Abstract
IMPORTANCE
Depression is frequently undiagnosed in patients with chronic rhinosinusitis (CRS) and affects quality of life, productivity, and health care use.
OBJECTIVE
To examine depression-specific outcomes after medical or surgical treatment of CRS.
DESIGN, SETTING, AND PARTICIPANTS
A multi-institutional, prospective study of patients with refractory CRS treated at tertiary academic rhinology centers was performed from March 1, 2011, to November 1, 2015. Data analysis was performed from October 1, 2015, to November 1, 2015.
INTERVENTIONS
Patients self-selected to undergo continued medical management or endoscopic sinus surgery for refractory CRS.
MAIN OUTCOMES AND MEASURES
Patients completed the 22-item Sinonasal Outcome Test (SNOT22), Rhinosinusitis Disability Index (RSDI), Pittsburgh Sleep Quality Index (PSQI), and missed productivity and medication use questionnaires before and at least 6 months after treatment. Computed tomography and endoscopy scoring were performed with reviewers masked to patient-reported data. Depression-specific outcomes were recorded using the 2-item Patient Health Questionnaire (PHQ2).
RESULTS
Baseline data were available on 685 patients, with 167 (24.4%) having depression according to the PHQ2 scores. The mean (SD) age of the patients was 50.5 (15.0) years, and 332 (48.4%) were male. Revision surgery status was the only baseline factor associated with depression (53.9%vs 38.0%, P < .001). Patients with depression had worse baseline SNOT22 (mean, 64.5 vs 47.6), PSQI (mean, 12.8 vs 8.4), productivity (mean, 22.8 vs 5.2 days missed), and medication use scores for oral antibiotics (mean, 23.8 vs 14.8) and oral corticosteroids (mean, 17.8 vs 9.9) (P < .001 for all). Medical and surgical treatments had similar outcomes for patients with depression with mean improvement in the PHQ2 scores from 3.96 to 1.91 (P < .001), and 110 of 167 patients (65.9%) categorized as having depression at baseline were categorized as not having depression after treatment. Improvements in the PHQ2 scores were associated with improvements in the SNOT22, PSQI, oral antibiotic use, and productivity scores (P ≤ .001 for all).
CONCLUSIONS AND RELEVANCE
Depression is a common comorbidity in patients with CRS and affects numerous quality-of-life and health care outcomes. There are few objective baseline factors to aid physicians in identifying depression in patients with CRS. Medical and surgical treatments for CRS improve depression and related clinical outcomes.
Chronic rhinosinusitis (CRS) is a complex disease with broad effects throughout the body. In addition to triggering symptoms in the sinuses and upper airway, patients with CRS have comorbid systemic ailments, including depression, cognitive dysfunction, anxiety, and sleep disorders.1–3 We currently lack a thorough understanding of the association between CRS and systemic comorbidities and the effect of CRS-specific therapies on these comorbidities.
Depression is a highly prevalent chronic disease, and 9% to 25%of patients with CRS report a physician diagnosis of comorbid depression.4,5 Although this reported rate is similar to the population without CRS, validated screening instruments typically detect twice as many patients with CRS with previously undiagnosed depression.3,4,6 Comorbid depression in patients with CRS has been associated with worse baseline and posttreatment scores on sinus-specific quality-of-life (QOL) instruments.4–7 Therefore, identifying comorbid depression is important not only to improve patient counseling during the shared decision-making process regarding treatment selection but also to further elucidate how treatment of comorbid depression may influence sinus-specific outcomes for CRS.
To our knowledge, detailed studies have not been performed examining depression-specific outcomes after medical treatment of CRS, but studies8,9 have been performed after endoscopic sinus surgery (ESS) using the 21-item Beck Depression Inventory-II (BDI). Overall, ESS improves BDI scores by roughly 30%, and 26% to 49% of patients achieve a minimal clinically important difference (MCID).8,9 A limited number of CRS-specific factors have been associated with improved depression outcomes after ESS. Patients with CRS with nasal polyps (CRSwNP) achieved an MCID on the BDI more often after ESS than patients with CRS without nasal polyps (CRSsNP) (34 [61.8%] of 55 vs 21 [38.2%] of 55).8 Patients with hyposmia and anosmia also achieved an MCID more frequently (26 [54.2%] of 48 and 22 [61.1%] of 36, respectively) than normosmic patients (7 [25.9%] of 27), and improvement in objective olfactory test results correlated with improvement in BDI scores.9 Finally, nonsmokers have also been reported to have greater improvement in BDI scores after ESS.8
The purpose of this study was to investigate the association of CRS-specific patient factors with comorbid depression identified using the 2-item Patient Health Questionnaire (PHQ2), which is a rapid 2-question screening instrument. We also examined the association between comorbid depression and medical or surgical treatment outcomes, including CRS-specific QOL, sleep quality, productivity, and medication use. The results of this study may help physicians identify patients with CRS at risk for having undiagnosed depression and provide information to improve patient counseling for expected treatment outcomes for their CRS.
Methods
Study Population
A multi-institutional, prospective study of patients with refractory CRS treated at tertiary academic rhinology centers was performed from March 1, 2011, to November 1, 2015. Data analysis was performed from October 1, 2015, to November 1, 2015. Adult (≥18 years old) patients with CRS were recruited from rhinology clinics at Medical University of South Carolina, Oregon Health & Sciences University, Stanford University, and University of Calgary as part of an ongoing prospective cohort study. This study was approved in advance by the institutional review boards at the Medical University of South Carolina, Oregon Health & Sciences University, Stanford University, and Calgary University, and all participants provided written informed consent.
Each patient fulfilled diagnostic criteria for CRS according to the clinical practice guidelines of the American Academy of Otolaryngology–Head and Neck Surgery.10 Each study participant had undergone failed standard medical therapy with saline rinse, nasal corticosteroid spray, at least 3 weeks of broad-spectrum antibiotics, and 5 days of oral corticosteroids, then self-selected ESS or continued medical therapy for treatment of recalcitrant symptoms associated with CRS. With the use of standardized questionnaires, information on characteristics related to demographics and medical comorbidities was collected for each patient, including the presence of nasal polyposis, asthma, aspirin-exacerbated respiratory disease, allergic fungal rhinosinusitis, physician-diagnosed allergic rhinitis, physician-diagnosed depression, fibromyalgia, diabetes mellitus, tobacco use, oral corticosteroid dependency, and a history of previous sinus surgery. High-resolution computed tomography was performed on each patient during routine clinical care and before sinus surgery in all instances. Computed tomographic scans were evaluated by all enrolling physicians who were masked to patient-reported data, and the degree of sinus opacification was scored using the Lund-Mackay staging system, with the reviewer masked to other study data. Nasal endoscopy was performed on each patient, and those with visible polyps were classified as having CRSwNP and those without visible polyps as having CRSsNP. Nasal endoscopy examinations were scored using the Lund-Kennedy staging system. Olfactory function was quantified using the 12-item Brief Smell Identification Test (Sensonics Inc), which is a validated, forced-choice, scratch-and-sniff test that uses microencapsulated odorant strips (score range, 0–12). Olfaction scores were collected preoperatively and again after ESS or continued medical therapy (minimum of 6 months).
Several patient-reported outcome measures (PROMs) were assessed. Each patient completed 2 surveys of sinus-specific QOL, the 22-item Sinonasal Outcome Test (SNOT22) and its 5 subdomains (rhinologic, extranasal rhinologic, ear/facial, psychological, and sleep), and the Rhinosinusitis Disability Index (RSDI) and its 3 subdomains (emotional, functional, and physical).11 Sleep quality was assessed using the Pittsburgh Sleep Quality Index (PSQI), a self-reported questionnaire that measures sleep quality and disturbance in the preceding 1-month period.12,13 The PSQI has been used in a wide variety of clinical populations, including CRS, and has high internal consistency, reliability, and construct validity.1 It has 7 components (sleep quality, latency, duration, efficiency, disturbance, medication use, and daytime dysfunction) that are each scored from 0 to 3 for a total PSQI score of 0 to 21,with scores of 5 or higher indicating poor sleep. The PSQI is particularly useful because global scores are more highly correlated with sleep problems than depression, allowing sleep disturbance to be identified apart from depression. Depression was assessed using the PHQ2. This instrument inquires about the frequency of depressed mood and anhedonia in the preceding 2 weeks, scoring each question from 0 to 3, for a maximum score of 6. A cutoff of 3 or higher has a sensitivity of 83% and specificity of 92% for major depression.14 Medication use was measured as the number of days in the last 90 days that a specific medication was used. Missed productivity was number of days of work or school missed in the last 90 days. All patients had a minimum of 6 months of follow-up.
Statistical Analysis
Statistical analyses were performed using SAS statistical software, version 9.4 (SAS Institute Inc). Descriptive statistics for preoperative characteristics are reported as the mean (SD) number of responses for continuous measures. Categorical measures are reported as frequency (prevalence). Differences in baseline characteristics between patients categorized as having depression and not having depression by the PHQ2 were assessed using independent t tests and Pearson χ2 tests for continuous and categorical measures, respectively. To assess the association between the effect of CRS treatments on depression outcomes, mixed-effects linear models were constructed. To assess the association among age, race, treatment modality, and polyp status with achieving MCID, logistic regression was performed. Significance was assessed with an α of .05, and no correction for multiple comparisons was performed.
Results
Baseline Characteristics
Overall, baseline data were available for 685 patients (Table 1). Physician-diagnosed depression was present in 94 (13.7%). Scores on the PHQ2 of 3 or higher, indicating likely depression, were present in 167 patients (24.4%). When comparing patients with CRS with and without likely depression, as determined by the PHQ2, patients with depression drank less alcohol (14.1 vs 28.5 g/wk) and had higher rates of physician diagnosed depression (47 [28.1%] of 167 vs 47 [9.1%] of 518). Other demographic factors, including age, race, sex, and smoking status, and comorbidities, such as asthma, chronic obstructive pulmonary disease, or fibromyalgia, were not associated with depression. The only CRS-specific demographic characteristic that was associated with baseline depression was revision surgery status (P < .001). Sinus-specific QOL, as measured by SNOT22 and RSDI and their subdomains, was worse in patients with depression (P ≤ .001 for all) (Table 2). With regard to other PROMs, patients with depression had worse PSQI scores, missed nearly 4 times as many days of work, and used more oral antibiotics, oral corticosteroids, and decongestants than patients without depression (Table 2).
Table 1.
Baseline Demographic Characteristics, Comorbidities, and CRS-Related Measures in Patients With and Without Depressiona
| Variable | Total | No Depression (PHQ2 Score <3; Range, 0–6) |
Depression (PHQ2 Score ≥3; Range, 0–6) |
P Value |
|---|---|---|---|---|
| Total No. of patients | 685 | 518 | 167 | … |
| Demographic | ||||
| Age, mean (SD), y | 50.5 (15.0) | 50.8 (15.3) | 49.7 (14.4) | .38 |
| Male sex | 332 (48.4) | 260 (50.1) | 72 (43.1) | .12 |
| White race | 582 (85.0) | 440 (84.9) | 142 (85.0) | .72 |
| Smoking status, mean (SD), packs per day |
0.03 (0.17) | 0.03 (0.16) | 0.05 (0.19) | .20 |
| Alcohol consumption, mean (SD), g/wk |
25.0 (52.4) | 28.5 (56.5) | 14.1 (34.5) | .002 |
| Comorbidities | ||||
| Asthma | 255 (37.2) | 186 (35.8) | 69 (41.3) | .20 |
| AERD | 57 (8.3) | 41 (7.9) | 16 (9.6) | .49 |
| COPD | 31 (4.5) | 20 (3.9) | 11 (6.6) | .14 |
| Physician-diagnosed depression |
94 (13.7) | 47 (9.1) | 47 (28.1) | <.001 |
| Fibromyalgia | 24 (3.5) | 17 (3.3) | 7 (4.2) | .58 |
| CRS-related metrics | ||||
| CRS with polyps | 254 (37.1) | 194 (37.4) | 60 (35.9) | .74 |
| AFRS | 19 (2.8) | 14 (2.7) | 5 (3.0) | .84 |
| Allergy | 171 (24.9) | 130 (25.1) | 41 (24.6) | .90 |
| Revision surgery | 287 (41.8) | 197 (38.0) | 90 (53.9) | <.001 |
| Endoscopy score, mean (SD)b | 6.0 (3.9) | 5.9 (3.9) | 6.3 (3.7) | .23 |
| Total No. of patients who underwent endoscopy |
683 | 516 | 167 | … |
| CT score, mean (SD) (range, 0–24) |
11.8 (6.3) | 11.7 (6.35) | 12.2 (6.16) | .40 |
| Total No. of patients who underwent CT |
661 | 500 | 161 | … |
Abbreviations: AERD, aspirin-exacerbated respiratory disease; AFRS, allergic fungal rhinosinusitis (defined by the Bent-Kuhn criteria); COPD, chronic obstructive pulmonary disease; CRS, chronic rhinosinusitis; CT, computed tomography; PHQ2, 2-item Patient Health Questionnaire. Ellipses indicate data not applicable.
Data are presented as number (percentage) of patients unless otherwise indicated.
Endoscopy results are based on Lund-Kennedy staging system (score range, 0–20).
Table 2.
Baseline PROMs in Patients With and Without Depression
| Variable | Total No. | Mean (SD) | No Depression (PHQ2 Score <3; Range, 0–6) |
Depression (PHQ2 Score ≥3; Range, 0–6) |
P Value | ||
|---|---|---|---|---|---|---|---|
| Total No. | Mean (SD) | Total No. | Mean (SD) | ||||
| Sinus-specific PROMS | |||||||
| SNOT22 | |||||||
| Rhinologic (range, 0–30) | 686 | 16.2 (6.3) | 519 | 15.6 (6.2) | 167 | 17.9 (6.4) | <.001 |
| Extranasal rhinologic (range, 0–15) | 686 | 8.2 (3.6) | 519 | 7.9 (3.6) | 167 | 9.0 (3.6) | .001 |
| Ear/facial (range, 0–25) | 686 | 9.0 (5.3) | 519 | 8.3 (5.0) | 167 | 11.4 (5.5) | <.001 |
| Psychological (range, 0–35) | 686 | 15.3 (8.4) | 519 | 13.3 (7.7) | 167 | 21.6 (7.4) | <.001 |
| Sleep (range, 0–25) | 686 | 13.5 (6.9) | 519 | 12.3 (6.7) | 167 | 17.2 (5.9) | <.001 |
| Total (range, 0–110) | 686 | 51.7 (20.4) | 519 | 47.6 (19.0) | 167 | 64.5 (19.4) | <.001 |
| RSDI | |||||||
| Emotional (range, 0–40) | 684 | 12.3 (9.0) | 517 | 9.6 (7.4) | 167 | 20.9 (7.9) | <.001 |
| Functional (range, 0–36) | 684 | 14.6 (8.8) | 517 | 12.0 (7.5) | 167 | 22.6 (7.5) | <.001 |
| Physical (range, 0–44) | 684 | 18.7 (9.3) | 517 | 16.5 (8.4) | 167 | 25.6 (8.6) | <.001 |
| Total (range, 0–120) | 684 | 45.7 (24.8) | 517 | 38.1 (20.8) | 167 | 69.1 (21.2) | <.001 |
| Other PROMs | |||||||
| PHQ2 | 686 | 1.6 (1.6) | 519 | 0.8 (0.9) | 167 | 4.0 (1.0) | <.001 |
| Missed productivity (range, 0–90) | 660 | 9.3 (19.7) | 508 | 5.2 (13.6) | 152 | 22.8 (28.8) | <.001 |
| PSQI | |||||||
| Days (range, 0–3) | 676 | 1.3 (0.8) | 513 | 1.1 (0.7) | 163 | 2.0 (0.7) | <.001 |
| Disturbance (range, 0–3) | 676 | 1.9 (0.7) | 513 | 1.8 (0.6) | 163 | 2.3 (0.6) | <.001 |
| Duration (range, 0–3) | 675 | 1.0 (1.0) | 512 | 0.9 (1.0) | 163 | 1.4 (1.1) | <.001 |
| Efficiency (range, 0–3) | 675 | 1.0 (1.1) | 512 | 0.9 (1.5) | 163 | 1.5 (1.3) | <.001 |
| Latency (range, 0–3) | 675 | 1.5 (1.1) | 512 | 1.3 (1.0) | 163 | 2.0 (1.0) | <.001 |
| Medications (range, 0–3) | 676 | 1.1 (1.3) | 513 | 0.9 (1.2) | 163 | 1.6 (1.4) | <.001 |
| Sleep quality (range, 0–3) | 675 | 1.6 (0.8) | 512 | 1.4 (0.8) | 163 | 2.0 (0.8) | <.001 |
| Total (range, 0–21) | 674 | 9.4 (4.5) | 511 | 8.4 (4.1) | 163 | 12.8 (4.1) | <.001 |
| Medications used in the last 90 days | |||||||
| Oral | |||||||
| Antibiotics | 669 | 17.0 (22.0) | 509 | 14.8 (20.5) | 160 | 23.8 (25.0) | <.001 |
| Corticosteroids | 668 | 11.8 (19.5) | 509 | 9.9 (17.3) | 159 | 17.8 (24.4) | <.001 |
| Decongestants | 674 | 19.0 (30.8) | 512 | 17.5 (29.5) | 162 | 23.9 (34.2) | .02 |
| Leukotriene antagonists | 671 | 14.6 (31.2) | 511 | 13.7 (30.6) | 160 | 17.5 (32.8) | .18 |
| Oral antihistamines | 668 | 23.4 (35.3) | 509 | 23.6 (35.5) | 159 | 22.6 (34.8) | .74 |
| Saline rinse | 670 | 43.5 (37.9) | 509 | 43.7 (38.2) | 161 | 42.9 (37.2) | .82 |
| Corticosteroid drops and rinse | 675 | 13.5 (28.9) | 512 | 12.3 (27.8) | 163 | 17.3 (31.9) | .06 |
| Corticosteroid spray | 669 | 39.6 (38.4) | 509 | 39.1 (38.2) | 160 | 41.1 (39.1) | .57 |
Abbreviations: PHQ2, 2-item Patient Health Questionnaire; PROMs, patient-reported outcome measures; PSQI, Pittsburgh Sleep Quality Index; RSDI, Rhinosinusitis Disability Index; SNOT22, 22-item Sinonasal Outcome Test.
Treatment Outcomes
We next examined the effect of CRS treatments on depression outcomes. Of the 685 patients analyzed, 521 (76.1%) were treated with surgery, whereas 164 (23.9%) opted for continued medical management. Among patients with depression with PHQ2 scores of 3 or higher, the mean improvement in PHQ2 scores with either treatment was 1.91 (P < .001). After controlling for sex, age, polyp status, and baseline SNOT22 score, medical treatment improved the PHQ2 scores by 1.48 (P < .001), and surgery improved the PHQ2 scores by a mean of 2.03 (P < .001). No detectable difference in improvement by treatment modality was found (P = .14).
Among patients categorized as having depression at baseline, 110 (65.9%) achieved a PHQ2 score lower than 3 at follow-up, placing them in the nondepression category. Sex, age, polyp status, and treatment modality were not associated with achieving nondepression status. The MCID of the PHQ2 is not well established, but a typical definition is half the baseline SD. In this case, MCID would be approximately 0.8. Using an even more conservative threshold of a 1-point improvement in the PHQ2 score as our definition of MCID, 120 patients (71.9%) with baseline depression achieved an improvement of 1 or more. Sex, age, and treatment modality were not associated with achieving MCID; however, patients with CRSsNP had 3.48 times greater odds of achieving an MCID than patients with CRSwNP (odds ratio, 3.48; 95% CI, 1.21–10.01).
When examining changes in sinus-specific outcomes and other PROMs after medical or surgical therapy, change in the PHQ2 score was associated with improvement in the total SNOT22 score (P = .007) (Figure 1) and the PSQI score (P < .001). Because baseline depression was associated with increased medication use and missed productivity, we examined changes in these parameters with changes in the PHQ2 score. Associations were detected between changes in the PHQ2 score and fewer days of missed productivity (P = .004) (Figure 2) and less oral antibiotic use (P = .004) (Figure 3). No association was detected between changes in the PHQ2 score and changes in oral corticosteroid use (P = .92).
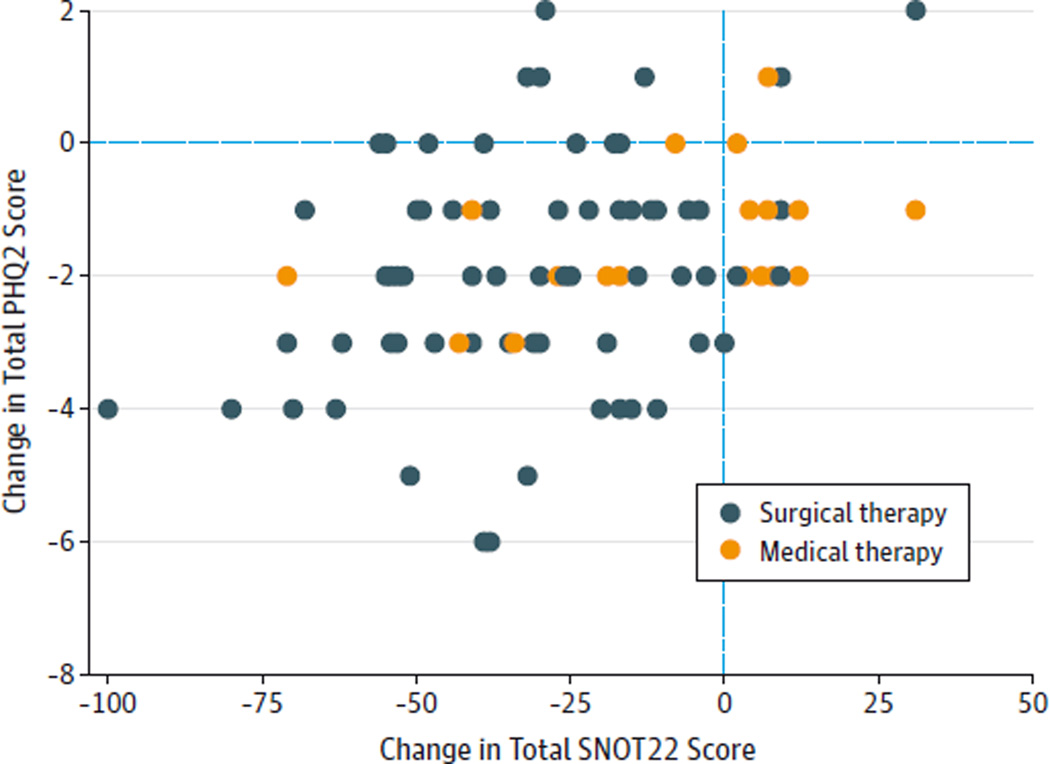
Figure 1. Change in Total 22-Item Sinonasal Outcome Test (SNOT22) vs Change in 2-Item Patient Health Questionnaire (PHQ2) Scores.
After medical or surgical therapy for chronic rhinosinusitis, improvements in the PHQ2 scores were associated with improvements in the total SNOT22 scores (P = .007). Decreasing PHQ2 and SNOT22 scores indicate clinical improvement.
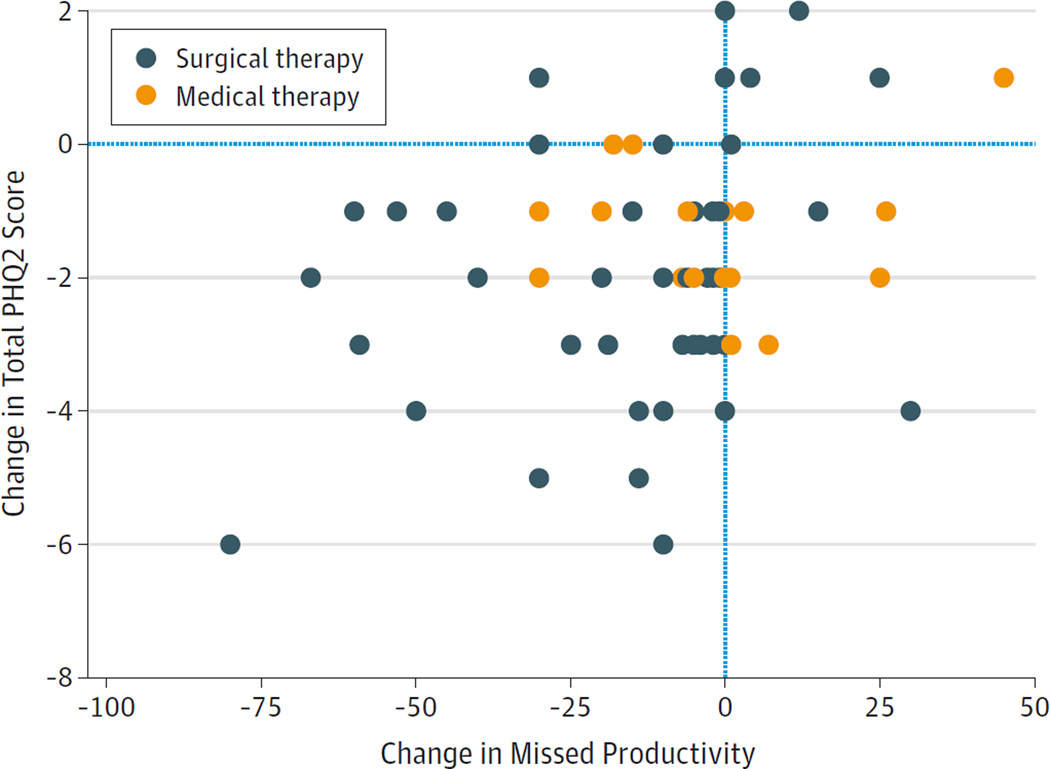
Figure 2. Change in Missed Productivity vs Change in the 2-Item Patient Health Questionnaire (PHQ2) Score.
After medical or surgical therapy for chronic rhinosinusitis, improvements in the PHQ2 scores were associated with improvements in missed productivity (P = .004). Decreasing PHQ2 scores and missed productivity values indicate clinical improvement.
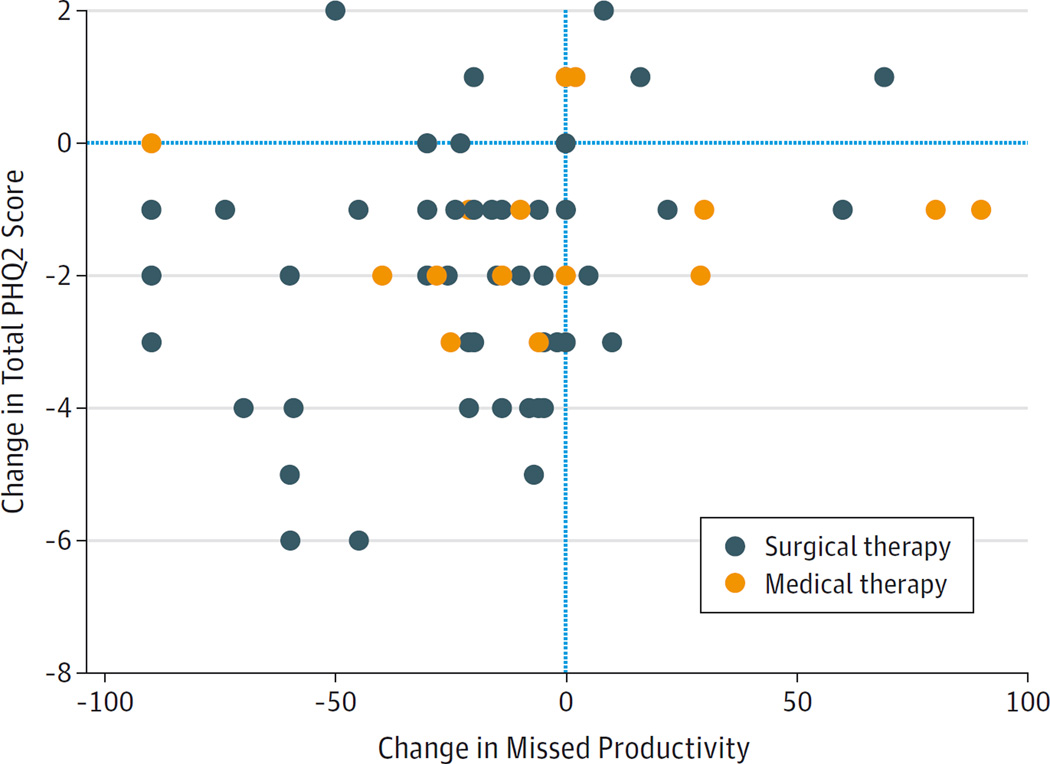
Figure 3. Change in Oral Antibiotic Use vs Change in the 2-Item Patient Health Questionnaire (PHQ2) Score.
After medical or surgical therapy for chronic rhinosinusitis, improvements in the PHQ2 scores were associated with improvements in days of oral antibiotic use (P = .004). Decreasing PHQ2 scores and days of antibiotic use indicate clinical improvement.
Discussion
Depression is associated with a variety of chronic medical illnesses, including coronary heart disease and diabetes.15 The bidirectional association between comorbid depression and chronic illness makes causality unclear, but it is known that comorbid depression negatively affects treatment outcomes. After coronary artery bypass, patients with depression have increased mortality and subsequent hospitalization.16 Given these reports,15,16 it is critical that we gain a better understanding of depression in CRS and its effect on treatment outcomes. Our overall prevalence of depression in patients with CRS was similar to prior reports,4,5 with 94 (13.7%) of 685 having physician-diagnosed depression and 167 (24.4%) of 685 having depression when using validated screening instruments. Similar to other chronic illnesses, detection of this comorbid depression is critical because we found that patients with depression have nearly 4 times as many days of missed work and use oral corticosteroids and oral antibiotics nearly twice as much as patients without depression. We found few associations between depression and patient demographic or objective CRS severity metrics, such as computed tomography or endoscopy, that would enable physicians to better predict comorbid depression. A prior report17 found that asthma, a common CRS-related comorbidity, was associated with depression. We examined asthma, fibromyalgia, chronic obstructive pulmonary disease, and aspirin-exacerbated respiratory disease as common CRS-related comorbidities and were unable to find an association with being classified as having depression by the PHQ2. A history of ESS was the only patient demographic associated with PHQ2-diagnosed depression (P < .001). Worse baseline PHQ2 scores were associated with worse scores on other QOL instruments, including sinus-specific and sleep-specific instruments. There is significant overlap between the various subdomains of these instruments; thus, an association is expected and has previously been reported for SNOT22, PSQI, BDI, and other questionnaires of fatigue and cognition.2
We found that medical and surgical therapies for CRS improve depression-specific PHQ2 scores by roughly 50% among patients with depression. With regard to ESS and depression outcomes, others have reported approximately 30% improvement using the BDI. It is not clear whether relative changes can be compared between instruments (ie, 50% improvement in the PHQ2 score does not necessarily mean greater success than 30% improvement in the BDI score). In addition, the mean baseline BDI score in these studies was between 9.5 and 12.4.8,9 Patients with depression generally have a BDI score of 14 or higher; thus, the actual prevalence of depression in these studies is not known.18,19 The magnitude of depression-specific QOL may be lower in other studies if most patients they included did not have depression before ESS and did not have significant room for improvement (ie, a floor effect). We also found that 120 patients (71.9%) in our study improved by 1 point or greater on the PHQ2, with 110 (65.9%) being categorized as not having depression after medical or surgical treatment. This finding compares favorably to other reported success rates of 26% to 49% for achieving MCID.8,9 Again, our success rate may be higher because we limited our analysis to patients who were categorized as having depression before treatment. We believe that patients who have baseline depression are the group of interest and represent the cohort with an opportunity for improvement.
To date, few studies have examined depression-specific outcomes after medical therapy for CRS. One study20 that examined the psychological subdomain of SNOT22 reported a 20% improvement with medical therapy and a significantly greater improvement of 43% for surgical therapy, although this is not a depression-specific outcome. At this point, it is unclear whether sinus surgery followed by medical therapy is more beneficial than continuing with medical therapy alone for CRS at improving depression-associated outcomes. Future studies using more detailed depression-specific instruments and standardized medical therapy are warranted.
Lack of nasal polyps was the only baseline factor associated with increased odds of achieving MCID in our study. This finding is in contrast to other studies8,9 reporting better depression outcomes in patients with CRSwNP, anosmia, or hyposmia. Unfortunately, we did not have detailed baseline olfactory studies for our patients, so the association between depression outcomes after surgery and objective olfaction is not clear. It is possible that the differences between our study and others based on polyp status may be owing to heterogeneous patient populations or different depression instruments and represent an interesting area for further study.
We found that improvement in the PHQ2 scores after medical or surgical therapy was associated with improvement in a number of PROMs. Not surprisingly, total SNOT22 and PSQI score changes were associated with PROM improvement. Improvements in the PHQ2 scores were also associated with improvements in missed work and oral antibiotic use. It is unclear whether independent treatment of comorbid depression in conjunction with continued medical therapy or surgery would result in even greater improvement, and the economic effect of improving CRS-associated depression with subsequent improvements in productivity and medication use remains to be determined.
Our study had a number of limitations. Although the PHQ2 instrument allows for rapid screening, its abbreviated nature lacks information on depression subscales provided with other more detailed instruments, such as the BDI, which includes somatic and cognitive subscales.21 Others have reported using thresholds of 1 or 2 to diagnose depression with the PHQ2.22 Lower thresholds obviously result in greater sensitivity for detecting depression but increase the potential to mistakenly classify a patient as having depression. To balance these findings, we opted for the commonly used cutoff of 3.14,23 In addition, the PHQ2 scores range from 0 to 6 only, so the ability of the PHQ2 to discriminate among varying severity of depression or changes with CRS treatments may be limited. We also lack detailed information regarding how preexisting depression was diagnosed and how many patients were taking antidepressant medications. Many antidepressants are contraindicated with alcohol, which may have contributed to our finding of less alcohol intake in the group with depression at baseline. Finally, patients in our study self-selected medical or surgical therapy for CRS, and a randomized prospective trial would be ideal.
Conclusions
Depression is commonly underdiagnosed in patients with CRS and has a significant effect on productivity and medication use. Few objective metrics are available to assist physicians in identifying at-risk patients, but revision surgery status may play a role and should alert physicians to possible depression. Medical and surgical therapies for CRS improve comorbid depression, productivity, and medication use.
Acknowledgments
Dr Soler reported working as a consultant for Olympus and receiving grant support from Intersect ENT, Entellus, and Optinose. Dr Schlosser reported working as consultant for Olympus and Meda and receiving grant support from Intersect ENT, Entellus, and Optinose.
Funding/Support: This study was supported by grant R01 DC005805 from the National Institutes of Health (Drs Smith, Soler, and Mace) and by grants R01 DC005805 and R03 DC013651-01 from the National Institute on Deafness and Other Communication Disorders (Dr Soler).
Role of the Funder/Sponsor: The funding sources had no role in the design and conduct of the study; collection, management, analysis, and interpretation of the data; preparation, review, or approval of the manuscript; and the decision to submit the manuscript for publication.
Footnotes
Author Contributions: Dr Schlosser and Mr Hyer had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Study concept and design: Schlosser, Hyer, Soler.
Acquisition, analysis, or interpretation of data: Schlosser, Hyer, Smith, Mace, Cortese, Uhde, Rudmik, Soler.
Drafting of the manuscript: Schlosser, Hyer, Smith.
Critical revision of the manuscript for important intellectual content: Schlosser, Hyer, Smith, Mace, Cortese, Uhde, Rudmik, Soler.
Statistical analysis: Hyer, Uhde, Rudmik, Soler.
Obtained funding: Smith, Mace.
Administrative, technical, or material support: Smith, Mace.
Study supervision: Schlosser.
Conflict of Interest Disclosures: No other disclosures were reported.
REFERENCES
- 1.Alt JA, Smith TL, Schlosser RJ, Mace JC, Soler ZM. Sleep and quality of life improvements after endoscopic sinus surgery in patients with chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(9):693–701. doi: 10.1002/alr.21364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Soler ZM, Eckert MA, Storck K, Schlosser RJ. Cognitive function in chronic rhinosinusitis: a controlled clinical study. Int Forum Allergy Rhinol. 2015;5(11):1010–1017. doi: 10.1002/alr.21581. [DOI] [PubMed] [Google Scholar]
- 3.Tomoum MO, Klattcromwell C, DelSignore A, Ebert C, Senior BA. Depression and anxiety in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5(8):674–681. doi: 10.1002/alr.21528. [DOI] [PubMed] [Google Scholar]
- 4.Litvack JR, Mace J, Smith TL. Role of depression in outcomes of endoscopic sinus surgery. Otolaryngol Head Neck Surg. 2011;144(3):446–451. doi: 10.1177/0194599810391625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mace J, Michael YL, Carlson NE, Litvack JR, Smith TL. Effects of depression on quality of life improvement after endoscopic sinus surgery. Laryngoscope. 2008;118(3):528–534. doi: 10.1097/MLG.0b013e31815d74bb. [DOI] [PubMed] [Google Scholar]
- 6.Nanayakkara JP, Igwe C, Roberts D, Hopkins C. The impact of mental health on chronic rhinosinusitis symptom scores. Eur Arch Otorhinolaryngol. 2013;270(4):1361–1364. doi: 10.1007/s00405-012-2230-1. [DOI] [PubMed] [Google Scholar]
- 7.Tomoum MO, Klattcromwell C, DelSignore A, Ebert C, Senior BA. Depression and anxiety in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2015;5(8):674–681. doi: 10.1002/alr.21528. [DOI] [PubMed] [Google Scholar]
- 8.Katotomichelakis M, Simopoulos E, Tripsianis G, et al. Predictors of quality of life outcomes in chronic rhinosinusitis after sinus surgery. Eur Arch Otorhinolaryngol. 2014;271(4):733–741. doi: 10.1007/s00405-013-2626-6. [DOI] [PubMed] [Google Scholar]
- 9.Katotomichelakis M, Simopoulos E, Tripsianis G, et al. Improvement of olfactory function for quality of life recovery. Laryngoscope. 2013;123(11):E10–E16. doi: 10.1002/lary.24113. [DOI] [PubMed] [Google Scholar]
- 10.Rosenfeld RM, Piccirillo JF, Chandrasekhar SS, et al. Clinical practice guideline (update): adult sinusitis. Otolaryngol Head Neck Surg. 2015;152((2) (suppl)):S1–S39. doi: 10.1177/0194599815572097. [DOI] [PubMed] [Google Scholar]
- 11.DeConde AS, Bodner TE, Mace JC, Smith TL. Response shift in quality of life after endoscopic sinus surgery for chronic rhinosinusitis. JAMA Otolaryngol Head Neck Surg. 2014;140(8):712–719. doi: 10.1001/jamaoto.2014.1045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Buysse DJ, Reynolds CF, III, Monk TH, Berman SR, Kupfer DJ. The Pittsburgh Sleep Quality Index: a new instrument for psychiatric practice and research. Psychiatry Res. 1989;28(2):193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- 13.Carpenter JS, Andrykowski MA. Psychometric evaluation of the Pittsburgh Sleep Quality Index. J Psychosom Res. 1998;45(1):5–13. doi: 10.1016/s0022-3999(97)00298-5. [DOI] [PubMed] [Google Scholar]
- 14.Kroenke K, Spitzer RL, Williams JB. The Patient Health Questionnaire-2: validity of a two-item depression screener. Med Care. 2003;41(11):1284–1292. doi: 10.1097/01.MLR.0000093487.78664.3C. [DOI] [PubMed] [Google Scholar]
- 15.van der Zwaan GL, van Dijk SE, Adriaanse MC, et al. Diagnostic accuracy of the Patient Health Questionnaire-9 for assessment of depression in type II diabetes mellitus and/or coronary heart disease in primary care. J Affect Disord. 2016;190:68–74. doi: 10.1016/j.jad.2015.09.045. [DOI] [PubMed] [Google Scholar]
- 16.Stenman M, Holzmann MJ, Sartipy U. Relation of major depression to survival after coronary artery bypass grafting. Am J Cardiol. 2014;114(5):698–703. doi: 10.1016/j.amjcard.2014.05.058. [DOI] [PubMed] [Google Scholar]
- 17.Katotomichelakis M, Simopoulos E, Zhang N, et al. Olfactory dysfunction and asthma as risk factors for poor quality of life in upper airway diseases. Am J Rhinol Allergy. 2013;27(4):293–298. doi: 10.2500/ajra.2013.27.3903. [DOI] [PubMed] [Google Scholar]
- 18.Beck AT, Steer RA, Ball R, Ranieri W. Comparison of Beck Depression Inventories -IA and -II in psychiatric outpatients. J Pers Assess. 1996;67(3):588–597. doi: 10.1207/s15327752jpa6703_13. [DOI] [PubMed] [Google Scholar]
- 19.Wang YP, Gorenstein C. Psychometric properties of the Beck Depression Inventory-II: a comprehensive review. Rev Bras Psiquiatr. 2013;35(4):416–431. doi: 10.1590/1516-4446-2012-1048. [DOI] [PubMed] [Google Scholar]
- 20.Deconde AS, Mace JC, Bodner T, et al. SNOT-22 quality of life domains differentially predict treatment modality selection in chronic rhinosinusitis. Int Forum Allergy Rhinol. 2014;4(12):972–929. doi: 10.1002/alr.21408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Huang C, Chen JH. Meta-analysis of the factor structures of the Beck Depression Inventory-II. Assessment. 2015;22(4):459–472. doi: 10.1177/1073191114548873. [DOI] [PubMed] [Google Scholar]
- 22.Arroll B, Goodyear-Smith F, Crengle S, et al. Validation of PHQ-2 and PHQ-9 to screen for major depression in the primary care population. Ann Fam Med. 2010;8(4):348–353. doi: 10.1370/afm.1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey M, Boyes A, Noble N, Waller A, Inder K. Validation of the PHQ-2 against the PHQ-9 for detecting depression in a large sample of Australian general practice patients [published online August 26, 2015] Aust J Prim Health. doi: 10.1071/PY14149. [DOI] [PubMed] [Google Scholar]