Abstract
Many studies lay emphasis on the clinical importance of perforating branches of the anterior communicating artery (ACoA) and report that vascular damage of the perforators from ACoA aneurysm during surgery cause subsequent postoperative amnesia. The purpose of our study was to analyze the safety of parent artery occlusion for ACoA aneurysm coiling based on the anatomical features of the ACoA complex in 13 patients with 13 ACoA aneurysms. All patients underwent coiling of the aneurysm sac and ACoA. Aneurysm characteristics including size, dome-to-neck ratio, anterior/posterior orientation of the aneurysm dome with respect to the axis of the pericallosal artery, location of the aneurysm neck with respect to the A1–A2 segment of the anterior cerebral artery (ACA) or the ACoA, and the presence of hypoplasia/aplasia of A1 segment were assessed. The aneurysm neck was located directly on the ACoA in five aneurysms (38%), whereas eight (62%) had the neck located at the A1–A2 junction. Of the five patients whose aneurysm neck was located in the ACoA, four patients had infarcts in the basal forebrain. Three of the patients complained of amnesia. None of the aneurysms with the neck located at the A1–A2 junction were associated with infarction. There has been little evidence thus far that parent vessel occlusion of ACoA aneurysms is a safe method for the treatment of aneurysms. Patients with the aneurysm neck located at the A1–A2 junction and without A1 aplasia, who were treated with aneurysm sac and ACoA embolism, were potentially safe.
Keywords: Aneurysm, anterior communicating artery, coiling
Introduction
Anterior communicating artery (ACoA) is the most common location for cerebral aneurysms and accounts for as much as 36% of aneurysms.1 Endovascular coil embolization is a less invasive alternative to surgical clipping of the aneurysm.2 Many studies have laid emphasis on the clinical importance of perforating branches of the ACoA and reported that vascular damage of the perforators from ACoA aneurysm during surgery cause subsequent postoperative amnesia.3–6 However, the effects of coil occlusion of ACoA in ACoA aneurysm coiling has scarcely been reported.7 The purpose of our study was to analyze the safety of parent artery occlusion for ACoA aneurysm coiling based on the anatomical features of the ACoA complex.
Materials and methods
Patients
From January 2008 to July 2014, 123 patients with 123 ACoA aneurysms were treated by endovascular coiling at our institution. Among the 123 patients, we retrospectively reviewed records of patients who were treated with aneurysm sac and ACoA coil embolism. Patients who had intraparenchymal hematoma revealed by preprocedure computed tomography (CT) scanning were excluded from the study. This study was approved by the local ethical committee. The requirement of informed consent was waived for this retrospective study.
Aneurysm characteristics
All patients underwent digital subtraction angiography with three-dimensional (3D) images reconstructed using a biplane angiographic unit (Axiom Artis Zee biplane; Siemens, Erlangen, Germany). Aneurysm characteristics including size, dome-to-neck ratio, anterior/posterior orientation of the aneurysm dome with respect to the axis of the pericallosal artery, location of the aneurysm neck with respect to the A1–A2 segment of the anterior cerebral artery (ACA) or the ACoA, and the presence of hypoplasia/aplasia of A1 segment were assessed.8 As the diameter below which the A1 segment could be classified as hypoplastic has not been well defined, we used 1.5 mm as the cutoff value.9 A wide aneurysm neck was defined as a neck of ≥4 mm or had a dome-to-neck ratio of <2, as measured by angiography.
Endovascular procedure
All patients underwent endovascular treatment during a single session under general anesthesia. If stent protection was anticipated, premedication was initiated in patients with unruptured aneurysm seven days prior to the procedure with 100 mg of aspirin and 75 mg of clopidogrel per day. For patients with ruptured aneurysms, a loading dose of clopidogrel and aspirin (300 mg each) was orally administered two hours before treatment. Unilateral femoral access was obtained through percutaneous femoral artery puncture by a 6 F guide catheter (Envoy; Cordis, Miami Lakes, FL, USA), which was inserted into the parent vessel. Catheterization of the aneurysm was performed using a 0.014-inch microcatheter (SL 10; Boston Scientific, Natick, MA). In circumstances where a stable coil frame could not be formed with a single microcatheter, 2 microcatheters or a stent-assisted technique was used. When the coil loop herniated into the ACoA, the ACoA was sacrificed. If the coil loop herniated into the A1 or A2 segments, we used balloon remodeling technique or a stent to protect the segments. Angiographic results were defined as complete, residual neck, or residual sac using the modified Raymond–Roy classification.10 Ipsilateral internal carotid artery (ICA) angiogram with contralateral ICA compression was performed to evaluate the ACoA patency. If a stent was used, patients were provided with daily doses of 100 mg of aspirin and 75 mg of clopidogrel for six months, followed by a daily dose of 100 mg of aspirin indefinitely.
Clinical and radiological follow-up
Neurological examinations were conducted throughout the hospitalization period and in the outpatient clinics postoperatively. Non-enhanced brain CT scans were performed immediately post-procedure. Follow-up magnetic resonance (MR) imaging with a 1.5-T or a 3-T system was scanned. The standard imaging protocol consisted of axial fluid-attenuated inversion recovery (FLAIR) imaging and MR angiography with 3D reconstruction. Infarction lesions with high signals detected by FLAIR imaging were analyzed.
Results
Patients
Of the 123 patients, 16 patients with 16 ACoA aneurysms underwent aneurysm sac and ACoA coiling. Two patients who had intraparenchymal hematoma in the frontal lobe of the brain and one patient who had acute thrombosis in stent with ipsilateral ACA occlusion were excluded. A total of 13 patients with 13 ACoA aneurysms were available for analysis, of whom five were women (38%) and eight were men (62%). The mean patient age was 59.4 ± 8.1 years (range, 44–74 years). There were four patients (31%) with unruptured aneurysms and nine patients (69%) with aneurysmal subarachnoid hemorrhage (SAH). Brain CT scan of all patients prior to treatment did not show evidence of infarct or intraparenchymal hematoma (Table 1).
Table 1.
Thirteen patients with 13 ACoA aneurysms treated by ACoA and aneurysm sac coil embolism.
| Patient | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 | 13 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Age/Sex | 61/F | 55/M | 57/M | 70/M | 52/F | 62/M | 55/F | 66/F | 44/M | 65/F | 54/M | 74/M | 57/M |
| Presentation | Unruptured | Ruptured | Ruptured | Unruptured | Unruptured | Unruptured | Ruptured | Ruptured | Ruptured | Ruptured | Ruptured | Ruptured | Ruptured |
| Aneurysm | |||||||||||||
| Size (mm) | 5.7 × 5.5 | 4.3 × 3.6 | 7.4 × 4.2 | 4.2 × 3.7 | 3.7 × 3.4 | 5.7 × 6 | 2.3 × 2.1 | 5 × 5.6 | 6.7 × 3.2 | 4 × 2 | 2.9 × 2.3 | 3.4 × 2.1 | 1.4 × 2 |
| Neck (mm) | 2.8 | 3.8 | 2.7 | 3.6 | 2.1 | 3.5 | 2 | 3 | 2.4 | 2.2 | 1.7 | 2 | 1.4 |
| Neck location | ACoA | ACoA | A1–A2 | A1–A2 | A1–A2 | ACoA | A1–A2 | ACoA | ACoA | A1–A2 | A1–A2 | A1–A2 | A1–A2 |
| Projection | Anterior | Anterior | Anterior | Anterior | Anterior | Posterior | Posterior | Posterior | Anterior | Posterior | Anterior | Anterior | Posterior |
| A1 segment | Normal | Dysplasia | Normal | Dysplasia | Normal | Dysplasia | Dysplasia | Normal | Normal | Normal | Normal | Normal | Normal |
| Assistance | None | None | None | Stent | None | None | None | None | Stent | None | None | None | Balloon |
| Result | Complete | Complete | Complete | Complete | Complete | Complete | RN | Complete | Complete | Complete | RN | Complete | Complete |
| Fisher grade | NA | 2 | 3 | NA | NA | NA | 2 | 2 | 3 | 2 | 2 | 3 | 2 |
| MRI F/U | |||||||||||||
| Period | Two weeks | Two weeks | Three weeks | Two weeks | Four weeks | Two weeks | Two weeks | Two weeks | Two weeks | Two weeks | One week | Two weeks | Two weeks |
| Lesion site | Lt BF | Rt BF | None | None | None | Rt BF | None | Rt BF | None | None | None | None | None |
| Clinical F/U | |||||||||||||
| Period (months) | 1 | 3 | 20 | 23 | 15 | 36 | 8 | 2 | 15 | 32 | 38 | 5 | 7 |
| Symptom | Amnesia | Amnesia | None | None | None | None | None | Amnesia | None | None | None | None | None |
ACoA: anterior communicating artery; BF: basal forebrain; MRI: magnetic resonance imaging; F/U: follow-up; RN: residual neck; M: male; F: female; Rt: right; Lt: left.
Aneurysm morphology
The mean maximum dimensions of patient aneurysms were 4.5 ± 1.7 mm. All but two cases were wide-necked and all cases were saccular aneurysms. Eight aneurysms had a dome-oriented anterior (62%), and five were posteriorly oriented (38%). The neck of the aneurysm was located directly on the ACoA in five aneurysms (38%), whereas eight (62%) had the neck located at the A1–A2 junction. One of the A1 segments was hypoplastic in four cases.
Immediate angiographic results
Aneurysm occlusions immediately post-procedure were complete occlusions in 11 cases (84.6%) and residual neck occlusions in two cases (15.4%). A stent-assisted technique was used in three patients for deploying the stent in the ACA. Immediate angiography with contralateral ICA compression did not reveal ACoA patency in all cases.
Immediate complications
Of the 13 patients, two patients (patients 3 and 9) developed communicating hydrocephalus. However, they did not require extraventricular drainage and were managed conservatively. None of the patients developed symptomatic vasospasm.
Clinical and radiological follow-up results
In all 13 patients, MR imaging was performed at a median of 2.2 weeks (range, 1–14 weeks) from aneurysm treatment. Of the five patients whose aneurysm neck was located in the ACoA, four patients (patients 1, 2, 6 and 8) had infarcts in the basal forebrain. Three of these patients (patients 1, 2 and 8) complained of amnesia. One patient (patient 2, Figure 1) had apparent memory impairment three months after treatment. The patient was alert and did not have any neurological deficits. The patient scored 26 of 30 in the Mini-Mental State Examination (MMSE). The Wechsler Memory Scale-revised score11 was 23, indicative that the patient had a memory impairment. The other patients (patients 1 and 8) had mild amnesia at one or two months post-treatment and were tested by MMSE. MMSE scores were 26, and 27, respectively. Delayed recall, which was assessed by asking the participants to recall three words (book, telephone, and penny) that they had been asked to repeat and memorize previously, was one of three in two patients. Patients whose aneurysm neck was located at the A1–A2 junction did not have infarcts (Figure 2).
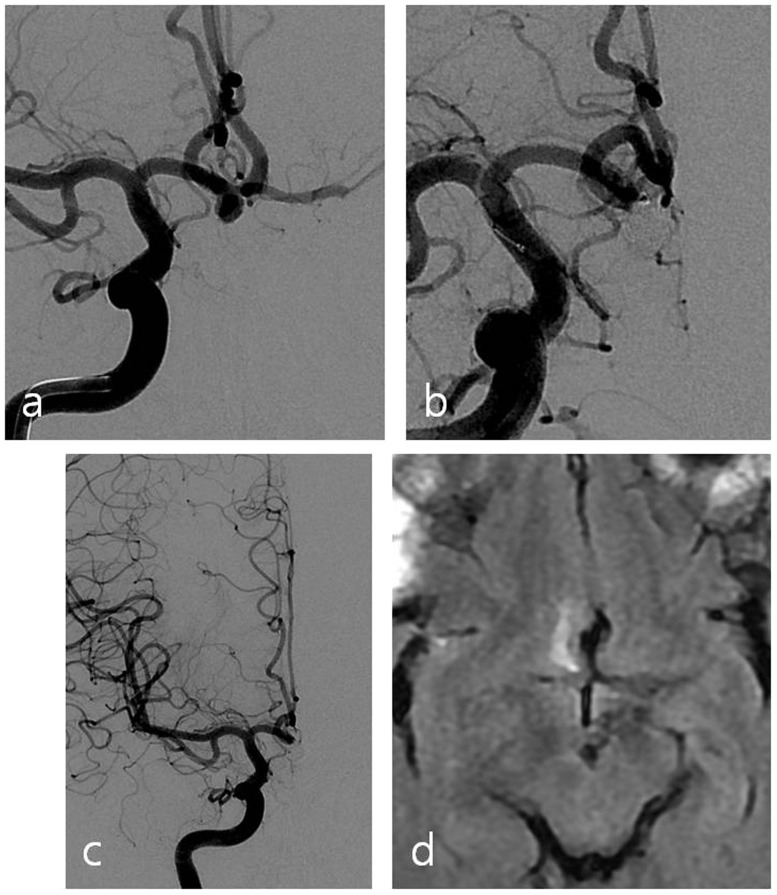
Figure 1.
(a) Working position angiography shows an aneurysm with a wide neck located at the anterior communicating artery. (b) Aneurysm and anterior communicating artery are both embolized with coils. (c) Angiography with left internal carotid artery compression after the procedure reveals occlusion of the anterior communicating artery. (d) Follow-up brain magnetic resonance imaging (MRI) shows infarction in right basal forebrain.
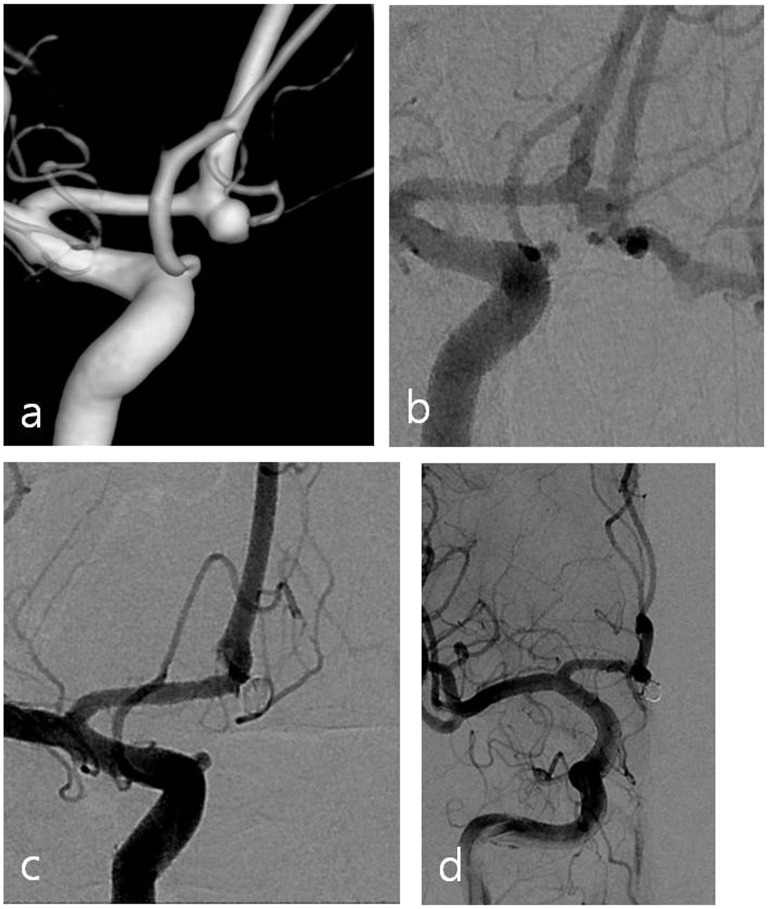
Figure 2.
(a) Three-dimensional reconstruction by angiography reveals an aneurysm with a wide neck located at the A1–A2 junction. (b) There is patency of anterior communicating artery during left internal carotid artery (ICA) compression test. (c) Aneurysm and the anterior communicating artery are both embolized with coils. (d) Angiography with left ICA compression after the procedure reveals occlusion of the anterior communicating artery. Follow-up brain magnetic resonance imaging (MRI) showed no infarctions.
Discussion
During endovascular treatment of ACoA aneurysms, aneurysms that are small, wide-necked, or complicated are likely to cause catheterization failure and coil herniation. Loose packing has been associated with recanalization.12
In this situation, multiple strategies for treating aneurysms include remodeling the neck using balloons, the simultaneous deployment of two coils at the beginning of the framing of the aneurysm, or by the use of stents.13–15 Stenting is particularly difficult and of limited use for ruptured aneurysms as the patient is placed in an antiplatelet regimen and thereby exposed to potential hemorrhagic complications.16 Bodily et al.17 reported that intracranial hemorrhagic and thromboembolic complications occurred in 8% and 6% of patients, respectively, and concluded that adverse outcomes were more common with stent-assisted coiling, as compared with conventional coiling.
Our priority was to obtain high packing density of coil for the treatment of aneurysms in order to minimize the recanalization or risk of rebleeding. It is likely that treatment of patients with ruptured wide neck ACoA aneurysms with either microsurgical repair, or acute, partial coil embolization of the dome/body, followed by either delayed stent-coiling or surgery after the SAH period, is more acceptable. However, these methods were not always possible. When putting more coils to aneurysm body, unanticipated coil loop herniation into the parent artery occurred in some cases. In the absence of A1 segment aplasia, aneurysm sac and ACoA embolization were performed when coil loop herniated into the ACoA. If the coil loop protruded into the A1 or A2 segments, we used balloon or stent to protect the segments.
ACoA is the origin of the perforating branches to the basal forebrain. It is often challenging to preserve perforators that surround or adhere to aneurysms during surgery. However, peri-aneurysmal perforators were not of primary concern, and only perforators arising from the necks of aneurysms were evaluated. In our study, there were no perforators arising from the necks of aneurysms in all patients. Li et al.7 evaluated the results of endovascular treatment in 16 ACoA aneurysm cases. In five of these cases, aneurysm sac and ACoA coil embolization was performed without complications, and those authors concluded that the aneurysm sac and ACoA embolism is potentially safer than traditional aneurysm sac embolism. In that study, diagnosis of infarction was made by non-enhanced brain CT. However, artifacts from the metallic coil can make identification of lesions difficult by this method. We could not evaluate basal forebrain lesions by CT and used MR imaging to examine the ischemic lesions related to the endovascular coiling procedure. In our study, five of 13 patients had infarcted foci in the basal forebrain or the rectus gyrus. We analyzed the aneurysm morphology by angiography at the time of embolization. ACoA aneurysms with the neck located at the ACoA had a higher tendency to infarct when the parent artery was occluded. Conversely, none of the aneurysms with the neck located at the A1–A2 junction was associated with infarction, probably because of the rich supply of multiple perforators that anastomose together in the basal forebrain. For example, multiple hypothalamic branches and a large subcallosal branch of the perforating arteries anastomose together.18,19
We presume that the ACoA was occluded in the case of A1–A2 aneurysms immediately adjacent to the neck of the lesion. Thus, the eccentric closure of the ACoA in these cases would allow a majority of ACoA perforators to receive blood flow from the contralateral side. In contrast, occlusion of the central aspect of the ACoA may not permit collateral blood flow, which would result in a higher rate of perforator closure.
In our study, three out of five patients with infarctions in the basal forebrain following ACoA aneurysm treatment displayed symptoms of amnesia. Since the 1950s, patients who survive ACoA aneurysms have been reported to experience amnesia similar to that of Korsakoff syndrome, with personality changes and decreased spontaneous activity.20 Memory disturbance is associated with infarcts of the perforating branches of the ACoA. Damage or occlusion of ACoA perforators can result in personality or memory disturbances.21,22
Two patients had infarcts in the basal forebrain by post-procedure MRI (each two weeks) after treatment. It may be possible that vasospasm associated SAH contributed to the infarcts. However, vasospasm following SAH primarily affects the larger intracerebral arteries and rarely causes isolated perforator vasospasm.23
In our study, two out of four patients who had a subsequent infarct after parent vessel occlusion, presented with unruptured intracranial aneurysm. Thus, we believe that parent artery occlusion rather than isolated perforator vasospasm may have contributed to the observed infarcts in the SAH patients.
We performed ipsilateral ICA angiogram with manual compression of the contralateral carotid artery to evaluate the ACoA patency. In untreated ACoA aneurysms this maneuver may increase the pressure to the aneurysm. Yoneda et al.24 measured changes in flow velocity in both internal carotid and vertebral arteries before and during digital compression of the common carotid artery. They noted increases in the flow velocity of patients with normal ACA after carotid compression, which ranged from 0 to 61%. Thus, manual carotid compression test should be performed with care.
Our study had several limitations. First, limitations were related to retrospective design. We did not utilize a more detailed memory test. In addition, it was difficult to measure other associated characteristics by angiography, such as ACoA length and the specific angles between ACA and ACoA. Second, we could not clarify the exact location of the infarct inside the basal forebrain as the 2D MR imaging was performed on relatively thick sections. Three-dimensional MR imaging with multiplanar reconstruction (MPR) would be able to better identify small structures in the basal forebrain.25 Third, the number of patients in our study was small. Hypoplasia of one of the A1 segments was observed in four cases. This is lower than many previous reports of ACoA aneurysms, which have reported the rate of unilateral hypoplasia to be as high as 80%.
Conclusion
Until recently, there has been little evidence that parent vessel occlusion of ACoA aneurysms is safe for the treatment of aneurysms. If alternative methods are not viable, ACoA aneurysm sac and ACoA embolism in patients with the aneurysm neck located at the A1–A2 junction without A1 aplasia may be considered.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Brisman JL, Song JK, Newell DW. Cerebral aneurysms. N Engl J Med 2006; 355: 928–939. [DOI] [PubMed] [Google Scholar]
- 2.O’Kelly CJ, Kulkarni AV, Austin PC, et al. The impact of therapeutic modality on outcomes following repair of ruptured intracranial aneurysms: An administrative data analysis. J Neurosurg 2010; 113: 795–801. [DOI] [PubMed] [Google Scholar]
- 3.Sekhar LN, Natarajan SK, Britz GW, et al. Microsurgical management of anterior communicating artery aneurysms. Neurosurgery 2007; 61: 273–290, discussion 290–292. [DOI] [PubMed] [Google Scholar]
- 4.Hernesniemi J, Dashti R, Lehecka M, et al. Microneurosurgical management of anterior communicating artery aneurysms. Surg Neurol 2008; 70: 8–28, discussion 29. [DOI] [PubMed] [Google Scholar]
- 5.DeLuca J, Diamond BJ. Aneurysm of the anterior communicating artery: A review of neuroanatomical and neuropsychological sequelae. J Clin Exp Neuropsychol 1995; 17: 100–121. [DOI] [PubMed] [Google Scholar]
- 6.Phillips S, Sangalang V, Sterns G. Basal forebrain infarction: A clinicopathologic correlation. Arch Neurol 1987; 44: 1134–1138. [DOI] [PubMed] [Google Scholar]
- 7.Li JW, Shi CH. Endovascular treatment of complicated ruptured anterior communicating artery aneurysms based on the anatomical features of the anterior communicating artery complex. Neurol India 2012; 60: 55–60. [DOI] [PubMed] [Google Scholar]
- 8.Gonzalez N, Sedrak M, Martin N, et al. Impact of anatomic features in the endovascular embolization of 181 anterior communicating artery aneurysms. Stroke 2008; 39: 2776–2782. [DOI] [PubMed] [Google Scholar]
- 9.Perlmutter D, Rhoton AL. Microsurgical anatomy of the anterior cerebral artery-anterior communicating artery-recurrent artery complex. J Neurosurg 1976; 45: 259–272. [DOI] [PubMed] [Google Scholar]
- 10.Roy D, Milot G, Raymond J. Endovascular treatment of unruptured aneurysms. Stroke 2011; 32: 1998–2004. [DOI] [PubMed] [Google Scholar]
- 11.Wechsler D. Wechsler Memory Scale Revised: Manual, San Antonio, TX: Psychological Corporation, 1987. [Google Scholar]
- 12.Kadirvel R, Ding YH, Dai D, et al. Proteomic analysis of aneurysm healing mechanism after coil embolization: Comparison of dense packing with loose packing. AJNR Am J Neuroradiol 2012; 33: 1177–1181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Moret J, Cognard C, Weill A, et al. Reconstruction technic in the treatment of wide-neck intracranial aneurysms. Long-term angiographic and clinical results. Apropos of 56 cases. J Neuroradiol 1997; 24: 30–44. [PubMed] [Google Scholar]
- 14.Turjman F. Combined stent implantation and endosaccular coil placement for treatment of experimental wide-necked aneurysms: A feasibility study in swine. AJNR Am J Neuroradiol 1994; 15: 1087–1090. [PMC free article] [PubMed] [Google Scholar]
- 15.Mericle RA, Wakhloo AK, Rodriguez R, et al. Temporary balloon protection as an adjunct to endosaccular coiling of wide-necked cerebral aneurysms: Technical note. Neurosurgery 1997; 41: 975–978. [DOI] [PubMed] [Google Scholar]
- 16.Mocco J, Snyder KV, Albuquerque FC, et al. Treatment of intracranial aneurysms with the Enterprise stent: a multicenter registry. J Neurosurg 2009; 110: 35–39. [DOI] [PubMed] [Google Scholar]
- 17.Bodily KD, Cloft HJ, Lanzino G, et al. Stent-assisted coiling in acutely ruptured intracranial aneurysms: A qualitative, systematic review of the literature. AJNR Am J Neuroradiol 2011; 32: 1232–1236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Marinković SV, Milisavljević MM, Marinković ZD. Microanatomy and possible clinical significance of anastomoses among hypothalamic arteries. Stroke 1989; 20: 1341–1352. [DOI] [PubMed] [Google Scholar]
- 19.Serizawa T, Saeki N, Yamaura A. Microsurgical anatomy and clinical significance of the anterior communicating artery and its perforating branches. Neurosurgery 1997; 40: 1211–1218. [DOI] [PubMed] [Google Scholar]
- 20.Norlen G, Olivecrona H. The treatment of aneurysms of the circle of Willis. J Neurosurg 1953; 10: 404–415. [DOI] [PubMed] [Google Scholar]
- 21.Damasio AR, Graff-Radford NR, Eslinger PJ, et al. Amnesia following basal forebrain lesions. Arch Neurol 1985; 42: 263–271. [DOI] [PubMed] [Google Scholar]
- 22.DeLuca J, Chiaravalloti N. The neuropsychological consequences of ruptured aneurysms of the anterior communicating artery. In: Harrison EJ, Owen AM. (eds). Cognitive deficits in brain disorders, London: Martin Duntz, 2002, pp. 17–36. [Google Scholar]
- 23.Ohkuma H, Itoh K, Shibata S, et al. Morphological changes of intraparenchymal arterioles after experimental subarachnoid haemorrhage in dogs. Neurosurgery 1997; 91: 230–236. [DOI] [PubMed] [Google Scholar]
- 24. Yoneda S, Nukada T, Kimura K, et al. Evaluation of cross-circulation through circle of Willis using an ultrasonic Doppler technique. Part I. Comparison between blood flow velocity by ultrasonic Doppler flowmetry and angiogram. Stroke 1981; 12: 478–484. [DOI] [PubMed]
- 25.Mugikura S, Kikuchi H, Fujii T, et al. MR imaging of subcallosal artery infarct causing amnesia after surgery for anterior communicating artery aneurysm. AJNR Am J Neuroradiol 2014; 35: 2293–2301. [DOI] [PMC free article] [PubMed] [Google Scholar]