Abstract
Ischaemic strokes are an uncommon occurrence in the setting of glioblastoma, and clinically challenging due to co-existing deficits from the tumour, but important to consider as a possible cause of clinical deterioration. Modern therapies and their associated improvements in survival may lead to a greater overall incidence. The possible underlying causes of ischaemia are multiple, and several factors may contribute in a given patient. This review discusses the causative mechanisms of ischaemic strokes in the setting of glioblastoma, with some illustrative cases.
Keywords: Glioblastoma, ischaemia, stroke
Introduction
Patients with glioblastoma most commonly present with a progressive neurologic deficit, usually developing over less than three months.1 An acute presentation may occur, for example, due to a seizure or haemorrhage. Similar aetiologies may also cause an acute deterioration in a patient with a known glioblastoma. In contrast, ischaemic strokes are an uncommon occurrence in the setting of glioblastoma, and can be clinically challenging due to co-existing deficits from the tumour. Frequently, the diagnosis of an ischaemic stroke is not considered until after imaging has been performed.2 Strokes may also be discovered incidentally on routine imaging.2 This review discusses the causative mechanisms of ischaemic strokes in the setting of glioblastoma, with some illustrative cases.
Case 1
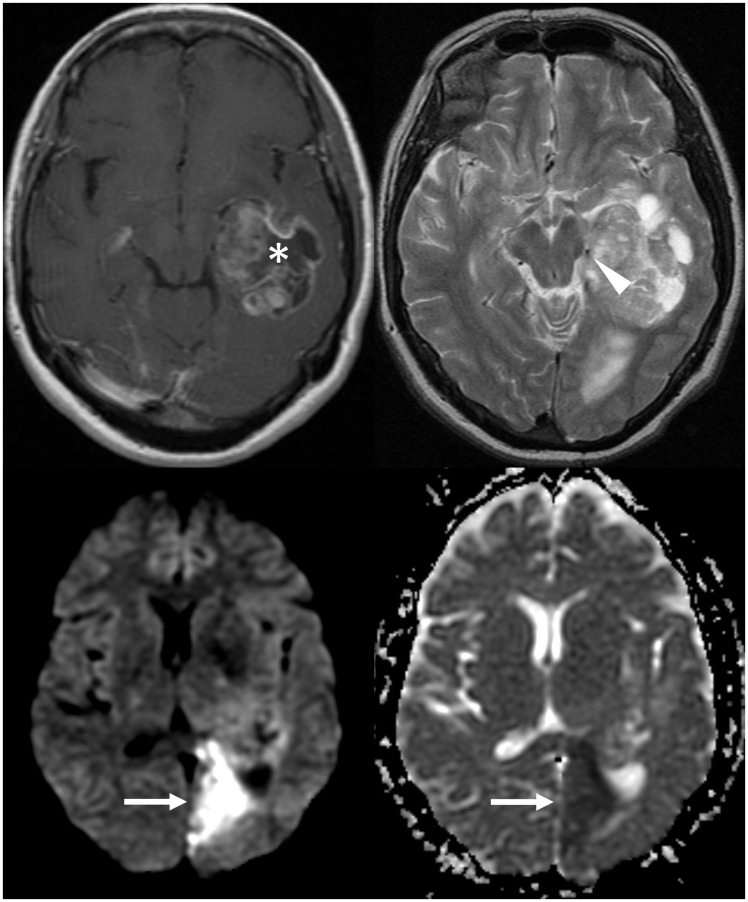
A 58-year-old female presented with recent-onset confusion and memory disturbance. Initial investigation with computed tomography (CT) was suggestive of glioblastoma, and subsequently characterised with magnetic resonance imaging (MRI). In additional to features consistent with a glioblastoma, the diffusion-weighted imaging (DWI) sequence demonstrated evidence of a left posterior cerebral artery territory infarct. The T2-weighted sequence showed that the cause of the ischaemic stroke was direct mass effect on the left posterior cerebral artery by the tumour (Figure 1). A craniotomy confirmed the suspicion of glioblastoma. Follow-up imaging eight weeks post-operatively demonstrated expected evolution of the infarct.
Figure 1.
The post-contrast image (top left) shows the rim-enhancing glioblastoma. On the T2-weighted sequence (top right), it is causing mass effect on the left posterior cerebral artery (arrowhead). The subsequent ischaemic stroke is demonstrated by diffusion restriction in the left posterior cerebral artery territory (arrows), with high signal on DWI (bottom left) and low signal on ADC (bottom right). DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient.
Case 2
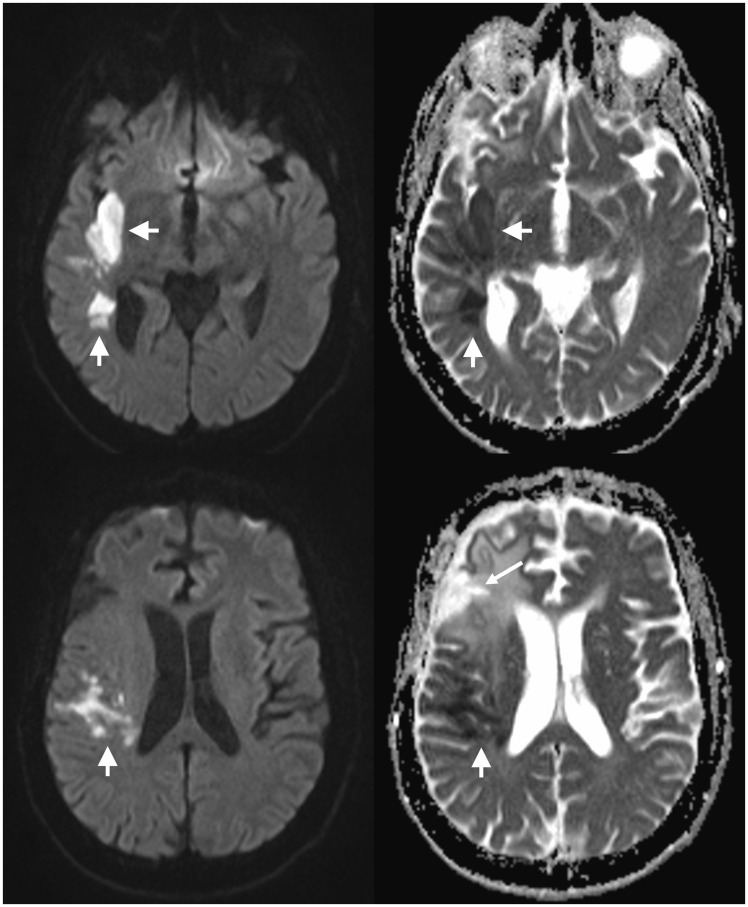
A 61-year-old male with a known glioblastoma presented with a sudden-onset left-sided facial droop. Examination also revealed left visual neglect and left upper limb weakness. The patient’s glioblastoma had been stable on imaging for several months, after adjuvant radiotherapy (a standard course of 60 Gy completed one year earlier) and a course of temozolamide chemotherapy, and the patient had been off treatment for several months at the time of presentation. An initial CT of the brain including CT perfusion demonstrated a large acute right middle cerebral artery (MCA) territory infarct. The infarct core occupied less than half of the overall perfusion abnormality, indicating a large ischaemic penumbra. The patient received intravenous thrombolysis with tissue plasminogen activator, with a door-to-needle time of 34 minutes and onset-to-needle time of 80 minutes. Thrombolysis resulted in significant improvement in the patient’s symptoms, and only minor residual visual neglect. The subsequent MRI demonstrated multiple areas of diffusion restriction in the right MCA territory (Figure 2), substantially smaller than the initial perfusion abnormality. Restricted diffusion subsided as these areas underwent expected evolution with resultant gliosis and encephalomalacia, consistent with infarcts. A later MRI, almost nine months after the right MCA stroke, demonstrated new enhancement at the margin of the right lateral ventricle, which raised concerns of disease progression. Imaging six weeks later, however, showed improvement in this enhancing area without any treatment, as well as the development of a new enhancing nodule nearby. The migratory nature of these later findings is most consistent with subclinical, subacute infarcts. MR perfusion (performed for assessment of the glioblastoma) demonstrated prolongation of mean transit time and time to peak persisting well beyond the acute presentation. Similarly, T1 post-contrast imaging showed attenuated enhancement of the right MCA branches compared to the contralateral side, providing further evidence of underlying radiation vasculopathy. The patient remained stable at review a further year after the initial MCA territory infarct.
Figure 2.
There are multiple areas of diffusion restriction in the right middle cerebral artery territory (short arrows), with high signal on DWI (left) and low signal on ADC (right). The surgical resection cavity (long arrow) and adjacent signal changes are seen a short distance anteriorly. DWI: diffusion-weighted imaging; ADC: apparent diffusion coefficient.
Discussion
MRI is the investigation of choice for diagnosing ischaemia, in particular in the setting of a glioblastoma, in which the appearances on CT may be masked by the tumour itself. Diffusion restriction is frequently observed in glioblastomas, related to increased cellularity;3 however, the degree of diffusion restriction typically seen in glioblastomas and acute ischaemia differs significantly. The apparent diffusion coefficient (ADC) values of high-grade gliomas are similar to those in normal adjacent white matter,4 but appear low compared to the surrounding vasogenic oedema, in which diffusion is facilitated (high ADC). In contrast, ADC values in the setting of acute ischaemia are lower than in normal adjacent parenchyma, as demonstrated in Figures 1 and 2. The distinction becomes more difficult in the setting of subacute infarction, when ADC normalises and enhancement may develop, concerning for disease progression. If doubt persists, follow-up imaging demonstrating rapid expected evolution of the infarct(s) and subsequent resolution of the diffusion restriction, with evolution to gliosis and encephalomalacia, allows the confident diagnosis of ischaemic stroke, while diffusion restriction related to the tumour itself will generally persist or evolve into an area of enhancement.
Acute ischaemic infarcts are rare in the setting of a previously unrecognised glioblastoma, with only a few cases reported in the literature. The most common cause reported is encasement of the relevant arterial branches by the tumour.5–7 Large-vessel occlusion may also be due to direct arterial infiltration,7 leptomeningeal involvement8 or, as in our first case, direct mass effect. The MCA branches are the most commonly affected.6 A generalised prothrombotic tendency related to the underlying malignancy may potentiate the above mechanisms, and a local procoagulant effect has also been postulated as a predisposing factor.6
In a study of 66 patients with ischaemic stroke in the setting of primary brain tumours, an acute operative complication was the cause in about half of the patients, with a variety of other aetiologies making up the remaining half.2 Other causes of treatment-related ischaemic strokes are radiation vasculopathy and anti-angiogenic chemotherapy. Radiation vasculopathy predominantly affects larger arteries, such as the internal carotid arteries or major cerebral arteries.9 It may manifest less frequently in glioblastoma patients compared to other intracranial neoplasms due to generally poor prognosis, but should be considered in longer-term survivors, as in our second case. In a large series of paediatric patients suffering strokes after cranial radiotherapy for brain tumours, the median duration from tumour diagnosis to stroke occurrence was 2.3 years, with a range of 0.3–15.8 years.9 Patients receiving ≥50 Gy are at greater risk,10 and of note, the standard radiotherapy dose for glioblastoma patients is 60 Gy. Similarly, in an adult series, the median interval between completion of radiotherapy to stroke onset was 3.2 years (range 0.5–30 years).2 In our second illustrative case, the time interval between the completion of radiotherapy and the development of ischaemia was relatively short, at one year, but this is within an acceptable time frame, and the dose of 60 Gy placed the patient in the high-risk category.
Anti-angiogenic chemotherapy agents are being increasingly used as an adjunct to radiotherapy, either first-line or after a failed response to temozolomide. The anti-angiogenic agent used most commonly in the treatment of glioblastoma is bevacizumab, a monoclonal antibody against vascular endothelial growth factor, a key promotor of angiogenesis. Bevacizumab has been shown to be associated with a mildly increased risk of both haemorrhagic and ischaemic stroke.11 Haemorrhagic strokes in patients treated with bevacizumab most commonly occur in the setting of disease progression.11 Bevacizumab may also result in the development of diffusion-restricting lesions, mimicking ischaemia, which evolve from an area of contrast-enhancement. The nature and mechanism of these bevacizumab-induced diffusion-restricting lesions remain unclear.12
The presence of an intracranial neoplasm is generally considered a contraindication to intravenous thrombolysis,13 and hence the reports on its administration to glioblastoma patients are few. Etgen et al. found only two cases of glioblastoma who had received thrombolysis. In both cases, the diagnosis of glioblastoma was not known at the time of presentation, and in retrospect the symptoms were probably due to seizures rather than ischaemia.14 In one of these two patients, thrombolysis was complicated by haemorrhage,15 not surprising given the propensity of glioblastomas to bleed spontaneously. More recently, a case of thrombolysis for pulmonary thromboembolism in the setting of recent neurosurgery for glioblastoma has been reported; in that instance, no complication of therapy was observed.16 The overlapping clinical manifestations of stroke and glioblastoma are well known, and may delay the diagnosis beyond the thrombolysis window. Even if an ischaemic stroke is diagnosed rapidly, one must consider whether it is likely to benefit from thrombolysis and whether the potential benefit is sufficient to allow for the increased risk. For example, the greater risk was considered warranted in the second case due to the presence of a large ischaemic penumbra and stability of the underlying glioblastoma.
Conclusion
Ischaemic infarcts are uncommon in the setting of glioblastoma, and frequently not suspected before being diagnosed on imaging. They are important to consider as a possible cause of an acute presentation or subsequent clinical deterioration, and modern therapies and their associated improvements in survival are likely to lead to a greater overall incidence. Infarcts are most commonly seen as an immediate post-operative complication. Other causes including a direct effect of the tumour on arterial branches, a prothrombotic tendency, radiation vasculopathy and anti-angiogenic chemotherapy. In addition, several factors may contribute in a given patient.
Acknowledgement
All procedures followed were in accordance with institutional guidelines.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Louis DN (ed.). WHO classification of tumours of the central nervous system. Lyon: International Agency for Research on Cancer, 2007.
- 2.Kreisl TN, Toothaker T, Karimi S, et al. Ischemic stroke in patients with primary brain tumors. Neurology 2008; 70: 2314–2320. [DOI] [PubMed] [Google Scholar]
- 3.Brandão LA, Shiroishi MS, Law M. Brain tumors: A multimodality approach with diffusion-weighted imaging, diffusion tensor imaging, magnetic resonance spectroscopy, dynamic susceptibility contrast and dynamic contrast-enhanced magnetic resonance imaging. Magn Reson Imaging Clin N Am 2013; 21: 199–239. [DOI] [PubMed] [Google Scholar]
- 4.Bulakbasi N, Kocaoglu M, Ors F, et al. Combination of single-voxel proton MR spectroscopy and apparent diffusion coefficient calculation in the evaluation of common brain tumors. AJNR Am J Neuroradiol 2003; 24: 225–233. [PMC free article] [PubMed] [Google Scholar]
- 5.Chen H, Cebula H, Schott R, et al. Glioblastoma multiforme presenting with ischemic stroke: Case report and review of the literature. J Neuroradiol 2011; 38: 304–307. [DOI] [PubMed] [Google Scholar]
- 6.Obeid M, Ulane C, Rosenfeld S. Pearls & Oy-sters: Large vessel ischemic stroke secondary to glioblastoma multiforme. Neurology 2010; 74: e50–e51. [DOI] [PubMed] [Google Scholar]
- 7.Pina S, Carneiro A, Rodrigues T, et al. Acute ischemic stroke secondary to glioblastoma. A case report. Neuroradiol J 2014; 27: 85–90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Herman C, Kupsky WJ, Rogers L, et al. Leptomeningeal dissemination of malignant glioma simulating cerebral vasculitis. Case report with angiographic and pathological studies. Stroke 1995; 26: 2366–2370. [DOI] [PubMed] [Google Scholar]
- 9.Bowers DC, Mulne AF, Reisch JS, et al. Nonperioperative strokes in children with central nervous system tumors. Cancer 2002; 94: 1094–1101. [PubMed] [Google Scholar]
- 10.Bowers DC, Liu Y, Leisenring W, et al. Late-occurring stroke among long-term survivors of childhood leukemia and brain tumors: A report from the Childhood Cancer Survivor Study. J Clin Oncol 2006; 24: 5277–5282. [DOI] [PubMed] [Google Scholar]
- 11.Fraum TJ, Kreisl TN, Sul J, et al. Ischemic stroke and intracranial hemorrhage in glioma patients on antiangiogenic therapy. J Neurooncol 2011; 105: 281–289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rieger J, Bähr O, Müller K, et al. Bevacizumab-induced diffusion-restricted lesions in malignant glioma patients. J Neurooncol 2010; 99: 49–56. [DOI] [PubMed] [Google Scholar]
- 13.Jauch EC, Saver JL, Adams HP, Jr, et al. Guidelines for the early management of patients with acute ischemic stroke: A guideline for healthcare professionals from the American Heart Association/American Stroke Association. Stroke 2013; 44: 870–947. [DOI] [PubMed] [Google Scholar]
- 14.Etgen T, Steinich I, Gsottschneider L. Thrombolysis for ischemic stroke in patients with brain tumors. J Stroke Cerebrovasc Dis 2014; 23: 361–366. [DOI] [PubMed] [Google Scholar]
- 15.Grimm SA, DeAngelis LM. Intratumoral hemorrhage after thrombolysis in a patient with glioblastoma multiforme. Neurology 2007; 69: 936. [DOI] [PubMed] [Google Scholar]
- 16.Bayram B, Oray NÇ, Korkmaz E, et al. Massive pulmonary embolism and cardiac arrest; thrombolytic therapy in a patient with recent intracranial surgery and glioblastoma multiforme. Am J Emerg Med 2014; 32: 1441.e1441–e1443. [DOI] [PubMed] [Google Scholar]