Abstract
The vast majority of antibody-drug conjugates (ADC) are prepared through amine-to-thiol conjugation. To date, N-Succinimidyl-4-(maleimidomethyl) cyclohexanecarboxylate (SMCC) has been one of the most frequently applied reagents for the preparation of ADC and other functional conjugates. However, SMCC-based conjugates suffer from limited stability in blood circulation and from a hydrophobic character of the linker, which may give rise to major pharmacokinetic implications. To address this issue, we have developed a heterobifunctional analogue of a SMCC reagent, i.e., sodium 4-(maleimidomethyl)-1,3-dioxane-5-carbonyl)oxy)-2,3,5,6- tetrafluorobenzenesulfonate (MDTF) for amine-to-thiol conjugation. By replacing the cyclohexyl ring in the SMCC structure with the 1,3-dioxane, we increased the hydrophilicity of the linker. A FRET probe based on MD linker was prepared and showed superior stability compared to the MCC linker in human plasma, as well as in a variety of aqueous buffers. A detailed investigation demonstrated an accelerated succinimide ring opening for MD linker, resulting in stabilized conjugates. Finally, the MDTF reagent was applied for the preparation of serum stable antibody-dye conjugate.
N-Succinimidyl 4-(maleimidomethyl)cyclohexanecarboxylate (SMCC) is one of the most used reagents in bioconjugation1,2. This heterobifunctional reagent contains an N-Succinimidyl (NHS) ester that reacts with amines, yielding a peptide bond and a maleimide group that reacts with thiols, resulting in the formation of a thioester. Both groups are joined together by a cyclohexyl ring, which has been shown to increase the aqueous stability of the maleimide group3. Due to the high abundance of both amines (e.g., lysine residues) and thiols (e.g., cysteine residues) in biological molecules, the SMCC reagent has become an indispensable tool for the modification of biomolecules.
The applications of SMCC include preparation of hapten-carrier conjugates4,5, antibody-enzyme conjugates6,7,8, immunotoxins9 and perhaps the most advanced application to date, generation of antibody-drug conjugates (ADC)10,11. Indeed, one of the two marketed ADCs, trastuzumab emtansine12, as well as other mertansine-based ADC in clinical development, are prepared via SMCC-mediated conjugation10,11,13, in which a highly potent drug is directly linked to an antibody through the MCC linker.
Despite its high applicability, some issues arise from the relatively hydrophobic character of SMCC. Precipitation of the linker in aqueous media, as well as aggregation and precipitation of resulting bioconjugates may occur, decreasing both conjugation efficiency and yield. This issue is of particular importance for the development of mertansine-based ADCs, where the drug is connected to an antibody through the MCC linker, without additional cleavable peptides or other elements that can increase water solubility.
To address the issue of reagent precipitation, a sulfo-SMCC linker containing a sulfonate group on the NHS ring was developed14. However, the linker structure remained unchanged and thus, the problem pertaining to linker innate hydrophobicity (causing aggregation and precipitation of bioconjugates) remained unsolved.
Results and Discussion
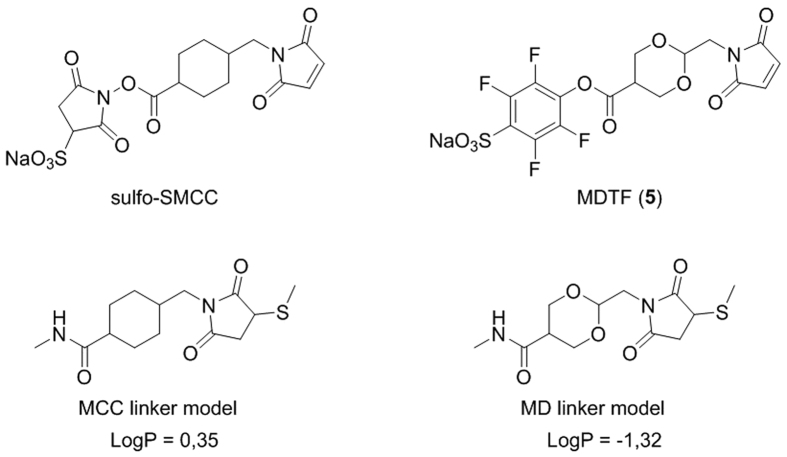
In an effort to address this issue, we designed a new SMCC-like reagent 5 with increased hydrophilicity of the linker core structure. This was achieved by substitution of the cyclohexyl ring by the 1,3-dioxane analogue (Fig. 1). By fitting two oxygen atoms into the structure, the calculated LogP value of the linker decreased by 1.67 units. Moreover, we replaced the sulfo-NHS-activated ester with the 4-sulfotetrafluorophenylester in order to increase the solubility of the final product in water, which is an important parameter for biological applications15.
Figure 1. SMCC and MDTF reagents, the resulting linker models and their calculated LogP values. LogP values indicate higher hydrophilicity of the MD linker model.
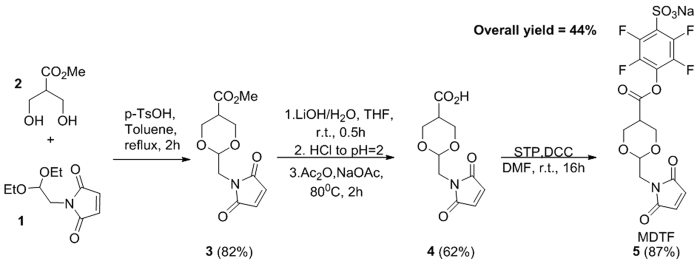
We developed a new heterobifunctional reagent, the sodium 4-(maleimidomethyl)-1,3-dioxane-5-carbonyl)oxy)-2,3,5,6-tetrafluorobenzenesulfonate 5 (MDTF) in three steps from readily available precursors 1 and 2 (Fig. 2). First, the reaction between 1 and 2 was carried out by refluxing their mixture in toluene, in the presence of a catalytic amount of p-TsOH in order to give 1,3-dioxane 3 in 82% yield. Then, hydrolysis of 3 with lithium hydroxide solution (THF/water) led to simultaneous de-esterification of carboxyl function and maleimide ring opening. The latter was then transformed to 4 (cis-isomer) using previously reported reaction conditions3 in 62% yield. Finally, the activation of the carboxylic function of 4 with sodium salt of 4-sulfo-2,3,5,6-tetrafluorophenol (STP) gave the targeted activated ester 5 in 44% overall yield. Reactions were reproduced three times on a scale ranging from hundreds of milligrams to several grams.
Figure 2. Synthesis of MDTF reagent.
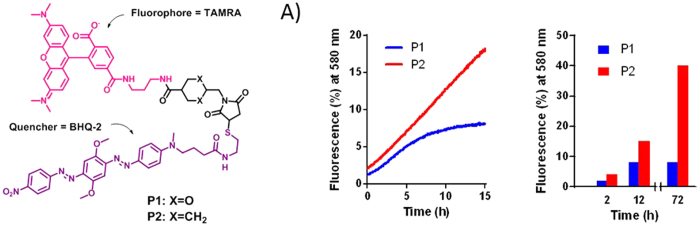
In order to assess the stability of the linker in biological media and at different pH we synthesized two FRET-based probes P1 and P2 using MDTF and sulfo-SMCC reagents respectively through amine-to-thiol conjugation of 1 eq. of fluorophore-amine (TAMRA-NH2) and 1 eq. of quencher-thiol (BHQ-2-SH). The probes were purified using semi-preparative HPLC in order to remove all traces of the starting materials (Fig. 3). These probes were not fluorescent, as the quencher and the fluorophore were linked together through MD- or MCC-linker, but cleavage of the linker or substitution of BHQ-2-SH by other thiol-containing molecules such as human serum albumin (HSA) resulted in the appearance of the fluorescence signal.
Figure 3. Structures of FRET-based probes P1 and P2 synthesized by amine-to-thiol coupling using MDTF and sulfo-SMCC reagents with 1 eq. of TAMRA-NH2 and 1 eq. of BHQ-2-SH.
(A) Side-by-side comparison of the stability of probes P1 and P2 (1 μM) in human plasma at 37 °C. Fluorescence of probes P1 and P2 was monitored at 580 nm and was normalized to the fluorescence of a solution of TAMRA-NH2 (1 μM) and BHQ-2-SH (1 μM) in human plasma.
To test the stability of the linkers we incubated probes P1 and P2 (1 μM) in different buffers (TRIS, PBS) at various pH (5.5 ÷ 9.0), as well as in human plasma and in 1 M HCl at 37 °C (see supporting information). The appearance of fluorescence was monitored at 580 nm over 15 h and normalized using a solution of TAMRA-NH2 (1 μM) and BHQ-2-SH (1 μM) in appropriate media as a positive control.
Interestingly, despite the presence of an acetal function in its structure, the MD linker appeared to be more stable than MCC, even at pH = 0 (see supporting information). We also found that the fluorescence observed during incubation of P1 in human plasma reached a plateau after 12 hours (Fig. 3), while P2 exhibited linearly increasing fluorescence. The latter was demonstrated as being the result of a gradual exchange of BHQ-2-SH by the thiol of human serum albumin (HSA) present in human plasma16. This linear fluorescence increase in P2 was maintained and after 72 hours provided 40% of linker cleavage. In contrast, the fluorescence of P1 remained unchanged after reaching a plateau.
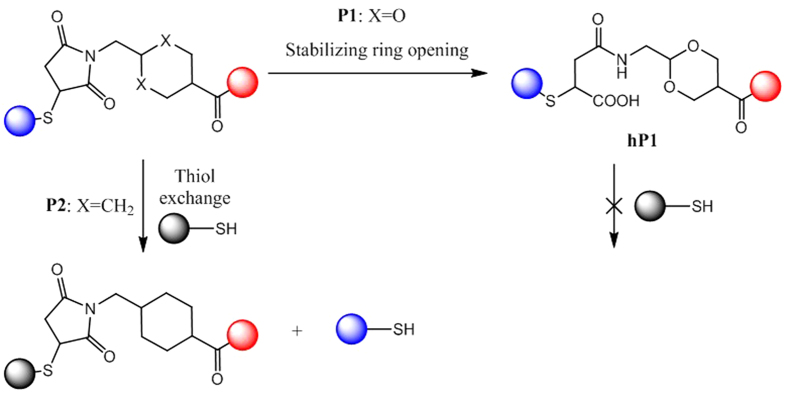
We hypothesized that the difference in behavior of similar scaffolds was due to the hydrolysis of the succinimide motif in the case of P1, which led to the succinamic acid hP1, which is known to be stable toward thiol exchange (Fig. 4).
Figure 4. The high rate of irreversible hydrolysis for the succinimide motif for P1 led to succinamic acid hP1, which does not undergo thiol exchange reaction. In the case of P2, thiol exchange occurred faster than the stabilizing succinimide hydrolysis.
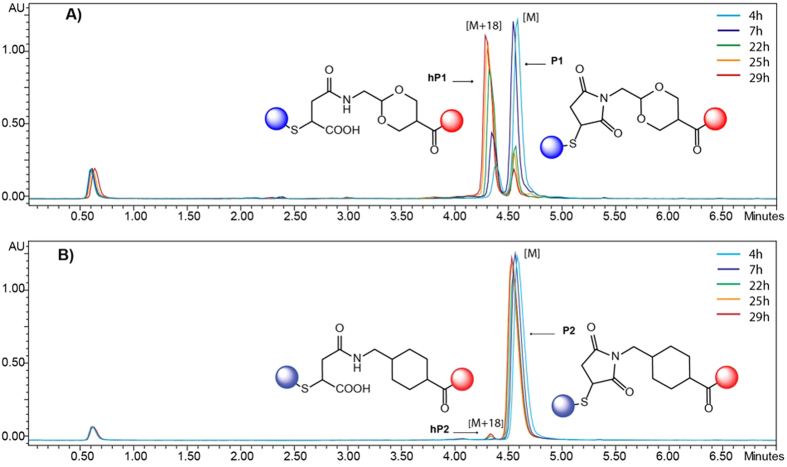
To confirm this hypothesis, we measured the succinimide hydrolysis rates in human plasma of P1 and P2 via LC-MS analysis. Hence, probes P1 and P2 (50 μL each) were incubated in human plasma containing 10% of DMSO at 37 °C. Aliquots were analyzed by HPLC after the precipitation of proteins by the addition of acetonitrile.
As expected, a peak corresponding to succinamic acid hP1 was observed for the probe P1 and the reaction was almost complete after 29 hours, while for the probe P2, only a trace amount of hydrolyzed product hP2 could be detected after 29 hours (Fig. 5).
Figure 5. Incubation of FRET probes P1 and P2(50 μM) in human plasma at 37 °C and subsequent analysis of mixture composition by LC-MS analysis at 550 nm.
(A) Hydrolysis of succinimide of the MD-based probe P1 yielding succinamic acid hP1. (B) Only trace amount of hydrolysed product was observed for the MCC-based probe P2.
MD-based linkers therefore appear to offer an interesting possibility for self-stabilization of the resulting conjugates via a succinimide ring opening. It is worth noting that it has earlier been reported that stabilization of maleimide-thiol conjugates, achieved by hydrolysis of the succinimide ring, can be induced by modulation of the site of conjugation to an antibody17,18 by an amino group adjacent to the maleimide19, by electron withdrawing N-substituents20,21 or by using N-aryl maleimides22. In most cases, buffers with high pH values are required to achieve hydrolysis20,21. Alternatively, to enable access to serum stable conjugates, maleimide can be replaced by other thiol-reactive groups such as 3-arylpropionitrile23,24 or phenyloxadiazole sulfones25; alternatively, bioorthogonal methods of conjugation can be applied26.
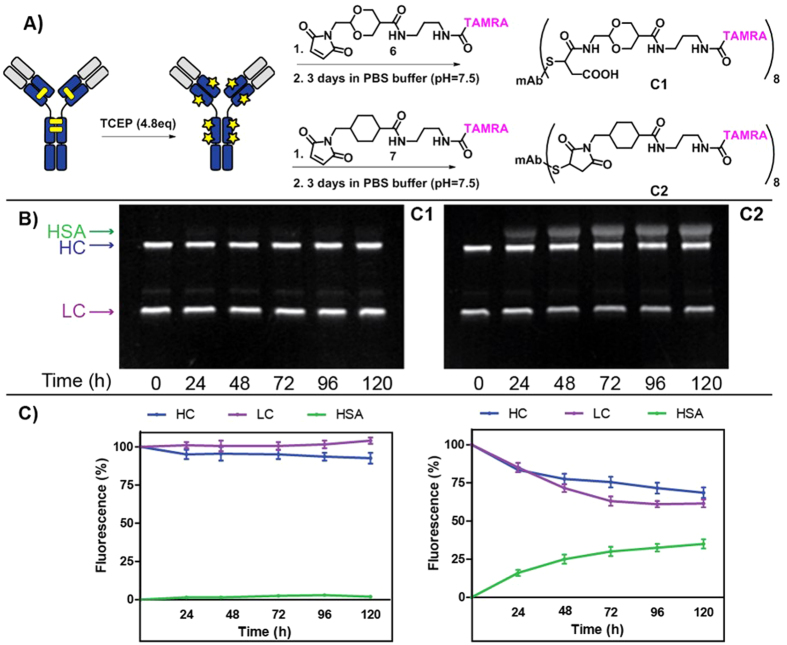
Encouraged by these results, we then decided to test the MDTF reagent for the preparation of an antibody-fluorophore (TAMRA) conjugate and to evaluate whether self-hydrolysis properties can be used to prepare stable conjugates. To this end, a side-by-side comparison with a sulfo-SMCC reagent was carried out (Fig. 6).
Figure 6.
(A) Preparation of Trastuzumab-TAMRA conjugates C1 and C2 with a dye-to-antibody ratio (DAR) = 8 via the maleimide-thiol reaction of reduced Trastuzumab with MD-TAMRA 6 and MCC-TAMRA 7, followed by the mild hydrolysis of succinimide in a PBS buffer (10 mM, pH = 7.5) at 37 °C (see supporting information). (B) Fluorescent SDS-PAGE analysis (excitation at 525 nm) of MD- and MCC-based antibody-dye conjugates after incubation in human plasma. Two lanes correspond to the labeled heavy (HC) and light chains (LC) of the antibody, the third line corresponds to the labeled human serum albumin (HSA) formed via thiol exchange reaction. (C) Quantitative analysis of conjugate stability in human plasma demonstrated 38% of payload transfer to HSA over 120 hours for the MCC-based conjugate C2 in contrast to 3% for the MD-based conjugate C1.
First, a reaction between MDTF or sulfo-SMCC and TAMRA-NH2 using classical conjugation conditions was conducted to yield payloads 6 and 7, respectively (see supporting information). Then, a complete reduction of the interchain disulfide bonds of Trastuzumab was performed by incubation with TCEP (4.8 equiv.) at 37 °C for 2 hours. The reduced antibody was then reacted with 6 or 7 at 4 °C for 1 hour to yield the corresponding conjugates as 5 mg/mL solutions in a PBS buffer (10 mM, pH = 7.5) after purification by gel filtration chromatography. The ESI-MS analysis27 confirmed the dye-to-antibody ratio value of 8 for both conjugates C1 and C2 (Fig. 6).To trigger succinimidyl hydrolysis, as well as conjugates C1 and C2 were maintained in the PBS buffer used for conjugation (10 mM, pH = 7.5) at 37 °С for 70 hours. Following stabilization, both conjugates were incubated in human plasma for five days. Aliquots were taken every 24 hours and analyzed by SDS PAGE. In addition to the two lanes corresponding to the labeled heavy and (HC) light chains (LC) of the antibody, the MCC-based conjugate showed the gradual appearance of a third lane, corresponding to the transfer of the fluorophore to the HSA16. Quantitative analysis via integration of the fluorescent signal of this lane showed 38% degradation over 120 hours for MCC-based conjugates. In contrast, the MD-based conjugate C1 did not show any noticeable transfer of payload to HSA and only 3% degradation was observed after 120 hours of incubation.
In summary, we have developed a new heterobifunctional reagent (MDTF) for amine-to-thiol conjugation that indicates similar reactivity towards sulfhydryl-containing molecules as the SMCC. Substitution of a cyclohexyl ring by a dioxine ring increased hydrophilic character in the new MD linker compared to the classical MCC linker. Interestingly, the MD linker underwent self-stabilization in mild conditions via a succinimide ring opening. The resulting succinamic acid-containing linker is not prone to the undesirable thiol exchange reaction. In fact, a MD-based FRET probe incubated in human plasma showed a considerably higher self-stabilization rate compared to the MCC-based probe. This hydrolytic stabilization process was shown to be efficient for the preparation of serum stable antibody conjugates.
Additional Information
How to cite this article: Dovgan, I. et al. 2-(Maleimidomethyl)-1,3-Dioxanes (MD): a Serum-Stable Self-hydrolysable Hydrophilic Alternative to Classical Maleimide Conjugation. Sci. Rep. 6, 30835; doi: 10.1038/srep30835 (2016).
Supplementary Material
Acknowledgments
This work was supported by CNRS, University of Strasbourg. The International Center for Frontier Research in Chemistry (icFRC) and Region Alsace are acknowledged for financial support.
Footnotes
Author Contributions I.D., S.K., O.K. and A.W. designed the study, I.D., S.K. and O.K. performed the experiments. All authors contributed to writing the manuscript.
References
- Koniev O. & Wagner A. Developments and recent advancements in the field of endogenous amino acid selective bond forming reactions for bioconjugation. Chem. Soc. Rev. 44, 5495–5551 (2015). [DOI] [PubMed] [Google Scholar]
- Hermanson G. T. Bioconjugate Techniques. Bioconjugate Techniques (Elsevier, 2013). [Google Scholar]
- Yoshitake S., Yamada Y., Ishikawa E. & Masseyeff R. Conjugation of glucose oxidase from Aspergillus niger and rabbit antibodies using N-hydroxysuccinimide ester of N-(4-carboxycyclohexylmethyl)-maleimide. Eur. J. Biochem. 101, 395–399 (1979). [DOI] [PubMed] [Google Scholar]
- Dewey R. E., Timothy D. H. & Levings C. S. A mitochondrial protein associated with cytoplasmic male sterility in the T cytoplasm of maize. Proc. Natl. Acad. Sci. USA 84, 5374–8 (1987). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peeters J. M., Hazendonk T. G., Beuvery E. C. & Tesser G. I. Comparison of four bifunctional reagents for coupling peptides to proteins and the effect of the three moieties on the immunogenicity of the conjugates. J. Immunol. Methods 120, 133–43 (1989). [DOI] [PubMed] [Google Scholar]
- Hashida S. & Ishikawa E. Use of Normal IgG and its Fragments to Lower the Non-Specific Binding of Fab’-Enzyme Conjugates in Sandwich Enzyme Immunoassay. Anal. Lett. 18, 1143–1155 (1985). [Google Scholar]
- Gould J. B. & Marks V. Nonisotopic Immunoassay. (Springer: US,, 1988). [Google Scholar]
- Uto I. et al. Determination of urinary Tamm-Horsfall protein by ELISA using a maleimide method for enzyme-antibody conjugation. J. Immunol. Methods 138, 87–94 (1991). [DOI] [PubMed] [Google Scholar]
- Vogel C.-W. Immunoconjugates: Antibody Conjugates in Radioimaging and Therapy of Cancer. (Oxford University Press, 1987). [Google Scholar]
- Erickson H. K. et al. Antibody-maytansinoid conjugates are activated in targeted cancer cells by lysosomal degradation and linker-dependent intracellular processing. Cancer Res. 66, 4426–33 (2006). [DOI] [PubMed] [Google Scholar]
- Polson A. G. et al. Antibody-drug conjugates targeted to CD79 for the treatment of non-Hodgkin lymphoma. Blood 110, 616–23 (2007). [DOI] [PubMed] [Google Scholar]
- Ballantyne A. & Dhillon S. Trastuzumab emtansine: first global approval. Drugs 73, 755–65 (2013). [DOI] [PubMed] [Google Scholar]
- Jain N., Smith S. W., Ghone S. & Tomczuk B. Current ADC Linker Chemistry. Pharm. Res. 32, 3526–3540 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tournier E. J. M., Wallach J. & Blond P. Sulfosuccinimidyl 4-(N-maleimidomethyl)-1-cyclohexane carboxylate as a bifunctional immobilization agent. Optimization of the coupling conditions. Anal. Chim. Acta 361, 33–44 (1998). [Google Scholar]
- Gee K. R., Archer E. A. & Kang H. C. 4-Sulfotetrafluorophenyl (STP) esters: New water-soluble amine-reactive reagents for labeling biomolecules. Tetrahedron Lett. 40, 1471–1474 (1999). [Google Scholar]
- Xu K. et al. Characterization of the drug-to-antibody ratio distribution for antibody-drug conjugates in plasma/serum. Bioanalysis 5, 1057–71 (2013). [DOI] [PubMed] [Google Scholar]
- Shen B.-Q. et al. Conjugation site modulates the in vivo stability and therapeutic activity of antibody-drug conjugates. Nat. Biotechnol. 30, 184–9 (2012). [DOI] [PubMed] [Google Scholar]
- Strop P. et al. Location matters: Site of conjugation modulates stability and pharmacokinetics of antibody drug conjugates. Chem. Biol. 20, 161–167 (2013). [DOI] [PubMed] [Google Scholar]
- Lyon R. P. et al. Self-hydrolyzing maleimides improve the stability and pharmacological properties of antibody-drug conjugates. Nat. Biotechnol. 32, 1059–62 (2014). [DOI] [PubMed] [Google Scholar]
- Tumey L. N. et al. Mild method for succinimide hydrolysis on ADCs: impact on ADC potency, stability, exposure, and efficacy. Bioconjug. Chem. 25, 1871–80 (2014). [DOI] [PubMed] [Google Scholar]
- Fontaine S. D., Reid R., Robinson L., Ashley G. W. & Santi D. V. Long-term stabilization of maleimide-thiol conjugates. Bioconjug. Chem. 26, 145–52 (2015). [DOI] [PubMed] [Google Scholar]
- Christie R. J. et al. Stabilization of cysteine-linked antibody drug conjugates with N-aryl maleimides. J. Control. Release 220, 660–670 (2015). [DOI] [PubMed] [Google Scholar]
- Kolodych S. et al. CBTF: New amine-to-thiol coupling reagent for preparation of antibody conjugates with increased plasma stability. Bioconjug. Chem. 26, 197–200 (2015). [DOI] [PubMed] [Google Scholar]
- Koniev O. et al. Selective irreversible chemical tagging of cysteine with 3-arylpropiolonitriles. Bioconjug. Chem. 25, 202–206 (2014). [DOI] [PubMed] [Google Scholar]
- Patterson J. T., Asano S., Li X., Rader C. & Barbas C. F. Improving the serum stability of site-specific antibody conjugates with sulfone linkers. Bioconjug. Chem. 25, 1402–1407 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lhospice F. et al. Site-Specific conjugation of monomethyl auristatin e to Anti-CD30 antibodies improves their pharmacokinetics and therapeutic index in rodent models. Mol. Pharm. 12, 1863–1871 (2015). [DOI] [PubMed] [Google Scholar]
- Beck A. et al. Cutting-edge mass spectrometry methods for the multi-level structural characterization of antibody-drug conjugates. Expert Rev. Proteomics 13, 157–83 (2015). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.