Abstract
Vitamin D is an important modulator of the immune response. It acts over several immune cell types where the Vitamin D receptor (VDR) is expressed. Due to the high relevance of this signaling pathway, several studies have investigated the possible influence of genes involved in the metabolism of Vitamin D and its receptor in different human diseases. Here, we analyzed whether four single-nucleotide polymorphisms of the VDR gene (rs731236, rs7975232, rs1544410 and rs2228570) are involved in the susceptibility to infection by Trypanosoma cruzi and/or to chronic Chagas cardiomyopathy (CCC) in a Colombian endemic population for this parasite. Our results showed that the rs2228570*A allele is associated with CCC development (P = 4.46E−03, OR = 1.51). In summary, the data presented in this report suggest that variation within the VDR gene may affect the immune response against T. cruzi, increasing the probability of cardiac complications in infected individuals.
Vitamin D is an important modulator of the immune system1,2. This molecule is involved in both the innate and adaptive responses acting on a wide spectrum of immune cells, in which the Vitamin D receptor (VDR) is expressed3. VDR is a member of the nuclear receptor superfamily. Binding of this molecule with the activated form of Vitamin D (1,25(OH)2D3) causes its dimerization with retinoid X receptor (RXR), which control the transcription of specific genes by interacting with the RNA polymerase II1,4.
Chagas disease represents an infectious condition caused by the flagellated protozoan Trypanosoma cruzi that currently affects around 6 million people in Latin America5. Although infected people do not usually develop further complications after the acute phase of disease, up to one third will suffer from chronic Chagas cardiomyopathy (CCC) a condition that affects the life quality and could lead to premature death6,7. The exact causes of the differential disease outcomes are largely unknown, but increasing knowledge suggests that immune gene variants could play a relevant role by influencing the adequate inflammatory response against the parasitic invasion8,9,10,11,12. Consistent with this, several members of the inflammatory pathway have been recently associated with risk of infection by T. cruzi and/or development of CCC, including genes encoding for HLA class-II molecules (DRB1 and DQB1), the chemokine receptor CCR5, interferon-gamma (IFN-γ), tumor necrosis factor alpha (TNF-α), migration inhibitory factor (MIF), and the interleukins IL-1, IL-12B, IL-17A, IL-18, among others13,14,15,16,17,18,19,20.
Due to the high relevance of Vitamin D and its receptor in the immune homeostasis, several studies have investigated the possible influence of genes involved in their metabolism in the development of autoimmune conditions and infectious diseases2,21. In this regard, some polymorphisms of the VDR gene have been reported to be associated with different outcomes in autoimmune and infectious diseases, especially in tuberculosis4,21.
Taking the above into consideration, we aimed to explore for the first time the possible implication of VDR genetic variation in Chagas disease susceptibility and clinical manifestation.
Results
The genotype frequencies of the four analyzed VDR variants did not deviate from Hardy-Weinberg equilibrium in the different subsets (P > 0.01), and their genotyping success rate was over 95%. The statistical power of this study is detailed in Table 1. The SNPs rs731236, rs7975232 and rs1544410 showed a relatively high LD in our study population (Fig. 1). Particularly, rs731236 and rs1544410 had an r2 value = 0.93. On the contrary, rs2228570 had an r2 value <0.10 with the other SNPs.
Table 1. Statistical power calculation of our study considering three different OR.
| Statistical power calculation | ||
|---|---|---|
| T. cruzi infection (436/547)* | Chronic Chagasic cardiomyopathy (171/376)** | |
| OR = 1.50 | 98% | 82% |
| OR = 1.25 | 60% | 35% |
| OR = 1.10 | 16% | 10% |
The estimation was performed considering a prevalence of 1.44% and a minor allele frequency of 25%.
*Analysis performed by using 436 seronegative vs. 547 seropositive individuals.
**Analysis performed by using 171 asymptomatic vs. 376 chronic Chagas cardiomyopathy individuals.
Figure 1.
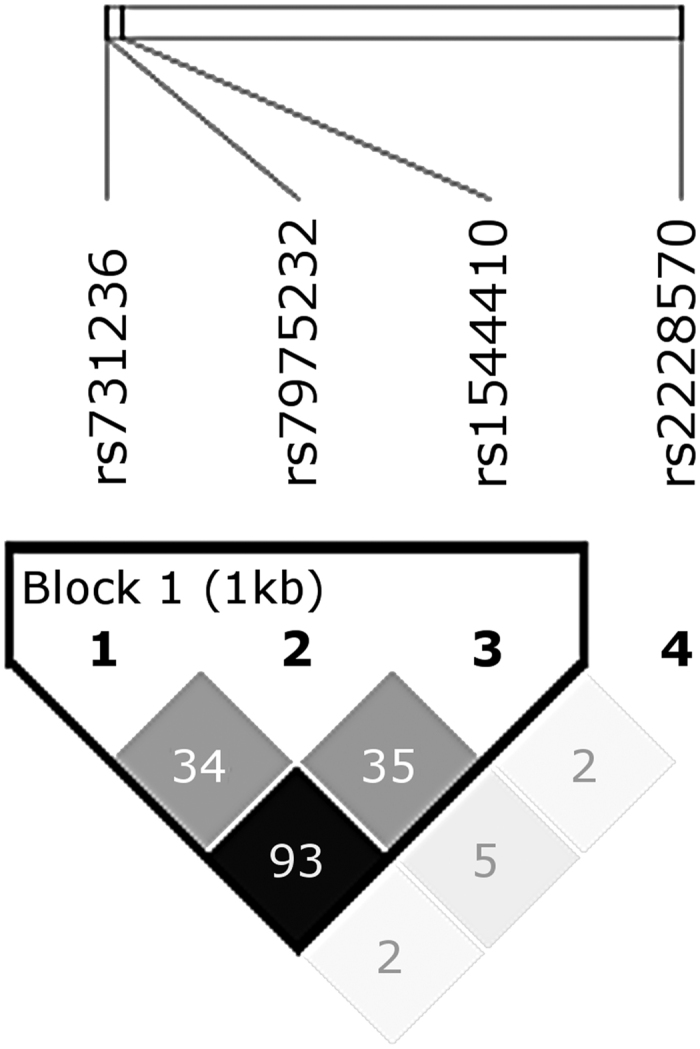
R-Squared plot of analyzed VDR gene variants estimated by using expectation-maximization algorithm in Haploview V4.2.
First, to analyze the possible implication of the VDR polymorphisms in the susceptibility to infection by T. cruzi, the allelic and genotypic frequencies of seronegative and seropositive individuals were compared (Table 2). No statistical significance was observed for rs731236, rs7975232 and rs1544410, indicating that these variants may not influence the risk of infection by T. cruzi in the studied population. On the other hand, the allele frequencies of rs2228570 differed significantly between the seronegative and seropositive groups (P = 0.0287, OR = 0.81, 95% CI = 0.67−0.98). The minor allele rs2228570*A was overrepresented in the seronegative subset (45.41% vs. 39.94%), suggesting a possible protective effect of this variant against infection by T. cruzi. However, the statistical significance was lost after correction for multiple testing (P = 0.1147).
Table 2. Logistic regression analysis of VDR polymorphisms in seronegative and seropositive individuals including age as covariate.
| SNP | 1|2 | Group (N) | Genotype. N (%) |
MAF % | Allele test/Age |
||||
|---|---|---|---|---|---|---|---|---|---|
| 1|1 | 1|2 | 2|2 | P | PFDR | OR [95% CI] | ||||
| rs731236 | G|A | Seronegative (435) | 42 (9.66) | 166 (38.16) | 227 (52.18) | 28.74 | |||
| Seropositive (545) | 43 (7.89) | 228 (41.83) | 274 (50.28) | 28.81 | 0.9024 | 0.9024 | 1.01 [0.83–1.24] | ||
| rs7975232 | C|A | Seronegative (436) | 96 (22.02) | 233 (53.44) | 107 (24.54) | 48.74 | |||
| Seropositive (532) | 97 (18.23) | 291 (54.70) | 144 (27.07) | 45.58 | 0.2206 | 0.4412 | 0.88 [0.72–1.08] | ||
| rs1544410 | T|C | Seronegative (434) | 39 (8.99) | 166 (38.25) | 229 (52.76) | 28.11 | |||
| Seropositive (535) | 43 (8.04) | 227 (42.43) | 265 (49.53) | 29.25 | 0.4677 | 0.6236 | 1.08 [0.88–1.33] | ||
| rs2228570 | A|G | Seronegative (436) | 89 (20.41) | 218 (50.00) | 129 (29.59) | 45.41 | |||
| Seropositive (542) | 83 (15.31) | 267 (49.26) | 192 (35.42) | 39.94 | 0.0287 | 0.1147 | 0.81 [0.67–0.98] | ||
Next, we evaluated the possible association between the VDR SNPs and the susceptibility to develop CCC. For that, we compared the allelic and genotypic frequencies of asymptomatic and CCC patients (Table 3). Similar to that observed in the previous analysis, no differences in the allele frequencies of both subgroups of patients were observed for the analyzed SNPs except for rs2228570 (P = 4.46E−03, OR = 1.51, 95% CI = 1.14−2.00). This association was maintained after controlling for multiple testing (P = 0.0178). In this case, the frequency of the rs2228570*A allele was reduced in the asymptomatic patients (34.41% vs. 42.47%), suggesting a putative protective role of this variant against CCC development.
Table 3. Logistic regression analysis of VDR polymorphisms in asymptomatic and chronic Chagas cardiomyopathy (CCC) individuals. including age as covariate.
| SNP | 1|2 | Group (N) | Genotype. N (%) |
MAF % | Allele test/Age |
||||
|---|---|---|---|---|---|---|---|---|---|
| 1|1 | 1|2 | 2|2 | P | PFDR | OR [95% CI] | ||||
| rs731236 | G|A | Asymptomatic (170) | 17 (10.00) | 69 (40.59) | 84 (49.41) | 30.29 | |||
| CCC (375) | 26 (6.93) | 159 (42.40) | 190 (50.67) | 28.13 | 0.4406 | 0.4633 | 0.89 [0.67–1.19] | ||
| rs7975232 | C|A | Asymptomatic (164) | 28 (17.07) | 88 (53.66) | 48 (29.27) | 43.90 | |||
| CCC (368) | 69 (18.75) | 203 (55.16) | 96 (26.09) | 46.33 | 0.4304 | 0.4633 | 1.12 [0.84–1.49] | ||
| rs1544410 | T|C | Asymptomatic (168) | 16 (9.52) | 72 (42.86) | 80 (47.62) | 30.95 | |||
| CCC (367) | 27 (7.36) | 155 (42.23) | 185 (50.41) | 28.47 | 0.4633 | 0.4633 | 0.90 [0.67–1.20] | ||
| rs2228570 | A|G | Asymptomatic (170) | 21 (12.35) | 75 (44.12) | 74 (43.53) | 34.41 | |||
| CCC (372) | 69 (16.67) | 192 (51.61) | 118 (31.72) | 42.47 | 4.46E-03 | 0.0178 | 1.51 [1.14–2.00] | ||
On the other hand, a possible haplotypic effect of the studied VDR SNPs was also tested. Due to the high LD in this population (Fig. 1), only three haplotypes were observed in the studied individuals (rs731236|rs7975232|rs1544410: ACC, GAT and AAC). No evidence of association was observed for any haplotype in the different tests performed (seronegative vs. seropositive and asymptomatic vs. CCC, data not shown).
Discussion
In this study, four genetic variants of the VDR gene were tested for association with risk of infection by T. cruzi and/or development of CCC in a population from a Colombian endemic region of Chagas disease. Our data provides strong evidence that the VDR SNP rs2228570 is associated with CCC in infected individuals, as the odds of having the minor allele was significantly increased in symptomatic individuals as compared to asymptomatic patients. This polymorphism is located at the 5′ end of the VDR coding sequence. It has been reported that the presence of the minor allele originates an alternative starting transcription site, which leads to a longer isoform with a reduced transcription activity. Therefore, it has been proposed that the rs2228570 variant may affect the responsiveness to vitamin D22,23.
Vitamin D is a key molecule of the immune system. The active form of this compound seems to potentiate phagocytosis by macrophages and the production of antimicrobial peptides1,2. Additionally, it has been observed that it shifts the immune response from TH1/TH17 towards TH2 by inhibiting the production of IFN-γ, IL-12, IL-17, and IL-21, among other cytokines2. In Chagas disease, the infection by T. cruzi causes a coordinated immune response. That is, in the first line of defense, the innate response is initiated by dendritic cells and macrophages, which produce IL-12 and TNF-α after recognizing the parasite. These cytokines activate natural killer cells that, in turn, enhance the production on IFN-γ. As a consequence, the parasite clearance is facilitated by shifting to a TH1 dominant response that controls the infection8. Hence, proinflammatory cytokines, such as IFN-γ, IL-12, IL-17 and TNF-α, are essential for controlling the parasite24,25,26,27,28. In addition, several studies have suggested that the persistence of T. cruzi in the organism is directly related to the development of severe complications in Chagas disease29,30,31. Our results are in agreement with the hypothesis that a stronger immune response may protect patients against the persistence of the parasite. Our data showed that the rs2228570*A allele, which allows the transcription of the enlarged VDR isoform, is more prevalent in CCC patients compared with asymptomatic individuals. We speculate that vitamin D could influence the inflammatory response against T. cruzi by downregulating the expression of pro-inflammatory cytokines such as IFN-γ, IL-12 and IL-17. As a consequence, the parasitic persistence could be favored thus increasing the predisposition to develop cardiac complications in Chagas patients. The analysis of vitamin D profiles in individuals exposed to T. cruzi infection with different degrees of cardiac involvement would shed light into this idea.
On the other hand, the frequency of the VDR allele rs2228570*A was increased in the seronegative group compared to the seropositive one. In principle, this may be contradictory to the above, as a more potent innate response should lead to a quicker clearance of the parasite in infected individuals before the production of antibodies. However, the statistical significance of this result was lost after correction for multiple testing, which indicates a lack of consistency of this putative association. Further analyses in larger populations are required to clarify this issue.
The role of vitamin D in other protozoan infections is not clear. In malaria, for instance, two different studies indicated that this molecule plays an important role in the control of the immune pathogenesis and cerebral malaria32,33,34. Vitamin D was observed to reduce the risk of cerebral malaria in mice, thus suggesting that it may attenuate the inflammatory response leading to an increase of survival rates32. However, evidences also point to vitamin D deficiency as directly responsible for severe cerebral malaria in children coming from Uganda33. To our knowledge, only one study has evaluated the possible association between VDR genetic variation and malaria. Specifically, two VDR SNPs, rs731236 and rs1544410, were shown to affect gametocytemia levels in individuals infected with Plasmodium vivax34. In Leishmania, two different studies indicated that suppression of both vitamin D and VDR in mice favor the parasite eradication in a TH1 dependent manner35,36, supporting our hypothesis that vitamin D likely promotes parasite clearance in Chagas.
Regarding the study of VDR gene polymorphisms in other infectious diseases, rs2228570 has been associated with tuberculosis in populations of different ethnicities, although the results are contradictory. For instance, a meta-analysis and a case/control study showed that the rs2228570*A allele conferred risk to this condition in Chinese and Iranian patients, respectively37,38. However, other studies reported associations of this same allele with protection against pulmonary tuberculosis in a Moroccan population39. VDR rs2228570 has been evaluated also in leprosy, an infectious disease caused by another species of Mycobacterium. In this case, homozygosity of rs2228570*A was associated with a higher risk to develop leprosy in an Indian population40.
Thus, it seems clear that the vitamin D signaling has a major role in the immune response against different infectious diseases, and this has led to propose that controlling the uptake and metabolic status of vitamin D may be useful to improve the current therapeutic strategies in these conditions1,41. Consequently, future studies aimed to analyze the levels of vitamin D in the different groups of individuals included in this study, could represent an important step forward towards the understanding of Chagas disease development and treatment. Replication of the genetic results reported here in larger independent cohorts would be also desirable to confirm our findings.
In conclusion, we have observed a genetic association between the VDR gene polymorphism rs2228570 and risk to develop CCC in Chagas patients. Although this association has a clear functional implication, more powered studies and functional experiments are needed to definitively confirm the involvement of the vitamin D signaling in the development of this severe complication after infection by T. cruzi.
Material and Methods
Study subjects
For this study, 1,172 individuals from the endemic regions for T. cruzi Guanentina and Comunera at the Santander Department, Colombia (localized between 5°26′ and 8°08′ north and 72°26′ and 74°32′ west) (Supplementary Figure S1) were enrolled. These provinces are located on the north-east side of the country, and their population is a homogeneous mix with no specific concentration of any ethnicity. Regarding the selection criteria, participants were recruited either after a medical visit to the endemic area or after attending to “Fundación Cardiovascular de Colombia”, a medical institution specialized in cardiovascular disorders situated in the city of Floridablanca (Supplementary Figure S1), where they were diagnosed with Chagas disease. Almost all invited individuals agreed to participate in this study. Since Chagas disease is a chronical disorder in which symptoms may appear after several years of infection7,8, we decided to exclude those individuals younger than 30 years old (189 in total) in order to perform a more consistent analysis of possible resistance to the infection (as the selected individuals had a longer time of exposure to vectorial infection). In total, 983 individuals were finally included in the analyses. Then, the individuals were classified as seronegative or seropositive (n = 436 and 547, respectively) for T. cruzi antigens accordingly to two different commercial immunological tests, the enzyme-linked immunosorbent assay (ELISA) (BioELISA Chagas, Biokit, Lliçà d’Amunt, Barcelona, Spain) and indirect hemagglutination (Chagatest IHA, Wiener Lab, Rosario, Argentina). All seronegative individuals were negative for both tests. Seropositive patients underwent an exhaustive clinical evaluation and were subsequently subdivided into asymptomatic and chronic Chagas cardiomyopathy (CCC) patients (n = 171 and 376, respectively) based on electrocardiogram and echocardiogram information. This classification is based in the guidelines from the World Health Organization (WHO) (http://www.who.int/mediacentre/factsheets/fs340/en/), the Pan-American Health Organization (PAHO) (http://www.paho.org/hq/index.php?option=com_topics&view=article&id=10&Itemid=40743), and the classification established by the international consensus of Buenos Aires of 2010 (http://www.fac.org.ar/7cvc/llave/c016/mordinio.pdf). The mean age of participants was 52.11 years for seronegative individuals, 56.67 for asymptomatic individuals and 62.66 for CCC patients. The sex distribution for the entire group was 55% female and 45% male.
Ethics statement
All participants signed an informed consent. This study was approved by the Act No. 15 of 2005 by the Ethics Committees from “Universidad Industrial de Santander” and “Fundación Cardiovascular de Colombia” in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki.
SNP selection
Following a candidate gene strategy, four single-nucleotide polymorphisms (SNPs) of the VDR gene, previously described to have a functional implication on gene expression and function, were selected for this study22,23,42,43. These variants include rs731236 (Taq1), rs7975232 (Apa1), rs1544410 (Bsm1), and rs2228570 (Fok1). The three first SNPs have been related to a differential expression of VDR in different cells, whereas the latter represents a cytosine to thymine change in an ACG codon, which creates an alternative start site that produces a longer VDR protein with a reduced transcriptional activity22,23,42,43.
DNA extraction and Genotyping
Genomic DNA was isolated from 5–10 ml of EDTA anticoagulated blood samples using standard salting-out techniques as previously described44. The four SNPs were genotyped using TaqMan allelic discrimination assays from Applied Biosystems (Foster City, California, USA; catalog numbers: C___2404008_10, C__28977635_10, C___8716062_10, C__12060045_20). The genotyping was performed on a LightCycler 480 real-time PCR system (Roche Diagnostics, Basel, Switzerland).
Statistical analysis
All the statistical analyses were performed with the statistical software package Plink V1.07 (http://pngu.mgh.harvard.edu/purcell/plink)45. For all groups of individuals, possible deviance from Hardy-Weinberg equilibrium was determined in every SNP at the 1% significance level. To test for possible allelic and genotypic associations with disease susceptibility and clinical complications, we compared the allelic, genotypic and haplotypic frequencies of the VDR variants between seronegative vs. seropositive individuals and asymptomatic vs. CCC individuals by logistic regression assuming an additive model and using age as covariate (as in Chagas disease symptoms may appear many years after infection). The Benjamini & Hochberg step-up false discovery rate (FDR) correction was used in all analyses to control for possible multiple testing effects. Odds ratios (OR) and 95% confidence intervals (CI) were calculated according to Woolf’s method. P-values lower than 0.05 were considered as statistically significant. Pairwise linkage disequilibrium (LD) (D’ and r2) and haplotypic blocks were estimated using an expectation–maximization algorithm as implemented in Haploview v4.246. The statistical power of our study was calculated with the Power Calculator for Genetic Studies 2006 (CaTS) software (http://www.sph.umich.edu/csg/abecasis/CaTS/)47.
Additional Information
How to cite this article: Leon Rodriguez D. A. et al. Evaluation of VDR gene polymorphisms in Trypanosoma cruzi infection and chronic Chagasic cardiomyopathy. Sci. Rep. 6, 31263; doi: 10.1038/srep31263 (2016).
Supplementary Material
Acknowledgments
This work is part of Doctoral Thesis “Estudio de las bases genéticas de la enfermedad de Chagas” of the Biomedicine PhD program of the Universidad de Granada, Spain. This work was supported by donations through the project no. 1102-633-38974 approved by Departamento Administrativo de Ciencia, Tecnología e Innovación, Colciencias, and Universidad Industrial de Santander, Bucaramanga, Colombia. We thank to Luis Eduardo Echeverria (from the Fundación Cardiovascular de Colombia Floridablanca, Santander, Colombia) for the care and management of patients, Sofía Vargas, Sonia García and Gema Robledo (from the Instituto de Parasitología y Biomedicina ‘López-Neyra’, CSIC, Spain) for their excellent technical assistance and all of the patients and healthy controls for kindly providing their essential collaboration.
Footnotes
Author Contributions D.A.L.R. made the experiments; D.A.L.R. and F.D.C. performed the statistical analyses; D.A.L.R. and F.D.C. wrote the manuscript; C.I.G. and J.M. reviewed the manuscript.
References
- Baeke F., Takiishi T., Korf H., Gysemans C. & Mathieu C. Vitamin D: modulator of the immune system. Current opinion in pharmacology 10, 482–496, doi: 10.1016/j.coph.2010.04.001 (2010). [DOI] [PubMed] [Google Scholar]
- Van Belle T. L., Gysemans C. & Mathieu C. Vitamin D in autoimmune, infectious and allergic diseases: a vital player? Best practice & research. Clinical endocrinology & metabolism 25, 617–632, doi: 10.1016/j.beem.2011.04.009 (2011). [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhu J. & DeLuca H. F. Where is the vitamin D receptor? Archives of biochemistry and biophysics 523, 123–133, doi: 10.1016/j.abb.2012.04.001 (2012). [DOI] [PubMed] [Google Scholar]
- Haussler M. R. et al. The nuclear vitamin D receptor: biological and molecular regulatory properties revealed. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 13, 325–349, doi: 10.1359/jbmr.1998.13.3.325 (1998). [DOI] [PubMed] [Google Scholar]
- Chagas disease in Latin America: an epidemiological update based on 2010 estimates. Releve epidemiologique hebdomadaire/Section d’hygiene du Secretariat de la Societe des Nations = Weekly epidemiological record/Health Section of the Secretariat of the League of Nations 90, 33-43 (2015). [PubMed]
- Rassi A. Jr., Rassi A. & Marin-Neto J. A. Chagas disease. Lancet 375, 1388–1402, doi: 10.1016/S0140-6736(10)60061-X (2010). [DOI] [PubMed] [Google Scholar]
- Bern C. Chagas’ Disease. The New England journal of medicine 373, 456–466, doi: 10.1056/NEJMra1410150 (2015). [DOI] [PubMed] [Google Scholar]
- Junqueira C. et al. The endless race between Trypanosoma cruzi and host immunity: lessons for and beyond Chagas disease. Expert reviews in molecular medicine 12, e29, doi: 10.1017/S1462399410001560 (2010). [DOI] [PubMed] [Google Scholar]
- Andrade D. V., Gollob K. J. & Dutra W. O. Acute chagas disease: new global challenges for an old neglected disease. PLoS neglected tropical diseases 8, e3010, doi: 10.1371/journal.pntd.0003010 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasconcelos R. H., Montenegro S. M., Azevedo E. A., Gomes Y. M. & Morais C. N. Genetic susceptibility to chronic Chagas disease: an overview of single nucleotide polymorphisms of cytokine genes. Cytokine 59, 203–208, doi: 10.1016/j.cyto.2012.04.035 (2012). [DOI] [PubMed] [Google Scholar]
- Ayo C. M. et al. Genetic susceptibility to Chagas disease: an overview about the infection and about the association between disease and the immune response genes. BioMed research international 2013, 284729, doi: 10.1155/2013/284729 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cunha-Neto E. & Chevillard C. Chagas disease cardiomyopathy: immunopathology and genetics. Mediators of inflammation 2014, 683230, doi: 10.1155/2014/683230 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nieto A. et al. HLA haplotypes are associated with differential susceptibility to Trypanosoma cruzi infection. Tissue antigens 55, 195–198 (2000). [DOI] [PubMed] [Google Scholar]
- Calzada J. E., Nieto A., Beraun Y. & Martin J. Chemokine receptor CCR5 polymorphisms and Chagas’ disease cardiomyopathy. Tissue antigens 58, 154–158 (2001). [DOI] [PubMed] [Google Scholar]
- Florez O., Zafra G., Morillo C., Martin J. & Gonzalez C. I. Interleukin-1 gene cluster polymorphism in chagas disease in a Colombian case-control study. Human immunology 67, 741–748, doi: 10.1016/j.humimm.2006.06.004 (2006). [DOI] [PubMed] [Google Scholar]
- Zafra G., Morillo C., Martin J., Gonzalez A. & Gonzalez C. I. Polymorphism in the 3’ UTR of the IL12B gene is associated with Chagas’ disease cardiomyopathy. Microbes and infection/Institut Pasteur 9, 1049–1052, doi: 10.1016/j.micinf.2007.04.010 (2007). [DOI] [PubMed] [Google Scholar]
- Torres O. A. et al. Role of the IFNG +874T/A polymorphism in Chagas disease in a Colombian population. Infection, genetics and evolution: journal of molecular epidemiology and evolutionary genetics in infectious diseases 10, 682–685, doi: 10.1016/j.meegid.2010.03.009 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Criado L., Florez O., Martin J. & Gonzalez C. I. Genetic polymorphisms in TNFA/TNFR2 genes and Chagas disease in a Colombian endemic population. Cytokine 57, 398–401, doi: 10.1016/j.cyto.2011.12.007 (2012). [DOI] [PubMed] [Google Scholar]
- Leon Rodriguez D. A., Echeverria L. E., Gonzalez C. I. & Martin J. Investigation of the role of IL17A gene variants in Chagas disease. Genes and immunity 16, 536–540, doi: 10.1038/gene.2015.42 (2015). [DOI] [PubMed] [Google Scholar]
- Machuca M. A., Suarez E. U., Echeverria L. E., Martin J. & Gonzalez C. I. SNP/haplotype associations of CCR2 and CCR5 genes with severity of chagasic cardiomyopathy. Human immunology 75, 1210–1215, doi: 10.1016/j.humimm.2014.09.023 (2014). [DOI] [PubMed] [Google Scholar]
- Adams J. S., Liu P. T., Chun R., Modlin R. L. & Hewison M. Vitamin D in defense of the human immune response. Annals of the New York Academy of Sciences 1117, 94–105, doi: 10.1196/annals.1402.036 (2007). [DOI] [PubMed] [Google Scholar]
- Saijo T. et al. A unique mutation in the vitamin D receptor gene in three Japanese patients with vitamin D-dependent rickets type II: utility of single-strand conformation polymorphism analysis for heterozygous carrier detection. American journal of human genetics 49, 668–673 (1991). [PMC free article] [PubMed] [Google Scholar]
- Arai H. et al. A vitamin D receptor gene polymorphism in the translation initiation codon: effect on protein activity and relation to bone mineral density in Japanese women. Journal of bone and mineral research: the official journal of the American Society for Bone and Mineral Research 12, 915–921, doi: 10.1359/jbmr.1997.12.6.915 (1997). [DOI] [PubMed] [Google Scholar]
- Hunter C. A., Slifer T. & Araujo F. Interleukin-12-mediated resistance to Trypanosoma cruzi is dependent on tumor necrosis factor alpha and gamma interferon. Infection and immunity 64, 2381–2386 (1996). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holscher C. et al. Defective nitric oxide effector functions lead to extreme susceptibility of Trypanosoma cruzi-infected mice deficient in gamma interferon receptor or inducible nitric oxide synthase. Infection and immunity 66, 1208–1215 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- da Matta Guedes P. M. et al. IL-17 produced during Trypanosoma cruzi infection plays a central role in regulating parasite-induced myocarditis. PLoS neglected tropical diseases 4, e604, doi: 10.1371/journal.pntd.0000604 (2010). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyazaki Y. et al. IL-17 is necessary for host protection against acute-phase Trypanosoma cruzi infection. J Immunol 185, 1150–1157, doi: 10.4049/jimmunol.0900047 (2010). [DOI] [PubMed] [Google Scholar]
- Pissetti C. W. et al. Genetic and functional role of TNF-alpha in the development Trypanosoma cruzi infection. PLoS neglected tropical diseases 5, e976, doi: 10.1371/journal.pntd.0000976 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Girones N. & Fresno M. Etiology of Chagas disease myocarditis: autoimmunity, parasite persistence, or both? Trends in parasitology 19, 19–22 (2003). [DOI] [PubMed] [Google Scholar]
- Gutierrez F. R., Guedes P. M., Gazzinelli R. T. & Silva J. S. The role of parasite persistence in pathogenesis of Chagas heart disease. Parasite immunology 31, 673–685, doi: 10.1111/j.1365-3024.2009.01108.x (2009). [DOI] [PubMed] [Google Scholar]
- Higuchi Mde L., Benvenuti L. A., Martins Reis M. & Metzger M. Pathophysiology of the heart in Chagas’ disease: current status and new developments. Cardiovascular research 60, 96–107 (2003). [DOI] [PubMed] [Google Scholar]
- He X. et al. Vitamin D inhibits the occurrence of experimental cerebral malaria in mice by suppressing the host inflammatory response. J Immunol 193, 1314–1323, doi: 10.4049/jimmunol.1400089 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cusick S. E., Opoka R. O., Lund T. C., John C. C. & Polgreen L. E. Vitamin D insufficiency is common in Ugandan children and is associated with severe malaria. PloS one 9, e113185, doi: 10.1371/journal.pone.0113185 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sortica V. A. et al. Role of IL6, IL12B and VDR gene polymorphisms in Plasmodium vivax malaria severity, parasitemia and gametocytemia levels in an Amazonian Brazilian population. Cytokine 65, 42–47, doi: 10.1016/j.cyto.2013.09.014 (2014). [DOI] [PubMed] [Google Scholar]
- Ehrchen J. et al. Vitamin D receptor signaling contributes to susceptibility to infection with Leishmania major. FASEB journal: official publication of the Federation of American Societies for Experimental Biology 21, 3208–3218, doi: 10.1096/fj.06-7261com (2007). [DOI] [PubMed] [Google Scholar]
- Whitcomb J. P. et al. The Role of Vitamin D and Vitamin D Receptor in Immunity to Leishmania major Infection. Journal of parasitology research 2012, 134645, doi: 10.1155/2012/134645 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C., Liu Q., Zhu L., Yang H. & Lu W. Vitamin D receptor gene polymorphisms on the risk of tuberculosis, a meta-analysis of 29 case-control studies. PloS one 8, e83843, doi: 10.1371/journal.pone.0083843 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salimi S., Farajian-Mashhadi F., Alavi-Naini R., Talebian G. & Narooie-Nejad M. Association between vitamin D receptor polymorphisms and haplotypes with pulmonary tuberculosis. Biomedical reports 3, 189–194, doi: 10.3892/br.2014.402 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arji N. et al. Genetic diversity of TLR2, TLR4, and VDR loci and pulmonary tuberculosis in Moroccan patients. Journal of infection in developing countries 8, 430–440, doi: 10.3855/jidc.3820 (2014). [DOI] [PubMed] [Google Scholar]
- Neela V. S. et al. Association of Taq I, Fok I and Apa I polymorphisms in Vitamin D Receptor (VDR) gene with leprosy. Human immunology 76, 402–405, doi: 10.1016/j.humimm.2015.04.002 (2015). [DOI] [PubMed] [Google Scholar]
- Korf H., Decallonne B. & Mathieu C. Vitamin D for infections. Current opinion in endocrinology, diabetes, and obesity 21, 431–436, doi: 10.1097/MED.0000000000000108 (2014). [DOI] [PubMed] [Google Scholar]
- Ma J. et al. Vitamin D receptor polymorphisms, circulating vitamin D metabolites, and risk of prostate cancer in United States physicians. Cancer epidemiology, biomarkers & prevention: a publication of the American Association for Cancer Research, cosponsored by the American Society of Preventive Oncology 7, 385–390 (1998). [PubMed] [Google Scholar]
- Morrison N. A. et al. Prediction of bone density from vitamin D receptor alleles. Nature 367, 284–287, doi: 10.1038/367284a0 (1994). [DOI] [PubMed] [Google Scholar]
- Miller S. A., Dykes D. D. & Polesky H. F. A simple salting out procedure for extracting DNA from human nucleated cells. Nucleic Acids Res 16, 1215 (1988). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purcell S. et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. American journal of human genetics 81, 559–575, doi: 10.1086/519795 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barrett J. C., Fry B., Maller J. & Daly M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265, doi: 10.1093/bioinformatics/bth457 (2005). [DOI] [PubMed] [Google Scholar]
- Skol A. D., Scott L. J., Abecasis G. R. & Boehnke M. Joint analysis is more efficient than replication-based analysis for two-stage genome-wide association studies. Nature genetics 38, 209–213, doi: 10.1038/ng1706 (2006). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


