in fluctuating irradiance (hereafter: ‘dynamic photosynthesis’) have mainly come from biochemical studies7,13,14, with less use being made of genetic diversity. Naturally occurring ecotypes, mutations, cultivars and genetically modified accessions offer a range of genotypes with specific properties, that could be used to study dynamic photosynthesis5. Arabidopsis thaliana possesses a wide, well documented genotypic diversity, which has been extended by selecting for mutations and by transgenic modifications.
Rubisco catalyses CO2 assimilation and its activation limits An after irradiance increases13,15. In the chloroplast stroma, several inhibitory compounds are present and bind to Rubisco. To maintain sufficient Rubisco activity, these inhibitors must be removed from the active sites by the ATPase Rubisco activase (Rca16). In Arabidopsis thaliana, there are two isoforms of Rca, the larger α-isoform and the smaller β-isoform17. In plants containing both isoforms, redox-regulation of the α-isoform affects the ADP sensitivity of the holoenzyme (composed of both isoforms18,19). In low irradiance (i.e. high ADP/ATP ratio), the α-isoform is less active and the rate of overall Rubisco activation is low. Since Rca is a central regulator of Rubisco activity, how these isoforms, or their concentration affect dynamic photosynthesis is an important yet unresolved question.
After CO2 assimilation by Rubisco, a fraction of the triose phosphates leaves the chloroplast in exchange for orthophosphate (Pi) from the cytosol. In the cytosol, triose phosphate is converted to sucrose, and sucrose phosphate synthase (SPS) plays a central role in this pathway20. In certain circumstances, such as photosynthetic induction in saturating CO2, irradiance-dependent activation of SPS can be slower than that of Calvin cycle enzymes, making the Calvin cycle transiently Pi-limited14. Furthermore, after irradiance decreases, an overshoot in sucrose synthesis can transiently drain metabolites from the Calvin cycle, transiently decreasing An21. Plants with reduced SPS concentration may therefore exhibit slower increases in An after irradiance increases, and a smaller transient dip in An after irradiance decreases.
Leaves protect themselves from absorbed irradiance that is in excess of the capacity of photochemistry using non-photochemical quenching (NPQ). This protection, however, may come at a price. Sustained high levels of NPQ after irradiance decreases may result in transient limitations of the quantum efficiency of photosystem II for electron transport (ϕPSII). Model calculations indicate that slow relaxation of NPQ could decrease canopy photosynthesis by ~13–24%22. NPQ has been shown to limit An in genotypes with faster NPQ buildup after irradiance increases23 or slower NPQ relaxation after irradiance decreases24. Thus, genotypes with constitutively low NPQ may have increased dynamic photosynthesis rates, principally as a result of less limitation on An following a decrease in irradiance.
In many plants, stomata open when irradiance increases. Typically, stomatal opening is slow, transiently limiting An during the irradiance increase25. Genotypes with constitutively high gs may not experience this limitation26, and may therefore be more productive in environments with a high proportion of fluctuating irradiance, provided that water is not limiting.
We used several genotypes, i.e. plants containing point mutations, transformants, T-DNA insertion lines (SALK lines27) and naturally occurring accessions of A. thaliana, to analyse how metabolic (Rubisco activation, sucrose synthesis, NPQ) and diffusional (gs) limitations affect dynamic photosynthesis. In addition to measuring their steady-state photosynthetic irradiance and CO2 responses, we exposed these genotypes to stepwise increases and decreases in irradiance, while measuring gas exchange and chlorophyll fluorescence. To investigate the effects of Rca regulatory properties or concentrations, we used the transformant rwt43 (lacks the α-isoform of Rca and is therefore ADP-insensitive19) and the mutant rca-2, which is due to a leaky allele mutation (decreased Rca concentration28). To analyze the effect of SPS, we studied the T-DNA mutant line spsa1 (80% reduction in maximum SPS activity29). The effect of low NPQ was investigated by using npq4-1 (lacks PsbS, greatly diminishing NPQ30) and npq1-2 (lacks zeaxanthin deepoxidase and therefore violaxanthin, diminishing NPQ31). Effects of high and low gs were analyzed by using aba2-1 (impaired abscisic acid (ABA) synthesis, leading to constitutively high gs32) and the natural accession C24 (low gs33), respectively. The accession Col-0 is the wildtype background to all mutants and transformants used in this study and acts as a control line. This study indicates that wildtype isoform composition and amount of Rca, as well as gs limit dynamic photosynthesis in A. thaliana, while wildtype levels of SPS and NPQ do not.
Results
Steady-state responses to irradiance and CO2 confirm genotypic effects on Rubisco activation state, sugar metabolism and stomatal conductance
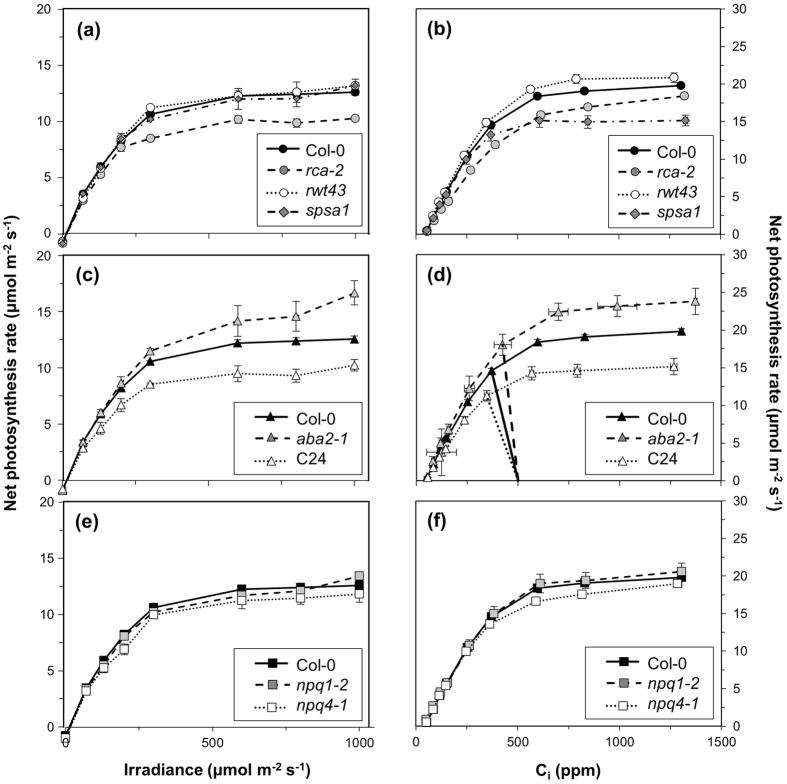
To characterize the steady-state behaviour of the different A. thaliana genotypes we measured their responses to irradiance and leaf internal CO2 concentration (Ci). Rates of An in Col-0 were comparable to studies using plants grown under similar conditions34,35,36,37. In the mutant containing less Rca, rca-2, irradiance-saturated An was lower than for Col-0, and saturation occurred around 600 μmol m−2 s−1 (Fig. 1a). The lower Ci response on An in rca-2 (Fig. 1b) resulted in significantly decreased maximum carboxylation rate by Rubisco (Vcmax; −23%), maximum rate of electron transport (Jmax; −14%) and maximum rate of triose phosphate utilisation (TPU; −7%) compared to Col-0 (Table 1). Assimilation in the transformant lacking the α-isoform of Rca, rwt43, exhibited similar irradiance and Ci responses as in Col-0 (Fig. 1). In the mutant with less SPS (spsa1), An did not differ from Col-0 in its irradiance response (Fig. 1a), but was strongly reduced at high Ci (Fig. 1b), resulting in decreased Jmax (−14%) and TPU (−23%). The ABA-deficient mutant, aba2-1, showed larger irradiance- and CO2-saturated photosynthesis rates compared to Col-0, while the accession C24 showed the opposite (Fig. 1c,d). Some parameters derived from Ci response curves were therefore larger in aba2-1 (Jmax: +18%, TPU: +19%), while they were smaller in C24 (Vcmax: −17%, Jmax: −20%, TPU: −22%). The supply lines38 (Fig. 1d) emphasize differences in gs between C24, Col-0 and aba2-1: the steeper the slope, the smaller the difference between external CO2 concentration (Ca) and Ci, and the larger gs. Irradiance and Ci responses of photosynthesis of low-NPQ mutants (npq1-2, npq4-1) were similar to Col-0 (Fig. 1e,f), except for lower Jmax in npq4-1 (−7%). The response of quantum yield of photosystem II (ϕPSII) to Ci largely paralleled that of An, with the exception that ϕPSII decreased at high Ci in many genotypes (except rca-2 and npq4-1; see Supplementary Fig. 1). This decrease in ϕPSII was most marked, and started at a lower Ci, in spsa1 (Supplementary Fig. 1a).
Figure 1.
Irradiance and CO2 response of net photosynthesis rates in rca-2, rwt43 and spsa1 (a,b), aba2-1 and C24 (c,d) and npq1-2 and npq4-1 (e,f). Col-0 is included in each panel for ease of comparison. In (d), supply lines38 between Ca = 500 and the corresponding Ci response curve of An are shown to emphasize stomatal effects of aba2-1, C24 and Col-0 on Ci. Averages ± SEM, n = 5–15.
Table 1. Parameters derived from Ci response curves of An.
| Vcmax | Jmax | TPU | RMSE | |
|---|---|---|---|---|
| Col-0 | 53 ± 1 | 100 ± 2 | 7.1 ± 0.1 | 0.93 ± 0.04 |
| rca-2 | 40 ± 1*** | 86 ± 2*** | 6.7 ± 0.1 n.s. | 0.95 ± 0.11 n.s. |
| rwt43 | 57 ± 3 n.s. | 105 ± 5 n.s. | 7.5 ± 0.2 n.s. | 0.98 ± 0.07 n.s. |
| spsa1 | 54 ± 4 n.s. | 86 ± 5** | 5.5 ± 0.3*** | 0.85 ± 0.06 n.s. |
| aba2-1 | 58 ± 3 n.s. | 118 ± 6*** | 8.5 ± 0.6** | 1.12 ± 0.11 n.s. |
| C24 | 44 ± 2** | 79 ± 4*** | 5.5 ± 0.4*** | 0.76 ± 0.07* |
| npq1-2 | 52 ± 3 n.s. | 101 ± 5 n.s. | 7.4 ± 0.4 n.s. | 0.95 ± 0.08 n.s. |
| npq4-1 | 53 ± 1 n.s. | 92 ± 2* | 6.8 ± 0.2 n.s. | 0.98 ± 0.03 n.s. |
Vcmax, maximum caboxylation rate by Rubisco (μmol CO2 m−2 s−1); Jmax, maximum rate of electron transport in the absence of regulation (μmol electrons m−2 s−1); TPU, maximum rate of triose phosphate utilisation (μmol CO2 m−2 s−1). The root mean squared error (RMSE, μmol CO2 m−2 s−1) of the differences between measurement and model during curve fitting55 is shown as an estimation of the overall goodness of fit. Averages ± SEM, n = 5–15. Stars within columns denote significance levels compared to Col-0: ***P < 0.0001, **P < 0.01, *P < 0.05. Absence of stars denotes lack of significant difference with Col-0 (P > 0.05).
Larger Rubisco activation state and gs accelerate photosynthetic induction, while lower NPQ does not
Next, we characterised the dynamic behaviour of leaf gas exchange by inducing photosynthesis in dark-adapted leaves using a stepwise increase to saturating irradiance (1000 μmol m−2 s−1). Rates of photosynthetic induction were initially similar between all genotypes (except rwt43) until ~60% induction was reached (Fig. 2). rwt43 reached 50% of photosynthetic induction (tA50) significantly faster than Col-0 (Table 2). Induction remained faster in rwt43 until it reached ~80% (Fig. 2a). In rca-2, the rate of induction slowed after 60% completion and then increased in a nearly linear fashion rather than the more exponential increase shown by all other genotypes (Fig. 2a). This increased the time to reach 90% of photosynthetic induction (tA90) by ~10 minutes compared to Col-0. spsa1 showed slightly slower induction rates (Fig. 2a), increasing tA90 by ~5 min compared to Col-0. aba2-1 exhibited faster induction, halving the tA90 of Col-0, while induction in C24 was identical to that of Col-0 (Fig. 2b). Induction in npq1-2 and npq4-1 was identical to Col-0 (Fig. 2c).
Figure 2.
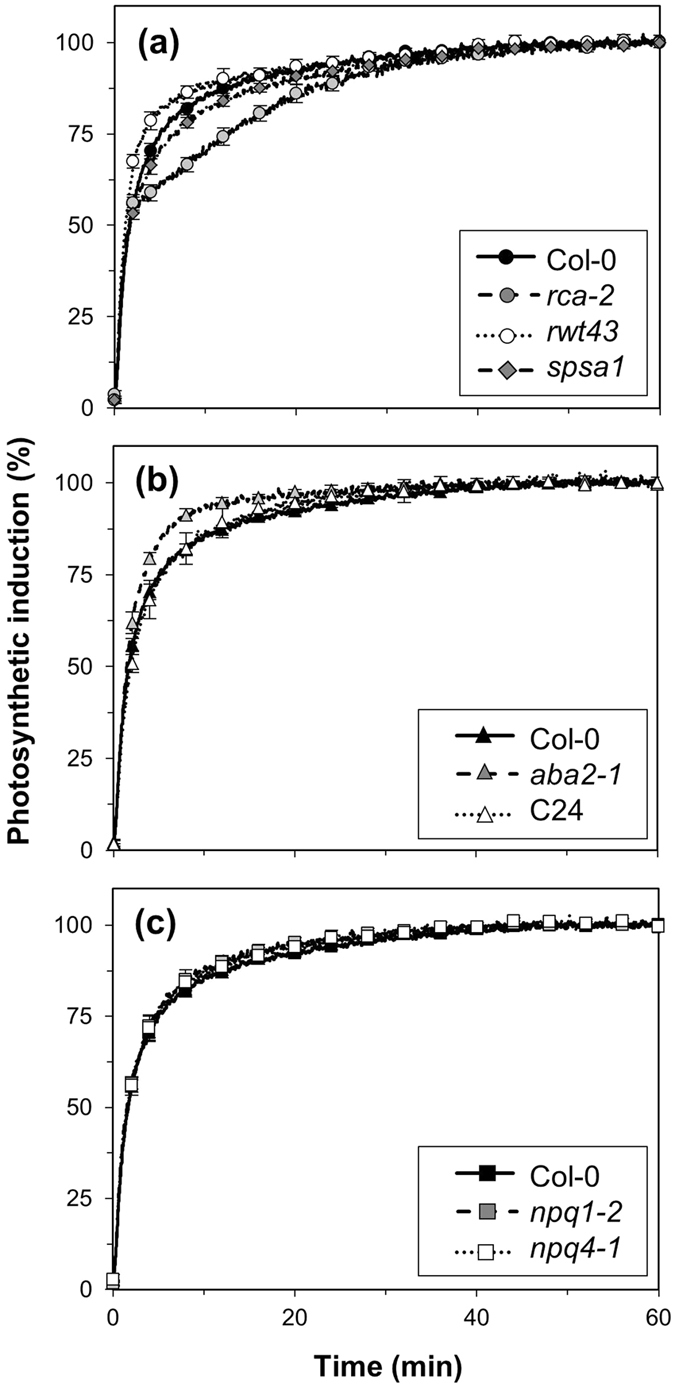
Photosynthetic induction after a step increase in irradiance from 0 to 1000 μmol m−2 s−1 in rca-2, rwt43 and spsa1 (a), aba2-1 and C24 (b) and npq1-2 and npq4-1 (c). Col-0 is included in each panel for ease of comparison. Averages ± SEM, n = 5–15.
Table 2. Time (minutes) to reach 50 and 90% of steady-state photosynthesis rates (tA50, tA90) after step increases in irradiance.
| Genotype | 0 → 1000 μmol m−2 s−1 |
70 → 800 μmol m−2 s−1 |
130 → 600 μmol m−2 s−1 |
|||
|---|---|---|---|---|---|---|
| tA50 | tA90 | tA50 | tA90 | tA50 | tA90 | |
| Col-0 | 1.6 ± 0.1 | 14.7 ± 1.2 | 1.3 ± 0.1 | 10.2 ± 1.1 | 0.6 ± 0.0 | 9.0 ± 2.2 |
| rca-2 | 1.5 ± 0.2 | 25.5 ± 1.5*** | 6.3 ± 0.4*** | 30.9 ± 2.0*** | 4.0 ± 0.7*** | 29.8 ± 1.7*** |
| rwt43 | 1.2 ± 0.1** | 14.2 ± 2.6 | 0.5 ± 0.0*** | 16.2 ± 6.1 | 0.3 ± 0.0*** | 18.8 ± 6.1 |
| spsa1 | 1.6 ± 0.1 | 19.5 ± 1.3* | 1.3 ± 0.1 | 14.1 ± 7.2 | 0.6 ± 0.1 | 13.7 ± 6.9 |
| aba2-1 | 1.4 ± 0.1 | 7.3 ± 0.5** | 1.3 ± 0.1 | 7.7 ± 2.6 | 0.8 ± 0.1 | 15.1 ± 5.8 |
| C24 | 1.9 ± 0.1 | 15.0 ± 3.2 | 1.7 ± 0.3* | 13.3 ± 2.7 | 0.9 ± 0.2* | 29.4 ± 5.1*** |
| npq1-2 | 1.4 ± 0.1 | 11.7 ± 1.7 | 1.3 ± 0.1 | 10.7 ± 2.9 | 0.7 ± 0.0 | 14.6 ± 8.6 |
| npq4-1 | 1.5 ± 0.1 | 14.8 ± 2.6 | 1.1 ± 0.1 | 6.1 ± 0.7 | 0.6 ± 0.0 | 15.3 ± 11.0 |
Averages ± SEM, n = 5–15. Stars within columns denote significance levels compared to Col-0: ***P < 0.0001, **P < 0.01, *P < 0.05. Absence of stars denotes lack of significant difference with Col-0 (P > 0.05).
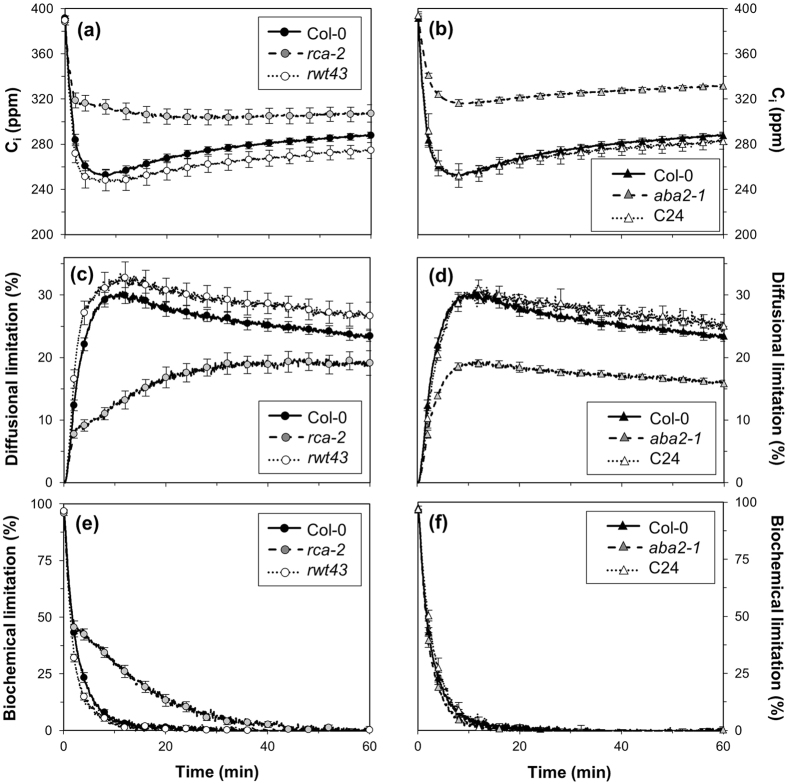
To explain the differences between genotypes affecting Rubisco activation and gs, we looked at the time courses of Ci, diffusional limitation and biochemical limitation. While Ci in Col-0 and rwt43 dropped by ~130 ppm within 10 minutes and then increased by 30–40 ppm following stomatal opening, in rca-2 it never dropped below its final value (Fig. 3a). Diffusional limitation reached its maximum within ~10 minutes in Col-0 and rwt43 and then relaxed, while in rca-2 its increase was much slower and levelled off after ~30 minutes (Fig. 3c). Biochemical limitation during induction relaxed almost completely within ~10 minutes in Col-0 and rwt43, while in rca-2 it was generally greater and the same extent of relaxation took ~40 minutes (Fig. 3e). Comparing Col-0 and C24, the responses of Ci were almost indistinguishable, while in aba2-1 the initial decrease in Ci was smaller, ranging from 50–60% of that found in Col-0 (Fig. 3b). Buildup and relaxation of diffusional limitation were much smaller in aba2-1 (Fig. 3d), while relaxation of biochemical limitation was similar between Col-0, aba2-1 and C24 (Fig. 3f).
Figure 3.
Leaf internal CO2 concentration (Ci), diffusional limitation and biochemical limitation after a step increase in irradiance from 0 to 1000 μmol m−2 s−1 in Col-0, rca-2 and rwt43 (a,c,e) and Col-0, aba2-1 and C24 (b,d,f). Averages ± SEM, n = 5–15.
Next to the dark-light transition discussed above, we also exposed leaves that had been adapted to low irradiance (hereafter: background irradiance) to stepwise increases in irradiance, namely 70 → 800 and 130 → 600 μmol m−2 s−1. The responses of An to these increases were qualitatively similar to those seen after the dark-light transition (Supplementary Fig. 2). rwt43 exhibited a faster increase, and rca-2 a much slower increase than Col-0 (Supplementary Fig. 2a,b). This reduced tA50, but not tA90, in rwt43, while tA50 and tA90 in rca-2 were larger than Col-0 (Table 2). C24 tended to increase photosynthesis more slowly compared to Col-0 (Supplementary Fig. 2c,d), leading to a larger tA50 after the 70 → 800 μmol m−2 s−1 step increase and larger tA50 and tA90 after the 130 → 600 μmol m−2 s−1 step increase. Assimilation responses in NPQ and SPS mutants to those intermediate irradiance increases were similar to Col-0.
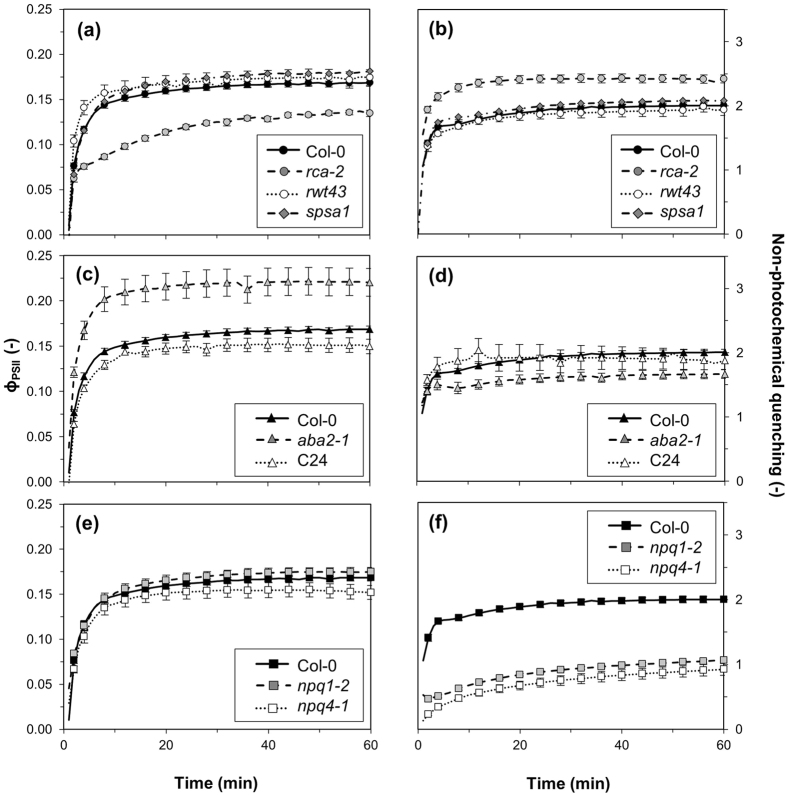
Apart from gas exchange dynamics, we also characterised changes in electron transport parameters after the stepwise 0–1000 μmol m−2 s−1 transition. Changes in ϕPSII largely paralleled those of An (Fig. 4). In rwt43, the increase in ϕPSII was slightly faster than in Col-0, while in rca-2, it was slower and steady-state ϕPSII was lower (Fig. 4a), paralleling its lower steady-state An (Fig 1a). Despite slightly larger ϕPSII throughout induction in spsa1, final values were not significantly different from Col-0 (P = 0.09, Fig. 4a). aba2-1 showed increased steady-state ϕPSII levels, while in C24 they were reduced compared to Col-0 (Fig. 4c), similar to the differences in steady-state assimilation (Fig. 1c). In npq4-1, ϕPSII was slightly lower during induction than in npq1-2 and Col-0 (npq1-2 had similar ϕPSII trends and values during induction as Col-0; Fig. 4e). NPQ in rca-2 increased more quickly to its steady-state level, which was larger than that of Col-0, spsa1 and rwt43 (Fig. 4b). NPQ in aba2-1 was lower than in Col-0 and C24 (which were not significantly different from each other, Fig. 4d). As expected, npq1-2 and npq4-1 developed much lower NPQ levels than Col-0, and NPQ buildup was slower compared to Col-0, but similar in both npq1-2 and npq4-1 (Fig. 4f). Dark-adapted Fv/Fm was 0.805 ± 0.002 (Avg ± standard error of the mean, SEM) in Col-0. In rca-2, C24 and npq4-1, Fv/Fm was marginally, but significantly, smaller, possibly due to photoinhibition that was not completely removed by dark adaptation. In spsa1, it was slightly but significantly higher than in Col-0 (Supplementary Fig. 3).
Figure 4.
Quantum yield of photosystem II (ϕPSII) and non-photochemical quenching (NPQ) after a step increase in irradiance from 0 to 1000 μmol m−2 s−1 in rca-2, rwt43 and spsa1 (a,b), aba2-1 and C24 (c,d) and npq1-2 and npq4-1 (e,f). Col-0 is included in each panel for ease of comparison. Averages ± SEM, n = 5–15.
Isoform, amount and initial activation state of Rca affect the rate of Rubisco activation
The apparent time constants of Rubisco activation (τR, the time to reach 63% of total change in Rubisco activation state), decreased with increasing background irradiance (Fig. 5). Genotypes differing in gs, NPQ and SPS did not differ from Col-0 in τR. However, τR tended to be 17–28% larger in spsa1 than in Col-0; P-values ranged from 0.07 to 0.09. Of the genotypes affecting Rca regulation, rca-2 exhibited the biggest differences in τR, both compared with Col-0 (P < 0.001 in all cases) and between background irradiances, with a τR of ~22 minutes in dark-adapted leaves decreasing to ~4 minutes in leaves adapted to an irradiance of 130 μmol m−2 s−1 (Fig. 5a). In rwt43, τR of dark-adapted leaves (2.3 min) was not significantly different to that of Col-0 (3.0 min; P = 0.08), but was significantly (P < 0.001) smaller at 70 and 130 μmol m−2s−1 background irradiance (Fig. 5b).
Figure 5.
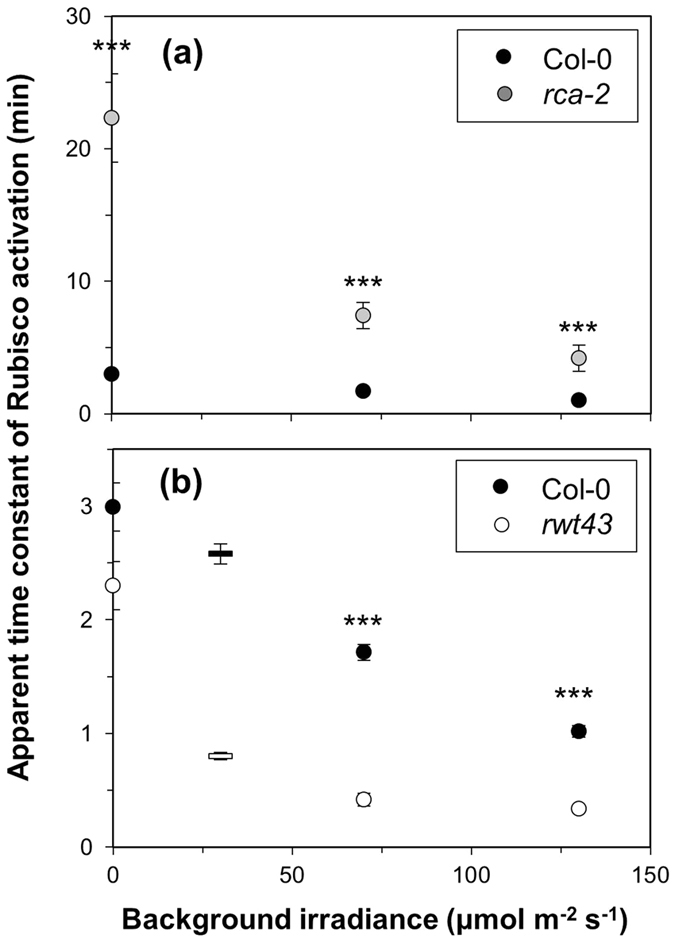
Apparent time constant of Rubisco activation in rca-2 (a) and rwt43 (b), compared to Col-0. Note the different scales of Y-axes in (a,b). Averages ± SEM, n = 5–15. Bars in (b) at 30 μmol m−2 s−1 background irradiance included from Carmo-Silva and Salvucci42. Stars denote significance levels of single genotypes compared to Col-0: ***P < 0.001.
Increases in initial gs up to a threshold value accelerate photosynthetic induction
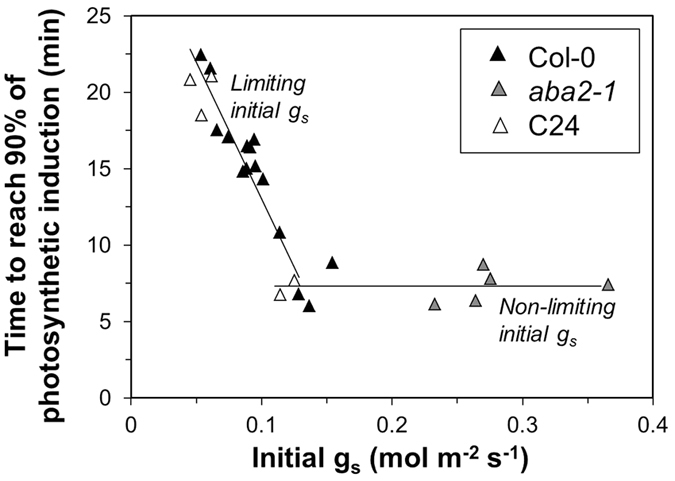
Before and after stepwise increases in irradiance, gs was considerably higher in aba2-1 than in Col-0 and C24 (Supplementary Fig. 4). In dark-adapted leaves of Col-0 and C24, gs was similar, but in leaves adapted to 70 or 130 μmol m−2 s−1, it was almost twice as high in Col-0 compared to C24. This spread in gs was used to explore the threshold between a limiting and a non-limiting initial gs for the subsequent rates of An increase. For example, after the 0 → 1000 μmol m−2 s−1 increase, tA90 was lower in plants with initially higher gs up to ~0.13 mol m−2 s−1, but above 0.13 mol m−2 s−1 there was no further decrease in tA90 (Fig. 6). This shows that an initial gs > 0.13 mol m−2 s−1 was non-limiting in this case. We also looked at various time points (tA10, tA20, etc.) after different low-to-high irradiance transitions (i.e. 0 → 1000, 70 → 800 and 130 → 600 μmol m−2 s−1) and found that the threshold between limiting and non-limiting initial gs was between 0.09 and 0.17 mol m−2 s−1, with no discernible trend between time points or background irradiance levels.
Figure 6. Relationship between initial gs and the time to reach 90% of final photosynthesis rates after a step increase in irradiance (0–1000 μmol m−2 s−1) in single replicates of Col-0, aba2-1 and C24.
Apart from the effect of initial gs on the rate of An increase, we also analysed the effects of gs increase after stepwise increases in irradiance (Supplementary Fig. 4). In C24 and Col-0, the increase in gs after the 0 → 1000 μmol m−2 s−1 increase (until 60 minutes after the start of illumination) and tA90 correlated positively (Supplementary Fig. 5). Because initial gs in aba2-1 was high, it was non-limiting to rates of increase in photosynthesis after irradiance increases, and stomatal opening did not correlate with tA90 (data not shown).
Lower NPQ and SPS do not increase transient photosynthesis after a decrease in irradiance
After step decreases in irradiance (600 → 200, 800 → 130 μmol m−2 s−1), relative changes in An were similar for all genotypes (Supplementary Fig. 6), and there were no significant differences in either post-illumination CO2 fixation or the post-illumination CO2 burst, including the NPQ mutants and spsa1 (Supplementary Fig. 7).
Discussion
Making use of the genetic diversity available for A. thaliana, we explored several possible physiological limitations of dynamic photosynthesis. This analysis revealed that altered Rubisco activation kinetics or stomatal conductance affect photosynthesis in a dynamic irradiance environment greatly, while alterations in non-photochemical quenching or sucrose synthesis do not.
Changes affecting Rca concentration (rca-2) or regulation (rwt43) had strong effects on dynamic photosynthesis. The observed effects were likely caused by different kinetics of Rubisco activation, as the initial increase in assimilation after dark-light transitions (first minute in Fig. 2a) was similar between genotypes, implying a similar limitation due to activation of RuBP regeneration (Sassenrath-Cole and Pearcy7 provided biochemical evidence for this). Furthermore, these genotypes had similar gs (Supplementary Fig. 8). Lower steady-state irradiance and CO2 responses in rca-2 may have been caused by a reduced steady-state activation of Rubisco39. Based on the dependency between maximum Rubisco activation state and Rca concentration reported by Mott and Woodrow40 and our estimation of Vcmax for rca-2 (Table 1), we estimate that rca-2 contains ~22% of wildtype Rca levels (Supplementary text 1). The effects on the rate of Rubisco activation of such low Rca content are apparent. In antisense or overexpressors of Rca in rice, a positive linear relationship between Rca concentration and the rate of photosynthetic induction was shown for various temperatures41, demonstrating the role of Rca concentration in controlling dynamic photosynthesis. Intriguingly, in our study τR decreased with background irradiance (Fig. 5). While this decrease was linear in Col-0, it resembled a negative exponential in rwt43. This is in agreement with data of Carmo-Silva and Salvucci42 (Fig. 5b). Previous studies have shown that Rubisco activation in Col-0 increased linearly with irradiance42,43,44, while in rwt43, Rubisco activation state did not change with increasing irradiance42; it was similar to Col-0 in dark-adapted leaves, but close to full activation in low irradiance19,42,44. Most likely differences in the activation state of Rca, rather than that of Rubisco, caused τR to decrease with background irradiance. Rca activity increased linearly between 0 and 300 μmol m−2 s−1 in intact spinach leaves45, and should be high in rwt43 except in darkness (see above).
Compared to natural fluctuations in irradiance, stomata open and close slowly46. Low initial gs can become a limitation to carbon fixation after a step change in irradiance2, because of comparably rapid activation of RuBP regeneration and Rubisco. The peak of this limitation is typically reached within ~10 minutes due to Rubisco activation without similarly large increases in gs, after which it relaxes due to stomatal opening (Fig. 3d). We note that the index of diffusional limitation should be refined with respect to changes in Rubisco activation during photosynthetic induction, as well as possible changes in mesophyll conductance (gm) during transients. With respect to gm, contrasting responses to irradiance have been reported (cf. refs 47 and 48); we therefore refrain from speculations on how it may have changed in our measurements but note that it may have affected the index of diffusional limitation. Nevertheless, we believe that diffusional limitation provides a useful qualitative tool to analyse the differences between the genotypes affecting Rubisco activation kinetics and gs.
The mutant with high initial gs (aba2-1) did not show such large differences in stomatal opening (i.e. difference between initial and final gs; Supplementary Fig. 4), but still had much higher rates of An increases when irradiance was raised. Therefore, we argue that increasing the initial gs is a simpler route to increasing dynamic photosynthesis than is increasing the rate of stomatal opening. Stomatal closure in low irradiance is an adaptive response to changing water supply and logical under non-irrigated field conditions, however for crops in well-watered situations, increasing gs at the expense of water use may be a reasonable target to increase rates of dynamic photosynthesis. Also, the threshold between limiting and non-limiting gs for rates of photosynthesis increase could be used as a phenotypic marker for breeding of cultivars with non-limiting gs in fluctuating irradiance. In our analysis, this threshold proved to be consistent, independent of the time point after stepwise increases in irradiance and level of background irradiance. Previous findings indicate that this threshold shows no diurnal variation26, and that it is unchanged by water stress26 or growth light conditions49. An open question that remains is whether the threshold is species-specific26 or not49. It is likely that a high initial gs correlates with constitutively high gs (i.e. stomata are more open and less sensitive to changes in irradiance), and faster responses of An to an increasing irradiance could be reached at the expense of lower intrinsic water use efficiency. Rapid screening for high gs could be achieved by thermal imaging50.
In Col-0, rates of NPQ buildup after a dark-light transition were similar to those seen in previous studies51,52, while mutants npq1-2 (lacking violaxanthin de-epoxidase31) and npq4-1 (lacking PsbS30) exhibited a much lower buildup of NPQ. However, they showed negligible differences in gas exchange to Col-0, neither in their steady-state responses to irradiance and CO2 (Fig. 1e,f) nor in their responses to step increases in irradiance (Fig. 2c, Supplementary Fig. 2e,f). Similar to our findings, reduced PsbS content in transgenic rice plants strongly reduced NPQ but had limited effects on carbon gain during a 5-min induction period23. In contrast, overexpressors with 2–4 fold increases in PsbS showed ~15% lower An during induction, demonstrating that increased energy dissipation can have adverse effects on assimilation23. Antisense mutants with reduced thylakoid membrane K+ flux capacities showed less rapid relaxation of NPQ after irradiance decreases, reducing electron transport and assimilation24. Our data revealed no differences between npq1-2, npq4-1 and Col-0 with respect to post-illumination CO2 fixation (Supplementary Fig. 7), and therefore show that unlike the rate of NPQ relaxation22,24, an initially low level of NPQ does not increase carbon gain directly after decreases in irradiance.
Irradiance-dependent activation of SPS is genotype-specific, and A. thaliana belongs to a group of species with low light/dark modulation of the enzyme53. This suggests that in the wildtype, SPS activity does not limit photosynthetic induction–however, in a plant with strongly reduced SPS concentration it might. We tested this possibility in the T-DNA mutant spsa1, which has a 80% lower maximum SPS activity than Col-029. Similar to our findings, Sun et al.29 found no photosynthetic differences between spsa1 and Col-0, except for a strong reduction in CO2-saturated An (−23%). Importantly, the decrease in SPS hardly affected photosynthetic responses to fluctuating irradiance. The only significant difference was a longer time to reach 90% of full induction after dark-light transitions (Table 2). However, no such differences were observed in transitions from low to higher irradiance. spsa1 would probably show decreased rates of dynamic photosynthesis in elevated CO2 concentrations. Furthermore, it may be that the absence of a measurable effect of spsa1 on the post-illumination CO2 burst, which is partly affected by the rate of sucrose synthesis21, was masked by the photorespiratory portion of the CO2 burst, which is most pronounced in C3 plants5. Also, reduced levels of SPS in species that exhibit strong light/dark modulation of SPS (e.g. barley, maize, spinach and sugarbeet53) would probably have a stronger negative effect on photosynthetic induction than shown here for A. thaliana.
The relationship between ϕPSII and Ci in C3 photosynthesis contains three phases: When An is (a) limited by Rubisco, ϕPSII increases with Ci; when An is (b) limited by RuBP regeneration, ϕPSII is constant with increases in Ci and when An is (c) limited by TPU, ϕPSII decreases with increasing Ci54,55. Most genotypes in our study did not show the plateau in ϕPSII that would signify a phase of RuBP regeneration limitation, with spsa1 showing an extreme form of that behaviour (Supplementary Fig. 1). This suggests that (a) TPU occurs at a lower Ci than visible from gas exchange, (b) different limitations occur simultaneously within different layers of the leaf, (c) changes in the rate of cyclic electron transport around photosystem I and/or strength of alternative electron sinks or (d) with increasing Ci during the phase of limitation by RuBP regeneration photosynthetic electron transport is sometimes restricted, and ϕPSII is reduced, due to the increased inhibition of starch synthesis following the inhibition of phosphoglucoisomerase by phosphoglycerate56. However, these results have to be interpreted with caution because the number of data points between the end of Rubisco limitation and the onset of TPU was limited and more data may lead to different conclusions.
In conclusion, in A. thaliana, the presence of the redox-regulated α-isoform of Rca in the wildtype, and wildtype levels of gs, are limiting for dynamic photosynthesis. Furthermore, reductions in Rca strongly decrease (dynamic) photosynthesis. We also show that wildtype levels of NPQ and SPS are not limiting in A. thaliana. This suggests Rca and gs as targets for improvement of photosynthesis in fluctuating irradiance.
Methods
Plant material
Seeds of npq4-1, spsa1 (SALK_148643C) and rca-2 (SALK_003204C) were obtained from NASC (University of Nottingham, Loughborough, UK57). C24 (CS76106) was obtained from the Arabidopsis Biological Resource Center (ABRC, Ohio State University, USA). Seeds of Col-0 and aba2-1 were obtained from Corrie Hanhart (Wageningen University, the Netherlands), npq1-2 was obtained from Dr. Shizue Matsubara (Forschungszentrum Jülich, Germany) and rwt43 was obtained from Dr. Elizabete Carmo-Silva (Rothamsted Research, UK).
Growth conditions
Plants were grown in 0.37 L pots using soil with a 4:1 peat:perlite mixture. Pots were placed on irrigation mats, and mats were saturated daily to full capacity. Plants were fertilized weekly using a nutrient solution especially developed for Arabidopsis58. To inhibit algal growth, the soil was covered with black plastic film. Plants were grown in a growth chamber in short-day conditions (8 hours of light) to delay flowering59 and thus ensure that leaves were large enough for gas-exchange measurements. Irradiance was 172 ± 4 μmol m−2 s−1 as supplied by LED lights (GreenPower LED production module deep red/white 120; Philips, Eindhoven, the Netherlands; Supplementary Fig. 9). Temperature was 23/18 °C (day/night) and relative humidity was 70%. Mutants lacking ABA (aba2-1) were sprayed with an aqueous solution containing 10 μmol mol−1 ABA (Sigma, St. Louis, U.S.A.) when plants were 2, 4 and 6 weeks old. This increases rosette growth compared to untreated aba2-1 plants (data not shown). There was a period of 15 days between the last application of ABA and the first measurements on aba2-1 plants.
Single genotypes were grown sequentially (approx. one batch per week). Five plants per batch were used for measurements. To monitor the quality of the growth system over time, Col-0 was grown in three batches, each batch separated by several weeks. The number of replicates was therefore 15 for Col-0, and 5 for all other genotypes. The growth system produced very reproducible photosynthetic phenotypes of Col-0 (Supplementary Fig. 10).
Measurements
Measurements were performed using the LI-6400 portable photosynthesis system (Li-Cor Biosciences, Lincoln, Nebraska, USA) equipped with the leaf chamber fluorometer (Part No. 6400-40) on single leaves of plants that were 6–8 weeks old. Leaves large enough to cover the leaf chamber gasket (area: 2 cm2, diameter: 1.6 cm) were used. Conditions in the cuvette were as follows: 23 °C air temperature, 70% relative humidity, 90/10% red/blue light mixture and 500 μmol s−1 air flow rate. The choice of flow rate was a compromise between getting a fast time response of the measuring system (necessary in dynamic gas exchange studies), and the difference in CO2 concentration between sample and reference air stream. Except for the CO2-response curves, the external CO2 mole fraction was kept at 400 ppm. The oxygen mole fraction was always 21%.
Stepwise increases in irradiance
Leaves were adapted to several background irradiances (0, 70 or 130 μmol m−2 s−1) for 30–60 minutes (until An and gs had visibly reached a steady state), and then exposed to single-step increases in irradiance, namely 0 → 1000, 70 → 800 and 130 → 600 μmol m−2 s−1. These intensities were chosen, after preliminary irradiance-response curves on Col-0 had shown that all but the highest (1000 μmol m−2 s−1) intensity were in the sub-saturating range (Supplementary Fig. 11). Gas exchange was logged nominally every second. Logging was stopped when gs reached a new steady state (this was assessed visually, and took a minimum of 30 minutes after the step increase), or 60 minutes after switching to 1000 μmol m−2 s−1. Before and after the 0 → 1000 μmol m−2 s−1 increase, ϕPSII and NPQ were measured, using a measuring beam intensity of ~1 μmol m−2 s−1 and a saturating pulse of ~7600 μmol m−2 s−1 intensity and 1 s duration. In preliminary measurements on Col-0, the saturating pulse was sufficient to saturate Fm’ in leaves adapted to 1000 μmol m−2 s−1 (assessed following the manufacturer’s recommendations for calibrating the saturating pulse: Fm’ was not increased when using saturating pulses of intensity higher than 7600 μmol m−2 s−1). The Fo and Fm relative fluorescence yields were measured in dark-adapted leaves. After the increase in irradiance, the Fm’ relative fluorescence yield was measured every minute for the first ten minutes, and every two minutes thereafter. The regular application of saturating flashes transiently increased the leaf temperature by 0.4–0.7 °C across genotypes (temperature traces of Col-0 are representative of all genotypes, Supplementary Fig. 12). Also, our data (Kaiser et al., unpublished) indicate that the regular application of saturating flashes of similar intensity and frequency in tomato (Lycopersicon esculentum) had no effects on leaf gas exchange during photosynthetic induction. The steady-state relative fluorescence yield, Fs, was measured continuously. Dark-adapted Fv/Fm, ϕPSII and NPQ were calculated as Fv/Fm = (Fm − Fo)/Fm, ϕPSII = (Fm’ − Fs)/Fm’ and NPQ = (Fm − Fm’)/Fm’, respectively.
During transients, gm and mitochondrial respiration (Rd) were assumed to be constant because, to our knowledge, changes in gm and Rd have never been assessed for irradiance transients. Rd in the light was considered similar to genotype-specific steady-state respiration in the dark; this assumption is supported by measurements on several species60. For gm, a value of 0.2 mol m−2 s−1 was assumed for all genotypes, which is an average of three values determined on Col-0 of comparable photosynthetic capacity35,61.
The time to reach 50 and 90% (i.e. t50 and t90) of steady-state An was calculated for each irradiance increase. To increase robustness of these indices to experimental noise and outliers, time series were smoothed using a local polynomial regression62 with a span of 5%. This means that, for each point in the time series, a polynomial of degree two was fitted using weighted least squares to a data window of size equal to 5% of the total size of the time series; the weight assigned to each point decreases with the distance from the central point.
Calculation of diffusional limitation, biochemical limitation and the apparent time constant of Rubisco activation
To calculate several parameters, An was corrected for transient changes in chloroplast CO2 concentration (Cc). For diffusional limitation, An was multiplied by the relative rate by which An would increase if Cc during induction was equal to ambient CO2 concentration, Ca (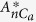 ):
):
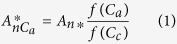 |
Where f(Ca) is the steady-state value of An at Ca (i.e. at 400 ppm), and f(Cc) is the steady-state value of An at Cc. The relative effects of Cc on An were taken from steady-state An/Cc response curves by fitting local polynomial regressions (LOESS) in the range 50–500 ppm (Supplementary Fig. 13). Diffusional limitation was then determined as:
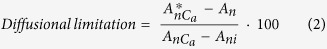 |
Where AnCa is the steady-state value of An at Ca and Ani is the initial steady-state rate of An. Diffusional limitation is therefore a combination of possible limitations due to gs and gm during induction and in the steady state (i.e. it does not decrease to 0% at the end of the time course). For biochemical limitation and τR, An was multiplied (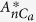 ) by the relative rate by which An would increase if transient Cc was equal to final, steady-state Cc (Ccf), following Woodrow and Mott15:
) by the relative rate by which An would increase if transient Cc was equal to final, steady-state Cc (Ccf), following Woodrow and Mott15:
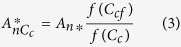 |
Where f(Ccf) is the solution for An at Ccf. Biochemical limitation was calculated after Allen and Pearcy63:
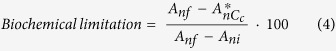 |
Throughout induction, biochemical limitation decreases from 100 to 0%, and therefore indicates the additional limitation imposed on An due to incomplete activation of several enzymes. Biochemical and diffusional limitations do not sum up to 100%, and are distinct. The apparent time constant of Rubisco activation (τR) was calculated after Woodrow and Mott15:
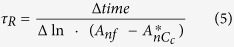 |
The range of timepoints (Δtime) for calculating τR differed between background irradiances (Supplementary Fig. 14), and in some cases between genotypes. This was due to differences in the rate of change of photosynthesis, and included 120 data points in the case of 0 → 1000 μmol m−2 s−1 (all genotypes) and 40 (for rwt43) or 60 (all other genotypes) in the case of 70 → 800 and 130 → 600 μmol m−2 s−1. These ranges were selected by visual inspection. The average root mean squared error of the linear fits was 1.2 μmol m−2 s−1 (range: 1.0–3.0 μmol m−2 s−1).
Stepwise decreases in irradiance
Irradiance was decreased in the following steps: 800 → 130 and 600 → 200 μmol m−2 s−1. From the CO2 exchange data, post-illumination CO2 fixation64 and post-illumination CO2 bursts65 were quantified. The former implies that photosynthesis is above the final steady-state value during the transient, while the latter implies a lower assimilation rate than at steady state. Values were estimated by integrating the difference between time series of photosynthesis and the final steady-state value66.
Irradiance response curves
When An was at a steady state, i.e. before step changes in irradiance or at the end of a measurement sequence, 120 data points were used to extract average An at a given irradiance. The resulting values were used to construct steady-state irradiance response curves.
CO2 response curves
Leaves were adapted to 1000 μmol m−2 s−1 for ~30 min and 500 ppm Ca. Ca was then decreased stepwise until 50 ppm, each step taking 2–3 minutes. Thereafter, Ca was raised to 500 ppm, and after waiting for ~15 minutes, leaves were exposed to stepwise increases in Ca until 1500 ppm, each step taking ~4 minutes. Values were logged every 5 s and the last 60 s of every CO2 step used to calculate average ± SEM of Ci and An. ϕPSII was determined at the end of each step as described above. Photosynthesis in all genotypes was corrected for CO2 leaks using dried leaves of Col-054. Parameters Vcmax, Jmax, and TPU were calculated after Sharkey et al.55.
Statistical analysis
Each genotype was compared to Col-0 using a Student’s t-test (Microsoft Excel, function t.test, assuming 2-tailed distribution and two-sample equal variance).
Additional Information
How to cite this article: Kaiser, E. et al. Metabolic and diffusional limitations of photosynthesis in fluctuating irradiance in Arabidopsis thaliana. Sci. Rep. 6, 31252; doi: 10.1038/srep31252 (2016).
Supplementary Material
Acknowledgments
We thank the Nottingham Arabidopsis Stock Centre, Corrie Hanhart, Elizabete Carmo-Silva and Shizue Matsubara for providing A. thaliana seeds. Also, we thank Sasan Aliniaeifard for help with the growth system. We thank Shizue Matsubara and Tom Sharkey for helpful discussions. This work was carried out within the BioSolar Cells programme. It was funded by the Dutch Ministry of Economic Affairs and Powerhouse.
Footnotes
Author Contributions Design of experiment: all authors. Execution of experiment: E.K. and A.M. Data analysis and interpretation: A.M. and E.K. Writing of manuscript: all authors.
References
- Pearcy R. W., Roden J. S. & Gamon J. A. Sunfleck dynamics in relation to canopy structure in a soybean (Glycine max (L.) Merr.) canopy. Agric. For. Meteorol. 52, 359–372 (1990). [Google Scholar]
- Pearcy R. W. Sunflecks and photosynthesis in plant canopies. Annu. Rev. Plant Physiol. 41, 421–453 (1990). [Google Scholar]
- Pearcy R. W., Krall J. P. & Sassenrath-Cole G. F. Photosynthesis in fluctuating light environments. Photosynth. Environ. 321–346 (1996). [Google Scholar]
- Kono M. & Terashima I. Long-term and short-term responses of the photosynthetic electron transport to fluctuating light. J. Photochem. Photobiol. B Biol. 137, 89–99 (2014). [DOI] [PubMed] [Google Scholar]
- Kaiser E. et al. Dynamic photosynthesis in different environmental conditions. J. Exp. Bot. 66, 2415–2426 (2015). [DOI] [PubMed] [Google Scholar]
- Yamori W. & Shikanai T. Physiological Functions of Cyclic Electron Transport Around Photosystem I in Sustaining Photosynthesis and Plant Growth. Annu. Rev. Plant Biol. 67, annurev-arplant-043015-112002 (2016). [DOI] [PubMed] [Google Scholar]
- Sassenrath-Cole G. F. & Pearcy R. W. The role of Ribulose-1,5-Bisphosphate regeneration in the induction requirement of photosynthetic CO2 exchange under transient light conditions. Plant Physiol. 99, 227–234 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Way D. A. & Pearcy R. W. Sunflecks in trees and forests: from photosynthetic physiology to global change biology. Tree Physiol. 32, 1066–1081 (2012). [DOI] [PubMed] [Google Scholar]
- Küppers M. & Pfiz M. Role of photosynthetic induction for daily and annual carbon gains of leaves and plant canopies. Photosynth. silico Underst. Complex. from Moelcules to Ecosyst. 417–440 (2009). [Google Scholar]
- Naumburg E. & Ellsworth D. S. Short-term light and leaf photosynthetic dynamics affect estimates of daily understory photosynthesis in four tree species. Tree Physiol. 22, 393–401 (2002). [DOI] [PubMed] [Google Scholar]
- Murchie E. H. & Niyogi K. K. Manipulation of photoprotection to improve plant photosynthesis. Plant Physiol. 155, 86–92 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carmo-Silva E., Scales J. C., Madgwick P. J. & Parry M. A. J. Optimizing Rubisco and its regulation for greater resource use efficiency. Plant. Cell Environ. 38, 1817–1832 (2015). [DOI] [PubMed] [Google Scholar]
- Seemann J. R., Kirschbaum M. U. F., Sharkey T. D. & Pearcy R. W. Regulation of Ribulose-1,5-Bisphosphate Carboxylase Activity in Alocasia macrorrhiza in Response to Step Changes in Irradiance. Plant Physiol. 88, 148–152 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M. & Grosse H. Interactions between sucrose synthesis and CO2 fixation I. Secondary kinetics during photosynthetic induction are related to a delayed activation of sucrose synthesis. J. Plant Physiol. 133, 129–137 (1988). [Google Scholar]
- Woodrow I. E. & Mott K. A. Rate limitation of non-steady-state photosynthesis by Ribulose-1,5-bisphosphate Carboxylase in spinach. Aust. J. Plant Physiol. 16, 487–500 (1989). [Google Scholar]
- Salvucci M. E., Portis A. R. J. & Ogren W. L. A soluble chloroplast protein catalyzes ribulosebisphosphate carboxylase/oxygenase activation in vivo. Photosynth. Res. 7, 193–201 (1985). [DOI] [PubMed] [Google Scholar]
- Salvucci M. E., Werneke J. M., Ogren W. L. & Portis A. R. Purification and species distribution of rubisco activase. Plant Physiol. 84, 930–936 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N. & Portis A. R. Mechanism of light regulation of Rubisco: A specific role for the larger Rubisco activase isoform involving reductive activation by thioredoxin-f. Proc. Natl. Acad. Sci. USA 96, 9438–9443 (1999). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang N., Kallis R. P., Ewy R. G. & Portis A. R. Light modulation of Rubisco in Arabidopsis requires a capacity for redox regulation of the larger Rubisco activase isoform. Proc. Natl. Acad. Sci. USA 99, 3330–3334 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stitt M., Lunn J. & Usadel B. Arabidopsis and primary photosynthetic metabolism - more than the icing on the cake. Plant J. 61, 1067–1091 (2010). [DOI] [PubMed] [Google Scholar]
- Prinsley R. T., Hunt S., Smith A. M. & Leegood R. C. The influence of a decrease in irradiance on photosynthetic carbon assimilation in leaves of Spinacia oleracea L. Planta 167, 414–420 (1986). [DOI] [PubMed] [Google Scholar]
- Zhu X.-G., Ort D. R., Whitmarsh J. & Long S. P. The slow reversibility of photosystem II thermal energy dissipation on transfer from high to low light may cause large losses in carbon gain by crop canopies: a theoretical analysis. J. Exp. Bot. 55, 1167–1175 (2004). [DOI] [PubMed] [Google Scholar]
- Hubbart S., Ajigboye O. O., Horton P. & Murchie E. H. The photoprotective protein PsbS exerts control over CO2 assimilation rate in fluctuating light in rice. Plant J. 71, 402–412 (2012). [DOI] [PubMed] [Google Scholar]
- Armbruster U. et al. Ion antiport accelerates photosynthetic acclimation in fluctuating light environments. Nat. Commun. doi: 10.1038/ncomms6439 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vico G., Manzoni S., Palmroth S. & Katul G. Effects of stomatal delays on the economics of leaf gas exchange under intermittent light regimes. New Phytol. 192, 640–652 (2011). [DOI] [PubMed] [Google Scholar]
- Allen M. T. & Pearcy R. W. Stomatal behavior and photosynthetic performance under dynamic light regimes in a seasonally dry tropical rain forest. Oecologia 122, 470–478 (2000). [DOI] [PubMed] [Google Scholar]
- Alonso J. M. et al. Genome-wide insertional mutagenesis of Arabidopsis thaliana. Science. 301, 653–657 (2003). [DOI] [PubMed] [Google Scholar]
- Shan X. et al. The role of Arabidopsis Rubisco activase in jasmonate-induced leaf senescence. Plant Physiol. 155, 751–764 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun J., Zhang J., Larue C. T. & Huber S. C. Decrease in leaf sucrose synthesis leads to increased leaf starch turnover and decreased RuBP regeneration-limited photosynthesis but not Rubisco-limited photosynthesis in Arabidopsis null mutants of SPSA1. Plant, Cell Environ. 34, 592–604 (2011). [DOI] [PubMed] [Google Scholar]
- Li X.-P. et al. A pigment-binding protein essential for regulation of photosynthetic light harvesting. Nature 403, 391–395 (2000). [DOI] [PubMed] [Google Scholar]
- Niyogi K. K., Grossman A. R. & Björkman O. Arabidopsis mutants define a central role for the xanthophyll cycle in the regulation of photosynthetic energy conversion. Plant Cell 10, 1121–1134 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leon-Kloosterziel K. et al. Isolation and characterisation of abscisic acid-deficient Arabidopsis mutants at two new loci. Plant J. 10, 655–661 (1996). [DOI] [PubMed] [Google Scholar]
- Brosché M. et al. Natural variation in ozone sensitivity among Arabidopsis thaliana accessions and its relation to stomatal conductance. Plant, Cell Environ. 33, 914–925 (2010). [DOI] [PubMed] [Google Scholar]
- Veljovic-Jovanovic S. D., Pignocchi C., Noctor G. & Foyer C. H. Low Ascorbic Acid in the vtc-1 Mutant of Arabidopsis is Associated with Decreased Growth and Intracellular Redistribution of the Antioxidant System. Plant Physiol. 127, 426–435 (2001). [PMC free article] [PubMed] [Google Scholar]
- Flexas J. et al. Mesophyll conductance to CO2 in Arabidopsis thaliana. New Phytol. 175, 501–511 (2007). [DOI] [PubMed] [Google Scholar]
- Xing H. T., Guo P., Xia X. L. & Yin W. L. PdERECTA, a leucine-rich repeat receptor-like kinase of poplar, confers enhanced water use efficiency in Arabidopsis. Planta 234, 229–241 (2011). [DOI] [PubMed] [Google Scholar]
- Bates G. W. et al. A Comparative Study of the Arabidopsis thaliana Guard-Cell Transcriptome and Its Modulation by Sucrose. Plos One 7 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farquhar G. D. & Sharkey T. D. Stomatal Conductance and Photosynthesis. Annu. Rev. Plant Physiol. 33, 317–345 (1982). [Google Scholar]
- Mate C. J., Hudson G. S., von Caemmerer S., Evans J. R. & Andrews T. J. Reduction of Ribulose Bisphosphate Carboxylase Activase levels in tobacco (Nicotiana tabacum) by Antisense RNA reduces Ribulose Bisphosphate Carboxylase carbamylation and impairs photosynthesis. Plant Physiol. 102, 1119–1128 (1993). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mott K. A. & Woodrow I. E. Modelling the role of Rubisco activase in limiting non-steady-state photosynthesis. J. Exp. Bot. 51, 399–406 (2000). [DOI] [PubMed] [Google Scholar]
- Yamori W., Masumoto C., Fukayama H. & Makino A. Rubisco activase is a key regulator of non-steady-state photosynthesis at any leaf temperature and, to a lesser extent, of steady-state photosynthesis at high temperature. Plant J. 71, 871–880 (2012). [DOI] [PubMed] [Google Scholar]
- Carmo-Silva A. E. & Salvucci M. E. The regulatory properties of Rubisco activase differ among species and affect photosynthetic induction during light transitions. Plant Physiol. 161, 1645–1655 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks A. & Portis A. R. Protein-bound ribulose bisphosphate correlates with deactivation of ribulose bisphosphate carboxylase in leaves. Plant Physiol. 87, 244–249 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scales J. C., Parry M. A. J. & Salvucci M. E. A non-radioactive method for measuring Rubisco activase activity in the presence of variable ATP: ADP ratios, including modifications for measuring the activity and activation state of Rubisco. Photosynth. Res. 119, 355–365 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lan Y., Woodrow I. E. & Mott K. A. Light-dependent changes in ribulose bisphosphate carboxylase activase activity in leaves. Plant Physiol. 99, 304–309 (1992). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fay P. A. & Knapp A. K. Photosynthetic and stomatal responses of Avena sativa (Poaceae) to a variable light environment. Am. J. Bot. 80, 1369–1373 (1993). [Google Scholar]
- Tholen D. et al. The chloroplast avoidance response decreases internal conductance to CO2 diffusion in Arabidopsis thaliana leaves. Plant, Cell Environ. 31, 1688–1700 (2008). [DOI] [PubMed] [Google Scholar]
- Flexas J., Ribas-Carbó M., Diaz-Espejo A., Galmés J. & Medrano H. Mesophyll conductance to CO2: current knowledge and future prospects. Plant, Cell Environ. 31, 602–621 (2008). [DOI] [PubMed] [Google Scholar]
- Valladares F., Allen M. T. & Pearcy R. W. Photosynthetic responses to dynamic light under field conditions in six tropical rainforest shrubs occuring along a light gradient. Oecologia 111, 505–514 (1997). [DOI] [PubMed] [Google Scholar]
- McAusland L., Davey P. A., Kanwal N., Baker N. R. & Lawson T. A novel system for spatial and temporal imaging of intrinsic plant water use efficiency. J. Exp. Bot. 64, 4993–5007 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X.-P., Muller-Moule P., Gilmore A. M. & Niyogi K. K. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl. Acad. Sci. USA 99, 15222–15227 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nilkens M. et al. Identification of a slowly inducible zeaxanthin-dependent component of non-photochemical quenching of chlorophyll fluorescence generated under steady-state conditions in Arabidopsis. Biochim. Biophys. Acta 1797, 466–475 (2010). [DOI] [PubMed] [Google Scholar]
- Huber S. C., Nielsen T. H., Huber J. L. & Pharr D. M. Variation among species in light activation of sucrose-phosphate synthase. Plant Cell Physiol. 30, 277–285 (1989). [Google Scholar]
- Long S. P. & Bernacchi C. J. Gas exchange measurements, what can they tell us about the underlying limitations to photosynthesis? Procedures and sources of error. J. Exp. Bot. 54, 2393–2401 (2003). [DOI] [PubMed] [Google Scholar]
- Sharkey T. D., Bernacchi C. J., Farquhar G. D. & Singsaas E. L. Fitting photosynthetic carbon dioxide response curves for C3 leaves. Plant, Cell Environ. 30, 1035–1040 (2007). [DOI] [PubMed] [Google Scholar]
- Sharkey T. D. What gas exchange data can tell us about photosynthesis. Plant, Cell Environ. doi: 10.1111/pce.12641 (2015). [DOI] [PubMed] [Google Scholar]
- Scholl R. L., May S. T. & Ware D. H. Seed and molecular resources for Arabidopsis. Plant Physiol. 124, 1477–1480 (2000). [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Rooijen R., Aarts M. G. M. & Harbinson J. Natural genetic variation for acclimation of photosynthetic light use efficiency to growth irradiance in Arabidopsis thaliana. Plant Physiol. 167, pp.114.252239 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibeaut D. M., Hulett J., Cramer G. R. & Seemann J. R. Maximal biomass of Arabidopsis thaliana using a simple, low-maintenance hydroponic method and favorable environmental conditions. Plant Physiol. 115, 317–319 (1997). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin X., Sun Z., Struik P. C. & Gu J. Evaluating a new method to estimate the rate of leaf respiration in the light by analysis of combined gas exchange and chlorophyll fluorescence measurements. J. Exp. Bot. 62, 3489–3499 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walker B., Ariza L. S., Kaines S., Badger M. B. & Cousins A. B. Temperature response of in vivo Rubisco kinetics and mesophyll conductance in Arabidopsis thaliana: comparisons to Nicotiana tabacum. Plant. Cell Environ. 36, 2108–2119 (2013). [DOI] [PubMed] [Google Scholar]
- Cleveland W. S., Grosse E. & Shyu W. M. In Stat. Model. S (Chambers J. M. & Hastie T. J.) (Wadsworth & Brooks/Cole, 1992). [Google Scholar]
- Allen M. T. & Pearcy R. W. Stomatal versus biochemical limitations to dynamic photosynthetic performance in four tropical rainforest shrub species. Oecologia 122, 479–486 (2000). [DOI] [PubMed] [Google Scholar]
- Pons T. L., Pearcy R. W. & Seemann J. R. Photosynthesis in flashing light in soybean leaves grown in different conditions. I. Photosynthetic induction state and regulation of ribulose-1,5-bisphosphate carboxylase activity. Plant, Cell Environ. 15, 569–576 (1992). [Google Scholar]
- Vines H. M., Tu Z.-P., Armitage A. M., Chen S.-S. & Black C. C. J. Environmental responses of the post-lower illumination CO2 burst as related to leaf photorespiration. Plant Physiol. 73, 25–30 (1983). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomimatsu H. et al. High CO2 concentration increases relative leaf carbon gain under dynamic light in Dipterocarpus sublamellatus seedlings in a tropical rain forest, Malaysia. Tree Physiol. 34, 944–954 (2014). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.