Summary
The identification of human papillomavirus (HPV) as an etiological factor for HPV-associated malignancies creates the opportunity to control these cancers through vaccination. Currently, available preventive HPV vaccines have not yet demonstrated strong evidences for therapeutic effects against established HPV infections and lesions. Furthermore, HPV infections remain extremely common. Thus, there is urgent need for therapeutic vaccines to treat existing HPV infections and HPV-associated diseases. Therapeutic vaccines differ from preventive vaccines in that they are aimed at generating cell-mediated immunity rather than neutralizing antibodies. The HPV-encoded early proteins, especially oncoproteins E6 and E7, form ideal targets for therapeutic HPV vaccines since they are consistently expressed in HPV-associated malignancies and precancerous lesions, playing crucial roles in the generation and maintenance of HPV-associated disease. Our review will cover various therapeutic vaccines in development for the treatment of HPV-associated lesions and cancers. Furthermore, we review strategies to enhance vaccine efficacy and the latest clinical trials on therapeutic HPV vaccines.
Keywords: HPV, human papillomavirus, immunotherapy, therapeutic vaccines, cervical cancer, clinical trials, HPV E6, HPV E7, adjuvant, combinatorial approach
Introduction
Human papillomavirus (HPV) is the causative agent in a host of conditions including warts, cancer, and other diseases. HPV is best known for its role in causing nearly 100% of cases of cervical cancer, which is the fourth most common cancer in women [1,2]. In addition, HPV has been indicated to be a human biologic carcinogen for 5 other types of cancers: penile, vaginal, vulvar, anal, and oropharynx including the base of the tongue and tonsils, with a high percentage of oropharyngeal cancer cases in the United States being attributed to HPV [1–4].
The identification of HPV as an etiological factor for HPV-associated malignancies creates the opportunity for the control of these cancers through immunization and other target therapies. Traditionally, vaccines have been mainly used as a preventative measure against infectious diseases. Indeed, there have been several successes in the development of prophylactic vaccines against disease-causing HPV types, targeting the major capsid protein L1 of the viral particle (for review see [5,6]). However, even though the prophylactic vaccines have been proven effective in preventing vaccinated, healthy patients from acquiring HPV infections or previously infected patients who does not have active infection (HPV seropositive but HPV DNA negative) from being re-infected by the same HPV type, there are no strong evidence to demonstrate the therapeutic effects of these prophylactic vaccines in treating and clearing established HPV infections and HPV-associated lesions (for review see [7,8]). In addition, the HPV infections remain extremely common globally, representing a significant health burden [2]. Thus, there remains an important need to develop effective treatments for established HPV infections and associated diseases. One potential treatment method involves the use of therapeutic vaccines that, unlike the preventive vaccines that intend to generate neutralizing antibodies against viral particles, aim to stimulate cell-mediated immune responses to specifically target and kill the infected cells.
In general, most of the sexually active women are infected by HPV during their lifetime. However, majority of these infections remain asymptomatic and are readily cleared by the immune system. A small fraction of the infected women whose immune system fails to clear the infection will develop persistent HPV infection, which may progress into low and high grade cervical intraepithelial neoplasia (CIN) and cervical carcinoma, or regress at any step of this process (for review see [9,10]). It is very often that as HPV-associated lesions progress into cancer, HPV viral DNA will be integrated into the genome of the host [11]. The integration process often leads to the deletion of many early (E1, E2, E4, E5) and late (L1, L2) genes. The deletion of L1 and L2 genes during the integration renders the prophylactic vaccines ineffective in targeting these infected cells. Importantly, E2 is a negative regulator of the HPV oncogenes E6 and E7. The deletion of the E2 gene during integration leads to the elevated expression of these oncoproteins, which is a process thought to contribute to the carcinogenesis of HPV-associated lesions (for review see [12,13]). It has been reported that in certain subset of HPV-associated cervical cancer, the HPV DNA remains episomal and is not integrated into host genome (for review see [14–16]). Nonetheless, the deregulated expression of E6 and E7 remains a common biological hallmark of HPV-associated cancers.
Since HPV oncoproteins E6 and E7 are required for the generation and maintenance of HPV-associated malignancies, they are consistently expressed and remain present and transcriptionally active in the transformed cells in HPV-induced cancer and precancerous lesions. Furthermore, because HPV E6 and E7 are foreign proteins, they can circumvent the issue of immune tolerance against self-antigens, a challenge presented by many other cancers, and thus they serve as ideal targets for therapeutic HPV vaccines (for review see [8]). These findings have inspired many attempts to create an optimal immunotherapeutic strategy against HPV infections and disease. Our review will cover various therapeutic HPV vaccines in development for the treatment of HPV associated diseases, in particular, cancers and lesions. Furthermore, we will review strategies designed to enhance the efficacy of these vaccines and review the latest clinical trials on therapeutic HPV vaccines.
Types of Therapeutic HPV vaccines
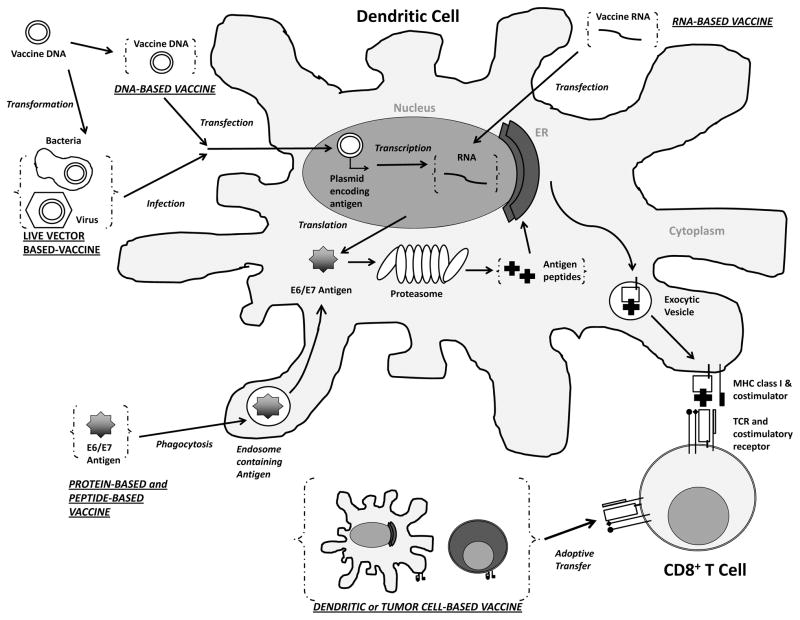
There are multiple types of therapeutic vaccines, mainly targeting HPV oncoproteins E6 and E7, that have been developed and tested in preclinical and clinical trials. These therapeutic vaccines include live vector, protein or peptide, nucleic acid, and cell-based vaccines. Generally, therapeutic vaccines will contain E6 and E7 antigens in various forms and aim to deliver these antigens to antigen presenting cells (APCs) to stimulate antigen presentation through major histocompatibility complex (MHC) class I and MHC class II, leading to the generation of CD8+ cytotoxic T cell or CD4+ helper T cell responses respectively. Before the E6 and E7 antigens can be presented on the MHC class I molecule of the APCs for the stimulation of CD8+ T cell responses, they are first processed and digested by the proteasome in the APCs into smaller peptides. Importantly, not all peptide fragments derived from the digestion of antigen can be successfully loaded onto MHC molecule and recognized by antigen-specific T cells [17]. Only some of these short peptides that contain the sequence of antigenic fragments (epitopes) can bind to the MHC molecule with high affinity and interact with the T cell receptor (TCR) of antigen-specific T cells for the elicitation of immune responses [18–20]. Most therapeutic HPV vaccines focus on eliciting immune responses against the E7 antigen because it is well characterized immunologically compared to E6 in preclinical model. The following sections will describe the characteristics of various therapeutic HPV vaccines, which are also summarized in Table 1. Figure 1 depicts the mechanisms of different types of therapeutic HPV vaccines in eliciting antigen-specific cell-mediated immune response.
Table 1.
Characteristics of current therapeutic HPV vaccines
| Advantages | Disadvantages | Reference | |
|---|---|---|---|
| Bacterial vector based |
|
|
[21–31] |
| Viral vector based |
|
|
[21–22, 32–46] |
| Peptide based |
|
|
[47–50] |
| Protein based |
|
|
[47, 49] |
| DNA based |
|
|
[51–56] |
| RNA replicon based |
|
|
[34, 57–63] |
| DC based |
|
|
[64–72] |
| Tumor cell based |
|
|
[8, 73–74] |
APCs - antigen presenting cells; MHC - major histocompatibility complex; DC - dendritic cell
Figure 1. Immune activation by therapeutic HPV vaccination.
Administration of various types of therapeutic HPV vaccines leads to introduction of antigen into the body in different forms. DNA plasmids encoding antigens (HPV oncoprotein E6 and E7) can be transfected into dendritic cells through DNA vaccination or infection of modified live vector vaccine. The DNA encoding antigens will be transcribed into RNA, which can also be introduced into the cell through RNA vaccination. The transcribed RNA will be further translated into antigen proteins, or long peptides, which can also be taken up by the dendritic cells through phagocytosis following protein-based vaccination or peptide-based vaccination. The antigen proteins or long peptides are then processed by the proteasomes into short peptides, loaded onto class one major histocompatibility complex (MHC I) inside the endoplasmic reticulum (ER) to be presented to T cell receptors (TCR) of CD8+ T cells. Alternatively, dendritic cells or tumor cells can be prepared ex vivo to express target antigen on MHC I molecules as well as the necessary co-stimulatory molecules and be vaccinated back to the body (a process called adoptive transfer) as whole cell-based vaccines for the priming of T cells. (ER – Endoplasmic Reticulum; MHC – Major Histocompatibility Complex; TCR – T Cell Receptor; E6/E7 – Human Papillomavirus E6 and E7 Protein.)
Live vector-based therapeutic HPV vaccines
Live vector-based vaccines can be categorized as bacterial or viral vectors, both capable of replicating within the body to express and spread the antigen. Live vector-based vaccines are highly immunogenic, and there are wide ranges of vectors to be selected to deliver the antigen in a desired manner. However, lived-vector based vaccines face a major drawback in that they pose potential safety risks, particularly for individuals with impaired immune system. In addition, the efficacy of immune response of repeated immunization using the same vector is limited due to the induction of vector-specific neutralizing antibodies and other pre-existing vector-specific immunity (for review see [8,21,22]). Solving these issues can further enhance the efficacy of live vector-based vaccines.
Bacterial vectors
Various bacterial vectors have been investigated for therapeutic HPV vaccines including Listeria monocytogenes [23], Lactobacillus lactis [24], Lactobacillus plantarum [25], and Lactobacillus casei [26].
Among the bacterial vectors, listeria is a promising vector due to its ability to infect macrophages and secrete a pore-forming toxin listeriolysin O (LLO) to evade phagosomal lysis and replicate in the cytoplasm of the host cell [27]. This ability allows the bacteria to be present in both cytoplasm and endosomal compartments, and the antigen peptides in the bacteria can thus be presented via MHC Class I to cytotoxic T cells as well as MHC Class II to helper T cells [28]. Listeria based E7 vaccines have been shown to cause regression of implanted, E6/E7 expressing solid tumors as well as to limit the growth of spontaneous E6/E7 expressing tumor in HPV-16 E6/E7 transgenic mice [23]. Furthermore, another study has demonstrated that listeriolysin induces a decrease in regulatory T cell population by preferentially expanding CD4+ FoxP3− T cells and CD8+ T cells in tumor bearing mice, thus augmenting the therapeutic and antitumor effects of vaccine against E6/E7 expressing TC-1 tumors [29].
Other attenuated bacterial vectors can also be generated by transforming them with plasmids containing the genes of interest. Examples of this includes mutant strains of Salmonella, Shigella, and E. coli, which can serve as carriers for the delivering of plasmid encoding genes of interest to APCs. Among them, the natural route of infection of Salmonella makes it an advantageous carrier that may even allow for oral vaccination [30]. Previous studies have tested the use of Salmonella as carrier for the delivery of HPV-16 E7 protein or E7 epitopes to generate E7-specific responses [31].
Viral vectors
Viral vectors have the ability to effectively infect and express encoded antigens, which makes them attractive for the development of therapeutic HPV vaccines. Several viral vectors have been investigated to deliver HPV E6 and E7 antigens, including adenoviruses [32], adeno-associated viruses [33], alphaviruses [34], and vaccinia virus [35].
Vaccinia virus is an enveloped, double-stranded DNA virus belonging to the poxvirus family. With a large genome and highly infectious nature, in addition to the low likelihood of aberrant integration of foreign DNA into its genome, vaccinia makes a promising vector [36]. Vaccinia has been used in animal models, utilizing intracellular targeting strategies to enhance antigen presentation by dendritic cells (DCs). Vaccinia-based vaccines include vaccinia encoding the E7 fused to calreticulin (CRT), a protein capable of enhancing MHC class I antigen presentation while minimizing the risk of potential malignant transformations of target cells [35], vaccinia expressing E7 fused to LLO [37], and vaccinia expressing E7 linked to sorting signals and lysosomal-associated membrane protein (SigE7-LAMP-1) [37]. Vaccinia-based vaccines encoding SigE7LAMP and expressing human Fms-like tyrosine kinase 3 ligand displayed protective anti-tumor effects by increasing antigen specific CD8+ T cell responses [38].
Adenoviruses have also shown potential as effective vectors in preclinical studies. A study showed that vaccination with replication-deficient adenoviruses encoding CRT linked to E7 (CRT/E7) fusion protein successfully eradicated the E7 expressing established tumors in mice [32]. Adenovirus vaccine encoding chimeric hepatitis B virus surface antigen (HBsAg) and HPV-16 E7 proteins have also been shown to induce enhanced E7-specific antibody and cytotoxic T lymphocyte (CTL) responses in vaccinated mice [39].
An alphavirus vector based immunotherapeutic vaccine encoding a fusion protein of HPV16 E6 and E7 into the recombinant Semliki Forest virus (SFV) particles (SFVeE6,7) was demonstrated to be unhampered by regulatory T cells, indicating that it may be a potential candidate for treatment of HPV as it may not require additional immune interventions to modulate Treg activity [40].
In another study, SFV expressing HPV-16 E7 was able to induce E7 specific cytotoxic T cells in HPV-transgenic mice [41]. The response can be enhanced through the co-administration of interleukin 2 (IL-2), inhibiting tumor angiogenesis [42]. A more recent study further enhanced the potency of recombinant Semliki Forest virus (rSFV) vaccine by fusing rSFV replicon particles expressing HPV E6 and/or E7 to helper T-cell epitopes and an ER targeting signal, which they reported to generate potent antigen CD8+ T cell responses leading to TC-1 tumor clearance and prevent formation of TC-1 tumors in mice [43].
Lentiviruses are able to transduce a variety of cell lines including tumor cells and dendritic cells. A therapeutic vaccine based on integrase defective lentiviral vector (IDLV) has been designed to deliver a mutated form of HPV16 E7 protein fused with CRT. Previous study has shown that a single shot of vaccination with IDLV-CRT/E7 is capable of inducing E7-specific humoral and cellular immune responses eradicating preexisting E7-expressing TC-1 tumor and preventing tumor growth in mice for up to one year after vaccination [44].
As mentioned before, a problem with live vector-based vaccines is the generation of antiviral immune responses and neutralizing antibodies upon primary exposure to the vaccine, which limits the effectiveness of subsequent administrations of the vaccine. This issue was partially addressed by a previous study that demonstrated that cyclooxygenase 2 (COX-2) inhibitors such as celecoxib, may prevent the generation of neutralizing antibodies to vaccinia, preventing repeated administration of the vaccine from losing infectivity [45]. Another concern for the use of live vector based vaccine is the intrinsic pathogenic potential of viral and bacterial vectors, which can be a safety risk for immunocompromised or immunosenescent individuals who have impaired immune functions (for review see [46]). Due to these concerns, other forms of therapeutic vaccines have also been explored.
Protein and Peptide-Based Vaccine
Peptides and proteins derived from HPV antigens are processed by dendritic cells and presented on either MHC class I or II molecules to stimulate CD8+ or CD4+ T-cell immune responses [8,47].
Peptide-Based Vaccine
Peptide-based vaccines are stable, safe, and easy to produce. In general, peptide-based vaccines are of poor immunogenicity. To enhance vaccine potency, peptides are linked to lipids and adjuvants, such as chemokines, cytokines, and Toll-like receptor (TLR) ligands [47]. These methods enhance the vaccine’s ability to activate innate and adaptive immunity, boosting CD8+ T cell responses (for review see [8]). However, the short peptides may fail to incorporate the epitope that is required to stimulate an efficient immune response [48]. Peptide-based vaccines are MHC specific, where the specific immunogenic epitopes of HPV antigens need to be identified for each individual for the vaccine to be effective [49]. As a result, peptide-based vaccines may not be ideal for large-scale treatment [50]. A possible solution to improve peptide-based vaccines is to apply overlapping long-peptide vaccines, which has been proven effective in inducing antigen specific T cell responses in several preclinical models (for review see [47,49]).
Protein-Based Vaccine
Similar to peptide-based vaccines, protein-based vaccines are safe and easy to produce. The benefit of using protein-based vaccines is that they contain all possible human leukocyte antigen (HLA) epitopes, which circumvents the limitation of MHC restriction that arises in the peptide-based vaccines (for review see [47,49]).
The limitations of protein-based vaccines are that they also suffer from low immunogenicity and most of them are presented through MHC class II pathway that activates the production of antibody instead of generating CTL response due to their exogenous nature (for review see [49]). Possible solutions to overcome these problems focus on enhancing the MHC class I presentation. Adjuvants and immunostimulating molecules are added to enhance endogenous processing which will increase the protein uptake by MHC class I, and fusion proteins that target the antigen of DCs are created which increase MHC I presentation and activate CD8+ response [47].
Nucleic acid-based vaccines
DNA vaccine
DNA vaccines are safe, stable, easy to produce, and have the ability to sustain antigen expression in cells for longer duration than RNA or protein vaccines, making them an emerging, attractive, and potentially effective approach for antigen-specific immunotherapy. DNA vaccination involves the injection of plasmid DNA that encodes the antigen of interest (HPV E6 and E7 in this case) into the host cells. DNA vaccines do not lead to generation of neutralizing antibody against the injected plasmid, compared to live vector vaccine, and are thus capable of repeated vaccination [8]. There is a potential risk that administration of DNA encoding HPV oncogenes E6 and E7 may lead to cellular transformation, which was addressed by modifying E6 and E7 DNA that results in subsequent expression of proteins that are incapable of oncogenic transformation [51].
In general, a DNA vaccine is administered via intramuscular approach. However, myocytes are the cells that predominantly uptake the injected DNA after intramuscular injections. Even though myocytes transfected with injected DNA will express the target antigen, they cannot fully activate strong immune responses because they are not professional APCs [52]. DCs play a crucial role in presenting the antigen to naive CD8+ cytotoxic T cells, and do so through two possible pathways [53]. Firstly, DCs can uptake exogenous antigen released from transfected myocytes through phagocytosis and subsequently process and present the exogenous antigen on MHC I molecule through the cross presentation pathway [54]. Secondly, DCs can be directly transfected by vaccination and present target antigen expressed endogenously to CD8+ T cells through direct presentation [55].
There is an important limitation of DNA vaccines in that naked DNA has low intrinsic immunogenicity due to the inability to amplify and spread from transfected cells to surrounding cells in vivo as compared to live vector based vaccines. To overcome this, several strategies to enhance the potency of DNA vaccines have been developed, targeting the important role that DCs play in vaccine mediated immunotherapy, these strategies aims to: 1) increase the amount of DCs that express target antigen, 2) enhance antigen processing and presentation by DCs, and 3) improve interaction between DCs and T cells (for review, see [56]). The strategies to enhance vaccine potency will be discussed in greater detail in a later section.
RNA Vaccine
Naked RNA replicon vaccine can be derived from RNA viruses including Sindbis virus [57], Venezuelan Equine Encephalitis virus [34], as well as SFV [58]. RNA replicon is capable of self-replication, leading to sustained level of antigen expression and an enhanced immunogenicity. Since the viral structural genes have been excluded from the RNA replicon vectors, they do not form viral particles, and thus will not lead to the generation of neutralizing antibody, allowing for repeated administration. RNA replicons also alleviate the potential risk of chromosomal integration and cellular transformation that is associated with DNA vaccines, and are thus highly advantageous as vaccination method targeting potentially oncogenic proteins like E6 and E7.
One major disadvantage of the RNA replicons is its low stability. To overcome this issue, attempts have been made to combine RNA replicons and DNA vaccine into DNA-launched RNA replicon, which are also called ‘suicidal DNA’ because it triggers apoptosis in cells that uptake the injected DNA to prevent potential integration and transformation of the transfected cells. The ‘suicidal DNA’ has been applied in a therapeutic HPV vaccine in preclinical models, during which the generation of potent HPV antigen-specific CD8+ T cell responses and antitumor effects were observed [59]. However, because the ‘suicidal DNA’ will elicit apoptosis in transfected cells, including DCs transfected with injected DNA, this approach has led to poor immunogenicity. To address this issue, genes encoding antiapoptotic protein have been incorporated into the suicidal DNA vector to enhance survival of transfected antigen presenting cells, which have been shown to generate even greater antigen-specific CD8+ T cell responses and more potent antitumor effects than the suicidal DNA vector that only encodes for E7 antigen in preclinical models [60]. An alternative strategy to overcome the issue of apoptosis is to use flavivirus Kunjin (KUN) vector to deliver the replicons. KUN does not induce apoptosis in transfected cells, thus allowing direct presentation by transfected DCs compared to ‘suicidal DNA’ vector [61]. DNA-launched KUN replicons encoding HPV16-E7 antigen were capable of generating E7-specific T cell responses and antitumor effects in preclinical models [62]. Despite the promising results demonstrated by RNA replicon vaccines in the preclinical model and the ongoing clinical trials testing the efficacy of RNA vaccines in treating other types of cancer such as lung cancer [63], RNA vaccines targeting HPV antigens and HPV related diseases have not yet progressed to be tested in clinical settings.
Whole Cell-based vaccines
Dendritic cell-based vaccine
The knowledge in dendritic cell biology, together with improved methods for preparing DCs ex vivo, have paved the way for the development of DC-based vaccines as another plausible strategy for therapeutic vaccines against HPV-associated malignancies. Dendritic cell-based HPV vaccines involve loading the DCs with HPV antigens ex vivo by infecting [64] or transfecting DCs with DNA or RNA encoding HPV antigen [65,66] or by pulsing DCs with antigen protein, peptide, or tumor lysate [67–70], and subsequent delivery of DCs into patients. In addition, DCs are capable of serving as natural adjuvants to enhance the potency of antigen-specific immunotherapy against cancer (for review see [71]). Since T-cell mediated apoptosis may limit the lifespan of DCs and their ability to prime T cells, strategies to prolong DC survival have been incorporated to enhance the efficacy of DC-based vaccines. One of such strategies is to transfect DCs with siRNAs targeting pro-apoptotic molecules such as Bak, Bid, Bim, and Bax proteins. Such strategies have been shown to generate enhanced antigen-specific CD8+ T cell activation and antitumor effects in mice [68,69]. In a more recent study, siRNA cocktail targeting immunosuppressive factors including interleukin 10 (IL-10) and tumor growth factor beta (TGF-B) was introduced into DCs to enhance the potency of DC-based vaccine and was shown to be able to elicit strong antitumor effects against immune-resistant, HPV-16 E7 expressing TC-1 (P3) tumor model [72].
Similar to other types of vaccines, there are also several limitations for DC-based vaccine. Firstly, the use of autologous DCs for individualized therapy is technically demanding and thus difficult for large-scale production for general population. In addition, variations in culturing techniques used to prepare DCs may lead to varying vaccine quality and lacking of standard criteria for evaluating the vaccines. Also, because it is necessary for the DCs to enter lymphoid organs to generate an effective T-cell mediated immune response, determining the most effective route of DC vaccine administration is important to maximize the effects elicited by the vaccine.
Tumor cell-based vaccine
Tumor cells are isolated and manipulated ex vivo to express immune modulatory proteins, which enhances their immunogenicity in vivo. Cytokine genes IL-2, IL-12 [73], and granulocyte macrophage colony stimulating factor (GMCSF) [74] have been used in HPV transformed tumor cell-based vaccines for mice with HPV-16 induced tumors to promote the differentiation of naïve T cells into effector or helper T cells and to stimulate stem cells to produce granulocytes.
Although tumor cell-based vaccines have gone through clinical trials in melanoma, pancreatic cancer, renal cell carcinoma, and colorectal carcinoma, tests for HPV associated tumors have not been conducted. The advantage of these vaccines is that tumor antigens do not have to be well identified and they may be able to cover more tumor antigens. However, this approach would not be practical for HPV associated-cervical cancers, as HPV has well-known tumor-specific antigens.
In addition, using tumor-based vaccinations has the potential risk of implanting new cancers in patients, thus preventing clinical trials in patients with mild HPV-associated diseases or precancerous lesions. Knowing the problems involved in tumor cell-based vaccines, the potency and purity must be individually tailored. Comparing with the other options of vaccines, tumor cell-based vaccines are considerably more time consuming and expensive to prepare. As a result, tumor cell-based vaccines against HPV have yet to be utilized and tested in clinical trials.
Enhancing The Therapeutic Effects of the Vaccines
Because many of the therapeutic HPV vaccine designs described above, such as protein, peptide and DNA based vaccines, have intrinsically low immunogenicity, administration of these vaccines alone may not effectively elicit the desired antigen-specific immune responses and therapeutic effects. Therefore, strategies to enhance the therapeutic effects of therapeutic vaccines have been investigated. This section describes and discusses examples of strategies developed to improve vaccine efficacy.
Immunostimulanting Molecules / Adjuvants
Adjuvant is a term originated from Latin word adjuvare, which means to help or to aid [75]. Co-administration of antigen with adjuvant can enhance the antigen-specific immune response based on these immune functional activities: 1) Translocation of antigens to lymph nodes to be recognized by T cells, 2) protection of antigen to enable longer antigen exposure, 3) enhancement of local reaction at injection site, 4) induction of proinflammatory cytokines secretion, and 5) interaction with pattern recognition receptors such as Toll-Like Receptors [76]. In general, adjuvants help activate APCs to up-regulate molecules essential for antigen presentation, including MHC molecules, costimulatory molecules, and cytokines. These events allow more effective antigen presentation to the adaptive immune system, leading to better T cell activation and expansion (for review see [77]).
CRT in DNA vaccines is an example of immunostimulating molecule utilization [78]. CRT is a carrier protein associated with peptides delivered into the endoplasmic reticulum (ER) by transporters associated with antigen processing to aid antigen presentation. DNA vaccines encoding CRT linked to HPV antigen E7 led to expression of a fusion protein CRT/E7, which has a better homing ability into the ER of cells, including DCs, for better MHC-I antigen presentation and CD8+ CTL activation. In addition, the potency of CRT/E7 vaccine can be further enhanced when co-administered with additional adjuvants. Vaccination with CRT/E7 together with DNA encoding papillomavirus capsid proteins L1 and/or L2 generates even greater E7-specific CD8+ T cell responses than vaccination with CRT/E7 alone, resulting in potent antitumor effects, tumor control, and survival of tumor bearing mice [79].
Anti-apoptotic molecules, as mentioned in previous sections, are another example of adjuvant [60]. Administration of anti-apoptotic protein, such as BCL-xL, increase DC resistance to CD8+ cytotoxic T cell-mediated killing, allowing for longer antigen presentation by DCs. Co-vaccination with DNA encoding HPV E7 antigen and DNA encoding anti-apoptotic protein has been shown to enhance E7-specific CD8+ T cell responses and antitumor effects [80].
Interferon-stimulating gene 15 (ISG15) is an ubiquitin-like protein induced by type I interferon stimulation and is associated with production of interferon gamma (IFN-y). A study demonstrated that ISG15 could serve as a potent CD8+ T cell mediated adjuvant and, when vaccinated with DNA vaccine targeting HPV-16 E7, leads to substantial increase in E7-specific IFN-y responses, expansion of CD8+ T cells, and control of HPV-associated tumors in preclinical model [81].
A novel adjuvant consisting of a synthetic RNA cross-linked with polymeric carrier has been shown to be capable of activating murine TLR-7 or human TLR-8 pathway [82]. TC-1 tumor bearing mice vaccinated with HPV-16 E7 peptide and RNA adjuvant generated a greater E7-specific CD8+ CTL responses and antitumor effects compared to mice vaccinated with E7 peptide only or E7 peptide with immune stimulant polyinosinic:polycytidylic acid (Poly I:C).
Combinatorial Approaches
While strategies to enhance different types of therapeutic vaccines have been developed, it has been shown that integration of various therapeutic approaches can further increase immunogenicity and efficacy of therapeutic HPV vaccines. Recent developments of therapeutic HPV vaccines have shifted to focus on combinatorial approaches. The potential combinations of various strategies are described below.
Prime-boost regimen for therapeutic HPV vaccines
The variety of therapeutic vaccines available leads to the hypothesis that administration of different vaccines through prime-boost regimens can potentially enhance overall potency of therapeutic treatment. For example, because DNA vaccines often generate weak cell-mediated immune responses, a combinatorial vaccination approach can be used to overcome this limitation. Indeed, it has been shown that priming the immune system with DNA vaccine encoding HPV-16 E6/E7 followed by boosting with recombinant vaccinia based therapeutic HPV vaccine [83], adenovirus based vaccine [84], or HPV-16 E6/E7 expressing tumor based vaccine [85] led to the generation of greater HPV-16 E6/E7 specific CD8+ T cell responses than vaccination with one type of vaccine alone in preclinical models. Mice primed with Sindbis virus RNA replicon vaccine against E7 antigen and boosted with vaccinia vector vaccine encoding E7 antigen have also been shown to generate better CD8+ T cell immune responses and more potent antitumor effects than mice treated with single type of vaccination [86]. Another study also showed that even though vaccinating mice with E7 protein vaccine CyaA336/E7, a fusion protein consisting of full length HPV-16 E7 inserted at position 336 of the cell-invasive adenylate cyclase toxoid of Bordetella pertussis, can lead to the generation of E7 specific immune response and treatment effects against E7 expressing TC-1 tumor, the therapeutic efficacy of CyaA336/E7 vaccine could be further enhanced when boosted with attenuated modified vaccinia virus Ankara (MVA) that expressed E7 protein [87].
Combining therapeutic HPV vaccines with immunomodulatory agents
Modulation of the tumor microenvironment is an important factor contributing to effective immunotherapy. The tumor microenvironment usually contains many factors that can hinder the effectiveness of immune therapies. Example of this includes the release of immunosuppressive cytokines including IL-10 [88] and TGF-B [89] by regulatory T cells that resides in tumor microenvironment. Thus, depletion of T regulatory cells in tumor microenvironment can significantly enhance potency of therapeutic HPV vaccine [90]. Other immunosuppressive molecules present in tumor microenvironments include B7 homolog-1 (B7-H1), also known as program death ligand 1 (PD-L1) (for review see [91]), signal transducer and activator of transcription 3 (STAT3) (for review see [92]), indoleamine 2,3-dioxygenase (IDO) enzyme (for review see [93]), galectin-1 (for review see [94]), and MHC class I polypeptide-related sequence A and B (MICA / B) (for review see [95]). These immune suppressive molecules all serve as potential targets of immune modulation for enhancing treatment effect of therapeutic HPV vaccines (for review see [96]).
Vitamin E has been shown to have strong anticarcinogenic properties capable of inhibiting proliferation and inducing apoptosis in cancer cells [97]. Administration of vitamin E has been demonstrated to induce TC-1 necrosis in vivo as well as alleviating immune suppression by reducing the amount of myeloid derived suppressor cells (MDSCs) that resides in the tumor microenvironment, ultimately leading to potent antitumor effects when administered along with E7-specific adoptive T cell transfer [98].
In addition to reducing immune suppressive factors presented in tumor microenvironment, immunostimulants have also demonstrated success in enhancing the potency of immunotherapy. Administration of agonist antibody of costimulatory receptor 4-1BB along with E6/E7 peptide vaccine resulted in high CD8+ versus regulatory T cells ratio, infiltration of E7-specific CD8+ T cells, and regression of TC-1 tumors [99]. Histone deacetylase inhibitor (HDACi) has been shown to increase MHC I and II molecule expression on the surface of TC-1 tumor cells, making them more susceptible to E7-specific T cells generated through therapeutic HPV DNA vaccination [100]. Furthermore, local administration of molecules that activates innate immune responses such as imiquimod or GMCSF have been shown to lead to secretion of proinflammatory cytokines in the tumor location, which promotes DCs maturation, migration, and cross presentation, resulting in potent local antigen-specific immune responses against TC-1 tumors [101,102].
Combining therapeutic HPV vaccines with other therapeutic modalities
Therapeutic HPV vaccines may also be combined with other therapeutic modalities, including chemotherapy, radiation therapy, or other therapeutic agents to strengthen the overall therapeutic effects [103–111]. Several chemotherapies and radiotherapies have been shown to augment the potency of therapeutic HPV vaccines. For example, we showed that combination of radiation therapy with therapeutic HPV peptide vaccine improves potency of therapeutic HPV vaccine. Radiation therapy induces cancer cell deaths and release of tumor antigen and endogenous adjuvant within tumor microenvironment, which was sufficient to elicit priming and expansion of E7 specific CD8+ CTLs in TC-1 tumor bearing mice following vaccination with E7 peptide, leading to potent therapeutic antitumor effects against E7 expressing TC-1 tumor [112]. Likewise, treatment with chemotherapeutic agents cyclophosphamide [113] or cisplatin [114,115] have also been shown to improve therapeutic HPV vaccine potency.
Optimal Route of vaccination
Method of administration
The method of vaccination, particularly for DNA vaccine, is important in eliciting the desired therapeutic effects. Delivery methods targeting DNA to areas rich in DCs can increase the population of DCs presenting the antigen, leading to more T cell priming and activation. Intradermal administration via gene gun presents a potentially effective method of DNA vaccination. Gene gun ballistically delivers gold particles coated with DNA that encodes target antigen to the Langerhan cells, immature DCs of the skin. Vaccination via gene gun has been shown to require the least amount of DNA to generate potent antigen-specific CD8+ T cell immune responses compared to biojector or intramuscular injection [116]. A drawback of gene gun vaccination is that the amount of DNA that can be delivered with each shot of the gene gun is limited [117], thus may require multiple administrations, increasing the risk of local side effects. Intramuscular injection followed by electroporation is another effective administration method [118]. Electroporation applies a small electric current through the local area, enhancing DNA uptake by the muscle cells, creating large numbers of antigen expressing and releasing muscle cells. Local DCs can then process and present these antigens through the MHC class I pathway. In addition, electroporation induces cytokines release and creates a favorable environment for DCs. A study comparing various method of DNA vaccination showed that intramuscular injection with electroporation generated higher amount of E7-specific CD8+ T cells than conventional intramuscular injection without electroporation or epidermal delivery via gene gun [118]. Other alternative methods of vaccination include the use of laser beam [119] or microencapsulation of DNA [120].
Location of administration
There are increasing amounts of evidence suggesting that the delivery site of vaccines is an important factor contributing to the success of vaccines. Recent data suggest that targeted delivery of vaccines to tumor-draining lymph nodes (tDLNS) is a potential vaccination strategy that can enhance the efficacy of cancer vaccines [121]. We have performed many studies comparing the therapeutic effect of vaccine when vaccinated locally, near the tumor location, versus when vaccinated systemically, distant from tumor location. When all other conditions are similar, intratumoral administration of therapeutic HPV vaccine has been shown to generate more potent local antigen specific immune responses and antitumor effects compared to subcutaneous [112], intraperitoneal [115], or intramuscular [114] administration. Furthermore, we have demonstrated that intravaginal administration of therapeutic HPV DNA vaccine followed by electroporation leads to generation antigen-specific CD8+ T cells that express a4B7, a surface marker known to home T cells to mucosal areas including the cervicovaginal tract [122]. Thus, local vaccination proximal to the tumor or tDLNS may potentially be applied to enhance the potency of future therapeutic HPV vaccines as well as therapeutic vaccines targeting other types of cancer.
Therapeutic HPV Vaccines Clinical Trials
Clinical trials are essential for evaluating whether a therapeutic HPV vaccine is able to contain HPV infections and HPV-associated diseases in human. Here, we summarized a number of recent clinical trials that implemented various forms of therapeutic HPV vaccines against HPV-associated lesions and malignancies (Table 2).
Table 2.
Recent Therapeutic HPV Vaccine Clinical Trials Using Different Forms of Vaccines
| Vaccine | Antigen(s) | Construct | Organization | Trial Design | Outcome | Side Effects | Reference |
|---|---|---|---|---|---|---|---|
| Bacterial Vector Based | |||||||
| Lm-LLo-E7 (ADXS11-001; ADXS-HPV) | HPV-16 E7 | prfA-defective Listeria monocytogenes strain transformed with plasmid encoding HPV-16 E7 antigen fused to a fragment of nonhemolytic listeriolysin O (LLO) | Advaxis, Inc. | Phase I in patients with metastatic, refractory or recurrent, advanced squamous cell carcinoma of the cervix (15 patients) | Increase in E7-specific T cells detected in PBMCs of three patients. Reduction in tumor size observed in 4 patients. |
Pyrexia, vomiting, chills, headache, anemia, nausea, tachycardia, muscle and skeletal pain. | [123] |
| GLBL101c | HPV16-E7 | Recombinant Lactobacillus Casei expressing modified version of HPV16-E7 | GENOLAC BL Corp | Phase I/IIa in HPV16+ CIN3 patients (17 patients) | Significant increase in E7-CMI in cervical vaginal tract. 9 patients experienced disease regression to CIN2, and 5 further regressed to LSIL |
No major side effects observed. | [125] |
| Viral Vector Based | |||||||
| TG4001 | HPV-16 E6/E7 | Recombinant Modified Vaccinia Ankara expressing HPV-16 E6, E7, and IL-2 | Transgene/Roche | Phase I in HPV16+ CIN2/3 Patients (21 patients) | 10 of 21 (48%) showed disease regression. HPV DNA clearance in 8 patients and mRNA clearance in 7 patients. | Inflammation, pruritus, edema, lymphadenopathy, fever, headache, asthenia, bone pain, vaginal discharge | [126] |
| MVA E2 | HPV-16 E2 | Recombinant Modified Vaccinia Ankara encoding E2 from BPV | Instituto Mexicano del Seguro Social | Phase I/II in CIN1/2/3 Patients (78 patients) | 50% vaccinated patients were free of lesions at the end of treatment, and 87% were lesion free by 3 weeks after treatment. | Headache, flu-like symptom, fever, chills, abdominal pain, joint pain. | [128] |
| Phase I/II in male patients with flat condyloma lesions (30 patients) | No lesions or HPV detected in 28 of 30 patients 4 weeks after treatment. | No local or systemic adverse effects reported. | [129] | ||||
| Phase III in patients with HPV-induced AGIN (1176 female patients and 180 male patients) | 90% lesion clearance in female treated patient and 100% lesion clearance in male treated patients. Antibody and T cell responses observed in all tested patients. | Headache, flu-like symptom, fever, chills, abdominal pain, joint pain. | [130] | ||||
| Peptide / Protein Based | |||||||
| HPV16-SLP | HPV-16 E6/E7 | Combination of nine HPV-16 E6 and four HPV-16 E7 synthetic peptides with incomplete Freund’s adjuvant | ISA Pharmaceuticals | Phase II in patients with HPV16+ VIN3 (20 patients) | 15 patients had objective clinical response at 12 months. 9 complete response and 6 partial response. 85% with circulating HPV-16 specific T cells. 83% had CMI against HPV-16. | Local swelling, redness, increased skin temperature, pain at vaccination site, fever, flu like symptoms, chills, and tiredness. | [131] |
| Phase II study in patients with HPV16+ HSIL (9 patients) | All vaccinated patients showed strong HPV-specific T cell response after vaccination. Change in patterns of immune infiltrate. | Itching, redness, swelling, and pain at injection site, headache, diarrhea, fatigue/dizziness, nausea, chills, myalgia, rash, fever, urticarial, edema. | [132] | ||||
| Phase II in patients HPV16+ advanced or recurrent gynecological carcinoma (20 patients) | 9 patients with HPV-16 specific immune response. Duration of survival correlates with magnitude of T cell response. | Injection site reaction, fever, chills, fatigue, nausea, flue-like symptom | [133] | ||||
| Phase II in patients with low-grade abnormalities of the cervix (50 patients) | 97% of vaccinated patients generated HPV 16-specific CMI | Flu-like symptom, injection site reaction. | [135] | ||||
| GL-0810 | HPV-16 antigen | HPV-16 immunomodulatory peptide with adjuvant Montanide and GM-CSF | Gliknik Inc. | Phase I in patients with recurrent/metastatic squamous cell carcinoma of the head and neck (9 patients) | 80% of patients who received 4 vaccinated developed T cell and antibody response. Progression free and over all survival is 80 and 196 days respectively. | Erythema, itching, and pain at injection site | [136] |
| TA-CIN | HPV-16 E6/E7/L2 | HPV16 E6E7L2 fusion protein | Xenova Research Limited | Phase I in healthy patients (40 subjects) | TA-CIN specific IgG in 24 of 32 vaccinated patients. 25 of 32 vaccinated patients generated CMI. | Injection site reaction, tenderness. Headache and fatigue | [138] |
| Phase II with VIN2/3 patients (19 patients) | 63% lesion response 1 year after vaccination. Significant increase. Significant CMI observed in lesion responders. |
Local reaction associated with imiquimod. | [141] | ||||
| TA-CIN + TA-HPV | HPV-16/18 E6/E7/L2 | HPV16 E6E7L2 fusion protein and vaccinia virus with HPV16/18 E6/E7 | Celtic Pharma | Phase I with HPV16+ VIN patient (10 patients) | Partial or complete clinical response in 2 patients. All but 1 patient showed HPV-16 specific IgG and/or T cell responses. | Pain at injection site. | [139] |
| Phase II with HPV16+ high-grade AGIN Patients (29 patients) | 17 patients showed TA-CIN induced T cell responses. 11 generated HPV-16/18 E6 and/or E7 specific T cells. 14 with IgG response to HPV-16 E7. | N/A | [140] | ||||
| Nucleotide Based | |||||||
| pNGVL4a-sig/E7(detox)/HSP70 + TA-HPV | HPV-16/18 E6/E7 | Plasmid encoding mutated form of HPV16-E7 linked to sig and HSP70 and vaccinia virus with HPV16/18 E6/E7 | Sidney Kimmel Comprehensive Cancer Center | Phase I with HPV16+ CIN3 Patients (12 patients) | 58% vaccinated patients have generated HPV-16 E7-specific CMI. Increase CD8+ T cell infiltration to lesions. | Tenderness, local site reaction, blister, erythema, pruritus | [142] |
| GX-188E | HPV-16/18 E6/E7 | Plasmid encoding fusion protein of HPV 16/18 E6/E7 linked to Flt3L and tpa | Genexine, Inc | Phase I in patients with HPV 16/18+ CIN3 (9 patients) | All patients displayed enhanced HPV-specific CMI. 7 patients demonstrated complete lesion regression by the end of the trial. | Chills, injection site pain, swelling, hypoesthesia, headache, fatigue, rhinitis | [143] |
| VGX-3100 | HPV-16/18 E6/E7 | Mixture of two plasmids encoding optimized consensus of E6 and E7 antigen of HPV 16 and 18 | Inovio Pharmaceuticals | Phase I with HPV16/18 + CIN2/3 Patients (18 patients) | HPV-specific CMI observed in 78% patients and HPV-specific humoral response observed in all patients. | Injection site reaction, pain, fever, tenderness. | [146] |
| Phase IIb with HPV16/18 + CIN2/3 Patients (167 patients) | 49.5% vaccinated patient demonstrated regression compared to 30.6% in placebo group. Vaccinations enhance T cell and humoral response. | Injection site reaction, fatigues, headache, lyalgia, nausea, arthralgia, erythema | [147] | ||||
| Whole Cell Based | |||||||
| DC + KLH | HPV-16 and HPV-18 E7 | Dendritic Cells pulsed with HPV-16 and HPV-18 E7 and keyhole limpet hemocyanin | National Institute of Health | Phase I in patients with stage Ib or IIa cervical cancer (10 patients) | Increase in HPV-specific humoral and CD4+ T cell responses observed, but not CD8+ T cell responses. | Local site reaction, erythema, swelling, pruritus | [148] |
| DC | HPV antigens | DC pulsed with HPV+ tumor lysate | Department of Biotechnology (DBT, Govt. of India) | Phase I in in patients with HPV+ advanced, recurrent cervical cancer (14 patients) | No significant increase in lymphocyte proliferation observed. Lack of biopsy sample and small sample size prevent definite conclusions. | Local site reaction, fever, chills, abdominal discomfort, vomiting. | [149] |
CIN - Cervical intraepithelial neoplasia; AGIN - Ano Genital Intraepithelial Neoplasia; HSIL - High-grade squamous intraepithelial lesion; VIN - vulvar intraepithelial neoplasia
Live vector-based
In 2009, first clinical use of a listeria-based therapeutic HPV vaccine was reported [123]. The vaccine, Lm-LLo-E7 (also known as ADXs11-001 or ADXS-HPV) consists of a prfA-defective Listeria monocytogenes strain transformed with plasmid encoding HPV-16 E7 antigen fused to a fragment of nonhemolytic LLO [124]. The phase I trial tested the safety of this vaccine construct in 15 patients with metastatic, refractory, or recurrent advanced squamous cell carcinoma of the cervix who had failed prior chemotherapy, radiotherapy and/or surgery. The patients were vaccinated intravenously, followed by IV supplementation of 500mg ampicillin 5 days after vaccination, followed by a 10-day oral course of ampicillin (500mg QID). The study demonstrated that the vaccine was well tolerated by patients. Common adverse effects observed include pyrexia, vomiting, chills, headache and anemia, nausea and tachycardia, and musculoskeletal pain, with 6 patients (40%) experience vaccine related grade 3 adverse events and no drug-related grade 4 adverse events observed. The overall change in physical examination were considered not clinically significant and mainly attributed to underlying disease. Peripheral blood mononuclear cells (PBMCs) were collected from the patients, and of the three patients with PBMC samples viable for testing, an increase in E7-specific IFN-y+ T cells were detected in the PBMCs of these patients after vaccination. In addition, reduction in total tumor size was observed in 4 patients (30.8%), suggesting that the vaccine might have certain therapeutic effects in controlling the progression of cancer.
A more recent study tested the safety and efficacy of oral vaccination of a bacterial vector-based therapeutic HPV vaccine in a Phase I/IIa study involving 17 patients with HPV-16+ cervical intraepithelial neoplasia (CIN) 3 lesions [125]. The vaccine, GLBL101c, is generated from a recombinant Lactobacillus casei that expresses a modified version of HPV16-E7 antigen that is no longer carcinogenic [26]. The bacterial vector based vaccine was made into powder and enclosed in capsule and administered to patients through ingestions. None of the participating patients experienced serious adverse events. Although only two patients demonstrated a significant increase in E7-CMI in PBMCs, significant increase in E7-specific cell-mediated immune responses was observed in the cervical vaginal tract of all patients receiving the vaccine, with 9 of the 13 patients (69%) who received 4–6 capsules/day experienced pathological down-grade to CIN3 and were followed up for 12 months without additional treatment, during with 5 of the 9 patients (56%) showed further cytological regression to Low grade squamous intraepithelial lesion (LSIL).
TG4001 vaccine is a suspension of MVATG8042 particles consisting of attenuated recombinant MVA containing sequences encoding modified HPV-16 E6/E7 as well as human IL-2. Its safety and efficacy has been evaluated in twenty-one patients with HPV-16 related CIN2/3 lesions [126]. Each patient received three subcutaneous injection of 5 × 107 pfu of TG4001 on either the right or left thighs with 1-week interval between each administration. 19 patients (90%) reported at least 1 adverse event, with 65 of the 86 total AE reported were attributed to TG4001. Most of the AE were mild or moderate, with no grade-3 or 4 observed. The documented AE includes inflammation, pruritus, and edema at the injection site, and lymphadenopathy, fever, headache, asthenia, bone pain, and vaginal discharge for systemic reaction. At six months after the treatment, ten of the twenty one patients (48%) were evaluated as clinical responders with disease regression to LSIL. In addition, HPV-16 DNA clearance were observed in 8 of the 10 responders, while HPV-16 mRNA clearance were observed in 7, and no recurrence of high-grade lesion was observed in these responders at 12 months after treatment.
Another MVA based therapeutic HPV vaccine has been created and designed to target HPV E2 protein instead of E6 or E7 oncoprotein [127]. The safety and efficacy has been evaluated by multiple clinical trials [128,129]. Recently, the MVA E2 vaccine has been used in a phase III clinical study for the treatment of HPV-induced ano-genital intraepithelial lesions, including CIN1, 2, and 3 or condyloma lesions for females, and condyloma lesions in urethra or anal lesions for males [130]. A total of 1356 participating patients including 1176 females and 180 males participated in the study. The vaccine was injected locally to the lesion site, either into the uterus of female patients, instilled into the urethra of male patients, or injected locally into visible lesions. 825 of 870 (94.82%) female patients with low-grade lesions and 220 of 300 (73.33%) female patients with high-grade lesions demonstrated lesion clearance at the end of treatment. The overall efficacy of MVA E2 vaccine in treating HPV-induced CIN lesions in this study is around 90%. All of the male patients showed complete elimination of lesions. During the 5 years follow up period, only 5 out of the 141 vaccinated female patients with high-grade lesions and no male patients experienced lesion recurrence. In addition, antibodies against HPV-E2 proteins were detected in the serum of every treated patients and generation of cytotoxic T cell response specific against HPV antigens were observed in all randomly tested patients. Common adverse events observed during the study include headaches, flu symptoms, fever, chills, abdominal pain, and joint pain, which were all considered to be grade 1 / mild side effects. These encouraging results demonstrated the therapeutic potential of MVA E2 vaccine in stimulating the immune system to treat HPV associated intraepithelial lesions.
Peptide and Protein-Based Vaccine
The therapeutic potential of HPV16 synthetic long-peptide vaccine (HPV16-SLP) in human patients has been studied substantially in multiple clinical trials [131–133]. The vaccine, HPV16-SLP, consists of 9 E6 and 4 E7 peptides of 25 to 35 amino acids long with overlap of 10 to 14 amino acids between each subsequent peptide, as well as Montanide ISA-51 as adjuvant [134]. The ability of HPV16-SLP vaccines to establish long-term immunologic memory in patients with low-grade abnormalities of the cervix was further tested in a placebo-controlled, double-blinded phase II study [135]. 50 patients with LSIL or cervical abnormality were recruited. The patients were randomly assigned to receive either the vaccine or placebo on the arm or thigh followed by either vaccine or placebo booster one year after the first administration. Common AEs observed in this study include flu-like syndrome, injection side reactions. 97% of vaccinated patients generated significant HPV-16 specific immune responses. The study showed that two low-dose HPV16-SLP vaccinations could induce strong and stable HPV-16 specific T cell immune responses in vaccinated patients that last for at least one year.
The applicability of a therapeutic HPV peptide-based vaccine with adjuvant Montanide and GMCSF (GL-0810) has also been tested in patients with recurrent / metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN) in a phase I dose escalation trial [136]. The vaccination was well tolerated, with mainly grade 1 AE including erythema, pain, and itching at injection site. Out of the five patients with HPV-16+ RM SCCHN and received all four subcutaneous vaccinations, four (80%) of them generated T cell and antibody responses, and no dose limiting toxicities was observed. The median progression free survival and the median overall survival of patients who received vaccination were 80 and 196 days respectively. The study demonstrated that the vaccine was well tolerated by patients with late stage cancer and was capable of eliciting an immune response.
HPV therapeutic vaccine TA-CIN is a subunit vaccine comprising HPV16 E6E7L2 fusion protein [137]. This vaccine has been proven safe and immunogenic in multiple phase I and II trials [138–140]. The ability of the TA-CIN vaccine to be administered with topical immunomodulatory, imiquimod, for treating patients with grade 2 or 3 vulvar intraepithelial neoplasia (VIN) was further tested in another phase II study [141]. 19 Patients received imiquimod 5% cream as well as 3 times of intramuscular TA-CIN vaccination (128μg / time) to the deltoid muscle at 1 month interval. Common AE includes local inflammation, ulceration, malaise and flu-like symptoms after imiquimod cream application. No side effects or AEs associated with TA-CIN vaccination were identified. 20 weeks after vaccination, a significant increase in infiltrating CD8+ and CD4+ T cells were observed in lesion responders, and complete regression of VIN was observed in 63% of treated patients at week 52 after treatment, who also demonstrated the generation of immune responses at week 20. The study demonstrated that the ability to elicit antigen-specific immune responses in patients correlates with lesion regression.
Nucleic acid-based vaccines
Many clinical trials have been conducted recently evaluating the efficacy of therapeutic HPV DNA vaccines in human patients. One such trial incorporated the strategy of heterologous prime-boost vaccination to treat patients with HPV-16 associated CIN2/3 lesion [142]. The DNA vaccine used in this study, pNGVL4a-sig/E7(detox)/HSP70, is a plasmid encoding mutated form of HPV16-E7 linked to signal peptide and heat shock protein 70, which have been shown to enhance the immune response generated by the antigen [116]. Another vaccine used in the study, TA-HPV, is a recombinant HPV vaccinia virus based vaccine encoding the HPV-16 and 18 E6 and E7 protein [36]. 12 Patients were injected intramuscularly twice with pNGVL4a-sig/E7(detox)/HSP70 and boosted with intramuscular injection of TA-HPV, with each vaccination given in one month intervals. All reported AEs were considered to be mild. Noted side effects include tenderness, local reaction at injection site, blister with drainage, erythema and pruritus. 7 of the 12 (58%) vaccinated patients developed HPV 16-specific CMI, predominantly HPV-16 E7-specific responses. In addition, the study demonstrated that a significant increase in antigen-specific CD8+ T cell infiltrate in the HPV associated lesions could be observed, regardless of a lack in increase of antigen specific CD8+ T cell responses in the peripheral blood. Thus, the study suggested that local immune responses, instead of the traditionally monitored systemic immune responses, are ultimately responsible for the therapeutic effects against target lesions and better clinical outcomes.
A phase I trial was recently conducted testing the safety and efficacy of a therapeutic HPV DNA vaccine, GX-188E, in 9 patients with cervical intraepithelial neoplasia 3 (CIN3) [143]. The GX-188E DNA vaccine is engineered to express fusion protein of HPV16 and HPV18 E6 and E7 that is fused to the extracellular domain of Flt3L and signal sequence of plasminogen activator (tpa). Flt3L and tpa inclusion sought to promote trafficking and presentation of the fusion protein to the secretory pathway, thus enhancing the potency of the vaccine. Patients were vaccinated with GX-188E DNA through intramuscular injection into the deltoid muscle followed by electroporation. Among the 9 vaccinated patients, only grade 1 or lower adverse events associated with vaccination were observed, and the patients recovered quickly from the adverse event. These AEs associated with vaccination include chills, injection site pain, swelling and hypoesthesia. Some other AEs, including headache, rhinitis and fatigue, could potentially be associated with vaccination. After vaccination, no anti-DNA antibodies were detected in patients. These results indicate that GX-188E is safe and well tolerated by human patients. Furthermore, statistically significant cellular immune responses were observed in all vaccinated patients. GX-188E vaccination also elicits a weak antibody response against E7 protein in 3 of the 9 vaccinated patients. At 8 weeks post last vaccination, complete lesion regression was observed in 6 of the 9 patients, and no lesion recurrence was observed in these responders throughout the remaining period of the trial, while 1 additional patient demonstrated complete lesion regression at 36 weeks post vaccination. These findings encourage further clinical studies of GX-188E.
A therapeutic HPV DNA vaccine, VGX-3100, is a mixture of two plasmids encoding optimized consensus of E6 and E7 antigen of HPV 16 and 18 [144,145]. The vaccine has been administered to eighteen female patients with previously treated CIN2/3 lesions via intramuscular injection into deltoid muscle followed by electroporation [146]. All eighteen patients received three rounds of vaccination and the vaccine was well tolerated with no dose-limiting toxicity observed. AEs that were related to vaccination includes injection site reaction, fever, pain during electroporation, tenderness. Furthermore, induction of HPV-specific CD8+ T cells with full cytolytic function was detected in 14 of the 18 (78%) patients. In addition, 17 of 18 (94%) patients demonstrated increased HPV16 E7 antibody titers, and increased HPV18 E7 antibody titers were observed in all 18 patients. Increase in antibody titers against HPV16 and 18 E6 were observed in 12 (67%) and 7 (39%) patients respectively. These results demonstrated the potential of the vaccine to induce robust antigen specific immune responses and contribute to elimination of HPV-infected cells and subsequent regression of lesion. The encouraging results in this phase I study prompted the initiation of phase IIb trial, further verifying the therapeutic potential of VGX-3100 DNA vaccine for the treatment of CIN2/3 lesions in a randomized, double-blind, placebo-controlled study [147]. 167 patients were enrolled in the phase IIb study, with 125 patients receiving VGX-3100 vaccine and 42 patients receiving placebo. The vaccine was administered intramuscularly into deltoid muscle followed by electroporation. Common AEs observed include injection site reactions, fatigues, headache, myalgia, nausea, and arthralgia. The major side effect experienced by VGX-3100 recipients but not by placebo recipients is erythema (78.4% of patients in VGX-3100 group and 57.1% of patients in placebo group, p = 0.007). 49.5% of VGX-3100 recipients had histopathological regression compared to 30.6% for placebo recipients (p = 0.034), based on the per-protocol analysis. Concomitant regression and viral clearance were observed in 39.5% of vaccinated patients compared to 15.4% of patients who received placebo. The study has also demonstrated that higher HPV-specific T cell and humoral responses were elicited in the vaccinated cohort than the placebo cohort, and correlated the observed immune responses to lesion regression and viral clearance.
Whole Cell-based vaccines
The therapeutic potential demonstrated by DC-based vaccines in the preclinical models prompted studies to further test their efficacy in human patients. A phase I, dose escalation trial has been conducted to evaluate the safety, toxicity, and immunogenicity of a DC-based vaccine in patients with stage Ib or IIa cervical cancer [148]. The autologous dendritic cells were obtained from the patients and pulsed with full length HPV16/18-E7 oncoprotein and keyhole limpet hemocyanin (KLH) before they were vaccinated back into the patient via subcutaneous injection to the inguinal ligament of the anterior mid-thigh. 14 patients enrolled in the study, with 10 of the 14 patients completed the DC vaccine protocol with a total of 5 vaccinations. Among the 10 patients, 1 has HPV 18+ squamous cell carcinoma, 7 with HPV 16+ squamous cell carcinoma, and 2 harboring HPV16+ adenocarcinoma. The study reported that the DC-based vaccine was safe and well tolerated by the patients, with only minor local reactions, including erythema, swelling, and pruritus, observed after vaccination. Increases in HPV-specific humoral response were observed in patients after vaccination. The study also reported an increase in E7-specific CD4+ T cell immune responses in patients after vaccination, but no statistically significant increase in CD8+ T cell responses were observed.
Another phase I clinical trial evaluated the toxicity and immunogenicity of a DC-based vaccine in patients with HPV+ advanced, recurrent cervical cancer [149]. Fourteen participating patients were separated into three treatment arms: saline only control, unprimed mature DC, and autologous tumor lysate primed mature DC. Dendritic cells were collected from the patient and pulsed with or without tumor lysate obtained from the same patient. Saline control, unprimed mature DC, or autologous tumor lysate primed mature DCs were administered to patients via intra-dermal injection. Only grade 0 and grade 1 toxicity were observed in three of the fourteen patients, which includes itching at vaccination site, fever, chills, abdominal discomfort, and vomiting, suggesting that the DC based vaccine was well tolerated. However, no statistically significant increase proliferation of lymphocytes were observed in patients of all treatment arm were observed. In addition, biopsy samples could not be obtained from all patients after vaccination for detailed evaluation. Even though one of the patients treated with autologous tumor lysate primed mature DC subsequently received cisplatin chemotherapy showed complete clinical response and remained disease free for more than 72 months, the sample size of the study was too small for definite conclusions.
In addition to the results from various clinical trials described above, Table 3 listed and summarized the currently ongoing therapeutic HPV vaccine clinical trials for different HPV-associated diseases.
Table 3.
Currently Ongoing Therapeutic HPV Vaccine Clinical Trials for Different HPV-Associated Diseases
| Vaccine | Antigen(s) | Construct | Organization | Trial Design | Estimated Date of Trial Completion | Clinical Trials.gov Identifier |
|---|---|---|---|---|---|---|
| Persistent HPV Infection and Low-Grade Squamous Intraepithelial Lesion | ||||||
| PDS0101 | HPV-16 E6/E7 | R-enantiomer of 1,2-dioleoyl-3-trimethylammonium-propane chloride + Peptides HPV-16 E6 and E7 | PDS Biotechnology Corp. | Phase I in female patients with high risk HPV infection or CIN1 (18 estimated patients) | Information not provided | NCT02065973 |
| ProCervix | HPV-16/18 E7 | 2 recombinant adenylate cyclase (CyaA) proteins: CyaA-HPV 16E7 & CyaA-HPV 18E7 | Genticel | Phase II in female patients with HPV16/18+ infection or ASCUS/LSIL (220 estimated patients) | December 2016 | NCT01957878 |
| Cervical Intraepithelial Neoplasia (CIN) / High-Grade Squamous Intraepithelial Lesion | ||||||
| GX-188E | HPV-16/18 E6/E7 | Plasmid encoding fusion protein of HPV 16/18 E6/E7 linked to Flt3L and tpa | Genexine, Inc | Phase II in HPV 16/18+ CIN2, CIN2/3, and CIN3 patients in Eastern Europe (120 estimated patients) | December 2017 | NCT02596243 |
| Phase II in HPV 16/18+ CIN3 patients in South Korea (72 estimated patients) | June 2016 | NCT02139267 | ||||
| pNGVL4a-CRT/E7(detox) | HPV-16 E7 | Plasmid encoding mutated form of HPV16-E7 linked to calreticulin | Sidney Kimmel Comprehensive Cancer Center | Phase I in patients with HPV16+ CIN2/3 (39 estimated patients) | December 2015 | NCT00988559 |
| pNGVL4a-sig/E7(detox)/HSP70 + TA-HPV | HPV-16/18 E6/E7 | Plasmid encoding mutated form of HPV16-E7 linked to sig and HSP70 and vaccinia virus with HPV16/18 E6/E7 | Phase I in patients with HPV16+ CIN3 in combination with topical imiquimod (48 estimated patients) | July 2016 | NCT00788164 | |
| TVGV-1 + GPI-0100 | HPV-16 E7 | Fusion protein of HPV-16 E7 and ER targeting sequence | THEVAX Genetics Vaccine Co. | Phase IIa in patients with HPV induced cervical HSIL (51 estimated patients) | June 2017 | NCT02576561 |
| Pepcan + Candin | HPV-16 E6 | HPV16 E6 peptides combined with Candida skin testing reagent candin. | University of Arkansas | Phase II in patients with cervical HSIL (125 estimated patients) | August 2020 | NCT02481414 |
| Anal Intraepithelial Neoplasia (AIN) | ||||||
| ISA101 (SLP-HPV-01; HPV16-SLP) | HPV-16 E6/E7 | Combination of nine HPV-16 E6 and four HPV-16 E7 synthetic peptides with incomplete Freund’s adjuvant | ISA Pharmaceuticals | Phase I/II in HIV+ male patients with HPV-16+ AIN2/3 (45 estimated patients) | February 2018 | NCT01923116 |
| HPV-Associated Incurable Solid Tumors | ||||||
| ISA101 (SLP-HPV-01; HPV16-SLP) | HPV-16 E6/E7 | Combination of nine HPV-16 E6 and four HPV-16 E7 synthetic peptides with incomplete Freund’s adjuvant | ISA Pharmaceuticals | Phase II in patients with HPV-16+ Incurable solid tumors (oropharyngeal squamous cell carcinoma, cervical, vulvar, vaginal, anal, and penile cancer) as combination therapy with Nivolumab (28 estimated patients) | December 2018 | NCT02426892 |
| Head and Neck Cancer | ||||||
| ADXS11-001 (Lm-LLo-E7) | HPV-16-E7 | prfA-defective Listeria monocytogenes strain transformed with plasmid encoding HPV-16 E7 antigen fused to a fragment of nonhemolytic listeriolysin O (LLO) | Advaxis, Inc. | Phase II in patients with HPV+ Oropharyngeal Squamous Cell Carcinoma before robot-assisted resection (30 estimated patients) | March 2017 | NCT02002182 |
| INO-3112 (VGX-3100 + INO-9012) | HPV-16/18 E6/E7 | Mixture of three plasmids encoding optimized consensus of E6 and E7 antigen of HPV 16 and 18 and proprietary immune activator expressing IL-12 | Inovio Pharmaceuticals | Phase I/IIA in patients with HPV associated head and neck squamous cell carcinoma (25 estimated patients) | December 2017 | NCT02163057 |
| Cervical Cancer | ||||||
| ADXS11-001 (Lm-LLo-E7) | HPV-16-E7 | prfA-defective Listeria monocytogenes strain transformed with plasmid encoding HPV-16 E7 antigen fused to a fragment of nonhemolytic listeriolysin O (LLO) | Advaxis, Inc. | Phase II in patients with persistent or recurrent squamous or non-squamous cell carcinoma of the cervix (67 estimated patients) | October 2018 | NCT01266460 |
| INO-3112 (VGX-3100 + INO-9012) | HPV-16/18 E6/E7 | Mixture of three plasmids encoding optimized consensus of E6 and E7 antigen of HPV 16 and 18 and proprietary immune activator expressing IL-12 | Inovio Pharmaceuticals | Phase I/IIA in female patients with new, recurrent, or persistent cervical cancer (30 estimated patients) | April 2019 | NCT02172911 |
| Phase II in patients with locally advanced cervical cancer as combination therapy with chemoradiation (126 estimated patients) | May 2021 | NCT02501278 | ||||
| ISA101 (SLP-HPV-01; HPV16-SLP) | HPV-16 E6/E7 | Combination of nine HPV-16 E6 and four HPV-16 E7 synthetic peptides with incomplete Freund’s adjuvant | ISA Pharmaceuticals | Phase I/II in female patients with HPV-16+ advanced or recurrent cervical cancer (48 estimated patients) | December 2016 | NCT02128126 |
| TA-CIN + GPI-0100 | HPV-16 E6/E7/L2 | HPV16 E6E7L2 fusion protein + GPI-0100 adjuvant | Sidney Kimmel Comprehensive Cancer Center | Phase I in patients with HPV16 associated cervical cancer (30 estimated patients) | May 2020 | NCT02405221 |
AGIN - Ano Genital Intraepithelial Neoplasia; AIN – Anal intraepithelial Neoplasia; ASCUC – atypical squamous cells of undetermined significance; CIN - Cervical intraepithelial neoplasia; ER – Endoplasmic reticulum; HIV – Human immunodeficiency virus; HPV – Human papillomavirus; HSIL - High-grade squamous intraepithelial lesion; LSIL – Low-grade squamous intraepithelial lesion
Expert Commentary & Five-Year View
The identification and characterization of high-risk HPV as a causative agent for many cases of cancer provides an important rationale for the development of therapeutic HPV vaccines. The ongoing developments in the field provide encouraging evidence for the possibility of the eventual eradication of HPV-related diseases and malignancies. In the development of therapeutic HPV vaccines, we discussed various approaches targeting the most relevant antigens associated with HPV associated malignancies, the HPV E6 and E7 oncoproteins, which represent tumor-specific antigens and ideal targets for therapeutic HPV vaccines. Based on our studies and studies from other peers in the field, we conclude that current therapeutic HPV vaccines, including live vector based, peptide/protein based, nucleic acid based, and whole cell based immunization, are each associated with advantages and limitations. Many current studies focus on implementing strategies such as incorporation of adjuvants, prime-boost regimens, and other combinations strategies to further enhance the T cell immune responses. Increasing understanding of the tumor microenvironment has led to the use of therapeutic vaccines with chemo-radiotherapy as well as molecules capable of blocking negative regulations and immune suppressions present in the tumor microenvironment to enhance the therapeutic effect of treatment strategies. In addition, understanding the importance of local immune responses will lead to future studies identifying the ideal vaccination sites.
Interestingly, several preclinical studies testing the efficacy of potential therapeutic HPV vaccine candidates have reported the ability of therapeutic HPV vaccines in generating protective effects and prevent the development of cancer in vaccinated mice. However, most of the currently designed therapeutic HPV vaccine candidates mainly aimed to target cancer causing HPV types (16 and 18). In addition, T cell mediated immune responses is more specific than antibody mediated immune responses, and thus therapeutic HPV vaccines may not protect patients from other HPV types that are included in the currently available prophylactic vaccines (HPV 6 and 11 etc.). Thus, in the present state, one should still consider using prophylactic vaccinse for age-specific preventive vaccination and administer therapeutic HPV vaccines to those with detected lesions and/or infections. In the future, with further understandings and improvements, therapeutic HPV vaccines may potentially be administered to healthy patients as a mean to prevent the establishment of potential HPV infections and cancers.
With continued effort in the development of therapeutic HPV vaccines and the knowledge accumulated from recent and previous studies, we can foresee the continued success of therapeutic HPV vaccines over the next five years. We anticipate that, in the near future, therapeutic HPV vaccines will become a clinically available option, alongside other therapies including surgery, chemotherapy, and radiation therapy, for the control of HPV-associated diseases.
Key-issues.
Human papillomavirus (HPV) the key etiological factor for cervical cancer.
The current commercial preventive HPV vaccines have been shown to be efficacious in generating capsid-specific neutralizing antibody responses to HPV-16 and HPV-18, the two most common types associated with cervical cancer.
There are no strong evidence to show that prophylactic vaccines have definite therapeutic effects against pre-existing HPV infections and related diseases. HPV-induced neoplasia is highly prevalent globally and therefore, continued development of therapeutic vaccines is critical.
Antigen-specific immunotherapy generated by therapeutic HPV vaccines has focused on enhancing T cell-mediated killing of HPV-transformed tumor cells, which constitutively express HPV-encoded proteins, E6 and E7.
Encouraging success in preclinical studies has been demonstrated by various types of therapeutic HPV vaccines.
Each type of therapeutic HPV vaccine has its limitations, and strategies have been designed to overcome these limitations to further enhance the efficacy of the vaccines.
Therapeutic HPV vaccines can be synergized with additional therapeutic strategies to generate more potent therapeutic effects.
Delivery site of therapeutic vaccines may be crucial for eliciting the desired immune responses, which warrants for further investigation.
Various therapeutic HPV vaccines targeting HPV E6 and E7 have been explored in clinical trials.
Acknowledgments
The authors thank Emily Robitschek for her input and critical review of the manuscript. This review is not intended to be an encyclopedic one, and the authors apologize to those not cited.
List of Abbreviations
- APCs
antigen presenting cells
- CIN
cervical intraepithelial neoplasia
- COX
cyclooxygenase
- CRT
calreticulin
- CTL
cytotoxic T lymphocyte
- DCs
dendritic cells
- ER
endoplasmic reticulum
- GMCSF
granulocyte macrophage colony stimulating factor
- HBsAg
hepatitis B virus surface antigen
- HDACi
histone deacetylase inhibitor
- HLA
human leukocyte antigen
- HPV
human papillomavirus
- IDLV
integrase defective lentiviral vector
- IFN
interferon
- IL
interleukin
- ISG
interferon-stimulating gene
- KLH
keyhole limpet hemocyanin
- KUN
flavivirus Kunjin
- LAMP
lysosomal-associated membrane protein
- LLO
listeriolysin O
- LSIL
low grade squamous intraepithelial lesion
- MDSCs
myeloid derived suppressor cells
- MHC
major histocompatibility complex
- MVA
modified vaccinia virus Ankara
- RM
recurrent / metastatic
- SCCHN
squamous cell carcinoma of the head and neck
- SFV
Semliki Forest virus
- tDLNS
tumor-draining lymph nodes
- TCR
T cell receptor
- TGF
tumor growth factor
- TLR
Toll-like receptor
- VIN
vulvar intraepithelial neoplasia
Footnotes
Financial and competing interests disclosure
This work was funded by the United States National Institutes of Health (NIH) Cervical Cancer Specialized Program of Research Excellence (SPORE) (P50 CA098252) and R01 grant (CA114425-01). T-C Wu is a founder of and has an equity ownership interest in Papivax LLC. Also, he own Papivax Biotech Inc. stock options and is a member of Papivax Biotech Inc.’s Scientific Advisory Board. Additionally, T-C Wu and C-F Hung are entitled to royalties on an invention described in this article. This arrangement has been reviewed and approved by the Johns Hopkins University in accordance with its conflict of interest policies. The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
References
Reference annotations
* Of interest
** Of considerable interest
- 1.Wakeham K, Kavanagh K. The burden of HPV-associated anogenital cancers. Current oncology reports. 2014;16(9):402. doi: 10.1007/s11912-014-0402-4. [DOI] [PubMed] [Google Scholar]
- 2.Forman D, de Martel C, Lacey CJ, et al. Global burden of human papillomavirus and related diseases. Vaccine. 2012;30(Suppl 5):F12–23. doi: 10.1016/j.vaccine.2012.07.055. [DOI] [PubMed] [Google Scholar]
- 3.Maxwell JH, Grandis JR, Ferris RL. HPV-Associated Head and Neck Cancer: Unique Features of Epidemiology and Clinical Management. Annual review of medicine. 2015 doi: 10.1146/annurev-med-051914-021907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mehanna H, Beech T, Nicholson T, et al. Prevalence of human papillomavirus in oropharyngeal and nonoropharyngeal head and neck cancer--systematic review and meta-analysis of trends by time and region. Head & neck. 2013;35(5):747–755. doi: 10.1002/hed.22015. [DOI] [PubMed] [Google Scholar]
- 5.Harper DM. Currently approved prophylactic HPV vaccines. Expert review of vaccines. 2009;8(12):1663–1679. doi: 10.1586/erv.09.123. [DOI] [PubMed] [Google Scholar]
- 6.Kash N, Lee MA, Kollipara R, Downing C, Guidry J, Tyring SK. Safety and Efficacy Data on Vaccines and Immunization to Human Papillomavirus. Journal of clinical medicine. 2015;4(4):614–633. doi: 10.3390/jcm4040614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Harper DM, Williams KB. Prophylactic HPV vaccines: current knowledge of impact on gynecologic premalignancies. Discov Med. 2010;10(50):7–17. [PubMed] [Google Scholar]
- 8.Ma B, Maraj B, Tran NP, et al. Emerging human papillomavirus vaccines. Expert opinion on emerging drugs. 2012;17(4):469–492. doi: 10.1517/14728214.2012.744393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ostor AG. Natural history of cervical intraepithelial neoplasia: a critical review. Int J Gynecol Pathol. 1993;12(2):186–192. [PubMed] [Google Scholar]
- 10.Ghittoni R, Accardi R, Chiocca S, Tommasino M. Role of human papillomaviruses in carcinogenesis. Ecancermedicalscience. 2015;9:526. doi: 10.3332/ecancer.2015.526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klaes R, Woerner SM, Ridder R, et al. Detection of high-risk cervical intraepithelial neoplasia and cervical cancer by amplification of transcripts derived from integrated papillomavirus oncogenes. Cancer research. 1999;59(24):6132–6136. [PubMed] [Google Scholar]
- 12.zur Hausen H. Papillomaviruses and cancer: from basic studies to clinical application. Nat Rev Cancer. 2002;2(5):342–350. doi: 10.1038/nrc798. [DOI] [PubMed] [Google Scholar]
- 13.Doorbar J. Model systems of human papillomavirus-associated disease. J Pathol. 2016;238(2):166–179. doi: 10.1002/path.4656. [DOI] [PubMed] [Google Scholar]
- 14.Arias-Pulido H, Peyton CL, Joste NE, Vargas H, Wheeler CM. Human papillomavirus type 16 integration in cervical carcinoma in situ and in invasive cervical cancer. J Clin Microbiol. 2006;44(5):1755–1762. doi: 10.1128/JCM.44.5.1755-1762.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Pett M, Coleman N. Integration of high-risk human papillomavirus: a key event in cervical carcinogenesis? J Pathol. 2007;212(4):356–367. doi: 10.1002/path.2192. [DOI] [PubMed] [Google Scholar]
- 16.Gray E, Pett MR, Ward D, et al. In vitro progression of human papillomavirus 16 episome-associated cervical neoplasia displays fundamental similarities to integrant-associated carcinogenesis. Cancer research. 2010;70(10):4081–4091. doi: 10.1158/0008-5472.CAN-09-3335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yao Y, Huang W, Yang X, et al. HPV-16 E6 and E7 protein T cell epitopes prediction analysis based on distributions of HLA-A loci across populations: an in silico approach. Vaccine. 2013;31(18):2289–2294. doi: 10.1016/j.vaccine.2013.02.065. [DOI] [PubMed] [Google Scholar]
- 18.Feltkamp MC, Smits HL, Vierboom MP, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23(9):2242–2249. doi: 10.1002/eji.1830230929. [DOI] [PubMed] [Google Scholar]
- 19.Riemer AB, Keskin DB, Zhang G, et al. A conserved E7-derived cytotoxic T lymphocyte epitope expressed on human papillomavirus 16-transformed HLA-A2+ epithelial cancers. J Biol Chem. 2010;285(38):29608–29622. doi: 10.1074/jbc.M110.126722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kumar A, Hussain S, Yadav IS, et al. Identification of human papillomavirus-16 E6 variation in cervical cancer and their impact on T and B cell epitopes. J Virol Methods. 2015;218:51–58. doi: 10.1016/j.jviromet.2015.03.008. [DOI] [PubMed] [Google Scholar]
- 21.Mahdavi A, Monk BJ. Vaccines against human papillomavirus and cervical cancer: promises and challenges. Oncologist. 2005;10(7):528–538. doi: 10.1634/theoncologist.10-7-528. [DOI] [PubMed] [Google Scholar]
- 22.Bergot AS, Kassianos A, Frazer IH, Mittal D. New Approaches to Immunotherapy for HPV Associated Cancers. Cancers (Basel) 2011;3(3):3461–3495. doi: 10.3390/cancers3033461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sewell DA, Pan ZK, Paterson Y. Listeria-based HPV-16 E7 vaccines limit autochthonous tumor growth in a transgenic mouse model for HPV-16 transformed tumors. Vaccine. 2008;26(41):5315–5320. doi: 10.1016/j.vaccine.2008.07.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bermudez-Humaran LG, Cortes-Perez NG, Le Loir Y, et al. An inducible surface presentation system improves cellular immunity against human papillomavirus type 16 E7 antigen in mice after nasal administration with recombinant lactococci. Journal of medical microbiology. 2004;53(Pt 5):427–433. doi: 10.1099/jmm.0.05472-0. [DOI] [PubMed] [Google Scholar]
- 25.Cortes-Perez NG, Azevedo V, Alcocer-Gonzalez JM, et al. Cell-surface display of E7 antigen from human papillomavirus type-16 in Lactococcus lactis and in Lactobacillus plantarum using a new cell-wall anchor from lactobacilli. Journal of drug targeting. 2005;13(2):89–98. doi: 10.1080/10611860400024219. [DOI] [PubMed] [Google Scholar]
- 26.Adachi K, Kawana K, Yokoyama T, et al. Oral immunization with a Lactobacillus casei vaccine expressing human papillomavirus (HPV) type 16 E7 is an effective strategy to induce mucosal cytotoxic lymphocytes against HPV16 E7. Vaccine. 2010;28(16):2810–2817. doi: 10.1016/j.vaccine.2010.02.005. [DOI] [PubMed] [Google Scholar]
- 27.Schnupf P, Portnoy DA. Listeriolysin O: a phagosome-specific lysin. Microbes and infection / Institut Pasteur. 2007;9(10):1176–1187. doi: 10.1016/j.micinf.2007.05.005. [DOI] [PubMed] [Google Scholar]
- 28.Peters C, Paterson Y. Enhancing the immunogenicity of bioengineered Listeria monocytogenes by passaging through live animal hosts. Vaccine. 2003;21(11–12):1187–1194. doi: 10.1016/s0264-410x(02)00554-6. [DOI] [PubMed] [Google Scholar]
- 29.Chen Z, Ozbun L, Chong N, Wallecha A, Berzofsky JA, Khleif SN. Episomal expression of truncated listeriolysin O in LmddA-LLO-E7 vaccine enhances antitumor efficacy by preferentially inducing expansions of CD4+FoxP3− and CD8+ T cells. Cancer immunology research. 2014;2(9):911–922. doi: 10.1158/2326-6066.CIR-13-0197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Darji A, Guzman CA, Gerstel B, et al. Oral somatic transgene vaccination using attenuated S. typhimurium. Cell. 1997;91(6):765–775. doi: 10.1016/s0092-8674(00)80465-1. [DOI] [PubMed] [Google Scholar]
- 31.Krul MR, Tijhaar EJ, Kleijne JA, et al. Induction of an antibody response in mice against human papillomavirus (HPV) type 16 after immunization with HPV recombinant Salmonella strains. Cancer immunology, immunotherapy : CII. 1996;43(1):44–48. doi: 10.1007/s002620050302. [DOI] [PubMed] [Google Scholar]
- 32.Gomez-Gutierrez JG, Elpek KG, Montes de Oca-Luna R, Shirwan H, Sam Zhou H, McMasters KM. Vaccination with an adenoviral vector expressing calreticulin-human papillomavirus 16 E7 fusion protein eradicates E7 expressing established tumors in mice. Cancer immunology, immunotherapy : CII. 2007;56(7):997–1007. doi: 10.1007/s00262-006-0247-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Liu DW, Tsao YP, Kung JT, et al. Recombinant adeno-associated virus expressing human papillomavirus type 16 E7 peptide DNA fused with heat shock protein DNA as a potential vaccine for cervical cancer. J Virol. 2000;74(6):2888–2894. doi: 10.1128/jvi.74.6.2888-2894.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cassetti MC, McElhiney SP, Shahabi V, et al. Antitumor efficacy of Venezuelan equine encephalitis virus replicon particles encoding mutated HPV16 E6 and E7 genes. Vaccine. 2004;22(3–4):520–527. doi: 10.1016/j.vaccine.2003.07.003. [DOI] [PubMed] [Google Scholar]
- 35.Hsieh CJ, Kim TW, Hung CF, et al. Enhancement of vaccinia vaccine potency by linkage of tumor antigen gene to gene encoding calreticulin. Vaccine. 2004;22(29–30):3993–4001. doi: 10.1016/j.vaccine.2004.03.057. [DOI] [PubMed] [Google Scholar]
- 36.Borysiewicz LK, Fiander A, Nimako M, et al. A recombinant vaccinia virus encoding human papillomavirus types 16 and 18, E6 and E7 proteins as immunotherapy for cervical cancer. Lancet. 1996;347(9014):1523–1527. doi: 10.1016/s0140-6736(96)90674-1. [DOI] [PubMed] [Google Scholar]
- 37.Lamikanra A, Pan ZK, Isaacs SN, Wu TC, Paterson Y. Regression of established human papillomavirus type 16 (HPV-16) immortalized tumors in vivo by vaccinia viruses expressing different forms of HPV-16 E7 correlates with enhanced CD8(+) T-cell responses that home to the tumor site. J Virol. 2001;75(20):9654–9664. doi: 10.1128/JVI.75.20.9654-9664.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zurkova K, Babiarova K, Hainz P, et al. The expression of the soluble isoform of hFlt3 ligand by recombinant vaccinia virus enhances immunogenicity of the vector. Oncology reports. 2009;21(5):1335–1343. doi: 10.3892/or_00000359. [DOI] [PubMed] [Google Scholar]
- 39.Baez-Astua A, Herraez-Hernandez E, Garbi N, et al. Low-dose adenovirus vaccine encoding chimeric hepatitis B virus surface antigen-human papillomavirus type 16 E7 proteins induces enhanced E7-specific antibody and cytotoxic T-cell responses. J Virol. 2005;79(20):12807–12817. doi: 10.1128/JVI.79.20.12807-12817.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Walczak M, Regts J, van Oosterhout AJ, et al. Role of regulatory T-cells in immunization strategies involving a recombinant alphavirus vector system. Antiviral therapy. 2011;16(2):207–218. doi: 10.3851/IMP1751. [DOI] [PubMed] [Google Scholar]
- 41.Riezebos-Brilman A, Regts J, Freyschmidt EJ, Dontje B, Wilschut J, Daemen T. Induction of human papilloma virus E6/E7-specific cytotoxic T-lymphocyte activity in immune-tolerant, E6/E7-transgenic mice. Gene therapy. 2005;12(18):1410–1414. doi: 10.1038/sj.gt.3302536. [DOI] [PubMed] [Google Scholar]
- 42.Riezebos-Brilman A, Regts J, Chen M, Wilschut J, Daemen T. Augmentation of alphavirus vector-induced human papilloma virus-specific immune and anti-tumour responses by co-expression of interleukin-12. Vaccine. 2009;27(5):701–707. doi: 10.1016/j.vaccine.2008.11.032. [DOI] [PubMed] [Google Scholar]
- 43.Ip PP, Boerma A, Walczak M, et al. Antigen design enhances the immunogenicity of Semliki Forest virus-based therapeutic human papillomavirus vaccines. Gene therapy. 2015;22(7):560–567. doi: 10.1038/gt.2015.24. [DOI] [PubMed] [Google Scholar]
- 44.Grasso F, Negri DR, Mochi S, et al. Successful therapeutic vaccination with integrase defective lentiviral vector expressing nononcogenic human papillomavirus E7 protein. International journal of cancer. Journal international du cancer. 2013;132(2):335–344. doi: 10.1002/ijc.27676. [DOI] [PubMed] [Google Scholar]
- 45.Chang CL, Ma B, Pang X, Wu TC, Hung CF. Treatment with cyclooxygenase-2 inhibitors enables repeated administration of vaccinia virus for control of ovarian cancer. Molecular therapy : the journal of the American Society of Gene Therapy. 2009;17(8):1365–1372. doi: 10.1038/mt.2009.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Ginaldi L, Loreto MF, Corsi MP, Modesti M, De Martinis M. Immunosenescence and infectious diseases. Microbes and infection / Institut Pasteur. 2001;3(10):851–857. doi: 10.1016/s1286-4579(01)01443-5. [DOI] [PubMed] [Google Scholar]
- 47.Lin K, Doolan K, Hung CF, Wu TC. Perspectives for preventive and therapeutic HPV vaccines. Journal of the Formosan Medical Association = Taiwan yi zhi. 2010;109(1):4–24. doi: 10.1016/s0929-6646(10)60017-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48*.Rosales R, Rosales C. Immune therapy for human papillomaviruses-related cancers. World journal of clinical oncology. 2014;5(5):1002–1019. doi: 10.5306/wjco.v5.i5.1002. A comprehensive review for various preventative and therapeutic strategies against HPV associated diseases. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Su JH, Wu A, Scotney E, et al. Immunotherapy for cervical cancer: Research status and clinical potential. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2010;24(2):109–129. doi: 10.2165/11532810-000000000-00000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hung CF, Ma B, Monie A, Tsen SW, Wu TC. Therapeutic human papillomavirus vaccines: current clinical trials and future directions. Expert opinion on biological therapy. 2008;8(4):421–439. doi: 10.1517/14712598.8.4.421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Kim JW, Hung CF, Juang J, et al. Comparison of HPV DNA vaccines employing intracellular targeting strategies. Gene therapy. 2004;11(12):1011–1018. doi: 10.1038/sj.gt.3302252. [DOI] [PubMed] [Google Scholar]
- 52.Fu TM, Ulmer JB, Caulfield MJ, et al. Priming of cytotoxic T lymphocytes by DNA vaccines: requirement for professional antigen presenting cells and evidence for antigen transfer from myocytes. Mol Med. 1997;3(6):362–371. [PMC free article] [PubMed] [Google Scholar]
- 53.Porgador A, Irvine KR, Iwasaki A, Barber BH, Restifo NP, Germain RN. Predominant role for directly transfected dendritic cells in antigen presentation to CD8+ T cells after gene gun immunization. The Journal of experimental medicine. 1998;188(6):1075–1082. doi: 10.1084/jem.188.6.1075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Chattergoon MA, Robinson TM, Boyer JD, Weiner DB. Specific immune induction following DNA-based immunization through in vivo transfection and activation of macrophages/antigen-presenting cells. J Immunol. 1998;160(12):5707–5718. [PubMed] [Google Scholar]
- 55.Dupuis M, Denis-Mize K, Woo C, et al. Distribution of DNA vaccines determines their immunogenicity after intramuscular injection in mice. J Immunol. 2000;165(5):2850–2858. doi: 10.4049/jimmunol.165.5.2850. [DOI] [PubMed] [Google Scholar]
- 56.Tsen SW, Paik AH, Hung CF, Wu TC. Enhancing DNA vaccine potency by modifying the properties of antigen-presenting cells. Expert review of vaccines. 2007;6(2):227–239. doi: 10.1586/14760584.6.2.227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Cheng WF, Hung CF, Hsu KF, et al. Cancer immunotherapy using Sindbis virus replicon particles encoding a VP22-antigen fusion. Human gene therapy. 2002;13(4):553–568. doi: 10.1089/10430340252809847. [DOI] [PubMed] [Google Scholar]
- 58.Berglund P, Quesada-Rolander M, Putkonen P, Biberfeld G, Thorstensson R, Liljestrom P. Outcome of immunization of cynomolgus monkeys with recombinant Semliki Forest virus encoding human immunodeficiency virus type 1 envelope protein and challenge with a high dose of SHIV-4 virus. AIDS research and human retroviruses. 1997;13(17):1487–1495. doi: 10.1089/aid.1997.13.1487. [DOI] [PubMed] [Google Scholar]
- 59.Hsu KF, Hung CF, Cheng WF, et al. Enhancement of suicidal DNA vaccine potency by linking Mycobacterium tuberculosis heat shock protein 70 to an antigen. Gene therapy. 2001;8(5):376–383. doi: 10.1038/sj.gt.3301408. [DOI] [PubMed] [Google Scholar]
- 60.Kim TW, Hung CF, Juang J, He L, Hardwick JM, Wu TC. Enhancement of suicidal DNA vaccine potency by delaying suicidal DNA-induced cell death. Gene therapy. 2004;11(3):336–342. doi: 10.1038/sj.gt.3302164. [DOI] [PubMed] [Google Scholar]
- 61.Varnavski AN, Young PR, Khromykh AA. Stable high-level expression of heterologous genes in vitro and in vivo by noncytopathic DNA-based Kunjin virus replicon vectors. J Virol. 2000;74(9):4394–4403. doi: 10.1128/jvi.74.9.4394-4403.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Herd KA, Harvey T, Khromykh AA, Tindle RW. Recombinant Kunjin virus replicon vaccines induce protective T-cell immunity against human papillomavirus 16 E7-expressing tumour. Virology. 2004;319(2):237–248. doi: 10.1016/j.virol.2003.10.032. [DOI] [PubMed] [Google Scholar]
- 63.Sebastian M, Papachristofilou A, Weiss C, et al. Phase Ib study evaluating a self-adjuvanted mRNA cancer vaccine (RNActive(R)) combined with local radiation as consolidation and maintenance treatment for patients with stage IV non-small cell lung cancer. BMC cancer. 2014;14:748. doi: 10.1186/1471-2407-14-748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mackova J, Kutinova L, Hainz P, et al. Adjuvant effect of dendritic cells transduced with recombinant vaccinia virus expressing HPV16-E7 is inhibited by co-expression of IL12. International journal of oncology. 2004;24(6):1581–1588. [PubMed] [Google Scholar]
- 65.Wang TL, Ling M, Shih IM, et al. Intramuscular administration of E7-transfected dendritic cells generates the most potent E7-specific anti-tumor immunity. Gene therapy. 2000;7(9):726–733. doi: 10.1038/sj.gt.3301160. [DOI] [PubMed] [Google Scholar]
- 66.Benencia F, Courreges MC, Coukos G. Whole tumor antigen vaccination using dendritic cells: comparison of RNA electroporation and pulsing with UV-irradiated tumor cells. Journal of translational medicine. 2008;6:21. doi: 10.1186/1479-5876-6-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Murakami M, Gurski KJ, Marincola FM, Ackland J, Steller MA. Induction of specific CD8+ T-lymphocyte responses using a human papillomavirus-16 E6/E7 fusion protein and autologous dendritic cells. Cancer research. 1999;59(6):1184–1187. [PubMed] [Google Scholar]
- 68.Peng S, Kim TW, Lee JH, et al. Vaccination with dendritic cells transfected with BAK and BAX siRNA enhances antigen-specific immune responses by prolonging dendritic cell life. Human gene therapy. 2005;16(5):584–593. doi: 10.1089/hum.2005.16.584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Kim JH, Kang TH, Noh KH, et al. Enhancement of dendritic cell-based vaccine potency by anti-apoptotic siRNAs targeting key pro-apoptotic proteins in cytotoxic CD8(+) T cell-mediated cell death. Immunology letters. 2009;122(1):58–67. doi: 10.1016/j.imlet.2008.12.006. [DOI] [PubMed] [Google Scholar]
- 70.Adams M, Navabi H, Jasani B, et al. Dendritic cell (DC) based therapy for cervical cancer: use of DC pulsed with tumour lysate and matured with a novel synthetic clinically non-toxic double stranded RNA analogue poly [I]:poly [C(12)U] (Ampligen R) Vaccine. 2003;21(7–8):787–790. doi: 10.1016/s0264-410x(02)00599-6. [DOI] [PubMed] [Google Scholar]
- 71.Santin AD, Bellone S, Roman JJ, Burnett A, Cannon MJ, Pecorelli S. Therapeutic vaccines for cervical cancer: dendritic cell-based immunotherapy. Curr Pharm Des. 2005;11(27):3485–3500. doi: 10.2174/138161205774414565. [DOI] [PubMed] [Google Scholar]
- 72.Ahn YH, Hong SO, Kim JH, et al. The siRNA cocktail targeting interleukin 10 receptor and transforming growth factor-beta receptor on dendritic cells potentiates tumour antigen-specific CD8(+) T cell immunity. Clinical and experimental immunology. 2015;181(1):164–178. doi: 10.1111/cei.12620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Mikyskova R, Indrova M, Simova J, et al. Treatment of minimal residual disease after surgery or chemotherapy in mice carrying HPV16-associated tumours: Cytokine and gene therapy with IL-2 and GM-CSF. International journal of oncology. 2004;24(1):161–167. [PubMed] [Google Scholar]
- 74.Chang EY, Chen CH, Ji H, et al. Antigen-specific cancer immunotherapy using a GM-CSF secreting allogeneic tumor cell-based vaccine. International journal of cancer. Journal international du cancer. 2000;86(5):725–730. doi: 10.1002/(sici)1097-0215(20000601)86:5<725::aid-ijc19>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 75.Vogel FR. Immunologic adjuvants for modern vaccine formulations. Ann N Y Acad Sci. 1995;754:153–160. doi: 10.1111/j.1749-6632.1995.tb44448.x. [DOI] [PubMed] [Google Scholar]
- 76*.Guimaraes LE, Baker B, Perricone C, Shoenfeld Y. Vaccines, adjuvants and autoimmunity. Pharmacological research. 2015;100:190–209. doi: 10.1016/j.phrs.2015.08.003. A detailed review discussing the mechanism, advantages, and draw backs of adjuvants. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.O’Hagan DT, Valiante NM. Recent advances in the discovery and delivery of vaccine adjuvants. Nature reviews. Drug discovery. 2003;2(9):727–735. doi: 10.1038/nrd1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Cheng WF, Hung CF, Chai CY, et al. Tumor-specific immunity and antiangiogenesis generated by a DNA vaccine encoding calreticulin linked to a tumor antigen. The Journal of clinical investigation. 2001;108(5):669–678. doi: 10.1172/JCI12346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Yang B, Yang A, Peng S, et al. Co-administration with DNA encoding papillomavirus capsid proteins enhances the antitumor effects generated by therapeutic HPV DNA vaccination. Cell Biosci. 2015;5:35. doi: 10.1186/s13578-015-0025-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Kim TW, Hung CF, Zheng M, et al. A DNA vaccine co-expressing antigen and an anti-apoptotic molecule further enhances the antigen-specific CD8+ T-cell immune response. Journal of biomedical science. 2004;11(4):493–499. doi: 10.1007/BF02256098. [DOI] [PubMed] [Google Scholar]
- 81.Villarreal DO, Wise MC, Siefert RJ, Yan J, Wood LM, Weiner DB. Ubiquitin-like Molecule ISG15 Acts as an Immune Adjuvant to Enhance Antigen-specific CD8 T-cell Tumor Immunity. Molecular therapy : the journal of the American Society of Gene Therapy. 2015 doi: 10.1038/mt.2015.120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Heidenreich R, Jasny E, Kowalczyk A, et al. A novel RNA-based adjuvant combines strong immunostimulatory capacities with a favorable safety profile. International journal of cancer. Journal international du cancer. 2015;137(2):372–384. doi: 10.1002/ijc.29402. [DOI] [PubMed] [Google Scholar]
- 83.Chen CH, Wang TL, Hung CF, Pardoll DM, Wu TC. Boosting with recombinant vaccinia increases HPV-16 E7-specific T cell precursor frequencies of HPV-16 E7-expressing DNA vaccines. Vaccine. 2000;18(19):2015–2022. doi: 10.1016/s0264-410x(99)00528-9. [DOI] [PubMed] [Google Scholar]
- 84.Wlazlo AP, Deng H, Giles-Davis W, Ertl HC. DNA vaccines against the human papillomavirus type 16 E6 or E7 oncoproteins. Cancer gene therapy. 2004;11(6):457–464. doi: 10.1038/sj.cgt.7700723. [DOI] [PubMed] [Google Scholar]
- 85.Rittich S, Duskova M, Mackova J, Pokorna D, Jinoch P, Smahel M. Combined immunization with DNA and transduced tumor cells expressing mouse GM-CSF or IL-2. Oncology reports. 2005;13(2):311–317. [PubMed] [Google Scholar]
- 86.Lin CT, Hung CF, Juang J, et al. Boosting with recombinant vaccinia increases HPV-16 E7-Specific T cell precursor frequencies and antitumor effects of HPV-16 E7-expressing Sindbis virus replicon particles. Molecular therapy : the journal of the American Society of Gene Therapy. 2003;8(4):559–566. doi: 10.1016/s1525-0016(03)00238-7. [DOI] [PubMed] [Google Scholar]
- 87.Mackova J, Stasikova J, Kutinova L, et al. Prime/boost immunotherapy of HPV16-induced tumors with E7 protein delivered by Bordetella adenylate cyclase and modified vaccinia virus Ankara. Cancer immunology, immunotherapy : CII. 2006;55(1):39–46. doi: 10.1007/s00262-005-0700-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Yue FY, Dummer R, Geertsen R, et al. Interleukin-10 is a growth factor for human melanoma cells and down-regulates HLA class-I, HLA class-II and ICAM-1 molecules. International journal of cancer. Journal international du cancer. 1997;71(4):630–637. doi: 10.1002/(sici)1097-0215(19970516)71:4<630::aid-ijc20>3.0.co;2-e. [DOI] [PubMed] [Google Scholar]
- 89.Gorelik L, Flavell RA. Immune-mediated eradication of tumors through the blockade of transforming growth factor-beta signaling in T cells. Nature medicine. 2001;7(10):1118–1122. doi: 10.1038/nm1001-1118. [DOI] [PubMed] [Google Scholar]
- 90.Chuang CM, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Enhancing therapeutic HPV DNA vaccine potency through depletion of CD4+CD25+ T regulatory cells. Vaccine. 2009;27(5):684–689. doi: 10.1016/j.vaccine.2008.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Rice AE, Latchman YE, Balint JP, Lee JH, Gabitzsch ES, Jones FR. An HPV-E6/E7 immunotherapy plus PD-1 checkpoint inhibition results in tumor regression and reduction in PD-L1 expression. Cancer gene therapy. 2015 doi: 10.1038/cgt.2015.40. [DOI] [PubMed] [Google Scholar]
- 92.Yu H, Kortylewski M, Pardoll D. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature reviews. Immunology. 2007;7(1):41–51. doi: 10.1038/nri1995. [DOI] [PubMed] [Google Scholar]
- 93.Munn DH, Mellor AL. IDO and tolerance to tumors. Trends in molecular medicine. 2004;10(1):15–18. doi: 10.1016/j.molmed.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 94.Rubinstein N, Alvarez M, Zwirner NW, et al. Targeted inhibition of galectin-1 gene expression in tumor cells results in heightened T cell-mediated rejection; A potential mechanism of tumor-immune privilege. Cancer cell. 2004;5(3):241–251. doi: 10.1016/s1535-6108(04)00024-8. [DOI] [PubMed] [Google Scholar]
- 95.Groh V, Wu J, Yee C, Spies T. Tumour-derived soluble MIC ligands impair expression of NKG2D and T-cell activation. Nature. 2002;419(6908):734–738. doi: 10.1038/nature01112. [DOI] [PubMed] [Google Scholar]
- 96.Kim R, Emi M, Tanabe K, Arihiro K. Tumor-driven evolution of immunosuppressive networks during malignant progression. Cancer research. 2006;66(11):5527–5536. doi: 10.1158/0008-5472.CAN-05-4128. [DOI] [PubMed] [Google Scholar]
- 97.Prasad KN, Kumar B, Yan XD, Hanson AJ, Cole WC. Alpha-tocopheryl succinate, the most effective form of vitamin E for adjuvant cancer treatment: a review. Journal of the American College of Nutrition. 2003;22(2):108–117. doi: 10.1080/07315724.2003.10719283. [DOI] [PubMed] [Google Scholar]
- 98.Kang TH, Knoff J, Yeh WH, et al. Treatment of tumors with vitamin E suppresses myeloid derived suppressor cells and enhances CD8+ T cell-mediated antitumor effects. PloS one. 2014;9(7):e103562. doi: 10.1371/journal.pone.0103562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Bartkowiak T, Singh S, Yang G, et al. Unique potential of 4-1BB agonist antibody to promote durable regression of HPV+ tumors when combined with an E6/E7 peptide vaccine. Proceedings of the National Academy of Sciences of the United States of America. 2015;112(38):E5290–5299. doi: 10.1073/pnas.1514418112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Lee SY, Huang Z, Kang TH, et al. Histone deacetylase inhibitor AR-42 enhances E7-specific CD8(+) T cell-mediated antitumor immunity induced by therapeutic HPV DNA vaccination. J Mol Med (Berl) 2013;91(10):1221–1231. doi: 10.1007/s00109-013-1054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101*.Soong RS, Song L, Trieu J, et al. Toll-like receptor agonist imiquimod facilitates antigen-specific CD8+ T-cell accumulation in the genital tract leading to tumor control through IFNgamma. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(21):5456–5467. doi: 10.1158/1078-0432.CCR-14-0344. Study demonstrated the immune recruitment effect of local adjuvant application. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Lee SJ, Song L, Yang MC, et al. Local administration of granulocyte macrophage colony-stimulating factor induces local accumulation of dendritic cells and antigen-specific CD8+ T cells and enhances dendritic cell cross-presentation. Vaccine. 2015;33(13):1549–1555. doi: 10.1016/j.vaccine.2015.02.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Lu D, Hoory T, Monie A, Wu A, Wang MC, Hung CF. Treatment with demethylating agent, 5-aza-2′-deoxycytidine enhances therapeutic HPV DNA vaccine potency. Vaccine. 2009;27(32):4363–4369. doi: 10.1016/j.vaccine.2009.02.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Kang TH, Lee JH, Song CK, et al. Epigallocatechin-3-gallate enhances CD8+ T cell-mediated antitumor immunity induced by DNA vaccination. Cancer research. 2007;67(2):802–811. doi: 10.1158/0008-5472.CAN-06-2638. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Bae SH, Park YJ, Park JB, Choi YS, Kim MS, Sin JI. Therapeutic synergy of human papillomavirus E7 subunit vaccines plus cisplatin in an animal tumor model: causal involvement of increased sensitivity of cisplatin-treated tumors to CTL-mediated killing in therapeutic synergy. Clinical cancer research : an official journal of the American Association for Cancer Research. 2007;13(1):341–349. doi: 10.1158/1078-0432.CCR-06-1838. [DOI] [PubMed] [Google Scholar]
- 106.Ye GW, Park JB, Park YJ, Choi YS, Sin JI. Increased sensitivity of radiated murine cervical cancer tumors to E7 subunit vaccine-driven CTL-mediated killing induces synergistic anti-tumor activity. Molecular therapy : the journal of the American Society of Gene Therapy. 2007;15(8):1564–1570. doi: 10.1038/sj.mt.6300149. [DOI] [PubMed] [Google Scholar]
- 107.Tseng CW, Monie A, Wu CY, et al. Treatment with proteasome inhibitor bortezomib enhances antigen-specific CD8+ T-cell-mediated antitumor immunity induced by DNA vaccination. J Mol Med (Berl) 2008;86(8):899–908. doi: 10.1007/s00109-008-0370-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Tseng CW, Trimble C, Zeng Q, et al. Low-dose radiation enhances therapeutic HPV DNA vaccination in tumor-bearing hosts. Cancer immunology, immunotherapy : CII. 2009;58(5):737–748. doi: 10.1007/s00262-008-0596-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Chuang CM, Monie A, Wu A, Hung CF. Combination of apigenin treatment with therapeutic HPV DNA vaccination generates enhanced therapeutic antitumor effects. Journal of biomedical science. 2009;16:49. doi: 10.1186/1423-0127-16-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Patel D, Shukla S, Gupta S. Apigenin and cancer chemoprevention: progress, potential and promise (review) International journal of oncology. 2007;30(1):233–245. [PubMed] [Google Scholar]
- 111.Tseng CW, Monie A, Trimble C, et al. Combination of treatment with death receptor 5-specific antibody with therapeutic HPV DNA vaccination generates enhanced therapeutic anti-tumor effects. Vaccine. 2008;26(34):4314–4319. doi: 10.1016/j.vaccine.2008.06.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112**.Wu CY, Yang LH, Yang HY, et al. Enhanced cancer radiotherapy through immunosuppressive stromal cell destruction in tumors. Clinical cancer research : an official journal of the American Association for Cancer Research. 2014;20(3):644–657. doi: 10.1158/1078-0432.CCR-13-1334. Study suggested that radiotherapy converts the radiated tumor site to favor the generation of immune responses elicited by therapeutic vaccines. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Peng S, Lyford-Pike S, Akpeng B, et al. Low-dose cyclophosphamide administered as daily or single dose enhances the antitumor effects of a therapeutic HPV vaccine. Cancer immunology, immunotherapy : CII. 2013;62(1):171–182. doi: 10.1007/s00262-012-1322-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Peng S, Wang JW, Karanam B, et al. Sequential cisplatin therapy and vaccination with HPV16 E6E7L2 fusion protein in saponin adjuvant GPI-0100 for the treatment of a model HPV16+ cancer. PloS one. 2015;10(1):e116389. doi: 10.1371/journal.pone.0116389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Lee SY, Kang TH, Knoff J, et al. Intratumoral injection of therapeutic HPV vaccinia vaccine following cisplatin enhances HPV-specific antitumor effects. Cancer immunology, immunotherapy : CII. 2013;62(7):1175–1185. doi: 10.1007/s00262-013-1421-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Trimble C, Lin CT, Hung CF, et al. Comparison of the CD8+ T cell responses and antitumor effects generated by DNA vaccine administered through gene gun, biojector, and syringe. Vaccine. 2003;21(25–26):4036–4042. doi: 10.1016/s0264-410x(03)00275-5. [DOI] [PubMed] [Google Scholar]
- 117.Babiuk LA, van Drunen Littel-van den H, Babiuk SL. Immunization of animals: from DNA to the dinner plate. Veterinary immunology and immunopathology. 1999;72(1–2):189–202. doi: 10.1016/s0165-2427(99)00132-4. [DOI] [PubMed] [Google Scholar]
- 118.Best SR, Peng S, Juang CM, et al. Administration of HPV DNA vaccine via electroporation elicits the strongest CD8+ T cell immune responses compared to intramuscular injection and intradermal gene gun delivery. Vaccine. 2009;27(40):5450–5459. doi: 10.1016/j.vaccine.2009.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Tsen SW, Wu CY, Meneshian A, Pai SI, Hung CF, Wu TC. Femtosecond laser treatment enhances DNA transfection efficiency in vivo. Journal of biomedical science. 2009;16:36. doi: 10.1186/1423-0127-16-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Klencke B, Matijevic M, Urban RG, et al. Encapsulated plasmid DNA treatment for human papillomavirus 16-associated anal dysplasia: a Phase I study of ZYC101. Clinical cancer research : an official journal of the American Association for Cancer Research. 2002;8(5):1028–1037. [PubMed] [Google Scholar]
- 121.Jeanbart L, Ballester M, de Titta A, et al. Enhancing efficacy of anticancer vaccines by targeted delivery to tumor-draining lymph nodes. Cancer immunology research. 2014;2(5):436–447. doi: 10.1158/2326-6066.CIR-14-0019-T. [DOI] [PubMed] [Google Scholar]
- 122**.Sun Y, Peng S, Qiu J, et al. Intravaginal HPV DNA vaccination with electroporation induces local CD8+ T-cell immune responses and antitumor effects against cervicovaginal tumors. Gene therapy. 2015;22(7):528–535. doi: 10.1038/gt.2015.17. Study demonstrated that local administration of the therapeutic vaccine lead to more potent local immune responses and antitumor effects. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Maciag PC, Radulovic S, Rothman J. The first clinical use of a live-attenuated Listeria monocytogenes vaccine: a Phase I safety study of Lm-LLO-E7 in patients with advanced carcinoma of the cervix. Vaccine. 2009;27(30):3975–3983. doi: 10.1016/j.vaccine.2009.04.041. [DOI] [PubMed] [Google Scholar]
- 124.Gunn GR, Zubair A, Peters C, Pan ZK, Wu TC, Paterson Y. Two Listeria monocytogenes vaccine vectors that express different molecular forms of human papilloma virus-16 (HPV-16) E7 induce qualitatively different T cell immunity that correlates with their ability to induce regression of established tumors immortalized by HPV-16. J Immunol. 2001;167(11):6471–6479. doi: 10.4049/jimmunol.167.11.6471. [DOI] [PubMed] [Google Scholar]
- 125.Kawana K, Adachi K, Kojima S, et al. Oral vaccination against HPV E7 for treatment of cervical intraepithelial neoplasia grade 3 (CIN3) elicits E7-specific mucosal immunity in the cervix of CIN3 patients. Vaccine. 2014;32(47):6233–6239. doi: 10.1016/j.vaccine.2014.09.020. [DOI] [PubMed] [Google Scholar]
- 126.Brun JL, Dalstein V, Leveque J, et al. Regression of high-grade cervical intraepithelial neoplasia with TG4001 targeted immunotherapy. American journal of obstetrics and gynecology. 2011;204(2):169e161–168. doi: 10.1016/j.ajog.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 127.Rosales C, Graham VV, Rosas GA, Merchant H, Rosales R. A recombinant vaccinia virus containing the papilloma E2 protein promotes tumor regression by stimulating macrophage antibody-dependent cytotoxicity. Cancer immunology, immunotherapy : CII. 2000;49(7):347–360. doi: 10.1007/s002620000125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Corona Gutierrez CM, Tinoco A, Navarro T, et al. Therapeutic vaccination with MVA E2 can eliminate precancerous lesions (CIN 1, CIN 2, and CIN 3) associated with infection by oncogenic human papillomavirus. Human gene therapy. 2004;15(5):421–431. doi: 10.1089/10430340460745757. [DOI] [PubMed] [Google Scholar]
- 129.Albarran YCA, de la Garza A, Cruz Quiroz BJ, et al. MVA E2 recombinant vaccine in the treatment of human papillomavirus infection in men presenting intraurethral flat condyloma: a phase I/II study. BioDrugs : clinical immunotherapeutics, biopharmaceuticals and gene therapy. 2007;21(1):47–59. doi: 10.2165/00063030-200721010-00006. [DOI] [PubMed] [Google Scholar]
- 130**.Rosales R, Lopez-Contreras M, Rosales C, et al. Regression of human papillomavirus intraepithelial lesions is induced by MVA E2 therapeutic vaccine. Human gene therapy. 2014;25(12):1035–1049. doi: 10.1089/hum.2014.024. A phase III clinical trial to test the efficacy of therapeutic HPV vaccines in high grade CIN patients. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Kenter GG, Welters MJ, Valentijn AR, et al. Vaccination against HPV-16 oncoproteins for vulvar intraepithelial neoplasia. The New England journal of medicine. 2009;361(19):1838–1847. doi: 10.1056/NEJMoa0810097. [DOI] [PubMed] [Google Scholar]
- 132.de Vos van Steenwijk PJ, Ramwadhdoebe TH, Lowik MJ, et al. A placebo-controlled randomized HPV16 synthetic long-peptide vaccination study in women with high-grade cervical squamous intraepithelial lesions. Cancer immunology, immunotherapy : CII. 2012;61(9):1485–1492. doi: 10.1007/s00262-012-1292-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.van Poelgeest MI, Welters MJ, van Esch EM, et al. HPV16 synthetic long peptide (HPV16-SLP) vaccination therapy of patients with advanced or recurrent HPV16-induced gynecological carcinoma, a phase II trial. Journal of translational medicine. 2013;11:88. doi: 10.1186/1479-5876-11-88. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Zwaveling S, Ferreira Mota SC, Nouta J, et al. Established human papillomavirus type 16-expressing tumors are effectively eradicated following vaccination with long peptides. J Immunol. 2002;169(1):350–358. doi: 10.4049/jimmunol.169.1.350. [DOI] [PubMed] [Google Scholar]
- 135.de Vos van Steenwijk PJ, van Poelgeest MI, Ramwadhdoebe TH, et al. The long-term immune response after HPV16 peptide vaccination in women with low-grade pre-malignant disorders of the uterine cervix: a placebo-controlled phase II study. Cancer immunology, immunotherapy : CII. 2014;63(2):147–160. doi: 10.1007/s00262-013-1499-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Zandberg DP, Rollins S, Goloubeva O, et al. A phase I dose escalation trial of MAGE-A3- and HPV16-specific peptide immunomodulatory vaccines in patients with recurrent/metastatic (RM) squamous cell carcinoma of the head and neck (SCCHN) Cancer immunology, immunotherapy : CII. 2015;64(3):367–379. doi: 10.1007/s00262-014-1640-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.van der Burg SH, Kwappenberg KM, O’Neill T, et al. Pre-clinical safety and efficacy of TA-CIN, a recombinant HPV16 L2E6E7 fusion protein vaccine, in homologous and heterologous prime-boost regimens. Vaccine. 2001;19(27):3652–3660. doi: 10.1016/s0264-410x(01)00086-x. [DOI] [PubMed] [Google Scholar]
- 138.de Jong A, O’Neill T, Khan AY, et al. Enhancement of human papillomavirus (HPV) type 16 E6 and E7-specific T-cell immunity in healthy volunteers through vaccination with TA-CIN, an HPV16 L2E7E6 fusion protein vaccine. Vaccine. 2002;20(29–30):3456–3464. doi: 10.1016/s0264-410x(02)00350-x. [DOI] [PubMed] [Google Scholar]
- 139.Davidson EJ, Faulkner RL, Sehr P, et al. Effect of TA-CIN (HPV 16 L2E6E7) booster immunisation in vulval intraepithelial neoplasia patients previously vaccinated with TA-HPV (vaccinia virus encoding HPV 16/18 E6E7) Vaccine. 2004;22(21–22):2722–2729. doi: 10.1016/j.vaccine.2004.01.049. [DOI] [PubMed] [Google Scholar]
- 140.Smyth LJ, Van Poelgeest MI, Davidson EJ, et al. Immunological responses in women with human papillomavirus type 16 (HPV-16)-associated anogenital intraepithelial neoplasia induced by heterologous prime-boost HPV-16 oncogene vaccination. Clinical cancer research : an official journal of the American Association for Cancer Research. 2004;10(9):2954–2961. doi: 10.1158/1078-0432.ccr-03-0703. [DOI] [PubMed] [Google Scholar]
- 141.Daayana S, Elkord E, Winters U, et al. Phase II trial of imiquimod and HPV therapeutic vaccination in patients with vulval intraepithelial neoplasia. British journal of cancer. 2010;102(7):1129–1136. doi: 10.1038/sj.bjc.6605611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Maldonado L, Teague JE, Morrow MP, et al. Intramuscular therapeutic vaccination targeting HPV16 induces T cell responses that localize in mucosal lesions. Science translational medicine. 2014;6(221):221ra213. doi: 10.1126/scitranslmed.3007323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim TJ, Jin HT, Hur SY, et al. Clearance of persistent HPV infection and cervical lesion by therapeutic DNA vaccine in CIN3 patients. Nat Commun. 2014;5:5317. doi: 10.1038/ncomms6317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Yan J, Harris K, Khan AS, Draghia-Akli R, Sewell D, Weiner DB. Cellular immunity induced by a novel HPV18 DNA vaccine encoding an E6/E7 fusion consensus protein in mice and rhesus macaques. Vaccine. 2008;26(40):5210–5215. doi: 10.1016/j.vaccine.2008.03.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Yan J, Reichenbach DK, Corbitt N, et al. Induction of antitumor immunity in vivo following delivery of a novel HPV-16 DNA vaccine encoding an E6/E7 fusion antigen. Vaccine. 2009;27(3):431–440. doi: 10.1016/j.vaccine.2008.10.078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Bagarazzi ML, Yan J, Morrow MP, et al. Immunotherapy against HPV16/18 generates potent TH1 and cytotoxic cellular immune responses. Science translational medicine. 2012;4(155):155ra138. doi: 10.1126/scitranslmed.3004414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 147.Trimble CL, Morrow MP, Kraynyak KA, et al. Safety, efficacy, and immunogenicity of VGX-3100, a therapeutic synthetic DNA vaccine targeting human papillomavirus 16 and 18 E6 and E7 proteins for cervical intraepithelial neoplasia 2/3: a randomised, double-blind, placebo-controlled phase 2b trial. Lancet. 2015 doi: 10.1016/S0140-6736(15)00239-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Santin AD, Bellone S, Palmieri M, et al. Human papillomavirus type 16 and 18 E7-pulsed dendritic cell vaccination of stage IB or IIA cervical cancer patients: a phase I escalating-dose trial. J Virol. 2008;82(4):1968–1979. doi: 10.1128/JVI.02343-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Ramanathan P, Ganeshrajah S, Raghanvan RK, Singh SS, Thangarajan R. Development and clinical evaluation of dendritic cell vaccines for HPV related cervical cancer--a feasibility study. Asian Pacific journal of cancer prevention : APJCP. 2014;15(14):5909–5916. doi: 10.7314/apjcp.2014.15.14.5909. [DOI] [PubMed] [Google Scholar]