Abstract
Our previous work implied that interleukin 6 (IL6) may be a biological marker for major depressive disorder (MDD). In this study, we performed a comprehensive genetic study to determine the association between the gene encoding IL6 (IL6) and MDD in Han Chinese. There were 50 drug-naïve MDD patients and 50 healthy controls undergoing an mRNA expression study. A sample of 772 patients with MDD and 759 healthy controls were used for genetic analysis. Next, we performed an eQTL analysis to identify whether risk SNP(s) is associated with IL6 expression in brain. Our results showed that patients with MDD have higher levels of IL6 than healthy controls (P = 0.008). The SNP rs1800797 has a significant association with MDD (P = 0.01) in a dominant model. The eQTL analysis showed a marginally significant association between the rs1800797 and IL6 expression in the frontal cortex (P = 0.087). Our preliminary findings are suggestive of an association between rs1800797 and the risk of MDD. Further investigations are required to evaluate this association in larger samples to increase statistical power, and to examine the correlation between rs1800797 and IL6 methylation patterns.
Major depressive disorder (MDD) is a severe mental disorder, typically characterized by a cluster of emotional and somatic symptoms at psychological level1, and abnormal brain connectivity and functioning at physical level2. MDD is prevalent in excess of 17% among Han Chinese3 and also becoming a leading cause of disability and mortality in the worldwide. Thus, it is one of the most urgent challenges for current psychiatric research to understand the pathogenesis of MDD
It has been confirmed that the heritability of MDD is estimated up to 70%4 and recent studies into its genetic etiology have detected a number of susceptibility genes and chromosomal regions implicated with immune system5,6. Clinical studies have widely demonstrated aberrant inflammation profiles of MDD patients in either central neural system (CNS) or peripheral tissues7,8. Therefore, one emerging hypothesis for this association is that chronic low-grade activation of inflammation and the immune system likely contribute to some of the biological mechanisms in the development of MDD9.
Our previous work using whole-genome cRNA microarrays found that genes associated with MDD were enriched in interleukin 6 (IL6)-mediated signaling events10. Similar results were also reported in the Netherlands11. IL6 is a multifunctional cytokine that regulates the growth and differentiation of various tissues, and plays an important role in the immune response and acute phase reactions12. Goldsmith et al.13 performed a meta-analysis of blood cytokines in 18 studies for MDD. The authors observed enhanced level of IL6 in acutely MDD patients, and following treatment for acute phase, IL6 level significantly decreased in patients with MDD. In CNS, IL6 acts as a neurotrophic cytokine expressed in both neurons and glia14, whose level is also reported to be increased in the cerebrospinal fluid of MDD patients15. Such findings provided suggestive evidence for the role of IL6 in the pathophysiology of MDD.
At the molecular level, the human gene encoding IL6 (IL6) is mapped to chromosome 7p15, a candidate region previously implicated in MDD16. Unsurprisingly, genetic variations of IL6 have been reported to modulate the chronic stress exposure in the development of depressive symptoms17, and increase the risk of interferon-induced depression18. Based on this premise, it is plausible that IL6 is likely to be a promising candidate gene for MDD susceptibility.
In this study, we hypothesized that IL6 may be a susceptibility gene for MDD. First, we analyzed the IL6 mRNA expression difference between drug-naïve MDD patients and normal controls. Given the relevance of genetic variations of IL6 to depressive symptoms, we subsequently investigated whether IL6 is genetically associated with MDD among a Chinese Han population. As a third aim, we performed an eQTL (expression quantitative trait loci) analysis via an available database to investigate the potential role of the risk SNP in IL6 mRNA expression in brain.
Results
In total, 50 drug-naïve patients with MDD and 50 healthy controls were measured for peripheral IL6 mRNA expression. The two cohorts were well matched in terms of age, gender and smoking status, but significant difference was observed in body mass index (BMI) (P = 0.03, Supplementary Table S1). Figure 1 showed that patients with MDD have higher levels of IL6 than healthy controls (P = 0.008).
Figure 1. Expression levels of IL6 mRNA in peripheral blood in drug-naïve patients with major depressive disorder and healthy controls.
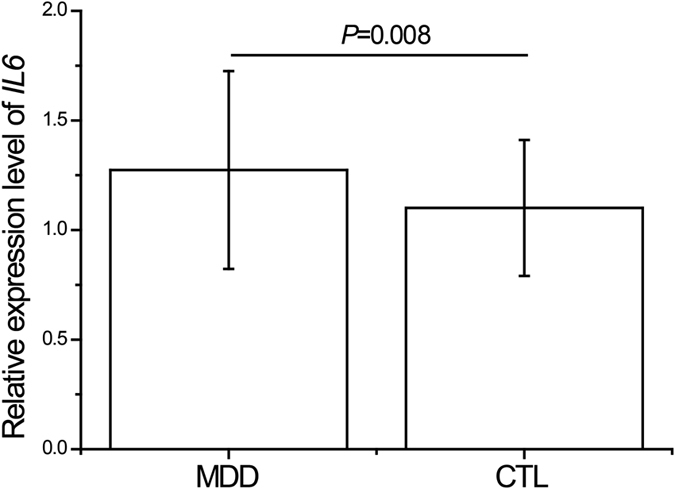
IL6 mRNA was normalized to that of GAPDH. MDD, major depressive disorder patients (n = 50); CTL, control controls (n = 50).
Genotype distributions of all studied polymorphisms in control group were consistent with the Hardy-Weinberg equilibrium (HWE) (P > 0.05). Statistical analyses of the data for the five polymorphisms were presented in Table 1. The SNP rs1800797 showed a significant association with MDD (P = 0.01) in a dominant model. The frequency of the A allele of rs1800797 was significantly higher among MDD patients than that among the controls (OR = 4.72, 95% CI: 1.60–13.89, P = 0.01). The pairwise LD among the 5 SNPs is presented in Supplementary Figure S1. Strong LD was observed between rs1524107 and rs2069837 (D’ = 1.00, r2 = 0.57). Supplementary Table S2 listed all P values corresponding to the haplotypes, with a haplotype frequency less than 3% being dropping. We did not find any significant association of the haplotypes consisting of rs1524107 and rs2069837 with MDD.
Table 1. Comparison of genotype and allele frequencies of IL6 SNPs between MDD and control groups.
| SNP | n | Genotype |
P | OR (95% CI) | P | P c | n | Allele |
OR (95% CI) | P | |||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| rs1800797 | AA | AG | GG | A | G | ||||||||
| Case | 772 | 0 | 19 (2.5) | 753 (97.5) | 0.002 | 4.76 (1.61–14.07)a | 0.002a | 1544 | 19 (1.2) | 1525 (98.8) | 4.72 (1.60–13.89) | 0.002 | |
| Control | 759 | 0 | 4 (0.5) | 755 (99.5) | NAb | NAb | 0.94 | 1518 | 4 (0.3) | 1514 (99.7) | |||
| rs1800796 | GG | GC | CC | G | C | ||||||||
| Case | 772 | 56 (7.3) | 346 (44.8) | 370 (47.9) | 0.48 | 1.06 (0.87–1.30)a | 0.57a | 1544 | 458 (29.7) | 1086 (70.3) | 1.01 (0.86–1.18) | 0.93 | |
| Control | 759 | 64 (8.4) | 320 (42.2) | 375 (49.4) | 0.85 (0.59–1.23)b | 0.39b | 0.72 | 1518 | 448 (29.5) | 1070 (70.5) | |||
| rs1800795 | CC | CG | GG | C | G | ||||||||
| Case | 772 | 0 | 16 (2.1) | 756 (97.9) | 0.06 | 2.27 (0.93–5.56)a | 0.09a | 1544 | 16 (1.0) | 1528 (99.0) | 2.26 (0.93–5.51) | 0.07 | |
| Control | 759 | 0 | 7 (0.9) | 752 (99.1) | NAb | NAb | 0.90 | 1518 | 7 (0.5) | 1511 (99.5) | |||
| rs2069837 | GG | GA | AA | G | A | ||||||||
| Case | 772 | 22 (2.8) | 269 (34.8) | 481 (62.3) | 0.70 | 1.06 (0.86–1.30)a | 0.60a | 1544 | 313 (20.3) | 1231 (79.7) | 1.03 (0.86–1.23) | 0.76 | |
| Control | 759 | 25 (3.3) | 251 (33.1) | 483 (63.6) | 0.86 (0.48–1.54)b | 0.66b | 0.27 | 1518 | 301 (19.8) | 1217 (80.2) | |||
| rs1524107 | CC | CT | TT | C | T | ||||||||
| Case | 772 | 58 (7.5) | 354 (45.9) | 360 (46.6) | 0.68 | 1.04 (0.85–1.27)a | 0.72a | 1544 | 470 (30.4) | 1074 (69.6) | 1.00 (0.86–1.17) | 1.00 | |
| Control | 759 | 64 (8.4) | 334 (44.0) | 361 (47.6) | 0.88 (0.61–1.28)b | 0.51b | 0.28 | 1518 | 462 (30.4) | 1056 (69.6) | |||
aP values in dominant model.
bP values in recessive model.
cP values for Hardy-Weinberg equilibrium in control group.
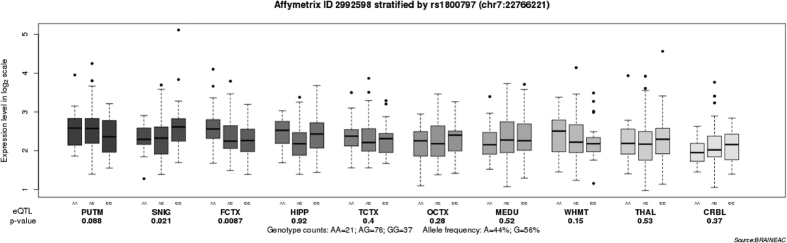
We then performed an eQTL analysis to investigate whether SNP rs1800797 influences the IL6 expression in the brain. As shown in Fig. 2, we observed a marginally significant association between the rs1800797 and IL6 expression in the frontal cortex (P = 0.087). Data showed that carriers with A allele have significantly higher levels of IL6 expression in frontal cortex that those without A allele.
Figure 2. Association of rs1800797 with IL6 mRNA expression levels in ten brain regions (Affymetrix ID 2992598).
Data were extracted from the BRAINEAC database (http://caprica.genetics.kcl.ac.uk/BRAINEAC/). SNIG, substantia nigra; PUTM, putamen (at the level of the anterior commissure); MEDU, the inferior olivary nucleus (sub-dissected from the medulla); THAL, thalamus (at the level of the lateral geniculate nucleus); OCTX, occipital cortex; HIPP, hippocampus; FCTX, frontal cortex; TCTX, temporal cortex; WHMT, intralobular white matter; CRBL, cerebellar cortex.
Discussion
As MDD etiology is known to be linked to inflammation in at least some cases and immune response has been proven to be genetically influenced19, it is speculated that genetic factors in immune dysfunction may be involved in the pathophysiology of MDD20. IL6, a key proinflammatory cytokine21, has been reported in the development of MDD in prior literature. Our previous work has provided suggestive evidence for its role in the etiology of MDD in Han Chinese. Herein, we performed a comprehensive analysis to investigate the association of IL6 with MDD in Chinese Han population. To the best of our knowledge, there is the first study to address this association.
In the first step, we tested the levels of IL6 mRNA expression in drug-naïve patients with MDD and healthy controls. Our results showed a significant higher level of IL6 expression in MDD group than that in control one. This is in line with previous reports22,23. On the other hand, the elevated level of IL6 in MDD patients can be reduced to a normal level after antidepressant treatment as the depressive symptoms improved23,24. Taken together, the above evidence implies that increased IL6 expression is possibly involved in the etiology of MDD.
Earlier studies have detected the genetic mechanism of IL6 in depressive symptoms. SNP rs1800795, a functional polymorphism in the promoter of IL6, was reported to increase the risk of stress-17 and interferon treatment-18 induced depression. However, it is not known about the role of IL6 variations in the etiology of MDD. Thus, we carried out a genetic study to evaluate the association of IL6 with MDD in Han Chinese. We found a MDD-associated SNP rs1800797 in a dominant model, and the frequency of the A allele of rs1800797 was significantly higher among MDD patients than that among the controls. The frequency of A allele in controls (0.5%) is similar to the reported data in Han Chinese (0.3%)25. Prior literature reported that rs1800797 polymorphism is associated with a number of human diseases, such as asthma26, type II diabetes27 and leprosy28 that were reported to have a high morbidity prevalence with MDD29,30,31. SNP rs1800797 presents in the promoter region of IL6. Variations in this region may lead to functional alteration of IL6 and ultimately influence the occurrence of human diseases32. Our eQTL analysis showed that the rs1800797 polymorphism have a regulatory effect on IL6 expression in the frontal cortex and individuals with A allele of rs1800797 have higher levels of IL6 expression than those without the A allele. These results imply that elevated levels of IL6 expression in the frontal cortex may be a risk factor for the development of MDD.
The neural network is believed to modulate aspects of normal emotional behavior and implicated in the pathophysiology of MDD33. Advances in neuroimaging techniques offer the potential to investigate the neural mechanism underlying MDD. Recently, we scanned patients with MDD and healthy controls using magnetic resonance imaging (MRI) to investigate the alterations in the cortical surface of MDD, and our findings suggest that frontal cortical alteration is a vulnerability to MDD during earlier neurodevelopmental process34. A functional MRI experiment showed that MDD patients exhibit abnormal long distance connectivity and dysregulation of large-scale neural networks in medial prefrontal cortex35. A number of positron emission tomography (PET) studies have repeatedly identified a decrease in metabolic activity in the prefrontal cortex in patients with MDD36,37,38. Hence, abnormalities in prefrontal cortex may be important for investigations of the pathophysiology of MDD. Furthermore, Setiawan et al.7 applied PET to examine a marker of neuroinflammation, translocator protein (TSPO) binding in vivo, in order to determine the neuroinflammatory hypothesis of MDD. Their results showed that the magnitidue of TSPO density elevation was 26% in the prefrontal cortex of patients with MDD than that of controls. This suggests that neuroinflammation activation leading to abnormal function in prefrontal cortex may contribute to the development of MDD. As such, it is remain unknown whether rs1800797 polymorphism is responsible for the MDD-related neuroinflammation in frontal cortex, and this will subsequently need to be investigated in future. Meanwhile, literature indicated that gene expression of IL6 is regulated by DNA methylation of its promoter region39. The region from positions −666 to −426 relative to the transcription state site in IL6 may be the potential binding sites for methylation40. SNP rs1800797 consists of a G to A substitution at the −597 site. We speculated that DNA methylation may explain the underlying mechanism of rs1800797 in the etiology of MDD. This also requires for further clarification.
There are two limitations to the present study. As known that MDD is a mental disorder that originates from brain dysfunction, we measured the peripheral level of IL6 mRNA expression in this study. Thus, further analyses using brain tissues are needed to validate our results. Second, our results showed that the A allele of rs1800797 was found in 4 out of 759 controls. Although the HWE P value for this SNP in controls is 0.94, such a low frequency in a small sample size could potentially bias the HWE and dilute the statistical power. Taking it into consideration, our findings should be considered only preliminary.
In conclusion, we performed a comprehensive analysis to detect the role of IL6 on the pathophysiology of MDD in Chinese Han population. Our preliminary findings are suggestive of an association between rs1800797 and the risk of MDD. Further investigations are required to evaluate this association in larger samples to increase statistical power, and to examine the correlation between rs1800797 and IL6 methylation patterns.
Methods
Participants
For the expression study, there were 50 drug-naïve MDD patients, and 50 healthy controls recruited from the Division of Mood Disorders, Shanghai Mental Health Center, Shanghai Jiao Tong University School of Medicine. Demographic data on age, gender, smoking status, BMI, alcoholic abuse, duration of illness prior to admission, number of episode, family history of mood disorders was collected. Assessments of the Hamilton Rating Scale for Depression −17 (HRSD-17) were conducted independently by two experienced psychiatrists (interrater reliability, kappa = 0.84)41.
For the genetic study, we enrolled the MDD samples from our previous clinical trials: the “OPERATION” (OPtimized trEatment stRAtergies for Treatment-resIstant depressiON) study42,43 and the “CARE-SSD/MDD” (Construct An Rough Evaluation index system for subsyndromal symptomatic depression and major depressive disorder) study. All patients were diagnosed with MDD strictly according to The Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) criteria. Standard diagnostic assessments were supplemented with clinical information obtained by a review of medical records and interviews with family informants. Patients were excluded on the following criteria: (1) those with a lifetime diagnosis of bipolar disorder, schizoaffective disorder, schizophrenia, or another psychotic disorder; as well as (2) female patients who were pregnant, planning to become pregnant, or breast-feeding during the study period.
Control subjects were enlisted from the hospital staff and students of the School of Medicine in Shanghai that were interviewed by a specialized psychiatrist with SCID-P. Subjects with any psychiatric disorder and chronic physical disease were excluded from our analysis44,45.
All the patients and control subjects were of Han Chinese origin from Shanghai. All procedures were reviewed and approved by Institutional Review Boards of Shanghai Mental Health Center and other participating institutions. This study was performed in accordance with the guidelines laid out in the Declaration of Helsinki as revised in 1989. All subjects were of Han Chinese origin and provided written informed consent before any study-related procedures were performed.
RNA preparation and Quantitative real-time polymerase chain reaction (qRT-PCR)
On admission, 20 ml peripheral blood of fasting patients and healthy controls were collected between 07:00 am and 09:00 am, to avoid potential diurnal influence. RNA preparation was carried out as previously described46.
Relative IL6 mRNA expression levels were assessed by qRT-PCR with commercially available TaqMan gene expression assays for target gene IL6 and glyceraldehydes-3-phosphate dehydrogenase (GAPDH) as reference gene (Applied Biosystems, CA, USA). All experiments were conducted as referring to our previous studies45,47,48. In each sample, the expression of IL6 was normalized to the expression of the reference gene GAPDH. Results were reported in fold change using 2−∆∆Ct.
SNP selection and Genotyping
We retrieved CHB data from the HapMap database (http://www.hapmap.org) and defined linkage disequilibrium (LD) blocks using Haploview 4.2 (Broad Institute, Cambridge, MA, USA) to set inclusion criteria for tagging SNPs. Haplotype-tagging single nucleotide polymorphisms (htSNPs) with r2 cutoff > 0.8 and minor allele frequency (MAF) > 0.1 were selected. In total, there are two tag SNPs (rs1524107 and rs2069837) of IL6 selected for genotyping. Three functional SNPs (rs1800797, −597G/A, rs1800796, −572G/C and rs1800795, −174G/C) within IL6 were also examined in this study, because the activity of the promoter region of IL6 is affected by the polymorphisms49. Detailed information for these selected SNPs is shown in Supplementary Table S3.
Genomic DNA was isolated from whole blood using a Tiangen DNA isolation kit (Tiangen Biotech, Beijing, China). The five SNPs were detected using multiplex PCR and the SNaPshot assay. The detailed experiment processes were described in our previous publication50. All of the sample call rates exceeded 99.7%. Of the collected samples, 10% were repeated for the genotyping assay to ensure quality-control, and the results were 100% concordant.
Brain eQTL analysis
Converging evidence suggests that MDD originates from abnormal brain functions51, and brain samples are presumably appropriate for eQTL analysis of risk SNP(s). Here, we performed an eQTL analysis to identify whether risk SNP(s) is associated with IL6 expression in brain, using the brain eQTL database (http://caprica.genetics.kcl.ac.uk/BRAINEAC/), a large exon-specific eQTL data set covering ten human brain regions. More detailed information can be found in the original study52.
Statistical analysis
The statistical differences in the characteristics between groups were compared using chi-square test or t test. For the expression analysis, ANCOVA was carried out with age, gender, BMI and smoking status as covariates controlled in the model, to minimize the potential effect of these factors on the expression levels of IL6 mRNA53. For the genetic analyses, HWE testing, genotype and allele frequency analyses were conducted using SHEsis (http://analysis.bio-x.cn)54. Pairwise linkage disequilibrium of all pairs of SNPs was assessed using Haploview 4.2 (Broad Institute, Cambridge, MA, USA)55, and the extent of linkage disequilibrium (LD) was measured by the standardized D’ and r2. Odds ratios (ORs) and the corresponding 95% confidence intervals (CIs) were used to measure the association of the selected SNPs with MDD in dominant and recessive models, respectively. Calculations were performed using SPSS 17.0 (SPSS Inc., Chicago, IL, USA). To adjust for multiple testing, the level of significance was corrected via Bonferroni correction. All tests were two-tails, and the significance level was set at 0.05.
Additional Information
How to cite this article: Zhang, C. et al. Identification of IL6 as a susceptibility gene for major depressive disorder. Sci. Rep. 6, 31264; doi: 10.1038/srep31264 (2016).
Supplementary Material
Acknowledgments
We are deeply grateful to all participants. This work was supported by the National Natural Science Foundation of China (81471358), the Shanghai Science and Technology Commission Foundation (14411969000), the Shanghai Municipal Education Commission—Gaofeng Clinical Medicine Grant Support (20152530), the Shanghai Municipal Commission of Health and Family Planning Foundation (201540029) and the Shanghai Mental Health Center Foundation (2014-FX-03).
Footnotes
Author Contributions C.Z. and Y.F. designed the study. C.Z., Z.W., G.Z. and F.W. acquired the data, which all of the authors analyzed. C.Z. drafted the manuscript. Y.F. supervised this work and edited the manuscript. All the authors critically reviewed the manuscript and gave final approval for its publication.
References
- Yi Z. & Fang Y. Are subsyndromal symptomatic depression and major depressive disorder distinct disorders? Shanghai Arch Psychiatry 24, 286–287 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller C. H., Hamilton J. P., Sacchet M. D. & Gotlib I. H. Meta-analysis of Functional Neuroimaging of Major Depressive Disorder in Youth. JAMA Psychiatry 72, 1045–1053 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips M. R. et al. Prevalence, treatment, and associated disability of mental disorders in four provinces in China during 2001–2005: an epidemiological survey. Lancet 373, 2041–2053 (2009). [DOI] [PubMed] [Google Scholar]
- Kendler K. S., Neale M. C., Kessler R. C., Heath A. C. & Eaves L. J. The lifetime history of major depression in women. Reliability of diagnosis and heritability. Arch Gen Psychiatry 50, 863–870 (1993). [DOI] [PubMed] [Google Scholar]
- Goodyer I.M. Genes, environments and depressions in young people. Arch Dis Child 100, 1064–1069 (2015). [DOI] [PubMed] [Google Scholar]
- Anders S., Tanaka M. & Kinney D. K. Depression as an evolutionary strategy for defense against infection. Brain Behav Immun 31, 9–22 (2013). [DOI] [PubMed] [Google Scholar]
- Setiawan E. et al. Role of translocator protein density, a marker of neuroinflammation, in the brain during major depressive episodes. JAMA Psychiatry 72, 268–275 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howren M. B., Lamkin D. M. & Suls J. Associations of depression with C-reactive protein, IL-1, and IL-6: a meta-analysis. Psychosom Med 71, 171–186 (2009). [DOI] [PubMed] [Google Scholar]
- Strawbridge R. et al. Inflammation and clinical response to treatment in depression: A meta-analysis. Eur Neuropsychopharmacol 25, 1532–1543 (2015). [DOI] [PubMed] [Google Scholar]
- Yi Z. et al. Blood-based gene expression profiles models for classification of subsyndromal symptomatic depression and major depressive disorder. PLoS One 7, e31283 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jansen R. et al. Gene expression in major depressive disorder. Mol Psychiatry 21, 444 (2016). [DOI] [PubMed] [Google Scholar]
- Kopf M. et al. Impaired immune and acute-phase responses in interleukin-6-deficient mice. Nature 368, 339–342 (1994). [DOI] [PubMed] [Google Scholar]
- Goldsmith D. R., Rapaport M. H. & Miller B. J. A meta-analysis of blood cytokine network alterations in psychiatric patients: comparisons between schizophrenia, bipolar disorder and depression. Mol Psychiatry 10.1038/mp.2016.3. (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loddick S. A., Turnbull A. V. & Rothwell N. J. Cerebral interleukin-6 is neuroprotective during permanent focal cerebral ischemia in the rat. J Cerebr Blood F Met 18, 176–179 (1998). [DOI] [PubMed] [Google Scholar]
- Kern S. et al. Higher CSF interleukin-6 and CSF interleukin-8 in current depression in older women. Results from a population-based sample. Brain Behav Immun 41, 55–58 (2014). [DOI] [PubMed] [Google Scholar]
- Camp N. J. et al. Genome-wide linkage analyses of extended Utah pedigrees identifies loci that influence recurrent, early-onset major depression and anxiety disorders. Am J Med Genet B 135B, 85–93 (2005). [DOI] [PubMed] [Google Scholar]
- Tartter M., Hammen C., Bower J. E., Brennan P. A. & Cole S. Effects of chronic interpersonal stress exposure on depressive symptoms are moderated by genetic variation at IL6 and IL1beta in youth. Brain Behav Immun 46, 104–111 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Udina M. et al. Serotonin and interleukin-6: the role of genetic polymorphisms in IFN-induced neuropsychiatric symptoms. Psychoneuroendocrinology 38, 1803–1813 (2013). [DOI] [PubMed] [Google Scholar]
- Fard D. et al. Candidate gene variants of the immune system and sudden infant death syndrome. Int J Legal Med 10.1007/s00414-016-1347-y (2016). [DOI] [PubMed] [Google Scholar]
- Zhang C. et al. Complement factor H and susceptibility to major depressive disorder in Han Chinese. Br J Psychiatry 208, 446–452 (2016). [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J. K. et al. Chronic stress and age-related increases in the proinflammatory cytokine IL-6. Proc Natl Acad Sci USA 100, 9090–9095 (2003). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvalho L. A. et al. Inflammatory activation is associated with a reduced glucocorticoid receptor alpha/beta expression ratio in monocytes of inpatients with melancholic major depressive disorder. Transl Psychiatry 4, e344 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahl J. et al. The plasma levels of various cytokines are increased during ongoing depression and are reduced to normal levels after recovery. Psychoneuroendocrinology 45, 77–86 (2014). [DOI] [PubMed] [Google Scholar]
- Yang J. J. et al. Serum interleukin-6 is a predictive biomarker for ketamine’s antidepressant effect in treatment-resistant patients with major depression. Biol Psychiatry 77, e19–e20 (2015). [DOI] [PubMed] [Google Scholar]
- Li F. et al. Association between interleukin-6 gene polymorphisms and rheumatoid arthritis in Chinese Han population: a case-control study and a meta-analysis. Sci Rep 4, 5714 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lajunen T. K., Jaakkola J. J. & Jaakkola M. S. Interleukin 6 SNP rs1800797 associates with the risk of adult-onset asthma. Genes Immun (2016). [DOI] [PubMed] [Google Scholar]
- Saxena M., Agrawal C. G., Srivastava N. & Banerjee M. Interleukin-6 (IL-6)-597 A/G (rs1800797) & -174 G/C (rs1800795) gene polymorphisms in type 2 diabetes. Indian J Med Res 140, 60–68 (2014). [PMC free article] [PubMed] [Google Scholar]
- Aggarwal S. et al. Genetic variations and interactions in anti-inflammatory cytokine pathway genes in the outcome of leprosy: a study conducted on a MassARRAY platform. J Infect Dis 204, 1264–1273 (2011). [DOI] [PubMed] [Google Scholar]
- Singh G. P. Psychosocial aspects of Hansen’s disease (leprosy). Indian Dermatol Online J 3, 166–170 (2012). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang W. et al. Prevalence and influencing factors of co-morbid depression in patients with type 2 diabetes mellitus: a General Hospital based study. Diabetol Metab Syndr 7, 60 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ciprandi G., Schiavetti I., Rindone E. & Ricciardolo F. L. The impact of anxiety and depression on outpatients with asthma. Ann Allergy Asthma Immunol 115, 408–414 (2015). [DOI] [PubMed] [Google Scholar]
- Fishman D. et al. The effect of novel polymorphisms in the interleukin-6 (IL-6) gene on IL-6 transcription and plasma IL-6 levels, and an association with systemic-onset juvenile chronic arthritis. J Clin Invest 102, 1369–1376 (1998). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets W. C., Price J. L. & Furey M. L. Brain structural and functional abnormalities in mood disorders: implications for neurocircuitry models of depression. Brain Struct Funct 213, 93–118 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peng D. et al. Surface vulnerability of cerebral cortex to major depressive disorder. PLoS One 10, e0120704 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xue S., Wang X., Wang W., Liu J. & Qiu J. Frequency-dependent alterations in regional homogeneity in major depression. Behav Brain Res 306, 13–19 (2016). [DOI] [PubMed] [Google Scholar]
- Baxter L. R. Jr. et al. Reduction of prefrontal cortex glucose metabolism common to three types of depression. Arch Gen Psychiatry 46, 243–250 (1989). [DOI] [PubMed] [Google Scholar]
- Biver F. et al. Frontal and parietal metabolic disturbances in unipolar depression. Biol Psychiatry 36, 381–388 (1994). [DOI] [PubMed] [Google Scholar]
- Kennedy S. H. et al. Changes in regional brain glucose metabolism measured with positron emission tomography after paroxetine treatment of major depression. Am J Psychiatry 158, 899–905 (2001). [DOI] [PubMed] [Google Scholar]
- Ma Y. et al. The effects of omega-3 polyunsaturated fatty acids and genetic variants on methylation levels of the interleukin-6 gene promoter. Mol Nutr Food Res 60, 410–419 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dandrea M., Donadelli M., Costanzo C., Scarpa A. & Palmieri M. MeCP2/H3meK9 are involved in IL-6 gene silencing in pancreatic adenocarcinoma cell lines. Nucleic Acids Res 37, 6681–6690 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Z. Z. et al. Brain-derived neurotrophic factor levels and bipolar disorder in patients in their first depressive episode: 3-year prospective longitudinal study. Br J Psychiatry 205, 29–35 (2014). [DOI] [PubMed] [Google Scholar]
- Fang Y. et al. A pilot study of the efficacy and safety of paroxetine augmented with risperidone, valproate, buspirone, trazodone, or thyroid hormone in adult Chinese patients with treatment-resistant major depression. J Clin Psychopharmacol 31, 638–642 (2011). [DOI] [PubMed] [Google Scholar]
- Fang Y. et al. Comparisons of the efficacy and tolerability of extended-release venlafaxine, mirtazapine, and paroxetine in treatment-resistant depression: a double-blind, randomized pilot study in a Chinese population. J Clin Psychopharmacol 30, 357–364 (2010). [DOI] [PubMed] [Google Scholar]
- Zhang C., et al. A comprehensive analysis of NDST3 for schizophrenia and bipolar disorder in Han Chinese. Transl Psychiatry 6, e701 (2016). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C. et al. ZNF804A Genetic Variation Confers Risk to Bipolar Disorder. Mol Neurobiol 53, 2936–2943 (2016). [DOI] [PubMed] [Google Scholar]
- Zhang C. et al. Influence of BCL2 gene in major depression susceptibility and antidepressant treatment outcome. J Affect Disord 155, 288–294 (2014). [DOI] [PubMed] [Google Scholar]
- Ni J. et al. A Preliminary Genetic Analysis of Complement 3 Gene and Schizophrenia. PLoS One 10, e0136372 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C., Zhang Y., Cai J., Chen M. & Song L. Complement 3 and metabolic syndrome induced by clozapine: a cross-sectional study and retrospective cohort analysis. Pharmacogenomics J. 10.1038/tpj.2015.68 (2015). [DOI] [PubMed] [Google Scholar]
- Fernandes M. T. et al. Association of interleukin-6 gene polymorphism (rs1800796) with severity and functional status of osteoarthritis in elderly individuals. Cytokine 75, 316–320 (2015). [DOI] [PubMed] [Google Scholar]
- Zhang Z. F. et al. A haplotype in the 5′-upstream region of the NDUFV2 gene is associated with major depressive disorder in Han Chinese. J Affect Disorders 190, 329–332 (2016). [DOI] [PubMed] [Google Scholar]
- Northoff G. How do resting state changes in depression translate into psychopathological symptoms? From ‘Spatiotemporal correspondence’ to ‘Spatiotemporal Psychopathology’. Curr Opin Psychiatr 29, 18–24 (2016). [DOI] [PubMed] [Google Scholar]
- Ramasamy A. et al. Genetic variability in the regulation of gene expression in ten regions of the human brain. Nat Neurosci 17, 1418–1428 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. S., Park J. Y. & Yu R. Relationship of obesity and visceral adiposity with serum concentrations of CRP, TNF-alpha and IL-6. Diabetes Res Clin Pract 69, 29–35 (2005). [DOI] [PubMed] [Google Scholar]
- Shi Y. Y. & He L. SHEsis, a powerful software platform for analyses of linkage disequilibrium, haplotype construction, and genetic association at polymorphism loci. Cell Res 15, 97–98 (2005). [DOI] [PubMed] [Google Scholar]
- Barrett J. C., Fry B., Maller J. & Daly M. J. Haploview: analysis and visualization of LD and haplotype maps. Bioinformatics 21, 263–265 (2005). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.