Abstract
Previously-asymptomatic intracranial aneurysm rupture during cerebral angiography is an extremely rare phenomenon. There is a small risk of rupture of these aneurysms during digital subtraction angiography (DSA), but no case of spontaneous rupture during computed tomographic angiography (CTA) has been reported. A concise case of a 40-year-old woman referred for workup of an asymptomatic intracranial aneurysm who experienced acute rupture at the time of identification of a second aneurysm is described. This case highlights the potential for rupture at the time of scan acquisition and the importance of maintaining a high level of suspicion in the setting of neurological deterioration, as well as the potential need by all medical imaging professionals to identify an emergency and activate an urgent response.
Keywords: Intracranial aneurysm, computed tomographic angiography, non-invasive imaging, rupture, critical care
Introduction
Rupture of a previously-asymptomatic intracranial aneurysm rupture is occasionally encountered during endovascular coiling, but is not considered a significant risk during invasive angiography and is seen rarely.1 Acute rupture at the exact time of computed tomographic angiography (CTA) has not previously been reported, and intravenous contrast injection is not felt to be a risk factor for aneurysm rupture. Typically, CTA acquisition is not supervised by a physician, and identification/reporting of acute phenomena by a radiographer is not currently standardized. We describe a case of rupture at the time of CTA, after which the patient experienced decompensation in an unsupervised waiting area.
Case report
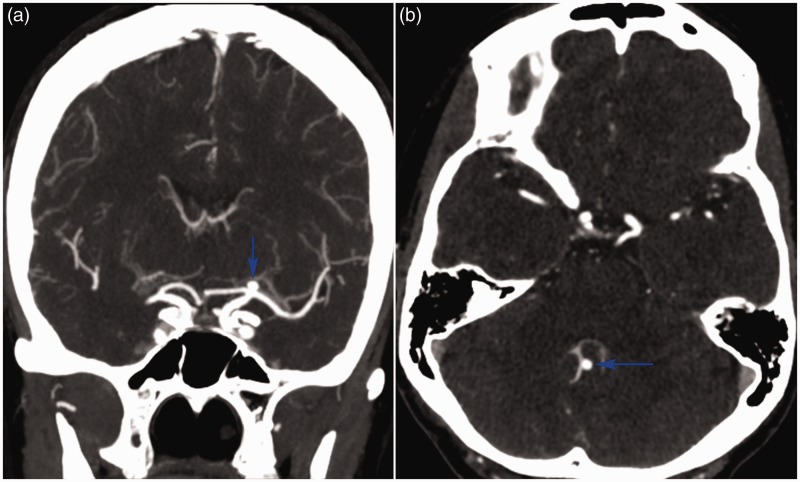
An asymptomatic 40-year-old woman was referred for evaluation of a left-sided unruptured carotid bifurcation aneurysm (Figure 1(a)) identified on workup for headaches. Upon returning from the CTA suite, she became progressively confused, and was immediately transferred to the emergency department. Rapid neurological deterioration ensued, as her Glasgow coma scale dropped to 3 and her pupils became unreactive at 6 mm. The question of contrast allergy was initially raised. CTA revealed a previously-undetected posterior inferior cerebellar artery (PICA) aneurysm (Figure 1(b)) which demonstrated active contrast extravasation (Figure 2(a)). She was urgently sedated, intubated, and infused with mannitol. A repeat unenhanced head CT (Figure 2(b)) showed a Fisher grade 4 subarachnoid haemorrhage with intraventricular extension and hydrocephalus, characteristic of ruptured PICA aneurysm. Bilateral external ventricular drains were placed, and a suboccipital craniectomy was performed expeditiously with successful haematoma evacuation and aneurysm clipping. She was transferred in stable condition to the neuro-intensive care unit, but unfortunately succumbed to vasospasm 10 days later.
Figure 1.
Coronal (a) and axial (b) CTAs showing unruptured aneurysms (blue arrows) at the left carotid bifurcation and the right PICA’s telovelotonsillar segment, respectively.
Figure 2.
Sagittal CTA (a) showing contrast extravasation (red arrow) into the fourth ventricle and the cisterna magna of the subarachnoid space. Unenhanced head CT (b) showing severe subarachnoid haemorrhage with intraventricular extension and resultant hydrocephalus.
Discussion
Currently, CTA is the preferred vascular neuroimaging modality in the initial evaluation of intracranial aneurysm.2 Approximately 20% of intracranial aneurysms are multiple. Therefore, all patients with a known aneurysm should be comprehensively evaluated for co-existing lesions. Had the additional aneurysm been noticed initially, her annual risk of rupture would have been determined to be higher (0.5% per additional aneurysm/year). It is unclear whether she would have been triaged higher in most centres for this, and debate is still ongoing as to whether patients with multiple unruptured aneurysms should be treated. While re-bleeding from ruptured aneurysms has been documented during CTA,3,4 the discovery of a new aneurysm as the result of its acute rupture during non-invasive imaging is an unusual presentation of a common event that has not been reported previously. Sudden neurological deterioration in a patient with a known aneurysm should be considered subarachnoid haemorrhage until proven otherwise.
Catheter angiography represents the current gold standard for the detection and characterization of intracranial aneurysms.2 20% of aneurysms are multiple. For each additional aneurysm a patient has, the calculated risk of rupture increases by 0.5%/aneurysm/year. In patients who have active extravasation at the time of imaging, those who present with poor neurological status fare worse (regardless of treatment) than those who are initially well and deteriorate.3 Aneurysm rupture has been associated occasionally with catheter angiography, presumably due to forceful injection.5,6 With the slow administration through a peripheral IV, it is felt that CTA does not increase the risk of rupture. Thus, the event itself may well be attributed to stunning coincidence.
Several known disorders are associated with increased rupture risk, including polycystic kidney disease, Marfan, Ehlers–Danlos, and Loeys–Dietz syndromes. In addition to number of aneurysms as aforementioned, aneurysm size can predict rupture and is an important aspect of the PHASES score. Significant differences were noted between aneurysms of <7.0, 7.0–9.9, 10–19.9, and ≥20 mm in size.7
This is the only case that has ever been observed in the careers of our staff of at our high-volume aneurysm centre. It is difficult to speculate, but the likelihood of this occurrence is certainly extraordinarily small. Nevertheless, high clinical suspicion is paramount for a fast, stepwise, and effective therapeutic response. The patient was unsupervised and it was only by chance that her deterioration was necessarily witnessed. In collaboration with out CT technologists, we held instituted in-services during which our staff were educated about ominous imaging findings and encouraged to emergently call a radiologist to confirm these. We propose that centres performing neuroimaging should at the very least be prepared to both supervise and initially manage patients who deteriorate. In addition, while many radiographers will inform an attending radiologist or another physician when an imaging finding appears concerning, standardization in the recognition and reporting of some acute pathological entities may improve detection and response in medical imaging departments.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Stockinger Z. Images in clinical medicine. Rupturing aneurysm of the posterior inferior cerebellar artery. N Engl J Med 1998; 339: 1758. [DOI] [PubMed] [Google Scholar]
- 2.Menke J, Larsen J, Kallenberg K. Diagnosing cerebral aneurysms by computed tomographic angiography: meta-analysis. Ann Neurol 2011; 69: 646–654. [DOI] [PubMed] [Google Scholar]
- 3.Kim E. Rupturing anterior communicating artery aneurysm during computed tomography angiography: three-dimensional visualization of bleeding into the septum pellucidum and the lateral ventricle. J Korean Neurosurg Soc 2014; 55: 357–361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Tsuang FY, Su IC, Chen JY, et al. Hyperacute cerebral aneurysm rerupture during CT angiography. J Neurosurg 2012; 116: 1244–1250. [DOI] [PubMed] [Google Scholar]
- 5.Sorimachi T, Takeuchi S, Koike T, et al. Intra-aneurysmal pressure changes during angiography in coil embolization. Surg Neurol 1997; 48: 451–457. [DOI] [PubMed] [Google Scholar]
- 6.Saitoh H, Hayakawa K, Nishimura K, et al. Intracarotid blood pressure changes during contrast medium injection. Am J Neuroradiol 1996; 17: 51–54. [PMC free article] [PubMed] [Google Scholar]
- 7.Backes D, Vergouwen MD, Tiel Groenestege AT, et al. PHASES score for prediction of intracranial aneurysm growth. Stroke 2015; 46: 1221–1226. [DOI] [PubMed] [Google Scholar]