Abstract
BACKGROUND
Recent evidence indicates that cancer stem cells play an important role in tumor initiation and maintenance. Additionally, the effect of tissue-resident stem cells located in the surrounding healthy tissue on tumor progression has been demonstrated. While most knowledge has been derived from studies of breast cancer cells, little is known regarding the influence of tissue resident stem cells on the tumor biology of prostate cancer.
METHODS
Twenty male athymic Swiss nu/nu mice (age: 6–8 weeks) were randomized into two treatment groups: 1) subcutaneous injection of 106 MDA PCa 118b human prostate cancer cells into the upper back or 2) subcutaneous injection of 106 MDA PCa 118b cells mixed directly with 105 GFP-labeled human adipose tissue-derived stem cells (hASCs). Tumor growth and volumes over the ensuing 3 weeks were assessed using calipers and micro-computed tomography. Immunohistochemistry was performed to identify engrafted hASCs in tumor sections.
RESULTS
At 3 weeks after injection, the mean tumor volume in the MDA PCa 118b/hASC co-injection group (1019.95 ± 73.49 mm3) was significantly higher than that in the MDA PCa 118b-only group (308.70 ± 21.06 mm3). Engrafted hASCs exhibited the nuclear marker of proliferation Ki67 and expressed markers for endothelial differentiation, indicating their engraftment in tumor vessels.
CONCLUSION
Our study revealed for the first time that ASCs subcutaneously co-injected with prostate cancer cells engraft and promote tumor progression. Further evaluation of the cross-talk between tumor and local tissue-resident stem cells may lead to new strategies for prostate cancer therapy.
Keywords: mesenchymal stem cell, prostate cancer cell, tumor growth
INTRODUCTION
Many human cancers contain reactive stroma which promotes tumorigenesis and angiogenesis. The reactive stroma is composed of inflammatory cells as well as fibroblasts. Picard et al. [1] demonstrated that the addition of fibroblasts to different cancer cell lines can reduce the minimum number of cells needed for tumor formation. Tuxhorn et al. [2] revealed that reactive stromal cells were vimentin and smooth muscle α-actin positive, indicating the myofibroblast phenotype. In a separate study, Tuxhorn et al. [3] further showed that LNCaP prostate carcinoma cells alone were essentially nontumorigenic, whereas combinations of LNCaP cells with three different human prostate stromal cell lines resulted in a tumor incidence of 50–63%. These tumors exhibited well-developed blood vessels at 2 weeks.
Stromal fibromuscular cells associated with primary prostate cancer differ from stromal cells in benign prostate tissue by an increased level of expression of the cell activation molecule, CD90 [4]. CD90 is a marker of mesenchymal stem cells (MSCs) and its expression is higher in prostate cancer stroma compared to normal tissue. Zhao et al. [5] compared the tumor promoting capacity of cultured prostate cancer-associated fibroblasts (CAFs) expressing high versus low levels of CD90 and found that CD90hi cells is more tumor-promoting than that of cells expressing low CD90. Recent studies have shown that at least a subset of cancer-associated myofibroblasts is derived from circulating bone marrow-derived cells [6, 7]. Furthermore, bone-marrow-derived human mesenchymal stem cells, when mixed with otherwise weakly metastatic human breast carcinoma cells, cause the cancer cells to increase their metastatic potency greatly in a tumour xenograft model [8]. Recently, we have shown that adipose tissue resident stem cells (ASCs) promote breast cancer growth and metastasis [9, 10]. Since the prostate is covered by the visceral pelvis fascia, which is a thick, connective, primarily adipose containing capsule, in this study we aimed to evaluate the role of ASCs in prostate cancer growth and progression.
MATERIALS AND METHODS
Cell Lines and Cell Culture
MDA PCa 118b [11] cells were propagated in BRFF-HPC1 medium (AthenaES, Baltimore, MD) with 20% fetal bovine serum (Atlanta Biologicals, Lawrenceville, GA), 2 mM glutamine, 100 U/ml penicillin, and 100 µg/ml streptomycin (Invitrogen, Carlsbad, CA).
Human adipose tissue was obtained with IRB approval from patients undergoing elective surgical procedures who gave written consent to undergo tissue harvesting for this research. The study was performed in accordance with guidelines of the Declaration of Helsinki for biomedical research and approved by the M. D. Anderson Institutional Review Board. ASCs were isolated from the tissue specimens, propagated, and characterized as described previously [12–14]. Briefly, adipose tissue specimens were rinsed in phosphate-buffered saline (PBS) and minced into 1 mm3 pieces. Blendzyme (Roche, Indianapolis, IN) was used for digestion of the extracellular matrix (4 U/1 g of fat) under gentle shaking for 40 min at 37°C followed by filtration through a 100-µm strainer. Released cells were pelleted, seeded in culture flasks, and incubated at 37°C in a humidified atmosphere containing 5% carbon dioxide. hASCs were cultured and expanded in minimum essential medium alpha 1 (Cellgro, Mediatech, Inc., Manassas, VA) supplemented with 20% heat-inactivated fetal bovine serum and antibiotics. Similarly prepared ASCs were previously demonstrated to be capable of multilineage differentiation in our laboratory [12–14]. Cells from passage 2 were used for all of our experiments.
Flow Cytometric Analysis of hASCs
hASCs from the second passage were harvested by treatment with 0.05% trypsin/0.53 mM ethylenediaminetetraacetic acid and then fixed for 10 min in 1% paraformaldehyde. Following fixation, cells were washed twice with PBS. Cell aliquots (1×106 cells/ml) were stained with primary antibodies at room temperature for 30 min. The primary antibodies were fluorescein isothiocyanate-conjugated anti-human CD44 (Chemicon, Billerica, MA), CD34, and CD90 (United States Biological, Swampscott, MA) and phycoerythrin-conjugated anti-human CD11b, CD105 (eBioscience, San Diego, CA), CD14, and CD45 (United States Biological). Isotype-matched normal mouse IgGs were used as controls (Figure 1). The antibodies were purchased from Chemicon (Millipore, Billerica, MA). Flow cytometry was performed using a fluorescence-activated cell sorter (FACSCalibur; BD Biosciences, San Jose, CA), and data analysis was performed using the CellQuest software program (Becton Dickinson, Franklin Lakes, NJ).
Figure 1. Characterization of surface marker expression in hASCs by flowcytometry.
The cells are positive for CD44 (99.76%), CD90 (99.61%) and CD105 (99.00%), and negative for CD11b (0.25%) and CD45 (0.00%).
Green Fluorescent Protein Lentiviral Construct
An enhanced green fluorescent protein (eGFP) lentiviral vector (provided by Dr. Ralph Arlinghaus, M. D. Anderson) was used to produce lentiviral particles via transient three-plasmid co-transfection of 293T human embryonic kidney cells (American Type Culture Collection, Manassas, VA) with a calcium phosphate transfection kit (Invitrogen). The lentiviral vector concentration was determined using a p24 enzyme-linked immunosorbent assay (Cell Biolabs, San Diego, CA), and 60% confluent hASCs were infected in passage one with 3×105 TU/ml virus and polybrene (8 µg/ml) (Chemicon). After 8 hours, the medium was replaced with fresh MEM alpha1 medium. Six days after lentiviral infection, GFP-positive cells were subjected to flow cytometric sorting with stringent gating for green fluorescent cells using a FACS Vantage SE cell sorter (Becton Dickinson).
Animal Model
Male 6- to 8-week-old athymic Swiss nu/nu mice were purchased from The Jackson Laboratory (Bar Harbor, ME). Twenty animals were randomized into two treatment groups: 1) subcutaneous injection of 106 MDA PCa 118b cells into the upper back or 2) subcutaneous injection of 106 MDA PCa 118b cells directly mixed with 105 eGFP-labeled hASCs in 100 µl of PBS into the upper back. Tumor growth was monitored and measured every third day after tumor-cell injection using calipers. On day 21 after injection, mice were imaged using high-resolution micro-computed tomography (CT) for exact tumor-volume assessment under anesthesia induced with 2–3% isoflurane. Animals were killed and tumors were resected immediately after micro-CT. This animal study was approved by and performed in accordance with the M. D. Anderson Institutional Animal Care and Use Committee.
Image Acquisition
The micro-CT images were obtained using a Locus RS-9 micro-CT scanner (GE Healthcare, London, Ontario, Canada) with the following imaging parameters: tube voltage, 80 kV; anode current, 470 µA; number of views, 360; exposure time, 600 msec; detector bin mode, 2×2; effective pixel size, 0.046 mm. Before imaging, animals were administered Fenestra vascular contrast solution (ART Advanced Research Technologies, Montreal, Quebec, Canada) via tail-vein injection. After injection of the contrast solution, anesthesia was induced using 5% isoflurane (Fisher Scientific, Pittsburgh, PA) and maintained using 1–2% isoflurane during micro-CT. The animals were killed and tumor dissection was performed immediately after micro-CT.
Image Evaluation
Imaging data were acquired and reconstructed using the MicroView software program (GE Healthcare). The micro-CT data for each mouse were analyzed using a commercially available visualization software program (Vital Images, Inc., Minnetonka, MN), which displayed the data as two-dimensional axial, sagittal, and coronal cross-sectional images. Evaluation of the images included assessment of tumor size and vascularity. Tumor volumes were calculated using the spherical volume equation:
Histology
Prostate tumor tissue specimens were obtained from the back of the mice with approximately 6-mm margins in sano and placed in either 10% formalin for fixation and subsequent paraffin processing and embedding or in molds for immersion in Tissue-Tek O.C.T. Compound (Sekura Finetek U.S.A., Torrance, CA) on dry ice for frozen-block preparation. Serial sectioning of specimen blocks was performed (5 µm for frozen sections and 4 µm for paraffin-embedded sections), and representative sections were stained with hematoxylin and eosin (H&E) for examination of the tissue architecture. Additional serial sections were prepared as unstained slides for subsequent immunofluorescent studies. Z-sections of H&E-stained slides were analyzed using an Axiovert microscope (Carl Zeiss AG, Oberkochen, Germany) equipped with a Canon G7 high-resolution digital camera adaptor for image acquisition (Canon, Lake Success, NY).
Immunofluorescence was carried out to identify engrafted hASCs based on the eGFP signal and to identify the phenotype of engrafted stem cells with specific antibodies against additional antigens. Primary antibodies against the following epitopes were used in these experiments: smooth muscle actin (SMA; ab32575, rabbit anti-human; 1:500), GFP (ab6556 and ab6673, rabbit anti-human and goat anti-human, respectively; 1:200), Ki67 (ab833 and ab15580, rabbit anti-human; 1:50), HSP47 (ab13510, mouse anti-human; 1:1000), cytokeratin 19 (ab15463, rabbit anti-human; 1:200; Abcam, Cambridge, MA), and von Willebrand factor (vWF; A0082, rabbit anti-human; 1:1000; Chemicon). Prior to antibody staining, slides were washed in PBS and fixed with 4% paraformaldehyde for 10 min at room temperature. Next, slides were washed with PBS containing 0.3% Triton X-100 (Sigma-Aldrich, St. Louis, MO) and subjected to a blocking solution of 10% donkey serum for 30 min at room temperature. Primary antibody incubation was then performed for 1 h at 37°C followed by washing with PBS and incubation with an Alexa Fluor 488 donkey anti-goat IgG secondary antibody (Invitrogen), Alexa Fluor 594 donkey anti-rabbit IgG, or Alexa Fluor 594 donkey anti-mouse IgG that was species-matched according to the primary antibody species used. Secondary antibodies were applied at a dilution factor of 1:1000 for 1 h at room temperature. 4’-6-Diamidino-2-phenylindole nuclear dye was applied for 15 min at room temperature, and slides were again washed and mounted with coverslips. The slides were analyzed and photomicrographs were taken using a confocal microscope (Olympus, Center Valley, PA). For each animal (five per group), slides containing different regions of tumors (n = 12) were screened in a meandering fashion. Slides with GFP signals were designated as positive for engrafted hASCs.
Statistical Analysis
Statistical analysis was conducted using the SPSS software program (version 17.0; SPSS Inc., Chicago, IL). Levene’s test for equality of variances and a t-test for equality of means were used to determine the statistical significance of all of the data. Values were depicted as the mean ± standard deviation, and P values less than or equal to 0.001 were considered statistically significant.
RESULTS
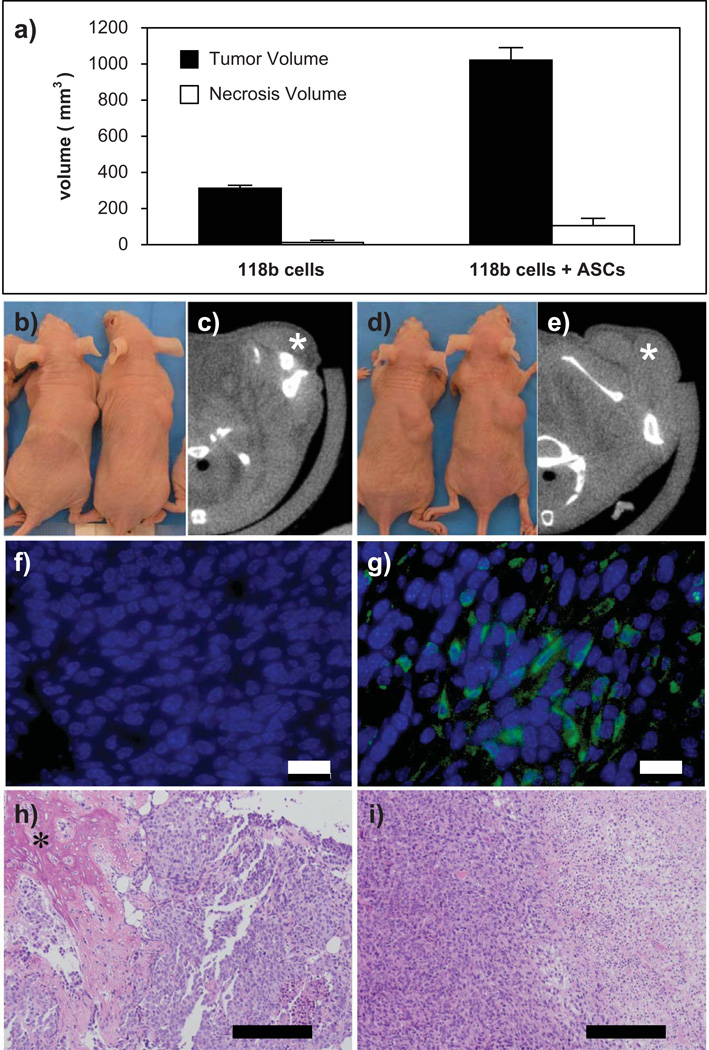
At 3 weeks after tumor-cell injection, the mean volume of prostate tumors induced by co-injection of MDA PCa 118b cells and hASCs (1019.95 ± 73.49 mm3) was more than three-fold higher compared to tumors induced by injection of MDA PCa 118b cells alone (308.70 ± 21.06 mm3) (P < 0.001). Furthermore, the mean volume of prostate tumor necroses was significantly higher (Fig. 2a, 2b, 2d) in mice that received co-injection of MDA PCa 118b cells and hASCs (106.11 ± 39.99 mm3) than in mice that received MDA PCa 118b cells alone (10.02 ± 13.99 mm3) (P < 0.001).
Figure 2. Effect of co-injection of hASCs and human prostate cancer cells.
a: Co-injection of hASCs and human prostate cancer line MDA PCa 118b significantly enhanced prostate tumor growth. b-e: Photographs of representative mice after (b) subcutaneous injection of human prostate cancer cells alone and (d) co-injection of hASCs and human prostate cancer cells into the upper back. Representative micro-CT images of a prostate tumor (*) on day 21 after (c) injection of human prostate cancer cells alone and (e) and co-injection of hASCs and human prostate cancer cells. f-g: Corresponding immunofluorescent images revealing that GFP-labeled hASCs engrafted into the tumor. Scale bar: 25 µm. h: H&E-stained sections of a prostate tumor induced by MDA PCa 118b cells alone showing differentiation in osteoblast-like cells with bone-like extracellular matrix formation (*). i: Necrotic and disorganized cells in the hASC / MDA PCa 118b co-injection group. Scale bar: 200 µm.
The volumes of prostate tumors and necroses detected using micro-CT correlated with histological observations from analysis of H&E-stained sections (Fig. 2c, 2e, 2h, 2i). We detected more necrotic and disorganized cells in prostate tumors induced by co-injection of MDA PCa 118b cells and hASCs than in those induced by injection of MDA PCa 118b cells alone. We observed ossification of tumor cells solely in tumors induced by MDA PCa 118b cells alone (Fig. 2h). We confirmed homing and engraftment GFP-labeled hASCs co-injected with MDA PCa 118b cells in all of the slides analyzed histologically (Fig. 2f, 2g). Furthermore, expression of Ki67 (Figure 3), endothelial marker vWF, and/or SMA was observed in samples that contained engrafted GFP-positive cells (Fig. 4a–c). In addition, we determined the quantitative contribution of hASCs to the overall tumor volume by enumerating the DAPI stained nuclei and GFP-positive cells per 25 high power field per tumor section. Using similar initial number of prostate cancer cells in both groups (106 MDA PCa 118b cells) we found two times more prostate cancer cells in the co-injection group when compared to injection of cancer cells alone (Table 1). To exclude that hASCs contribute to tumor volume by proliferation, we subcutaneously injected 105 luciferase-labeled hASCs as a control. Over a three week period, we observed a localized reduction in the bioluminescent signal (luciferase intensity) from 3.2 ± 1.0×105 to 1.2 ± 0.4×105 p/s/cm2/sr, indicating a decrease in hASC number. This data supports our finding that enhanced proliferation of the cancer cells, but not of the co-injected hASCs, is responsible for the observed increase in tumor volume.
Figure 3. Immunofluorescent staining for tumor biopsies showing expression of Ki67 in transplanted GFP-positive hASCs.
Overlay showing cells positive for both GFP (green) and Ki67 (red), indicating a proliferation of engrafted cells. Scale bar: 200 µm.
Figure 4. Immunofluorescent staining of tumor biopsies showing transplanted GFP-positive hASCs co-localizing with SMA-positive cells.
a: 4’-6-Diamidino-2-phenylindole-stained nuclei (blue). b: SMA (red). c: Overlay showing cells positive for both GFP (green) and SMA (red), indicating spontaneous differentiation into a vascular smooth muscle phenotype in tumor. Scale bar: 200 µm.
Table 1. Quantitative contribution of hASCs to overall tumor volume.
Prostate cancer cells and hASCs were determined by counting the DAPI-stained nuclei and the GFP-positive cells per 25 high power field per tumor section. The table gives the mean and SD count for the different cell types. The number of injected prostate cancer cells was the same in both groups (106 MDA PCa 118b cells). The number of prostate cancer (DAPI-positive) cells without the engrafted hASCs (GFP-positive cells) was more than twice as high in the co-injection group indicating increased tumor cell proliferation. The difference is statistically significant (P<0.001).
| 106 MDA PCa 118b cells only | 106 MDA PCa 118b cells + 105 GFP-positive hASCs |
|
|---|---|---|
| Tumor cells (DAPI+) | 31.0 ± 3.6 | 77.7 ± 10.8 |
| GFP-positive cells | 0 | 29.7 ± 5.7 |
| Total number of cells | 31.0 ± 3.6 | 107.4 ± 15.8 |
DISCUSSION
The present study revealed for the first time that in athymic Swiss nu/nu mice, hASCs subcutaneously co-injected with MDA PCa 118b prostate cancer cells engraft and promote tumor progression. Furthermore, we observed that engrafted hASCs express signals for the endothelial marker vWF and for SMA, indicating their involvement in formation of microvessels in tumor stroma.
Prostate cancer progression is associated with and dependent upon robust neovascularization. Cancer cells actively recruit stromal cells into the tumors and that this recruitment is essential for the generation of a microenvironment that promotes tumor growth [8, 15–17]. Increasing evidence indicates that bone marrow-derived mesenchymal cells support tumor angiogenesis by providing a supportive role as carcinoma-associated fibroblasts [18,19] or perivascular mural cells [20]. These cells express α-smooth muscle actin [19], Tie-2 [21], and other pericyte markers [22]. Furthermore, differentiation of MSC into endothelial cells has been demonstrated [23–25].
Emerging evidence suggest that tissue resident stem cells are also involved in tumor angiogenesis. Udagawa et al. [26] demonstrated that most of the tumor vessels were non-hematopoietic, tissue resident cells from the local environment rather than bone marrow-derived cells from the circulation. The findings from Udagawa et al. are in agreement with data from our present study as well as our previous report that ASCs are involved in tumor angiogenesis in a murine breast cancer model [9]. On the other hand, we found more necrotic cells in the coinjection group, indicating a lack of adequate blood supply. Such central necroses are often associated with aggressive, rapidly proliferating tumors. It is probable that the paracrine effects of the coinjected stem cells were predominant, leading to the observed increased proliferation and ultimately, necrosis of the tumor. Lin et al. [27] found that PC3 prostate cancer cells recruited adipose tissue-derived stem cells (ADSC) by the CXCL12/CXCR4 axis. This study revealed that tumors from ADSC-treated mice had twice as much CD31 staining as tumors from PBS-treated mice, and that FGF2 was expressed at a significantly higher level in the tumors of ADSC-treated mice. These data suggest that the ADSC-induced upregulation of FGF2 was probably responsible for the increased vascularity and tumor growth.
Overall, these findings shed new light on the support of cancer growth by tissue resident stem cells. More in-depth understanding of this interaction may lead to the development of a selective therapeutic treatment possibly targeting this interaction. Additionally, further studies should clarify the relative contributions of ASCs in periprostatic fat tissue as well as in stromal tissue adjacent to prostate tumor on this tumor-supporting effect of ASCs that is described for the first time in our study.
Acknowledgments
This work was supported in part by the Department of Defense Breast Cancer Research Program Grant W81XWH-08-1-0523 01 (to YHS) and the Alliance of Cardiovascular Researchers Grant 543102 (to EUA). We thank Dr. Xiaowen Bai and Yasheng Yan for technical assistance and Dr. Sebastian Gehmert, Dr. Sanga Gehmert and Dr. Andrew Altman for helpful discussion.
REFERENCES
- 1.Picard O, Rolland Y, Poupon MF. Fibroblast-dependent tumorigenicity of cells in nude mice: implication for implantation of metastases. Cancer Res. 1986;46(7):3290–3294. [PubMed] [Google Scholar]
- 2.Tuxhorn JA, et al. Reactive stroma in human prostate cancer: induction of myofibroblast phenotype and extracellular matrix remodeling. Clin Cancer Res. 2002;8(9):2912–2923. [PubMed] [Google Scholar]
- 3.Tuxhorn JA, et al. Stromal cells promote angiogenesis and growth of human prostate tumors in a differential reactive stroma (DRS) xenograft model. Cancer Res. 2002;62(11):3298–3307. [PubMed] [Google Scholar]
- 4.Liu AY, Roudier MP, True LD. Heterogeneity in primary and metastatic prostate cancer as defined by cell surface CD profile. Am J Pathol. 2004;165(5):1543–1556. doi: 10.1016/S0002-9440(10)63412-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Zhao H, Peehl DM. Tumor-promoting phenotype of CD90hi prostate cancer-associated fibroblasts. Prostate. 2009;69(9):991–1000. doi: 10.1002/pros.20946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chauhan H, et al. There is more than one kind of myofibroblast: analysis of CD34 expression in benign, in situ, and invasive breast lesions. J Clin Pathol. 2003;56(4):271–276. doi: 10.1136/jcp.56.4.271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ishii G, et al. Bone-marrow-derived myofibroblasts contribute to the cancer-induced stromal reaction. Biochem Biophys Res Commun. 2003;309(1):232–240. doi: 10.1016/s0006-291x(03)01544-4. [DOI] [PubMed] [Google Scholar]
- 8.Karnoub AE, et al. Mesenchymal stem cells within tumour stroma promote breast cancer metastasis. Nature. 2007;449(7162):557–563. doi: 10.1038/nature06188. [DOI] [PubMed] [Google Scholar]
- 9.Muehlberg F, et al. Tissue resident stem cells promote breast cancer growth and metastasis. Carcinogenesis. 2009;30(4):589–597. doi: 10.1093/carcin/bgp036. [DOI] [PubMed] [Google Scholar]
- 10.Pinilla S, et al. Tissue resident stem cells produce CCL5 under the influence of cancer cells and thereby promote breast cancer cell invasion. Cancer Lett. 2009;284(1):80–85. doi: 10.1016/j.canlet.2009.04.013. [DOI] [PubMed] [Google Scholar]
- 11.Li ZG, et al. Androgen receptor–negative human prostate cancer cells induce osteogenesis in mice through FGF9-mediated mechanisms. J. Clin. Invest. 2008;118(8):2697–2710. doi: 10.1172/JCI33093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bai X, et al. Electrophysiological properties of human adipose tissue-derived stem cells. Am J Physiol Cell Physiol. 2007;293(5):C1539–C1550. doi: 10.1152/ajpcell.00089.2007. [DOI] [PubMed] [Google Scholar]
- 13.Bai X, et al. Both cultured and freshly isolated adipose tissue-derived stem cells enhance cardiac function after acute myocardial infarction. Eur Heart J. 2010;31(4):489–501. doi: 10.1093/eurheartj/ehp568. [DOI] [PubMed] [Google Scholar]
- 14.Zhang L, et al. Chemoprevention of colorectal cancer by targeting APC-deficient cells for apoptosis. Nature. 2010 doi: 10.1038/nature08871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bhowmick NA, Neilson EG, Moses HL. Stromal fibroblasts in cancer initiation and progression. Nature. 2004;432(7015):332–337. doi: 10.1038/nature03096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Olumi AF, et al. Carcinoma-associated fibroblasts direct tumor progression of initiated human prostatic epithelium. Cancer Res. 1999;59(19):5002–5011. doi: 10.1186/bcr138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cornil I, et al. Fibroblast cell interactions with human melanoma cells affect tumor cell growth as a function of tumor progression. Proc Natl Acad Sci U S A. 1991;88(14):6028–6032. doi: 10.1073/pnas.88.14.6028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Haniffa MA, et al. Mesenchymal stem cells: the fibroblasts' new clothes? Haematologica. 2009;94(2):258–263. doi: 10.3324/haematol.13699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mishra PJ, et al. Carcinoma-associated fibroblast-like differentiation of human mesenchymal stem cells. Cancer Res. 2008;68(11):4331–4339. doi: 10.1158/0008-5472.CAN-08-0943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Au P, et al. Bone marrow-derived mesenchymal stem cells facilitate engineering of long-lasting functional vasculature. Blood. 2008;111(9):4551–4558. doi: 10.1182/blood-2007-10-118273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.De Palma M, et al. Tie2 identifies a hematopoietic lineage of proangiogenic monocytes required for tumor vessel formation and a mesenchymal population of pericyte progenitors. Cancer Cell. 2005;8(3):211–226. doi: 10.1016/j.ccr.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 22.Bexell D, et al. Bone marrow multipotent mesenchymal stroma cells act as pericyte-like migratory vehicles in experimental gliomas. Mol Ther. 2009;17(1):183–190. doi: 10.1038/mt.2008.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Oswald J, et al. Mesenchymal stem cells can be differentiated into endothelial cells in vitro. Stem Cells. 2004;22(3):377–384. doi: 10.1634/stemcells.22-3-377. [DOI] [PubMed] [Google Scholar]
- 24.Silva GV, et al. Mesenchymal stem cells differentiate into an endothelial phenotype, enhance vascular density, and improve heart function in a canine chronic ischemia model. Circulation. 2005;111(2):150–156. doi: 10.1161/01.CIR.0000151812.86142.45. [DOI] [PubMed] [Google Scholar]
- 25.Song YS, et al. Potential differentiation of human mesenchymal stem cell transplanted in rat corpus cavernosum toward endothelial or smooth muscle cells. Int J Impot Res. 2007;19(4):378–385. doi: 10.1038/sj.ijir.3901539. [DOI] [PubMed] [Google Scholar]
- 26.Udagawa T, et al. Analysis of tumor-associated stromal cells using SCID GFP transgenic mice: contribution of local and bone marrow-derived host cells. FASEB J. 2006;20(1):95–102. doi: 10.1096/fj.04-3669com. [DOI] [PubMed] [Google Scholar]
- 27.Lin G, et al. Effects of transplantation of adipose tissue-derived stem cells on prostate tumor. Prostate. 2010 doi: 10.1002/pros.21140. [DOI] [PMC free article] [PubMed] [Google Scholar]