Abstract
The obesity epidemic and the urgent need for effective and safe drugs to treat obesity-related diseases have greatly increased research interest in the metabolic hormones, fibroblast growth factor-19 (FGF19, FGF15 in mice) and FGF21. FGF19 and FGF21 function as endocrine hormones that play key roles in energy metabolism and counteract obesity. Importantly, in obese humans and lab animals, circulating FGF19 and FGF21 levels are elevated, and metabolic actions of these hormones are impaired but the underlying mechanisms remained unclear. Recent microRNA (miR) studies have revealed that aberrantly elevated miR-34a in obesity directly targets β-Klotho (βKL), the obligate co-receptor for both FGF19 and FGF21, and attenuates metabolic signaling of these hormones. In this review, we will discuss recent findings in the miR and FGF19/21 fields, emphasizing the novel function of obesity-associated miR-34a in attenuation of FGF19/21 metabolic actions, and further discuss miRs, including miR-34a, as potential drug targets for obesity-related diseases.
Keywords: miR-34a, βKlotho, FGF15, FGF19, FGF21, FGFR4, SIRT1, NAMPT
1. Introduction
The prevalence of obesity has been rapidly increasing at an alarming rate which correlates with life-style changes in modern society (Grundy, 2008). Disruption of energy balance due to excessive food intake and/or insufficient physical activity plays a pivotal role in development of this global epidemic (Spiegelman, and Flier, 2001). Obesity substantially increases risks for many human diseases, ranging from metabolic syndromes, including type 2 diabetes, fatty liver disease, and cardiovascular disease, to infertility, depression, neurological diseases, and even, certain types of cancer (Grundy, 2008; Kahn, and Flier, 2000). Not surprisingly, the American Medical Association has officially identified obesity as a medical disease. The obesity epidemic and the urgent need for effective drugs to treat obesity-related metabolic diseases have greatly increased research interest in two metabolic hormones which play key roles in energy metabolism and counteract obesity, fibroblast growth factor-19 (FGF19, FGF15 in mice) and FGF21 (Canto, and Auwerx, 2012; Potthoff, Kliewer, and Mangelsdorf, 2012).
The FGF19 subfamily members, FGF19, FGF21, and FGF23, are atypical FGFs that lack the conventional FGF heparin-binding domain and act as endocrine hormones (Beenken, and Mohammadi, 2009; Kuro-o, 2008). FGF19 and FGF21 play key roles in regulation of lipid and carbohydrate metabolism in response to nutritional status. Binding of FGF19 and FGF21 to the FGF receptors (FGFRs) and the obligate co-receptor for these hormones, βKL, triggers activation of intracellular signaling pathways to mediate metabolic actions of these hormones. Importantly, serum levels of FGF19 and FGF21 are highly elevated and metabolic signaling of these hormones is impaired in obese patients and lab animals (Dushay, Chui, et al, 2010; Mraz, Lacinova, et al, 2011), but the underlying mechanisms remain unclear.
MicroRNAs (miRs) are small non-coding RNAs that act as regulatory molecules that control expression of their target genes (Hobert, 2008). Mature miRs bind to the 3′ untranslated region (UTR) of target transcripts and inhibit protein translation and/or mRNA stability (Lewis et al., 2003). Nearly 50% of all human genes are predicted to be regulated by miRs (Hobert, 2008). MiRs have been shown to play crucial roles in diverse biological pathways, including development, differentiation, cell proliferation, and metabolism. Importantly, miRs are aberrantly expressed in human diseases (Rottiers, and Naar, 2012; Rottiers, et al., 2012; Lee and Kemper, 2010; Zhang et al., 2013), revealing the therapeutic potential of targeting miRs.
In this review, we will describe the emerging role of miRs as powerful regulators of lipid and glucose metabolism. We will also discuss atypical FGFs, focusing on metabolic hormones regulating energy metabolism, FGF19 and FGF21, and their obligate co-receptor βKL, and further discuss the role of an obesity-associated miR-34a in downregulating βKL expression, thus, attenuating metabolic action of FGF19 and FGF21. Finally, we will discuss the therapeutic potential of miR-34a as a novel drug target for treating obesity-related metabolic disorders, such as type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), and cardiovascular disease.
2. MicroRNAs as powerful regulators of lipid and glucose metabolism
Accumulating evidence indicates that miRs play critical roles in regulation of lipid and glucose metabolism. In their original studies, Stoffel and his colleague demonstrated the functional importance of a pancreatic islet-specific miR, miR-375, in regulation of glucose-stimulated insulin secretion and exocytosis by directly targeting myotrophin (Poy, Eliasson, et al, 2004). Esau et al. reported that miR-122, the most abundant miR in liver, plays important roles in liver cholesterol and fatty acid metabolism, and they further showed that miR-122 has a role in numerous hepatic functions, ranging from cholesterol metabolism, liver cancer, stress responses, and viral infection to circadian regulation (Esau, Davis, et al, 2006). Also, miR-33 has been shown to contribute to the regulation of cholesterol homeostasis in concert with master transcriptional activators of cholesterol/lipid synthesis, SREBPs, by targeting the cholesterol transporter genes, ABCA1 and ABCG1, and interestingly, miR-33’s is encoded within the introns of SREBP genes (Najafi-Shoushtari, Kristo, et al, 2010; Rayner, Suarez, et al, 2010). A recent study has shown that an SREBP-responsive miR operon expressing miR-182 and miR-96 constitutes a regulatory loop for intracellular lipid homeostasis (Jeon, Esquejo, et al, 2013).
Aberrant expression of miRs has been detected in pathological conditions, such as obesity and diabetic conditions. MiR microarray studies in two obese mouse models, the leptin-deficient ob/ob mice and diet-induce obese mice, identified miR-34a as the miR with the highest relative increase (Lee, Padhye, et al, 2010; Lee, and Kemper, 2010; Trajkovski, Hausser, et al, 2011). MiR-34a has been shown to act as a key regulator of hepatic lipid homeostasis (Choi, Fu, et al, 2013; Fu, Choi, et al, 2012) and also as a negative regulator of the formation of brown and beige fat in brown and white adipose tissue, respectively (Fu, Seok, et al, 2014). In this review, we will focus on miR-34a, but other miRs have altered levels in obesity as briefly summarized below and as has been recently reviewed (Rottiers, and Naar, 2012).
MiR-103 and 107 were upregulated in obese mice and silencing of miR-103/107 led to improved glucose homeostasis and insulin sensitivity (Trajkovski, Hausser, et al, 2011). Expression of both miR-378 and its host gene, PGC-1β, were elevated in obese mice. MiR-378 directly targets carnitine O-acetyltransferase, a key enzyme in mitochondrial metabolism of fatty acids (Carrer, Liu, et al, 2012). Additional miRs that regulate glucose metabolism and insulin sensitivity and are increased in obesity include let-7, miR-143, miR-145, and miR-802 (Jordan, Kruger, et al, 2011; Kornfeld, Baitzel, et al, 2013; Perez, Bernal, et al, 2013). MiR-155, miR-133, and miR-27 control adipocyte differentiation and that expression of miR-155 and miR-133 are increased, whereas that of miR-27 is decreased, in obesity (Chen, Siegel, et al, 2013; Sun, and Trajkovski, 2014; Trajkovski, Ahmed, et al, 2012).
3. Metabolic hormones FGF19/FGF21, and their obligate co-receptor β-Klotho
3-1. Atypical FGFs, FGF19, FGF21, and FGF23
FGFs are a family of growth factors that play critical roles in regulation of cell proliferation and differentiation, as well as normal development, tissue angiogenesis, and wound healing (Beenken, and Mohammadi, 2009). Twenty-two FGF members have been identified in humans and the FGF19 subfamily, the focus of this review, is comprised of the human FGF19 (FGF15 as mouse ortholog), FGF21, and FGF23 (Kir, Beddow, et al, 2011; Kuro-o, 2008). These FGF19 subfamily members act as endocrine hormones that control a variety of physiological processes, such as inhibiting bile acid synthesis in liver, promoting lipolysis in adipose tissue, and regulating vitamin D biosynthesis in kidney. The endocrine FGFs signal through cell surface receptor tyrosine kinases, FGFRs, including FGFR1, FGFR2, FGFR3, and FGFR4. The Klotho family members are membrane-spanning proteins that function as obligate co-receptors for the FGFRs and are required for the endocrine FGFs to bind to the receptor (Beenken, and Mohammadi, 2009)
Intriguingly, expression of FGF19, FGF21, and FGF23, is transcriptionally regulated by nutrient-sensing nuclear receptors. Synthesis of FGF19 is induced by the bile acid-activated FXR in the small intestine in response to a meal, and circulating FGF21 is induced by fatty acid-activated peroxisome proliferator-activated receptor-α (PPARα) in the liver in response to prolonged fasting (Badman, Pissios, et al, 2007; Inagaki, Dutchak, et al, 2007). FGF23 is induced by vitamin D nuclear receptor (Kuro-o, 2012). This review will focus on the physiological actions of FGF19 and FGF21 in regulation of lipid and carbohydrate metabolism in metabolic tissues and their dysregulation in obesity, in part due to elevated miR-34a.
3-2. βKL, the obligate co-receptor for FGF19 and FGF21
A unique feature of the endocrine FGFs is that they require the Klotho family proteins as co-receptors in order to exert their biological functions (Kuro-o, 2012; Ogawa, Kurosu, et al, 2007). There are two Klotho proteins, Klotho and β-Klotho, in the Klotho family. Klotho was initially discovered in 1997 in studies showing that mice with a mutation in this gene had shortened life spans and other phenotypes resembling human premature aging syndrome, “progeria” (Kuro-o, Matsumura, et al, 1997; Kuro-o, 2012). The Klotho-null mice also exhibited high phosphate and calcium levels in the serum. Moreover, FGF23-knockout mice displayed strikingly similar phenotypes as the Klotho-null mice, suggesting that they might function in a same signal transduction pathway. Indeed, Klotho protein was identified as a co-receptor of FGF23, which was important for calcium and phosphate metabolism (Kurosu, Ogawa, et al, 2006).
Beta-klotho (βKL) has 41% amino acid sequence similarity with Klotho (Kuro-o, 2012). It is also a transmembrane protein and mainly distributed in metabolic organs, like pancreas, liver and adipose tissue. However, βKL-null mice had a phenotype distinctly different from that of KL-null mice, most prominently, increased bile acid synthesis. In the βKL-null mice, expression of the Cyp7a1 gene, which encodes the rate-limiting enzyme of bile acid synthesis was increased (Ito, Fujimori, et al, 2005). Increased bile acid synthesis was also present in mice lacking FGF15 or its receptor, FGFR4, suggesting that the βKL-null phenotype was the result of impaired FGF15 signaling since βKL is the obligate co-receptor for FGF15 (FGF19 in human) in the liver. Beta-Klotho also functions as the co-receptor of FGF21 in the liver and adipose tissue. While FGFRs are widely expressed, βKL expression is restricted to liver, adipose tissue, pancreas and brain, and largely determines the tissue-specific metabolic actions of FGF15 and FGF21(Kurosu, Choi, et al, 2007).
4. MiR-34a and impaired hepatic FGF19 signaling in obesity
4.1 Role of the miR-34a/βKL/FGF19 axis in liver
The metabolic response to FGF19 signaling in liver is impaired in obese patients with NAFLD (steatosis) and steatohepatitis, despite relatively normal FGF19 production and highly elevated serum levels of FGF19 (Schreuder, Marsman, et al, 2010). In genetic or diet-induced obese mice, serum FGF15 levels were also increased (Fisher, Chui, et al, 2010; Fu, Seok, et al, 2014), suggestive of FGF15 resistance in obesity, but the underlying mechanisms remained unclear. Recent studies from our group have suggested that elevated miR-34a in obesity attenuates FGF19 signaling by directly targeting and downregulating βKL (Fu, Choi, et al, 2012). Remarkably, antisense inhibition of miR-34a in diet-induced obese mice partially restored βKL levels in the liver. Although FGFR4 is not a direct target of miR-34a, the stability of FGFR4 protein and localization of FGFR4 to cell membrane were also increased in a βKL-dependent manner (Fu and Kemper, unpublished data).
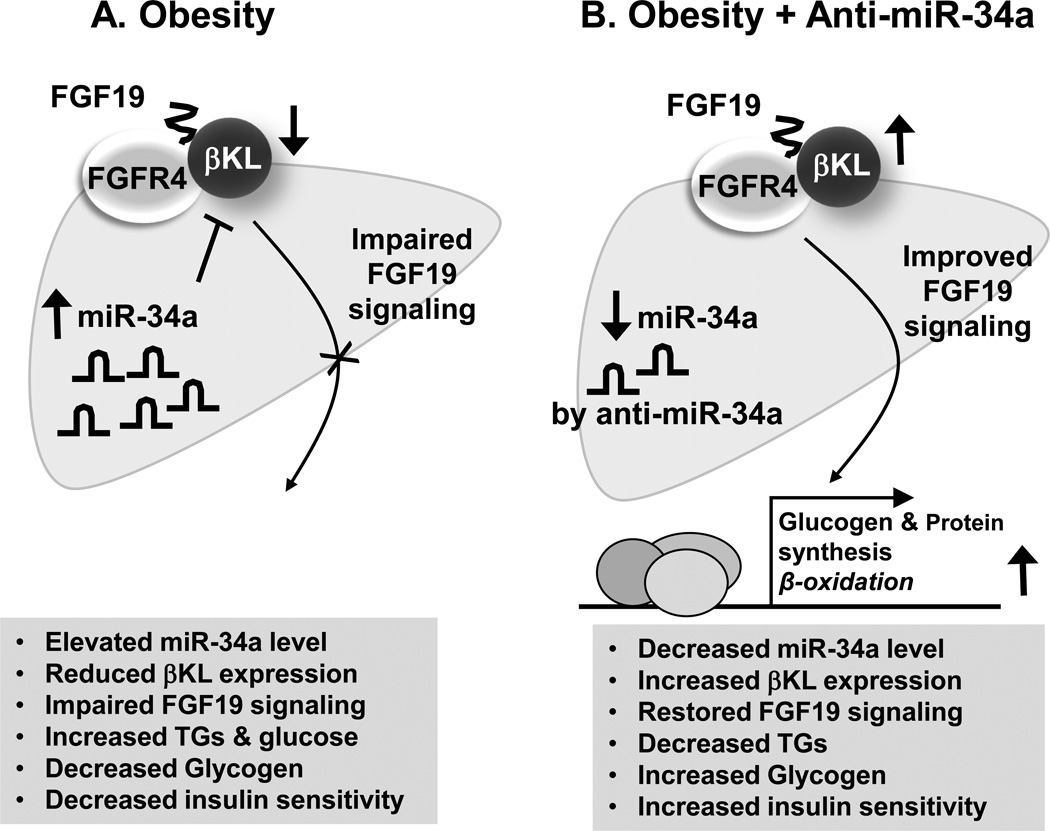
As illustrated in Fig. 1, treatment of dietary obese mice with antisense RNA for miR-34a (anti-miR-34a) resulted in improved metabolic outcomes, including decreased liver triglyceride and increased liver glycogen, which is consistent with improved regulation of expression of glucose and fatty acid metabolic genes by miR34a in the liver and increased insulin sensitivity. However, since miR-34a targets βKL and other potential components involved in FGF19 and FGF21 signaling in liver and other metabolic tissues, the effects of anti-miR34a in multiple tissues probably contribute to the overall beneficial outcome, including lipid-lowering effects and insulin sensitivity (Fu, Choi, et al, 2012; Fu, Seok, et al, 2014).
Fig. 1. Role of the miR-34a/βKL/FGF19 axis in liver.
(A) In diet-induced obese mice, aberrantly elevated hepatic miR-34a represses the expression of the obligate co-receptor for FGF19, βKL, by directly binding to the 3’UTR of the βKL transcript, resulting in attenuation of FGF19 signaling. The impaired FGF19 signaling in obesity leads to increased triglycerides/glucose levels, decreased glycogen levels in the liver, and decreased insulin sensitivity in fatty liver of obese mice. (B) Treatment with anti-miR-34a in dietary obese mice results in downregulation of the elevated miR-34a, increased expression of βKL, and subsequently, improves FGF19 signaling. The improved FGF19 signaling results in increased glycogen levels, decreased triglycerides levels in the liver, and increased insulin sensitivity.
Recent studies in FGF19 transgenic mice have revealed a new function for FGF19 in energy homeostasis. FGF19 transgenic mice have lower body weight, reduced fat, enhanced hepatic lipid oxidation, increased brown adipose tissue (BAT), and elevated energy expenditure (Wu, Ge, et al, 2010). These mice also had lower serum glucose and triglyceride levels, but normal insulin levels compared to control mice after feeding of a high fat diet. In addition, a recent study has demonstrated that treatment with a synthetic FXR agonist, Fex, in mice activates the FXR/FGF15 pathway selectively in the intestine, resulting in increased serum FGF15 levels and counteracting obesity in part via increasing energy expenditure and promotion of fat browning in adipose tissue (Fang, Suh, et al, 2015). It will be interesting to see whether miR-34a plays a role in these processes in the intestine and adipose tissue.
4.2 Regulation of hepatic miR-34a expression in physiology and obesity
It remains largely unknown how miR-34a expression is physiologically regulated and how miR-34a is abnormally upregulated in obesity, but recent studies from our group have provided evidence supporting the idea that the bile acid nuclear receptor FXR and SHP cascade pathway plays a role in inhibition of miR-34a expression in hepatocytes. In miR microarray studies, miR-34a exhibited the greatest fold-increase of liver miRs in FXR-KO mice compared to wild type mice, suggesting a potential role of FXR in inhibiting expression of miR-34a (Lee, Padhye, et al, 2010; Lee, and Kemper, 2010). We further showed that activation of FXR induces expression of SHP, an orphan nuclear receptor and metabolic repressor, and SHP is recruited to the promoter of miR-34a and inhibits binding of p53, a key activator miR-34a expression (Lee, Padhye, et al, 2010; Lee, and Kemper, 2010). Thus, activation of an FXR/SHP/p53 pathway appears to be involved in inhibition of miR-34a expression under physiological conditions.
In contrast, under pathophysiological conditions, such as NAFLD in obesity, this FXR/SHP/p53 cascade is defective and transcription of miR-34a is not inhibited. The underlying mechanism is not clear but decreased SIRT1 deacetylase and increased p300 acetylase function in fatty liver increase the acetylation status of p53 and FXR, which results in increased p53 and decreased FXR transcriptional activities(Kemper, Choi, and Kim, 2013). FXR, thus, appears to be a multi-functional regulator. On one hand, FXR ensures metabolic homeostasis by inducing the synthesis of a key metabolic hormone FGF19 in the intestine. On the other hand, FXR also inhibits expression of miR-34a in the liver, enhancing FGF19 signaling. However, a significant correlation between miR-34a and βKL levels was not observed in response to fasting/feeding in lean mice (Fu and Kemper, unpublished data), so the functional importance of this pathway in regulation of miR-34a expression in physiological conditions remains unclear and requires further study.
5. MiR-34a and impaired FGF21 signaling in obesity
Synthesis of FGF21 is induced mainly in the liver during prolonged fasting but also induced in other metabolic tissues, such as adipose tissue, pancreas, and muscle, in response to physiological or pathological stressors, such as cold exposure, exercise, and autophagy deficiency(Badman, Pissios, et al, 2007; Bookout, de Groot, et al, 2013; Fisher, Kleiner, et al, 2012; Inagaki, Dutchak, et al, 2007; Kim, Jeong, et al, 2013). FGF21 binds mainly to FGFR1 in adipose tissue and to FGFR3 in liver, both of which require βKL as the obligate co-receptor. Binding of FGF21 to the receptor complex stimulates downstream signaling kinases, including ERK and GSK, which mediate the metabolic actions of this hormone.
5.1. Role of miR-34a in the βKL/FGF21 axis in liver
FGF21 has received tremendous attention in the obesity field because of its beneficial lipid-lowering and insulin-sensitizing effects. Administration of recombinant FGF21 to obese individuals has beneficial outcomes, such as weight loss and increased insulin sensitivity, although it has side effects, such as fragile bones and water retention (Wei, Dutchak, et al, 2012). Physiologically, synthesis of FGF21 in the liver is induced during prolonged fasting through activation of a nuclear receptor, PPARα (Badman, Pissios, et al, 2007; Inagaki, Dutchak, et al, 2007). In response to prolonged fasting, PPARα is activated by fatty acids, which are mostly released from adipocytes and taken up by hepatocytes, and binds directly to the promoter of the Fgf21 gene to induce its transcription. Indeed, PPARα-KO mice are unable to catabolize fatty acids in the liver resulting in hepatic steatosis but these effects are partially reversed by administration of FGF21. In addition, hepatic FGF21 induction is also significantly increased after challenged with a ketogenic diet or in suckling mouse neonates (Badman, Pissios, et al, 2007; Inagaki, Dutchak, et al, 2007). In such conditions, fatty acids are elevated, which would active PPARα and induce synthesis of FGF21, resulting in increased fatty acid oxidation so that fatty acids are used as the primary metabolic fuel.
FGF21 signaling in the liver is mainly mediated by βKL and FGFR3(Owen, Mangelsdorf, and Kliewer, 2015). Since βKL was shown to be a target of miR-34a, it is possible that elevated miR-34a levels in obesity may attenuate hepatic FGF21 signaling. In our studies, overexpression of miR-34a compromised FGF21 signaling in hepatocytes and dysregulated its downstream target genes and conversely, downregulation of miR-34a by lentiviral-mediated expression of antisense-miR-34a in vivo resulted in improved hepatic FGF21 signaling (Fu, Seok, et al, 2014). In addition, the expression of FGF21 target genes involved in hepatic fatty acid oxidation (Cpt1, Mcad, Eci, CytC and Pparα) was increased, while that of lipogenic (Fas and Srebp-1c) and gluconeogenic (G6pase and Pepck) genes was decreased. These gene expression changes in the liver likely contribute to the systematic beneficial metabolic effects, such as weight loss, improved serum lipid and glucose profiles, and increased insulin sensitivity, which are observed after downregulation of miR-34a in obese mice.
MiR-34a likely targets multiple other genes in the liver in addition to βKL. We recently showed that hepatic miR-34a directly targets SIRT1, a NAD-dependent deacetylase, and NAMPT, a rate-limiting enzyme for NAD biosynthesis (Canto, Houtkooper, et al, 2012; Choi, Fu, et al, 2013; Kemper, Choi, and Kim, 2013). Hepatic overexpression of miR-34a reduced NAMPT and subsequently, hepatic NAD levels and decreased SIRT1 deacetylase activity, which results in increased acetylation of SIRT1 target transcriptional regulators, such as PGC1-α, SRBEP-1c, FXR and NF-κB (Choi, Fu, et al, 2013). The overall effect of overexpression of miR-34a was to mimic metabolic conditions present in obesity. Conversely, antagonism of miR-34a in obese mice restored NAMPT/NAD levels and alleviated hepatic steatosis, inflammation, and glucose intolerance. It was also shown that miR-34a directly targets and downregulates heaptic PPARα in obese mice (Ding, Li, et al, 2015). Further, a recent study has shown that miR-34a directly inhibits expression of HNF-4, resulting in inhibiiton of VLDL secretion and promoting NAFLD (Xu, Zalzala, et al, 2015). Therefore, a large network of genes regulated by the miR-34a/βKL axis may underlie the beneficial metabolic outcomes observed from the systematic anti-miR-34a experiments in diet-induced obese mice (Choi, Fu, et al, 2013; Fu, Choi, et al, 2012; Fu, Seok, et al, 2014).
5.2 Role of miR-34a in βKL/FGF21 signaling in adipose tissue
FGF21 is induced in the liver upon prolonged fasting (Inagaki, Dutchak, et al, 2007), but it is also induced in response to feeding in white adipose tissue (WAT) which mediates insulin-sensitizing effects in WAT by activating PPARγ (Dutchak, Katafuchi, et al, 2012). Indeed, PPARγ agonists, such as a thiazolidinedione (TZD), reversed the diet-induced obese phenotype of FGF21-KO mice. Moreover, FGF21 increased the expression of adiponectin, which acts as a beneficial adipokine and maintains systematic glucose and lipid homeostasis (Yamauchi, and Kadowaki, 2008). FGF21 expression is also induced upon cold exposure in both WAT and brown adipose tissue (BAT), which results in increased expression of thermogenic transcriptional activators, such as PGC1α, Prdm16, and mitochondrial uncoupling protein 1 (UCP1), and increased thermogenesis (Fisher, Kleiner, et al, 2012).
FGF21 levels are highly elevated and the expression of its receptor complex, both FGFR1 and βKL, are decreased in obesity, suggesting that FGF21 signaling may be impaired in obesity. In recent studies, our group has provided a mechanistic basis for FGF21 resistance by showing that elevated miR-34a contributes to attenuated FGF21 signaling in obese mice by downregulation of expression of the adipocyte FGF21 receptor complex components, both βKL and FGFR1, as illustrated in Fig. 2. Downregulation of miR-34a in obese mice had beneficial effects, including reduced adiposity, improved serum profiles, increased mitochondrial DNA copy number and increased oxidative function in adipose tissue (Fu, Seok, et al, 2014). Remarkably, downregulation of miR-34a increased co-expression of the beige fat-specific marker CD137 and the fat browning marker UCP1 in all types of white fat and also enhanced additional browning in brown adipose tissues.
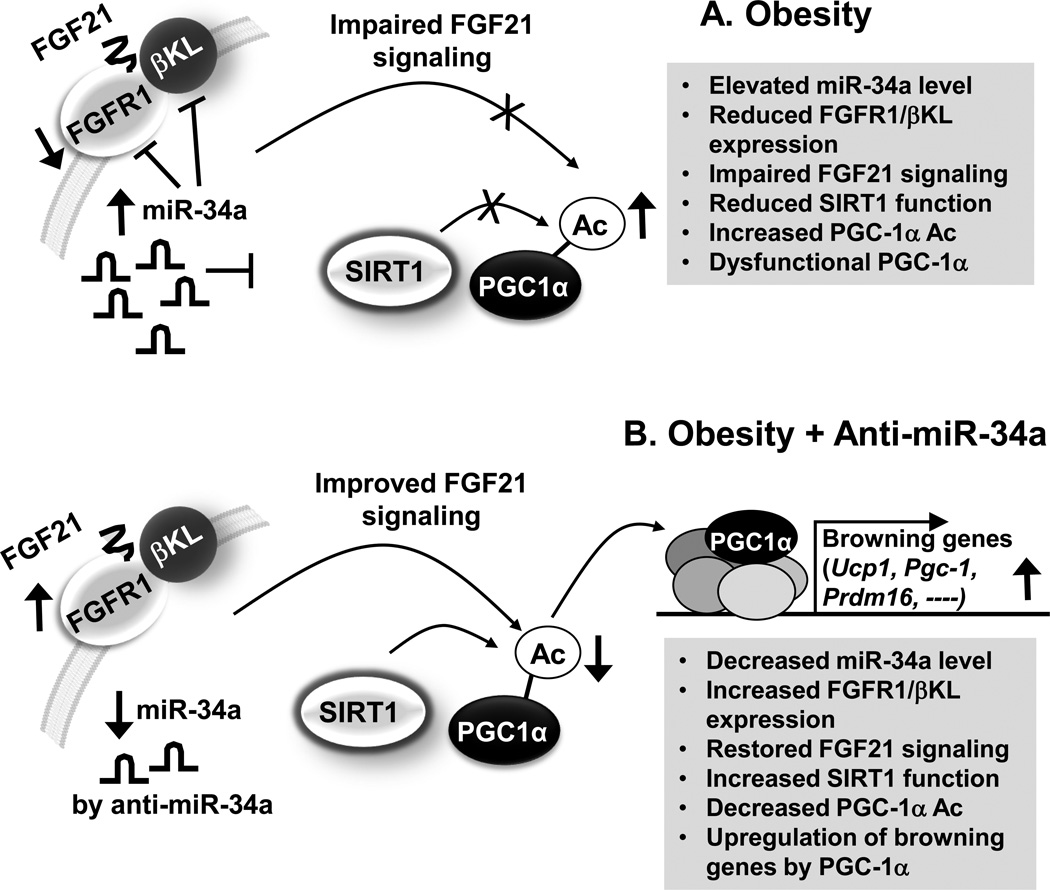
Fig. 2. Role of the miR-34a/βKL/FGF21 axis in adipose tissue.
(A) In diet-induced obese mice, elevated miR-34a in adipocytes attenuates FGF21 signaling, at least in part, by directly targeting both components of the FGF21 receptor complex, both FGFR1 and βKL, and also by targeting SIRT1 deacetylase. Decreased SIRT1 function results in increased acetylation levels of PGC1-α and subsequently decreased transcriptional activity of PGC1-α in regulation of PGC-1α target browning-related genes. (B) Downregulation of elevated miR-34a in obese mice by treatment with anti-miR-34a improves FGF21 signaling, at least in part, by increasing expression of the FGF21 receptor complex, both FGFR1 and βKL, and also increases expression and activity of SIRT1, which contributes to deacetylation of PGC-1α and induction of PGC-1α target browning-related genes.
In addition to increased FGF21 signaling, downregulation of miR-34a also increased SIRT1-dependent deacetylation of PGC-1α, which contributed to the induction of the browning genes, Ucp1, Pgc-1α and Prdm16 (Fisher, Kleiner, et al, 2012; Kajimura, Seale, and Spiegelman, 2010). PGC-1α was previously shown to be critical for FGF21-mediated fat browning upon cold exposure, and FGF21 was shown to increase PGC1-α protein levels without affecting protein stability(Fisher, Kleiner, et al, 2012), but the mechanism for the upregulation of PGC-1α activity during brown adipocyte differentiation was not determined. Our studies suggest that deacetylation of PGC-1α plays a role in linking FGF21 signaling with the induction of PGC-1α target browning genes (Fu, Seok, et al, 2014). Notably, PGC-1α acetylation levels were increased by downregulation of SIRT1 and also by downregulation of FGFR1/βKL, suggesting that both FGF21 signaling and SIRT1 function are important for deacetylation of PGC-1a and its browning gene activity (Fig. 2). Interestingly, each of these components, FGFR1, βKL and SIRT1, is a direct target of miR-34a, suggesting that miR-34a is a key upstream regulator of multiple target genes that control energy metabolism in multiple metabolic tissues.
In addition to fat browning, FGF21 has also been reported to increase glucose uptake in adipocytes by enhancing the expression of glucose transporter-1 (GLUT-1). FGF21 induces the phosphorylation of the transcription factors, serum response factor (SRF) and Ets-like protein 1 (Elk-1), which induce GLUT-1 expression (Ge, Chen, et al, 2011). However, these effects of FGF21 are impaired in adipose tissue of diet-induced obese mice. Taken together, in vivo silencing of miR-34a appears to be an appealing option for treatment of obesity by dramatically increasing beige-like fat depots in all types of WATs, as well as increasing brown fat depots in BAT as shown in Fig. 2, together with its beneficial metabolic effects on insulin sensitivity and its effects in liver that result in amelioration of obesity-induced NAFLD as illustrated in Fig. 1.
5.3. Role of miR-34a in the βKL/FGF21 axis in other tissues
FGF21, upon binding to its receptor, βKL and FGFR1, also exhibits beneficial metabolic actions in the pancreas. FGF21 improves pancreatic β-cell function and survival by activation of Erk and Akt signaling pathways (Wente, Efanov, et al, 2006). Studies from our group and others have shown that miR-34a levels are also elevated in pancreas of obese mice (Choi, Fu, et al, 2013; Fu, Seok, et al, 2014) and that elevated miR-34a leads to the dysfunction of pancreatic β-cell (Lovis, Roggli, et al, 2008). In pancreatic β-cell-derived MIN6B1 cells and pancreatic islets isolated from the leptin receptor-deficient db/db mice, chronic treatment with palmitic acid increased miR-34a levels in time- and dose-dependent manners, resulting in β-cell apoptosis and impaired insulin secretion (Lovis, Roggli, et al, 2008). Vesicle-associated membrane protein 2 (VAMP2), a direct target of miR-34a and a key player in β-cell exocytosis, was shown to mediate these effects. Further, FGF21 signaling in mouse pancreatic islets was impaired by high glucose levels which repressed expression of βKL, mediated by repression of PPARα, so that FGF21 signaling was attenuated (So, Cheng, et al, 2013). A role for miR-34a in regulation of βKL in the pancreas has not been reported, but since pancreatic miR-34a levels were abnormally increased in obesity (Fu, Seok, et al, 2014), it is possible that miR-34 may underlie the decreased βKL expression in the pancreas that results in compromised FGF21 signaling in obesity.
The FGF21/βKL pathway also orchestrates metabolism through regulation in the central nervous system. FGF21 has been reported to alter the brain circadian clock to coordinate activity and reproduction as well as to increase systemic glucocorticoid levels and suppressing physical activity, as part of the adaption to fasting. In brain-specific βKL-null mice, these effects of FGF21 were not observed showing that the FGF21 effects were mediated by its FGFR/βKL receptor (Bookout, de Groot, et al, 2013). In the hypothalamus, FGF21 acts at the suprachiasmatic nucleus to suppress vasopressin-kisspeptin signaling which blocks the luteinizing hormone surge (Owen, Bookout, et al, 2013). Since miR-34a levels are increased in brain in obesity (Li, Khanna, et al, 2011), especially in the aged brain, it will also be interesting to investigate the role of the brain FGF21/miR-34 axis in obesity and aging-related diseases.
It has been reported that FGF21 expression and secretion are induced upon prolonged starvation (Badman, Pissios, et al, 2007; Inagaki, Dutchak, et al, 2007). It was also shown that autophagy deficiency and subsequent mitochondrial dysfunction promote Fgf21 gene expression, and thus, promote protection from diet-induced obesity and insulin resistance(Kim, Jeong, et al, 2013). FGF21 also increases glucose uptake in muscle through upregulation of GLUT4 (Cuevas-Ramos, Almeda-Valdes, et al, 2012). Although miR-34a levels were not changed in skeletal muscle in obesity, it is possible that miR-34a expression is altered and thus, may affect FGF21 action in muscle in response to other biological cues or pathological conditions (Gan, Rumsey, et al, 2013).
6. Therapeutic and diagnostic potential of miRs
6.1 Therapeutic potential of miRs
Since expression of miRs is abnormally downregulated or upregulated in several disease states, efforts have been made to develop therapeutic agents that can mimic or downregulate miRs (Olson, 2014). Treatment with antagomirs, antisense miR inhibitors, of several miRs have shown beneficial effects, demonstrating the therapeutic potential of targeting miRs (Rottiers, Najafi-Shoushtari, et al, 2011). In general, there are three major antisense approaches, antagomiRs, which are conjugated to cholesterol to facilitate cellular uptake; locked nucleic acid (LNA) phosphorothioate chemistry; or chemical modification of the oligonucleotide at 2’-sugar and phosphate backbone moiety with MOE (2’-O-methoxyethylphosphorothioate) (Olson, 2014).
Antagonism of miR-122 in mice by systemically administered LNA-conjugated antisense miR resulted in upregulation of a large set of predicted target mRNAs in the liver (Esau, Davis, et al, 2006). Antagonism of miR-33 promoted cholesterol transport and had beneficial effects on atherosclerosis in mice (Najafi-Shoushtari, Kristo, et al, 2010) and importantly, inhibition of miR-33a/b raised plasma HDL and lowered VLDL in non-human primates (Rayner, Esau, et al, 2011). Antagonism of obesity-associated miR-103/miR-107 also resulted in improved insulin sensitivity (Trajkovski, Hausser, et al, 2011). Antagonism of miR-378 boosted activity of mitochondria, benefited obesity and cardiovascular disease (Trajkovski, Hausser, et al, 2011). Excitingly, recent in vivo antagonism or overexpression studies demonstrate that certain miRs are highly promising potential drug targets for treatment of metabolic diseases as summarized in Table 1.
Table 1.
Metabolic microRNAs currently in development as drug targets
| microRNA | Validated Target Genes |
Function (Key Ref) | Anti-miR Drugs |
|---|---|---|---|
| miR-122 |
Agpat1, Mogat1 MTTP, Klf6 |
Increase hepatic TG level and HCC development (Hsu et al; Tsai et al) |
RG-101 for Hepatitis C virus Infection |
| miR-33 | SREBP, HMGCR, LDL-R, ABCA1, ABCG1 |
Control cholesterol homeostasis (Esau, C. C et. al; Rayner, K. J. et. al; Najafi-Shoushtari et. al.) |
SPC-4955 for Cholesterol, HDL & Cardiovascular diseases |
| miR-103/107 | caveolin-1 | Regulate glucose homeostasis, insulin sensitivity (Trajkovski, M et. al.) |
RG-125 (AZD4076) for NASH in T2D patients/pre- diabetes |
| miR-378 | MED13 | Mitochondrial metabolism (Carrer et al.) |
Obesity, Cardiovascular Disease |
| miR-208 | Myh6, Myh7, Thrap1, Myostatin, MED13 |
Cardiac hypertrophy (Callis et. al.; Montgomery et. al; Grueter et.al.) |
Obesity, Cardiovascular Disease |
| miR-221 | DDIT4, CDKN1, p27, p57, |
Cell cycle arrest, Cell proliferation (Fornari et al. Pineaua et al.) |
Hepatocelluar Carcinoma |
| miR-21 | PTEN | Cell proliferation, Cell cycle arrest, Apoptosis (Meng et al. ) |
RG-012 for Hepatocelluar Carcinoma |
As described above, downregulation of miR-34a in mice resulted in decreased adiposity, increased glucose tolerance and insulin sensitivity, and improved lipid profiles (Fu, Seok, et al, 2014). Mechanistically, downregulation of miR-34a in obese mice resulted in improved FGF21 and FGF19 signaling by increasing expression of βKL and FGFR1. MiR-34a also has other targets, including SIRT1 and NAMPT, which would affect SIRT1 expression and activity (Choi, Fu, et al, 2013). Therefore, the beneficial effects of downregulation of miR-34a in obesity may involve increased expression of multiple gene targets in multiple tissues, which may provide an effective therapeutic advantage compared to the classical approach that targets only one molecule. However, this promiscuous targeting by miR-34a, as well as other miRs, could have detrimental side effects. To produce anti-miRs with acceptable therapeutic indexes, a thorough understanding of miR-34a regulatory networks, as aided by bioinformatics and systems biology approaches, will be essential.
Furthermore, abnormal activation of FGF19 is associated with colon and liver cancer so the extent of increased FGF19 signaling by downregulation of miR-34a must be carefully considered (Desnoyers, Pai, et al, 2008). Similarly, miR-34a is a well-known tumor suppressor, and deletion of the miR-34a gene was associated with tumorigenesis (Yamakuchi, Ferlito, and Lowenstein, 2008). Thus, targeting the miR-34a/ FGF19/21 axis will need a careful modulation of the extent of inhibition, because excess miR-34a is associated with metabolic diseases and extreme deficiency of miR-34a is associated with cancer.
6.2 Diagnostic potential of miRs
Although miRs in general act inside cells, several miRs, such as miR-34a and miR-155, have been detected circulating in the blood (Pogribny, Starlard-Davenport, et al, 2010). The circulating miRs have diagnostic potential since the levels correlated with tissue levels and they can, thus, serve as biomarkers for diseases. Although the mechanism of how these miRs travel through bloodstream is largely unknown, the discovery of circulating miRs has highlighted their potential as diagnostic markers for disease (Olson, 2014).
Human clinical studies show that miR-34a levels are increased more than 6-fold in patients with NALFD and with chronic hepatitis C (Cermelli, Ruggieri, et al, 2011). In patients with non-alcoholic steatohepatitis (NASH), miR-34a levels were increased ~100-fold. Levels of miR-34a have been reported to increase ~10-fold in ob/ob mice and diet-induced obese mice and ~6-fold in STZ-induced diabetic mice (Owen, Bookout, et al, 2013). Moreover, clinical data from our group also demonstrated that miR-34a levels are highly correlated with the BMI index of obese patients (Choi, Fu, et al, 2013). Strikingly, miR-34a levels in the serum of obese patients were significantly elevated compared to controls. In addition, it was shown that serum miR-34a levels correlate with progression of fatty liver disease, such as NAFLD, NASH and cirrhosis (Cermelli, Ruggieri, et al, 2011; Cheung, Puri, et al, 2008). This correlation suggests that serum miR-34a can serve as a potential diagnostic biomarker of liver diseases in place of liver biopsies.
7. Conclusion
Obesity has become a global pandemic, which has sparked research interest in energy metabolism. FGF19 and FGF21 are metabolic hormones that play key roles in energy metabolism and counteract obesity. Since βKL acts as the obligate co-receptor for both FGF19 and FGF21, βKL serves an effective potential therapeutic target for improving FGF19 and FGF21 signaling in obesity. Indeed, studies on agonist antibodies to FGFR1 or to both FGFR1 and βKL demonstrated that antibodies stably mimic the hormone action, resulting in beneficial metabolic effects in both mice and monkeys, although the antibody approach has the same side effects as recombinant FGF21, such as fragile bones and water retention (Foltz, Hu, et al, 2012; Wu, Kolumam, et al, 2011). As described above, targeting elevated miR-34a in obesity by anti-miR-34a holds promise as a potential anti-obesity drug by targeting a network of genes, including βKL, in multiple tissues. The striking role of downregulation of miR-34a in promoting fat browning in all types of WATs and BAT (Fu, Seok, et al, 2014), together with its beneficial effects on hepatic lipid metabolism, such as increased fatty acid oxidation, decreased lipogenesis, and improved steatosis (Fu, Choi, et al, 2012), suggest that targeting a single microRNA, miR-34a, may provide an effective therapeutic option for combating obesity and treating obesity-related metabolic disease. Other small miRs play important roles in metabolic regulation and may also serve as attractive therapeutic drug targets for treatment of obesity-related disease and disease biomarkers.
Acknowledgments
We thank Dr. Byron Kemper for critical reading of the manuscript. Research discussed from the authors’ laboratory was supported by grants DK062777 and NIH DK095842 from the National Institutes of Health.
References
- Badman MK, Pissios P, Kennedy AR, Koukos G, Flier JS, Maratos-Flier E. Hepatic fibroblast growth factor 21 is regulated by PPARalpha and is a key mediator of hepatic lipid metabolism in ketotic states. Cell. Metab. 2007;6:426–437. doi: 10.1016/j.cmet.2007.05.002. [DOI] [PubMed] [Google Scholar]
- Beenken A, Mohammadi M. The FGF family: biology, pathophysiology and therapy. Nat. Rev. Drug Discov. 2009;3:235–253. doi: 10.1038/nrd2792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookout AL, de Groot MH, Owen BM, Lee S, Gautron L, Lawrence HL, Ding X, Elmquist JK, Takahashi JS, Mangelsdorf DJ, Kliewer SA. FGF21 regulates metabolism and circadian behavior by acting on the nervous system. Nat. Med. 2013;9:1147–1152. doi: 10.1038/nm.3249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Auwerx J. Cell biology. FGF21 takes a fat bite. Science. 2012;6082:675–676. doi: 10.1126/science.1222646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canto C, Houtkooper RH, Pirinen E, Youn DY, Oosterveer MH, Cen Y, Fernandez-Marcos PJ, Yamamoto H, Andreux PA, Cettour-Rose P, et al. The NAD(+) precursor nicotinamide riboside enhances oxidative metabolism and protects against high-fat diet-induced obesity. Cell. Metab. 2012;6:838–847. doi: 10.1016/j.cmet.2012.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carrer M, Liu N, Grueter CE, Williams AH, Frisard MI, Hulver MW, Bassel-Duby R, Olson EN. Control of mitochondrial metabolism and systemic energy homeostasis by microRNAs 378 and 378*. Proc. Natl. Acad. Sci. U. S. A. 2012;38:15330–15335. doi: 10.1073/pnas.1207605109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cermelli S, Ruggieri A, Marrero JA, Ioannou GN, Beretta L. Circulating microRNAs in patients with chronic hepatitis C and non-alcoholic fatty liver disease. PLoS One. 2011;8:e23937. doi: 10.1371/journal.pone.0023937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Siegel F, Kipschull S, Haas B, Frohlich H, Meister G, Pfeifer A. miR-155 regulates differentiation of brown and beige adipocytes via a bistable circuit. Nat. Commun. 2013:1769. doi: 10.1038/ncomms2742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung O, Puri P, Eicken C, Contos MJ, Mirshahi F, Maher JW, Kellum JM, Min H, Luketic VA, Sanyal AJ. Nonalcoholic steatohepatitis is associated with altered hepatic MicroRNA expression. Hepatology. 2008;6:1810–1820. doi: 10.1002/hep.22569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SE, Fu T, Seok S, Kim DH, Yu E, Lee KW, Kang Y, Li X, Kemper B, Kemper JK. Elevated microRNA-34a in obesity reduces NAD+ levels and SIRT1 activity by directly targeting NAMPT. Aging Cell. 2013;6:1062–1072. doi: 10.1111/acel.12135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuevas-Ramos D, Almeda-Valdes P, Meza-Arana CE, Brito-Cordova G, Gomez-Perez FJ, Mehta R, Oseguera-Moguel J, Aguilar-Salinas CA. Exercise increases serum fibroblast growth factor 21 (FGF21) levels. PLoS One. 2012;5:e38022. doi: 10.1371/journal.pone.0038022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desnoyers LR, Pai R, Ferrando RE, Hotzel K, Le T, Ross J, Carano R, D'Souza A, Qing J, Mohtashemi I, Ashkenazi A, French DM. Targeting FGF19 inhibits tumor growth in colon cancer xenograft and FGF19 transgenic hepatocellular carcinoma models. Oncogene. 2008;1:85–97. doi: 10.1038/sj.onc.1210623. [DOI] [PubMed] [Google Scholar]
- Ding J, Li M, Wan X, Jin X, Chen S, Yu C, Li Y. Effect of miR-34a in regulating steatosis by targeting PPARalpha expression in nonalcoholic fatty liver disease. Sci. Rep. 2015:13729. doi: 10.1038/srep13729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dushay J, Chui PC, Gopalakrishnan GS, Varela-Rey M, Crawley M, Fisher FM, Badman MK, Martinez-Chantar ML, Maratos-Flier E. Increased fibroblast growth factor 21 in obesity and nonalcoholic fatty liver disease. Gastroenterology. 2010;2:456–463. doi: 10.1053/j.gastro.2010.04.054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutchak PA, Katafuchi T, Bookout AL, Choi JH, Yu RT, Mangelsdorf DJ, Kliewer SA. Fibroblast growth factor-21 regulates PPARgamma activity and the antidiabetic actions of thiazolidinediones. Cell. 2012;3:556–567. doi: 10.1016/j.cell.2011.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Esau C, Davis S, Murray SF, Yu XX, Pandey SK, Pear M, Watts L, Booten SL, Graham M, McKay R, et al. miR-122 regulation of lipid metabolism revealed by in vivo antisense targeting. Cell. Metab. 2006;2:87–98. doi: 10.1016/j.cmet.2006.01.005. [DOI] [PubMed] [Google Scholar]
- Fang S, Suh JM, Reilly SM, Yu E, Osborn O, Lackey D, Yoshihara E, Perino A, Jacinto S, Lukasheva Y, et al. Intestinal FXR agonism promotes adipose tissue browning and reduces obesity and insulin resistance. Nat. Med. 2015;2:159–165. doi: 10.1038/nm.3760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Chui PC, Antonellis PJ, Bina HA, Kharitonenkov A, Flier JS, Maratos-Flier E. Obesity is a fibroblast growth factor 21 (FGF21)-resistant state. Diabetes. 2010;11:2781–2789. doi: 10.2337/db10-0193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fisher FM, Kleiner S, Douris N, Fox EC, Mepani RJ, Verdeguer F, Wu J, Kharitonenkov A, Flier JS, Maratos-Flier E, Spiegelman BM. FGF21 regulates PGC-1alpha and browning of white adipose tissues in adaptive thermogenesis. Genes Dev. 2012;3:271–281. doi: 10.1101/gad.177857.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foltz IN, Hu S, King C, Wu X, Yang C, Wang W, Weiszmann J, Stevens J, Chen JS, Nuanmanee N, et al. Treating diabetes and obesity with an FGF21-mimetic antibody activating the betaKlotho/FGFR1c receptor complex. Sci. Transl. Med. 2012;162:162ra153. doi: 10.1126/scitranslmed.3004690. [DOI] [PubMed] [Google Scholar]
- Fu T, Choi SE, Kim DH, Seok S, Suino-Powell KM, Xu HE, Kemper JK. Aberrantly elevated microRNA-34a in obesity attenuates hepatic responses to FGF19 by targeting a membrane coreceptor beta-Klotho. Proc. Natl. Acad. Sci. U. S. A. 2012;40:16137–16142. doi: 10.1073/pnas.1205951109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fu T, Seok S, Choi S, Huang Z, Suino-Powell K, Xu HE, Kemper B, Kemper JK. MicroRNA 34a inhibits beige and brown fat formation in obesity in part by suppressing adipocyte fibroblast growth factor 21 signaling and SIRT1 function. Mol. Cell. Biol. 2014;22:4130–4142. doi: 10.1128/MCB.00596-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gan Z, Rumsey J, Hazen BC, Lai L, Leone TC, Vega RB, Xie H, Conley KE, Auwerx J, Smith SR, et al. Nuclear receptor/microRNA circuitry links muscle fiber type to energy metabolism. J. Clin. Invest. 2013;6:2564–2575. doi: 10.1172/JCI67652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ge X, Chen C, Hui X, Wang Y, Lam KS, Xu A. Fibroblast growth factor 21 induces glucose transporter-1 expression through activation of the serum response factor/Ets-like protein-1 in adipocytes. J. Biol. Chem. 2011;40:34533–34541. doi: 10.1074/jbc.M111.248591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grundy SM. Metabolic syndrome pandemic. Arterioscler. Thromb. Vasc. Biol. 2008;4:629–636. doi: 10.1161/ATVBAHA.107.151092. [DOI] [PubMed] [Google Scholar]
- Hobert O. Gene regulation by transcription factors and microRNAs. Science. 2008;5871:1785–1786. doi: 10.1126/science.1151651. [DOI] [PubMed] [Google Scholar]
- Inagaki T, Dutchak P, Zhao G, Ding X, Gautron L, Parameswara V, Li Y, Goetz R, Mohammadi M, Esser V, et al. Endocrine regulation of the fasting response by PPARalpha-mediated induction of fibroblast growth factor 21. Cell. Metab. 2007;6:415–425. doi: 10.1016/j.cmet.2007.05.003. [DOI] [PubMed] [Google Scholar]
- Ito S, Fujimori T, Furuya A, Satoh J, Nabeshima Y, Nabeshima Y. Impaired negative feedback suppression of bile acid synthesis in mice lacking betaKlotho. J. Clin. Invest. 2005;8:2202–2208. doi: 10.1172/JCI23076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeon TI, Esquejo RM, Roqueta-Rivera M, Phelan PE, Moon YA, Govindarajan SS, Esau CC, Osborne TF. An SREBP-responsive microRNA operon contributes to a regulatory loop for intracellular lipid homeostasis. Cell. Metab. 2013;1:51–61. doi: 10.1016/j.cmet.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jordan SD, Kruger M, Willmes DM, Redemann N, Wunderlich FT, Bronneke HS, Merkwirth C, Kashkar H, Olkkonen VM, Bottger T, et al. Obesity-induced overexpression of miRNA-143 inhibits insulin-stimulated AKT activation and impairs glucose metabolism. Nat. Cell Biol. 2011;4:434–446. doi: 10.1038/ncb2211. [DOI] [PubMed] [Google Scholar]
- Kahn BB, Flier JS. Obesity and insulin resistance. J. Clin. Invest. 2000;4:473–481. doi: 10.1172/JCI10842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kajimura S, Seale P, Spiegelman BM. Transcriptional control of brown fat development. Cell. Metab. 2010;4:257–262. doi: 10.1016/j.cmet.2010.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kemper JK, Choi SE, Kim DH. Sirtuin 1 deacetylase: a key regulator of hepatic lipid metabolism. Vitam. Horm. 2013:385–404. doi: 10.1016/B978-0-12-407766-9.00016-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim KH, Jeong YT, Oh H, Kim SH, Cho JM, Kim YN, Kim SS, Kim do H, Hur KY, Kim HK, et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nat. Med. 2013;1:83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- Kir S, Beddow SA, Samuel VT, Miller P, Previs SF, Suino-Powell K, Xu HE, Shulman GI, Kliewer SA, Mangelsdorf DJ. FGF19 as a postprandial, insulin-independent activator of hepatic protein and glycogen synthesis. Science. 2011;6024:1621–1624. doi: 10.1126/science.1198363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornfeld JW, Baitzel C, Konner AC, Nicholls HT, Vogt MC, Herrmanns K, Scheja L, Haumaitre C, Wolf AM, Knippschild U, et al. Obesity-induced overexpression of miR-802 impairs glucose metabolism through silencing of Hnf1b. Nature. 2013;7435:111–115. doi: 10.1038/nature11793. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Klotho and betaKlotho. Adv. Exp. Med. Biol. 2012:25–40. doi: 10.1007/978-1-4614-0887-1_2. [DOI] [PubMed] [Google Scholar]
- Kuro-o M. Endocrine FGFs and Klothos: emerging concepts. Trends Endocrinol. Metab. 2008;7:239–245. doi: 10.1016/j.tem.2008.06.002. [DOI] [PubMed] [Google Scholar]
- Kuro-o M, Matsumura Y, Aizawa H, Kawaguchi H, Suga T, Utsugi T, Ohyama Y, Kurabayashi M, Kaname T, Kume E, et al. Mutation of the mouse klotho gene leads to a syndrome resembling ageing. Nature. 1997;6655:45–51. doi: 10.1038/36285. [DOI] [PubMed] [Google Scholar]
- Kurosu H, Choi M, Ogawa Y, Dickson AS, Goetz R, Eliseenkova AV, Mohammadi M, Rosenblatt KP, Kliewer SA, Kuro-o M. Tissue-specific expression of betaKlotho and fibroblast growth factor (FGF) receptor isoforms determines metabolic activity of FGF19 and FGF21. J. Biol. Chem. 2007;37:26687–26695. doi: 10.1074/jbc.M704165200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurosu H, Ogawa Y, Miyoshi M, Yamamoto M, Nandi A, Rosenblatt KP, Baum MG, Schiavi S, Hu MC, Moe OW, Kuro-o M. Regulation of fibroblast growth factor-23 signaling by klotho. J. Biol. Chem. 2006;10:6120–6123. doi: 10.1074/jbc.C500457200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Kemper JK. Controlling SIRT1 expression by microRNAs in health and metabolic disease. Aging (Albany NY) 2010;8:527–534. doi: 10.18632/aging.100184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J, Padhye A, Sharma A, Song G, Miao J, Mo YY, Wang L, Kemper JK. A pathway involving farnesoid X receptor and small heterodimer partner positively regulates hepatic sirtuin 1 levels via microRNA-34a inhibition. J. Biol. Chem. 2010;17:12604–12611. doi: 10.1074/jbc.M109.094524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X, Khanna A, Li N, Wang E. Circulatory miR34a as an RNAbased, noninvasive biomarker for brain aging. Aging (Albany NY) 2011;10:985–1002. doi: 10.18632/aging.100371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovis P, Roggli E, Laybutt DR, Gattesco S, Yang JY, Widmann C, Abderrahmani A, Regazzi R. Alterations in microRNA expression contribute to fatty acid-induced pancreatic beta-cell dysfunction. Diabetes. 2008;10:2728–2736. doi: 10.2337/db07-1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mraz M, Lacinova Z, Kavalkova P, Haluzikova D, Trachta P, Drapalova J, Hanusova V, Haluzik M. Serum concentrations of fibroblast growth factor 19 in patients with obesity and type 2 diabetes mellitus: the influence of acute hyperinsulinemia, very-low calorie diet and PPAR-alpha agonist treatment. Physiol. Res. 2011;4:627–636. doi: 10.33549/physiolres.932099. [DOI] [PubMed] [Google Scholar]
- Najafi-Shoushtari SH, Kristo F, Li Y, Shioda T, Cohen DE, Gerszten RE, Naar AM. MicroRNA-33 and the SREBP host genes cooperate to control cholesterol homeostasis. Science. 2010;5985:1566–1569. doi: 10.1126/science.1189123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogawa Y, Kurosu H, Yamamoto M, Nandi A, Rosenblatt KP, Goetz R, Eliseenkova AV, Mohammadi M, Kuro-o M. BetaKlotho is required for metabolic activity of fibroblast growth factor 21. Proc. Natl. Acad. Sci. U. S. A. 2007;18:7432–7437. doi: 10.1073/pnas.0701600104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olson EN. MicroRNAs as therapeutic targets and biomarkers of cardiovascular disease. Sci. Transl. Med. 2014;239:239ps3. doi: 10.1126/scitranslmed.3009008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Bookout AL, Ding X, Lin VY, Atkin SD, Gautron L, Kliewer SA, Mangelsdorf DJ. FGF21 contributes to neuroendocrine control of female reproduction. Nat. Med. 2013;9:1153–1156. doi: 10.1038/nm.3250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owen BM, Mangelsdorf DJ, Kliewer SA. Tissue-specific actions of the metabolic hormones FGF15/19 and FGF21. Trends Endocrinol. Metab. 2015;1:22–29. doi: 10.1016/j.tem.2014.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perez LM, Bernal A, San Martin N, Lorenzo M, Fernandez-Veledo S, Galvez BG. Metabolic rescue of obese adipose-derived stem cells by Lin28/Let7 pathway. Diabetes. 2013;7:2368–2379. doi: 10.2337/db12-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pogribny IP, Starlard-Davenport A, Tryndyak VP, Han T, Ross SA, Rusyn I, Beland FA. Difference in expression of hepatic microRNAs miR-29c, miR-34a, miR-155, and miR-200b is associated with strain-specific susceptibility to dietary nonalcoholic steatohepatitis in mice. Lab. Invest. 2010;10:1437–1446. doi: 10.1038/labinvest.2010.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potthoff MJ, Kliewer SA, Mangelsdorf DJ. Endocrine fibroblast growth factors 15/19 and 21: from feast to famine. Genes Dev. 2012;4:312–324. doi: 10.1101/gad.184788.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poy MN, Eliasson L, Krutzfeldt J, Kuwajima S, Ma X, Macdonald PE, Pfeffer S, Tuschl T, Rajewsky N, Rorsman P, Stoffel M. A pancreatic islet-specific microRNA regulates insulin secretion. Nature. 2004;7014:226–230. doi: 10.1038/nature03076. [DOI] [PubMed] [Google Scholar]
- Rayner KJ, Esau CC, Hussain FN, McDaniel AL, Marshall SM, van Gils JM, Ray TD, Sheedy FJ, Goedeke L, Liu X, et al. Inhibition of miR-33a/b in non-human primates raises plasma HDL and lowers VLDL triglycerides. Nature. 2011;7369:404–407. doi: 10.1038/nature10486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rayner KJ, Suarez Y, Davalos A, Parathath S, Fitzgerald ML, Tamehiro N, Fisher EA, Moore KJ, Fernandez-Hernando C. MiR-33 contributes to the regulation of cholesterol homeostasis. Science. 2010;5985:1570–1573. doi: 10.1126/science.1189862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers V, Naar AM. MicroRNAs in metabolism and metabolic disorders. Nat. Rev. Mol. Cell Biol. 2012;4:239–250. doi: 10.1038/nrm3313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rottiers V, Najafi-Shoushtari SH, Kristo F, Gurumurthy S, Zhong L, Li Y, Cohen DE, Gerszten RE, Bardeesy N, Mostoslavsky R, Naar AM. MicroRNAs in metabolism and metabolic diseases. Cold Spring Harb. Symp. Quant. Biol. 2011:225–233. doi: 10.1101/sqb.2011.76.011049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schreuder TC, Marsman HA, Lenicek M, van Werven JR, Nederveen AJ, Jansen PL, Schaap FG. The hepatic response to FGF19 is impaired in patients with nonalcoholic fatty liver disease and insulin resistance. Am. J. Physiol. Gastrointest. Liver Physiol. 2010;3:G440–G445. doi: 10.1152/ajpgi.00322.2009. [DOI] [PubMed] [Google Scholar]
- So WY, Cheng Q, Chen L, Evans-Molina C, Xu A, Lam KS, Leung PS. High glucose represses beta-klotho expression and impairs fibroblast growth factor 21 action in mouse pancreatic islets: involvement of peroxisome proliferator-activated receptor gamma signaling. Diabetes. 2013;11:3751–3759. doi: 10.2337/db13-0645. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman BM, Flier JS. Obesity and the regulation of energy balance. Cell. 2001;4:531–543. doi: 10.1016/s0092-8674(01)00240-9. [DOI] [PubMed] [Google Scholar]
- Sun L, Trajkovski M. MiR-27 orchestrates the transcriptional regulation of brown adipogenesis. Metabolism. 2014;2:272–282. doi: 10.1016/j.metabol.2013.10.004. [DOI] [PubMed] [Google Scholar]
- Trajkovski M, Ahmed K, Esau CC, Stoffel M. MyomiR-133 regulates brown fat differentiation through Prdm16. Nat. Cell Biol. 2012;12:1330–1335. doi: 10.1038/ncb2612. [DOI] [PubMed] [Google Scholar]
- Trajkovski M, Hausser J, Soutschek J, Bhat B, Akin A, Zavolan M, Heim MH, Stoffel M. MicroRNAs 103 and 107 regulate insulin sensitivity. Nature. 2011;7353:649–653. doi: 10.1038/nature10112. [DOI] [PubMed] [Google Scholar]
- Wei W, Dutchak PA, Wang X, Ding X, Wang X, Bookout AL, Goetz R, Mohammadi M, Gerard RD, Dechow PC, et al. Fibroblast growth factor 21 promotes bone loss by potentiating the effects of peroxisome proliferator-activated receptor gamma. Proc. Natl. Acad. Sci. U. S. A. 2012;8:3143–3148. doi: 10.1073/pnas.1200797109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wente W, Efanov AM, Brenner M, Kharitonenkov A, Koster A, Sandusky GE, Sewing S, Treinies I, Zitzer H, Gromada J. Fibroblast growth factor-21 improves pancreatic beta-cell function and survival by activation of extracellular signal-regulated kinase 1/2 and Akt signaling pathways. Diabetes. 2006;9:2470–2478. doi: 10.2337/db05-1435. [DOI] [PubMed] [Google Scholar]
- Wu AL, Kolumam G, Stawicki S, Chen Y, Li J, Zavala-Solorio J, Phamluong K, Feng B, Li L, Marsters S, et al. Amelioration of type 2 diabetes by antibody-mediated activation of fibroblast growth factor receptor 1. Sci. Transl. Med. 2011;113:113ra126. doi: 10.1126/scitranslmed.3002669. [DOI] [PubMed] [Google Scholar]
- Wu X, Ge H, Lemon B, Vonderfecht S, Baribault H, Weiszmann J, Gupte J, Gardner J, Lindberg R, Wang Z, Li Y. Separating mitogenic and metabolic activities of fibroblast growth factor 19 (FGF19) Proc. Natl. Acad. Sci. U. S. A. 2010;32:14158–14163. doi: 10.1073/pnas.1009427107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu Y, Zalzala M, Xu J, Li Y, Yin L, Zhang Y. A metabolic stress-inducible miR-34a–HNF4alpha pathway regulates lipid and lipoprotein metabolism. Nat. Commun. 2015:7466. doi: 10.1038/ncomms8466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamakuchi M, Ferlito M, Lowenstein CJ. miR-34a repression of SIRT1 regulates apoptosis. Proc. Natl. Acad. Sci. U. S. A. 2008;36:13421–13426. doi: 10.1073/pnas.0801613105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamauchi T, Kadowaki T. Physiological and pathophysiological roles of adiponectin and adiponectin receptors in the integrated regulation of metabolic and cardiovascular diseases. Int. J. Obes. (Lond) 2008:S13–S18. doi: 10.1038/ijo.2008.233. [DOI] [PubMed] [Google Scholar]