Abstract
Objectives
The purpose of our study was to evaluate whether peritumoural perfusion weighted and proton spectroscopic magnetic resonance imaging can be used in differentiating between primary gliomas and solitary metastases.
Methods
Ten low-grade gliomas, eight high-grade gliomas and 10 metastases were prospectively evaluated with magnetic resonance imaging, dynamic susceptibility contrast enhanced perfusion imaging and single-voxel proton magnetic resonance spectroscopy before surgical resection or stereotactic biopsy. Maximal relative cerebral blood volume values were calculated drawing three regions of interest of 2 cm2 in the non-enhancing peritumoural areas. Maximal relative cerebral blood volume values were normalised to that of contralateral normal-appearing white matter. Maximal choline/creatine ratios were calculated from three voxels of 10 cm3 placed in the peritumoural areas defined as non-enhancing peritumoural white matter surrounding the tumour. The tumour grade presumed with these values was compared to histopathological grading. Differences in the study parameters between groups were assessed using the Mann–Whitney test. A receiver operating characteristic analysis was performed to determine cut-off values.
Results
A clear relative cerebral blood volume cut-off value of 1.88 was detected for differentiating low-grade gliomas from high-grade gliomas. A clear relative cerebral blood volume cut-off value of 1.20 was detected for differentiation of metastases from gliomas. The differences in the choline/creatine ratios in the peritumoural regions of high-grade gliomas and of solitary metastasis were statistically significant (P < 0.001) but a clear cut-off value was not found.
Conclusion
Our preliminary data support the hypothesis that peritumoural perfusion-weighted imaging can assist in preoperative differentiation between a glioma and a solitary metastasis.
Keywords: Brain glioma, brain metastases, perfusion weighted magnetic resonance imaging, magnetic resonance spectroscopy
Introduction
Primary gliomas and intracranial metastases are difficult to differentiate based on standard magnetic resonance imaging (MRI) alone. The ability to discern the two is often more difficult when the lesion is solitary.1,2
In the setting of a solitary mass in a patient with a known primary, rapid and accurate diagnosis of the lesion as either a solitary metastasis or a primary central nervous system tumour is fundamental, as it will lead to early treatment or intervention with potential positive impact on patient outcome.
Magnetic resonance perfusion-weighted imaging (PWI) and magnetic resonance spectroscopy (MRS) have been utilised to differentiate between a cerebral metastasis and a primary glioma. Dynamic susceptibility contrast PWI uses a T2*-weighted sequence during the first pass bolus of a paramagnetic contrast agent. In the evaluation of brain tumours, relative cerebral blood volume (rCBV) appears to be a highly useful perfusion parameter that reflects the underlying microvasculature and angiogenesis.1–8 MRS is also performed after paramagnetic contrast administration to evaluate metabolites within the area of the voxel. MRS has been demonstrated to be a useful tool in glioma grading, primarily through differences in choline metabolite ratios, which reflect the cellular density and rate of cellular membrane turnover.9,10
Several investigators have focused their studies on analysing the intratumoural perfusion pattern and spectroscopic pattern in attempting to differentiate between the two entities.1,11,12 There has been increasing research regarding the peritumoural differences among brain tumours over the past several years.1,3,13–17
Peritumoural oedema of metastases is purely vasogenic, and infiltrating tumour cells are not present outside its perivascular spaces; hence, the perilesional oedematous tissue requires fewer feeding blood vessels and is expected to have lower blood perfusion. Peritumoural oedema of high-grade gliomas is a mixture of vasogenic oedema and infiltration of neoplastic cells along the perivascular spaces associated with feeding blood vessels, and it is thus expected to have increased blood perfusion.1,3,16,17
This histopathological feature of primary tumours is not commonly seen with metastases and has led to speculation that the perilesional oedema can be studied with the aforementioned techniques to differentiate the perilesional white matter infiltration of the gliomas from the pure vasogenic oedema of the metastatic tumour.1,3,16,17
The utility of perfusion imaging in the peritumoural white matter in glioma grading has previously been evaluated. In their study, Law et al.3 demonstrated a higher rCBV in the peritumoural region of high-grade gliomas as compared to rCBV values in perilesional white matter in low-grade gliomas. Previous studies have also demonstrated an increased rCBV in the peritumoural region of a solitary glioma versus a metastasis.1,17 The use of MRS in differentiating gliomas from metastases has also been investigated by assessing peritumoural white matter and distinguishing the two pathological entities on the basis of higher choline levels present in the peritumoural regions of gliomas versus lower levels found in peritumoural white matter of metastatic lesions.1 The purpose of this study was to validate further the utility of PWI and MRS in the peritumoural regions of solitary tumours and assess whether these techniques can be used to differentiate gliomas from solitary metastases and, in the case of gliomas, establish if these techniques can distinguish between high and low-grade tumours.
Materials and methods
Patients
This study was approved by the institutional review board, with all patients providing written informed consent. All patients presented with a new diagnosis of a solitary brain mass in a single institution and had a histologically proved diagnosis.
There was a total of 28 patients (16 men, 12 women; mean age 56.5 years).
The malignancy of the glioma was graded as either high (grades III and IV) or low grade (grades I and II) according to the World Health Organization system.
Lesion diagnosis was proved for all patients by surgery or stereotaxic biopsy after MRI examination.
There were 10 low-grade gliomas (seven oligodendroglioma, three astrocytoma), eight high-grade gliomas (five anaplastic astrocytoma, one anaplastic oligodendroglioma and two glioblastoma multiforme), and 10 metastases (five breast carcinoma, three non-small cell lung carcinoma, two renal cell carcinoma).
Magnetic resonance imaging
All magnetic resonance examinations were performed prospectively from April 2014 to December 2014 using a 1.5 T unit (GE Signa Excite; General Electric Medical System, Milwaukee, USA).
Imaging protocol included axial and sagittal T2-weighted (5400/99 (TR/TE)) images, axial fluid-attenuated inversion recovery (FLAIR, 8000/120/2000 (TR/TE/TI)) images, along with axial, sagittal and coronal non-enhanced and contrast-enhanced T1-weighted (600/14 (TR/TE)) images.
Perfusion-weighted MRI was performed with a first-pass contrast-enhanced T2*-weighted single shot gradient-echo echo-planar (EPI) sequence. The parameters of the sequence were: TR/TE 1500 ms/54 ms, flip angle 90°, bandwidth 63.8 kHz, field of view 23 × 23 cm, matrix 126 × 128, number of excitations one, slice thickness 5 mm and section gap 1 mm, acquisition time 2.09 minutes.
We used 10 sections covering both the upper and the lower margins of the lesion observed in T2-weighted images. A series of images (10 sections, 40 images/section) were obtained at intervals of nearly 2 seconds before, during and after the administration of paramagnetic contrast agent. During the first 10 seconds before contrast agent administration, images were acquired to obtain baseline images. After the first 10 seconds a bolus of 0.2 mmol/kg of gadopentetate dimeglumine was injected through an 18 or 20 G intravenous line using an automatic injector (Medrad Inc., Warrendale, PA, USA) at 3 ml/s, followed by saline flush of an equal amount. We used this technique, as previously described by other authors.12,18,19
Each patient underwent single voxel water-suppressed point-resolved spectroscopy sequences (PRESS) TR/TE 1500 ms/35 ms, bandwidth 63.8 kHz, number of excitations eight, field of view 24 cm, acquisition time 3 minutes. The spectral data were obtained from an 8 mL voxel (2 × 2 × 2 cm3).
Automated optimisation of gradient shimming, transmitter pulse power and water suppression was used. All spectroscopic data were obtained after gadopentetate dimeglumine administration.
Post-processing steps including frequency shift, baseline correction, phase correction and peak fitting/analysis were performed first automatically and then manually if necessary using the software package provided by the manufacturer (PROBE-FuncTool 2; General Electric Medical System, Milwaukee, USA).
The spectra were automatically analysed for the relative signal intensities (areas under the fitted peaks in the time domain) of the following metabolites: choline-containing compounds (Cho), the creatine–phosphocreatine complex (Cr), and N-acetyl-aspartate (NAA). Lactate assignments were obtained observing the characteristic doublet caused by the inversion of the peak due to the J modulation; as PROBE/SVQ software does not report the lactate/Cr ratio, lactate was scored as: 0, absence; 1, trace; and 2, evidence. The same scores were assigned to the lipid parameter on the basis of the observation of the spectrum of each mobile lipid.
All spectral analyses were performed in a window from 0.50 to 4.30 ppm (using the standard method of assigning a shift value of 4.7 ppm to the measured unsuppressed water peak).
As previous authors20–26 have successfully evaluated Cho/Cr ratios of glial tumours and have found a direct correlation between such ratio and tumour aggressiveness, with higher grade tumours demonstrating higher Cho/Cr ratios, and other authors1 have also found the usefulness of Cho/Cr ratios in differentiating gliomas from metastases, we evaluated the ratios of Cho/Cr to establish the glioma grade and differentiate glioma from metastases.
Image postprocessing
EPI perfusion MRI was processed to obtain perfusion maps on an independent workstation using the software FuncTool 2 (General Electric Medical System, Milwaukee, USA). The precise algorithms for calculating rCBV have previously been described.15
Image analysis
The images were analysed in consensus by two neuroradiologists, each with at least 10 years experience and both blind to the histopathological analysis of the lesions.
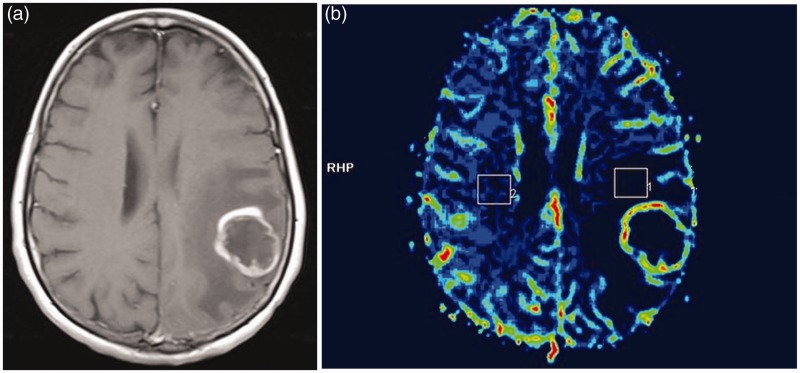
Maximal rCBV values from peritumoural areas were calculated using the mean rCBV values measured by drawing three regions of interest of 2 cm2 in the non-enhancing peritumoural white matter in a single axial T1-weighted image. Co-registration of the T1-weighted image with the corresponding rCBV map image was provided automatically by the software FuncTool 2. The maximal rCBV value was normalised to that of the contralateral normal-appearing white matter (Figure 1).
Figure 1.
A 44-year-old woman with a pathologically proved glioblastoma. (a) Contrast-enhanced axial T1-weighted image (600/14) demonstrating a peripherally enhancing lesion in the left parietal lobe. (b) Cerebral blood volume map with regions of interest of 2 cm2 drawn in the peritumoural white matter of the left parietal lobe and in the contralateral normal-appearing white matter.
The maximum values of Cho/Cr ratios were obtained from three different voxels placed in the non-enhancing peritumoural white matter, avoiding cystic or necrotic regions in an attempt to minimise the contribution of the partial volume effect, and from three different voxels placed in the contralateral normal-appearing white matter. The maximal Cho/Cr ratio was normalised to that of contralateral normal-appearing white matter. This method was used, as already used in other studies.8 MRS analysis was performed without knowledge of the final histological tumour diagnosis.
Tumour grade presumed on the rCBV values and Cho/Cr ratios from the peritumoural areas was compared to the histopathological grade.
Statistical analysis
Because of the small size of the sample and lack of normal distribution of data, we used a non-parametric test. The Mann–Whitney test was used for assessing statistical differences in the study parameters between groups.
A receiver operating characteristic (ROC) analysis was performed to determine cut-off values for rCBV values and Cho/Cr ratios to differentiate metastases from gliomas and to differentiate high-grade from low-grade gliomas.
For ROC analysis a larger area under the ROC curve (AUC) suggests better performance of a test. Sensitivity, specificity, AUC, true positive (TP), true negative (TN), false positive (FP), false negative (FN) fraction and positive predictive value (PPV), negative predictive value (NPV) as well as accuracy were provided for test performance evaluation. Differences were considered statistically significant at P < 0.05. The statistical software used was Analyse-it (Analyse-it Software Ltd., Leeds, UK) for Microsoft Excel (Microsoft Corporation, Redmond, Washington, USA).
Results
A total of 28 patients were analysed: 10 low-grade gliomas (seven oligodendroglioma, three astrocytoma), eight high-grade gliomas (five anaplastic astrocytoma, one anaplastic oligodendroglioma and two glioblastoma multiforme) and 10 metastases (five breast carcinoma, three non-small cell lung carcinoma, two renal cell carcinoma). All tumours demonstrated enhancement, with low-grade tumours having a tendency for minimal enhancement and high-grade tumours revealing more intense patterns of enhancement (Table 1).
Table 1.
Clinical data and contrast enhancement characteristics of patients with brain gliomas and solitary brain metastases.
| Case | Histology | Grade | Location | Contrast enhancement |
|---|---|---|---|---|
| 1 | Low-grade oligodendroglioma | I | Parietal | Minimal |
| 2 | Low-grade oligodendroglioma | I | Parietal | Minimal |
| 3 | Low-grade oligodendroglioma | I | Parietal | Minimal |
| 4 | Low-grade oligodendroglioma | I | Occipital | Moderate |
| 5 | Low-grade oligodendroglioma | I | Temporal | Moderate |
| 6 | Low-grade oligodendroglioma | I | Frontal | Moderate |
| 7 | Low-grade oligodendroglioma | I | Frontal | Moderate |
| 8 | Low-grade astrocytoma | II | Basal ganglia | Moderate |
| 9 | Low-grade astrocytoma | II | Thalamus | Moderate |
| 10 | Low-grade astrocytoma | II | Frontal | Moderate |
| 11 | Anaplastic astrocytoma | III | Parietal | Moderate |
| 12 | Anaplastic astrocytoma | III | Frontal | Intense |
| 13 | Anaplastic astrocytoma | III | Occipital | Intense |
| 14 | Anaplastic astrocytoma | III | Frontal | Moderate |
| 15 | Anaplastic astrocytoma | III | Frontal | Moderate |
| 16 | Anaplastic oligodendroglioma | III | Frontal | Intense |
| 17 | Glioblastoma | IV | Frontal | Intense |
| 18 | Glioblastoma | IV | Temporal | Intense |
| 19 | Breast carcinoma metastasis | – | Frontal | Intense |
| 20 | Breast carcinoma metastasis | – | Frontal | Intense |
| 21 | Breast carcinoma metastasis | – | Temporal | Intense |
| 22 | Breast carcinoma metastasis | – | Occipital | Intense |
| 23 | Breast carcinoma metastasis | – | Occipital | Intense |
| 24 | Non-small cell lung carcinoma | – | Frontal | Intense |
| 25 | Non-small cell lung carcinoma metastasis | – | Frontal | Intense |
| 26 | Non-small cell lung carcinoma metastasis | – | Frontal | Intense |
| 27 | Renal cell carcinoma metastasis | – | Temporal | Intense |
| 28 | Renal cell carcinoma metastasis | – | Occipital | Intense |
Differences in the mean rCBV values between low-grade gliomas (1.77) and high-grade gliomas (2.85) and between gliomas (2.25) and metastases (0.92) were statistically significant (P < 0.001).
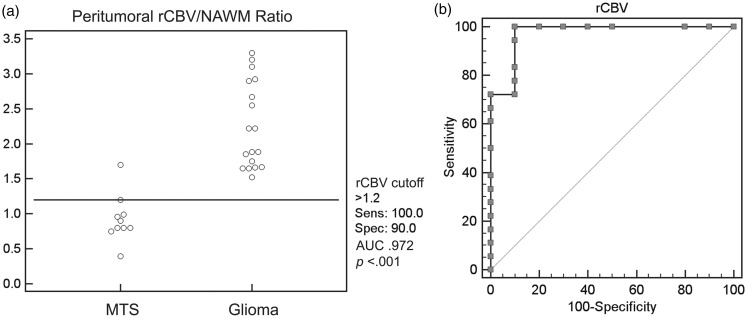
A cut-off rCBV value of 1.20 was effective in the differentiation of metastases from gliomas (sensitivity 100%, specificity 90%, AUC 0.972, TP 18, TN 9, FP 1, FN 0, PPV 94.7%, NPV 100%, accuracy 96.4%, P < 0.0001) (Figure 2).
Figure 2.
(a) Scatterplot demonstrating a relative cerebral blood volume (rCBV) cut-off value of 1.20 to differentiate between a metastasis (MTS) and a glioma. (b) Receiver operating characteristic curve demonstrating 100% sensitivity and 90% specificity of rCBV cut-off value of 1.20 to differentiate between a metastasis and a glioma. AUC: area under the curve; NAWM: normal-appearing white matter.
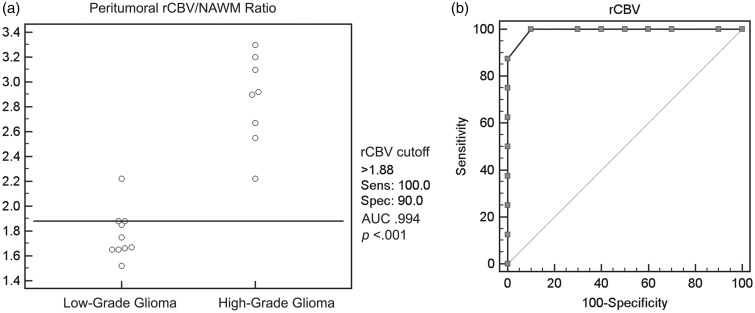
A cut-off rCBV value of 1.88 was detected for the differentiation of low-grade gliomas from high-grade gliomas (sensitivity 100%, specificity 90%, AUC 0.994, TP 8, TN 9, FP 1, FN 0, PPV 88.9%, NPV 100%, accuracy 94.4%, P < 0.0001) (Figure 3).
Figure 3.
(a) Scatterplot demonstrating the relative cerebral blood volume (rCBV) cut-off value of 1.88 to differentiate between high-grade gliomas and low-grade gliomas. (b) Receiver operating characteristic curve demonstrating 100% sensitivity and 90% specificity of rCBV cut-off value of 1.88 for differentiating between high-grade gliomas and low-grade gliomas. AUC: area under the curve; NAWM: normal-appearing white matter.
Differences in the Cho/Cr ratios in the peritumoural regions of high-grade gliomas (2.67) and of solitary metastases (0.87) were statistically significant (P < 0.001). However, a clear cut-off value to separate the two entities confidently could not be established.
Discussion
Brain gliomas and brain metastases are associated with high morbidity and mortality. Differentiation between brain gliomas and metastases as well as accurate grading of gliomas is important, as therapeutic approaches and prognosis for these lesions differ considerably.1,6,9,12,13,19,27
Conventional MRI techniques can be used to favour the diagnosis of a brain metastasis over that of a low-grade glioma, as these two different pathologies typically demonstrate substantial differences in their conventional imaging features, with a metastasis usually identified as an enhancing lesion with mass effect and surrounding vasogenic oedema, while low-grade gliomas appear as lacking significant oedema in the surrounding white matter and demonstrate milder degrees of mass effect. While conventional imaging features might readily distinguish between a solitary cerebral metastasis and a low-grade glial tumour, substantial overlap exists in conventional imaging features of a solitary cerebral metastasis and a higher grade glial neoplasm, with both lesions usually demonstrating contrast enhancement, mass effect and peritumoural oedema in the surrounding white matter. Despite such imaging similarities, high-grade gliomas and metastases are known to have significant histological differences in their peritumoural white matter. In particular, metastatic lesions are surrounded by pure vasogenic oedema, whereas high-grade gliomas demonstrate vasogenic oedema with microscopic cellular infiltration and proliferation in their peritumoural white matter. Indeed, a well-established histopathological feature of primary glial tumours is their propensity for microscopic infiltration of the white matter tracts surrounding the tumour.1–4 This aspect of glial neoplasia plays a critical role in the poor prognosis seen in this patient population,3 as several glioma patients who undergo complete surgical resections often succumb to recurrent neoplasm arising in the peritumoural white matter that was microscopically invaded prior to tumour resection. Such microscopic tumour infiltration of peritumoural white matter tracts is not appreciable on routine MRI techniques, but it is known to be present at the time of initial diagnosis and very often accounts for tumour recurrence. This well-established pattern of neoplastic infiltration of peritumoural white matter may be unique to glial tumours and is not typically observed in the setting of metastatic lesions. Hence, the peritumoural white matter oedema observed in the setting of cerebral metastases is thought to correspond to pure vasogenic white matter oedema, free of significant cellular infiltration.13,14,22,23 By contrast, the peritumoural white matter oedema surrounding primary glial neoplasms is thought to have a higher degree of cellularity, as it is thought to correspond to a combination of white matter fibres and microscopic tumour infiltration. Moreover, one can expect that the higher the grade of glial tumours, the more aggressive their growth pattern and, consequentially, the more robust angiogenesis seen with higher-grade tumours might lead to a greater tendency for microscopic invasion of their peritumoural white matter. The opposite might be expected with lower-grade glial neoplasms, as they demonstrate less aggressive growth patterns and can hence be expected to have more modest degrees of microscopic infiltration of their peritumoural white matter.1,3,12
These postulated differences in microscopic behaviour between low-grade and high-grade glial tumours versus metastases has led some investigators to seek advanced imaging techniques that might successfully demonstrate these histopathological distinctions.
In particular, some researchers have extended their analysis of perfusion patterns and metabolite ratios beyond the lesion itself and have begun studying the perilesional oedema with such advanced imaging techniques.1,3,16,17 For instance, Law et al.1 reported higher mean rCBV values and elevated Cho/Cr ratios in the peritumoural region to be associated with solitary high-grade glioma as opposed to metastases.
Our results support the hypothesis that peritumoural rCBV reflects the amount of microinfiltration of white matter tracts surrounding the gliomas. In particular, our results seem to confirm the hypothesis that higher grade gliomas, characterided by more aggressive angiogenesis, have a higher propensity for microinvasion of the peritumoural white matter tracts compared to their lower grade counterparts. The greater degree of microinvasion of the peritumoural white matter tracts was reflected by higher blood perfusion observed in the higher grade gliomas. Analyses of our data suggest a cut-off value for rCBV of 1.88, which could successfully differentiate between low-grade and high-grade gliomas. In addition, the unique tendency of glial neoplasia to infiltrate the peritumoural white matter tracts was also reflected in the substantially different rCBV patterns observed in primary glial tumours when compared to metastatic lesions. In our series, a cut-off value of 1.20 for rCBV in the peritumoural white matter was effective in differentiating gliomas from metastatic disease.
The high specificity and sensitivity for the cut-off values distinguishing metastasis from gliomas and high-grade gliomas from low-grade gliomas supports the potential clinical utility of these cut-off values in the diagnostic work-up of a of solitary intracranial lesion. This may be of particular value in patients whose conditions make surgical biopsy a very risky undertaking.
MRS has been successfully used to classify varying grades of brain gliomas.20–26 The metabolite pattern on MRS is a direct reflection of the aggressiveness of the tumour, with higher grade neoplasms demonstrating a higher degree of cell membrane turnover, which translates into larger peaks of choline metabolite and a reduction of NAA on magnetic resonance evaluation with spectroscopy.
Previous authors have successfully evaluated Cho/Cr ratios of glial tumours and have found a direct correlation between such ratio and tumour aggressiveness, with higher grade tumours demonstrating higher Cho/Cr ratios.20,22,25,26 If the efficacy of the Cho/Cr ratio in brain tumours is a well-established biomarker of the tumour grade, the utility of such a ratio in peritumoural oedema is less well established. Our study explored the utility of the Cho/Cr ratio in the peritumoural white matter in predicting the presence of a high-grade glioma or a metastasis. Based on the recognised differences of the peritumoural white matter of metastatic lesions and high-grade gliomas, we measured the Cho/Cr ratios in the peritumoural white matter of patients with high-grade gliomas and solitary cerebral metastases in our series. We observed a statistically significant difference in the Cho/Cr ratio between high-grade gliomas and solitary metastases with a mean Cho/Cr ratio value of 2.67 for high-grade gliomas and 0.87 for metastatic lesions. This difference suggests that there is a higher cell membrane turnover in the perilesional white matter of high-grade gliomas as compared to perilesional white matter in metastases, thus confirming the established micro-infiltrative tendency of higher grade glial neoplasms. The spectroscopic findings hence reflect the known histopathological tendencies of glial tumours to extend beyond the enhancing margins, with peritumoural white matter having greater tumour cellularity leading to higher cell membrane turnover and hence explaining the observed patterns of metabolite ratios. The lack of increased Cho/Cr in the peritumoural regions of metastases can be attributed to the purely vasogenic nature of their peritumoural oedema, lacking tumour infiltration and, therefore, void of substantial cellularity.1,8 While the difference of metabolite ratios between high-grade gliomas and metastasis was statistically significant, a clear cut-off Cho/Cr value to distinguish the two pathologies was not detected and should be further investigated in a large patient population.
The limitations of our study include the modest number of patients studied, in part due to the fact that some patients were excluded because they were unable to complete the perfusion-weighted and spectroscopic imaging.
Expanding this study to a larger population of patients presenting with a solitary enhancing neoplasm will lend additional validity to the conclusions we have reached.
An additional limitation could be that perfusion measurements were not corrected for contrast extravasation and contrast leakage in the perivascular spaces, which might cause inaccurate perfusion measurements.
While future research with a larger patient population will be necessary to increase our understanding of perilesional perfusion patterns in cerebral neoplasms, our preliminary results indicate the potential utility of this technique in preoperative grading of gliomas and separating primary glial tumours from solitary metastases.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Law M, Cha S, Knopp EA, et al. High-grade gliomas and solitary metastases: differentiation by using perfusion and proton spectroscopic MR imaging. Radiology 2002; 222: 715–721. [DOI] [PubMed] [Google Scholar]
- 2.Bulakbasi N, Kocaoglu M, Farzaliyev A, et al. Assessment of diagnostic accuracy of perfusion MR imaging in primary and metastatic solitary malignant brain tumors. AJNR Am J Neuroradiol 2005; 26: 2187–2199. [PMC free article] [PubMed] [Google Scholar]
- 3.Law M, Yang S, Wang H, et al. Glioma grading: sensitivity, specificity, and predictive values of perfusion MR imaging and proton MR spectroscopic imaging compared with conventional MR imaging. AJNR Am J Neuroradiol 2003; 24: 1989–1998. [PMC free article] [PubMed] [Google Scholar]
- 4.Law M, Yang S, Babb J, et al. Comparison of cerebral blood volume and vascular permeability from dynamic susceptibility contrast-enhanced perfusion MR imaging with glioma grade. AJNR Am J Neuroradiol 2004; 25: 746–755. [PMC free article] [PubMed] [Google Scholar]
- 5.Aprile I, Torni C, Fiaschini P, et al. High-grade cerebral glioma characterization: usefulness of MR spectroscopy and perfusion imaging associated evaluation. Neuroradiol J 2012; 25: 57–66. [DOI] [PubMed] [Google Scholar]
- 6.Sahin N, Melhem ER, Wang S, et al. Advanced MR imaging techniques in the evaluation of nonenhancing gliomas: perfusion-weighted imaging compared with proton magnetic resonance spectroscopy and tumor grade. Neuroradiol J 2013; 26: 531–541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Spampinato MV, Smith JK, Kwock L, et al. Cerebral blood volume measurements and proton MR spectroscopy in grading of oligodendroglial tumors. AJR Am J Roentgenol 2007; 188: 204–212. [DOI] [PubMed] [Google Scholar]
- 8.Zonari P, Baraldi P, Crisi G. Multimodal MRI in the characterization of glial neoplasms: the combined role of single-voxel MR spectroscopy, diffusion imaging and echo-planar perfusion imaging. Neuroradiology 2007; 49: 795–803. [DOI] [PubMed] [Google Scholar]
- 9.Dowling C, Bollen AW, Noworolski SM, et al. Preoperative proton MR spectroscopic imaging of brain tumors: correlation with histopathologic analysis of resection specimens. AJNR Am J Neuroradiol 2011; 22: 604–612. [PMC free article] [PubMed] [Google Scholar]
- 10.Falini A, Calabrese G, Origgi D, et al. Proton magnetic resonance spectroscopy and intracranial tumours: clinical perspectives. J Neurol 1996; 243: 706–714. [DOI] [PubMed] [Google Scholar]
- 11.Server A, Josefsen R, Kulle B, et al. Proton magnetic resonance spectroscopy in the distinction of high-grade cerebral gliomas from single metastatic brain tumors. Acta Radiol 2010; 51: 316–325. [DOI] [PubMed] [Google Scholar]
- 12.Hakyemez B, Erdogan C, Ercan I, et al. High-grade and low-grade gliomas: differentiation by using perfusion MR imaging. Clin Radiol 2005; 60: 493–502. [DOI] [PubMed] [Google Scholar]
- 13.Blasel S, Jurcoane A, Franz E, et al. Elevated peritumoral rCBV values as a mean to differentiate metastases from high-grade gliomas. Acta Neurochir 2010; 152: 1893–1899. [DOI] [PubMed] [Google Scholar]
- 14.Ricci R, Bacci A, Tugnoli V, et al. Metabolic findings on 3T 1H-MR Spectroscopy in peritumoral brain edema. AJNR Am J Neuroradiol 2007; 28: 1287–1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Svolos P, Kousi E, Kapsalaki E, et al. The role of diffusion and perfusion weighted imaging in the differential diagnosis of cerebral tumors: a review and future perspectives. Cancer Imaging 2014; 14: 20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rollin N, Guyotat J, Streichenberger N, et al. Clinical relevance of diffusion and perfusion magnetic resonance imaging in assessing intra-axial brain tumors. Neuroradiology 2006; 48: 150–159. [DOI] [PubMed] [Google Scholar]
- 17.Halshtok Neiman O, Sadetzki S, Chetrit A, et al. Perfusion weighted imaging of peritumoral edema can aid in the differential diagnosis of glioblastoma mulltiforme versus brain metastasis. Isr Med Assoc J 2013; 15: 103–105. [PubMed] [Google Scholar]
- 18.Knopp EA, Cha S, Johnson G, et al. Glial neoplasms: dynamic contrast enhanced T2*-weighted MR imaging. Radiology 1999; 211: 791–798. [DOI] [PubMed] [Google Scholar]
- 19.Lev MH, Ozsunar Y, Henson JW, et al. Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas. AJNR Am J Neuroradiol 2004; 25: 214–221. [PMC free article] [PubMed] [Google Scholar]
- 20.Tedeschi G, Lundbom N, Raman R, et al. Increased choline signal coinciding with malignant degeneration of cerebral gliomas: a serial proton magnetic resonance spectroscopy imaging study. J Neurosurg 1997; 87: 516–524. [DOI] [PubMed] [Google Scholar]
- 21.Rand SD, Prost R, Haughton V, et al. Accuracy of single-voxel proton MR spectroscopy in distinguishing neoplastic from non-neoplastic brain lesions. AJNR Am J Neuroradiol 1997; 18: 1695–1704. [PMC free article] [PubMed] [Google Scholar]
- 22.Sijens PE, Knopp MV, Brunetti A, et al. H-1 spectroscopy in patients with metastatic brain tumors: a multicenter study. Magn Reson Med 1995; 33: 818–826. [DOI] [PubMed] [Google Scholar]
- 23.Bruhn H, Frahm J, Gyngell ML, et al. Noninvasive differentiation of tumors with use of localized H-1 MR spectroscopy in vivo: initial experience in patients with cerebral tumors. Radiology 1989; 172: 541–548. [DOI] [PubMed] [Google Scholar]
- 24.Poptani H, Gupta RK, Roy R, et al. Characterization of intracranial mass lesions with in vivo proton MR spectroscopy. AJNR Am J Neuroradiol 1995; 16: 1593–1603. [PMC free article] [PubMed] [Google Scholar]
- 25.Pruel MC. Accurate noninvasive diagnosis of human brain tumors by using proton magnetic resonance spectroscopy. Nature Med 1996; 2: 323–325. [DOI] [PubMed] [Google Scholar]
- 26.Negendank WG, Sauter R, Brown TR, et al. Proton magnetic resonance spectroscopy in patients with glial tumors: a multicenter study. J Neurosurg 1996; 84: 449–458. [DOI] [PubMed] [Google Scholar]
- 27.Giese A, Westphal M. Treatment of malignant glioma: a problem beyond the margins of resection. J Cancer Res Clin Oncol 2001; 127: 217–225. [DOI] [PMC free article] [PubMed] [Google Scholar]