Abstract
We review the imaging appearance of connective tissue diseases of the head and neck. Bilateral sialadenitis and dacryoadenitis are seen in Sjögren’s syndrome; ankylosis of the temporo-mandibular joint with sclerosis of the crico-arytenoid joint are reported in rheumatoid arthritis and lupus panniculitis with atypical infection are reported in patients with systemic lupus erythematosus. Relapsing polychondritis shows subglottic stenosis, prominent ear and saddle nose; progressive systemic sclerosis shows osteolysis of the mandible, fibrosis of the masseter muscle with calcinosis of the subcutaneous tissue and dermatomyositis/polymyositis shows condylar erosions and autoimmune thyroiditis. Vascular thrombosis is reported in antiphospholipid antibodies syndrome; cervical lymphadenopathy is seen in adult-onset Still’s disease, and neuropathy with thyroiditis reported in mixed connective tissue disorder. Imaging is important to detect associated malignancy with connective tissue disorders. Correlation of the imaging findings with demographic data and clinical findings are important for the diagnosis of connective tissue disorders.
Keywords: Connective tissue, CT, MR imaging, malignancy, infection
Introduction
Connective tissue diseases are a heterogeneous group of acquired immunologically mediated inflammatory disorders characterized by abnormal function or structure of one or more of the elements of the connective tissue, such as collagen, elastin or the mucopolysaccharides. These disorders show widespread fibrinoid degeneration of the collagen fibers occurring in the mesenchymal tissue. They are characterized by autoantibody production and other immune-mediated dysfunction. There are common clinical and serological features of patients with connective tissue disorders. These disorders include Sjögren’s syndrome, rheumatoid arthritis, systemic lupus erythematosus, relapsing polychondritis, progressive systemic sclerosis, dermatomyositis/pyomyositis, antiphospholipid syndrome, adult-onset Still’s disease and mixed connective tissue disorder. Connective tissue disorders may affect different regions and compartments of the head and neck.1–5 These disorders may be associated with paranenopastic syndrome, especially hematologic tumors, or may increase the risk for the development of malignancies, predominantly lymphoproliferative disorders such as lymphomas and leukemias or solid organ tumors such head and neck, stomach, kidney and ovarian cancer.3–6 Also, these patients may be associated with infection and granulomatous lesions of the head and neck.7 Table 1 shows the imaging findings and associated malignancy in the head and neck in patients with connective tissue disorders.
Table 1.
Imaging findings of head and neck in connective tissue disorders.
| Connective tissue disorder | Head and neck imaging findings |
|---|---|
| Sjögren’s syndrome | Salivary gland: diffusely enlarged Lacrimal glands: diffusely enlarged Others: neuropathy and auto-immune thyroiditis Association: lymphoma |
| Rheumatoid arthritis | TMJ: ankylosis Larynx: crico-arytenoid sclerosis and erosion Cervical spine: atlanto-axial subluxation Association: lymphoma |
| Systemic lupus erythematosus | Subcutaneous tissue: lupus panniculitis Orbit: discoid lupus Parotid: necrosis Larynx: bamboo nodule Association: fungal infection, lymphoma |
| Relapsing polychondritis | Ear: linear calcification with prominent ear sign Nose: collapse with saddle nose Larynx: subglottic stenosis Association: myelodysplastic syndrome |
| Systemic sclerosis | Mandible: osteolysis Masséter and tongue: fibrosis Larynx: vocal cord polyp Subcutaneous tissue: calcinosis |
| Dermatomyositis/polymyositis | Mandible: condylar erosion Thyroid: autoimmune thyroiditis Orbit: myositis and infiltration Association: cancer tongue, squamous cell carcinoma |
| Antiphospholipid syndrome | Vascular: venous and arterial thrombosis |
| Adult-onset Still’s disease | Nodes: cervical lymphadenopathy Others: neuropathy, trochilitis and thyroiditis Association: hemophagocytic syndrome, lymphoma |
| Mixed connective tissue disorder | Neuropathy: optic and trigeminal nerves Thyroid: autoimmune thyroiditis Larynx: bamboo nodes Association: Castleman’s disease |
Different imaging modalities are used for the assessment of connective tissues diseases of head and neck. Ultrasound imaging with color duplex may have a role in the evaluation of salivary glands, cervical lymph nodes and soft tissue of the neck.8 Cross-sectional imaging modalities such as contrast-enhanced computed tomography (CT) and magnetic resonance (MR) imaging provides a high-quality assessment of structural abnormalities that is used for the assessment of the extent of lesion in different regions of the head and neck.6 Advanced MR imaging techniques such as diffusion weighted-MR, perfusion-weighted MR imaging, MR spectroscopy,9–11 and advanced CT imaging techniques such as dual-energy CT and CT perfusion may have a role in the assessment of malignancy associated with connective tissue disorders.12,13 However, functional imaging with fluorodeoxyglucose positron emission tomography-computed tomography (FDG-PET-CT) provide early functional information about the activity of connective tissue diseases.14
Sjögren’s syndrome
Sjögren’s syndrome is a chronic, systemic, autoimmune inflammatory disorder that usually affects women (female/male ratio, 9:1) in the fourth and fifth decades of life. This disorder is characterized by lymphocytic infiltration of the exocrine glands, primarily the salivary and lacrimal glands. Patients with Sjögren’s syndrome typically present with dry mouth and dry eyes.15–17 Imaging is helpful for staging of the disease, detection of the associated infection as well as a surveillance for lymphomas, as patients with Sjögren’s syndrome are at a higher risk (44 times) of developing lymphomas. The co-existence of neoplastic diseases in patients with Sjögren’s syndrome is a long-recognized phenomenon.15
In the early phase, parotid glands are diffusely enlarged with normal parenchyma. In the intermediate phase, the salivary glands are diffusely enlarged with multiple, scattered cysts and solid masses. The cysts range in size from less than 1 mm (micro-cysts) to macro-cysts and mixed solid-cystic masses of more than 2 cm with honeycomb or salt-and-pepper appearance. In the chronic phase, there is atrophy of the glands with low signal intensity.18 The apparent diffusion coefficient value of the parotid glands in Sjögren’s syndrome is well correlated with the salivary flow rate. In the early stages, the apparent diffusion coefficient of the salivary gland increased, whereas in the advanced stage, the apparent diffusion coefficient markedly decreased19 (Figure 1). FDG-PET typically shows high tracer localization in the salivary glands in Sjögren’s sialadenitis.14 Sjögren’s syndrome may result in chronic bilateral inflammation of the lacrimal glands. The lacrimal glands in patients with Sjögren’s syndrome have significantly lower apparent diffusion coefficient values than volunteers20 (Figure 2). In addition, Sjögren’s syndrome may be associated with cranial neuropathy and autoimmune thyroiditis.1–3
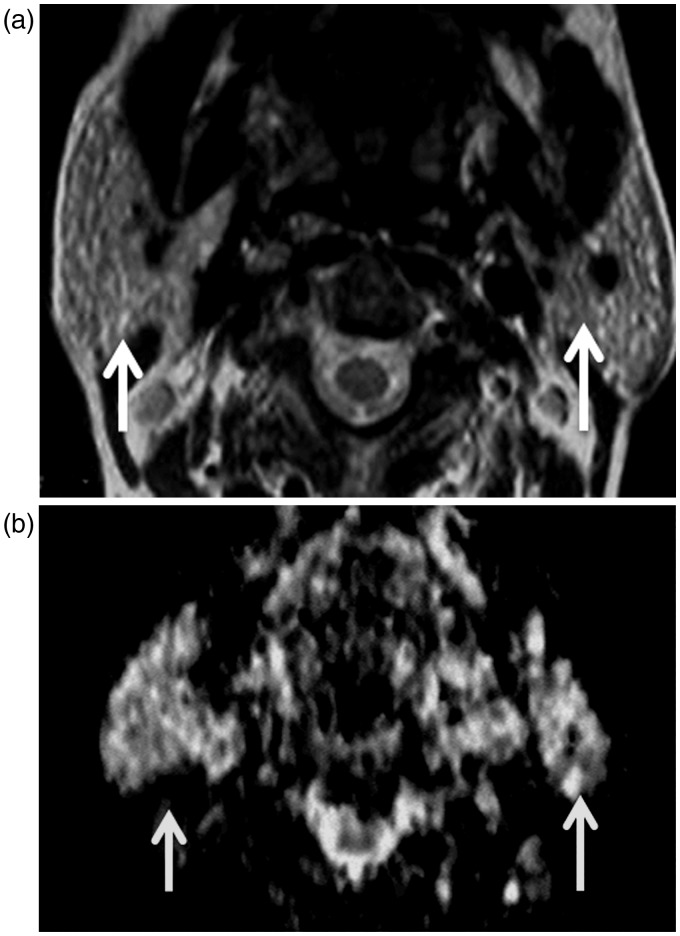
Figure 1.
Sjögren’s syndrome: (a) Axial T2-weighted image shows that multiple hyperintense macro-cysts (arrows) are seen scattered in both parotid glands; (b) Axial diffusion-weighted MR image shows unrestricted diffusion within the micro and macro-cysts (arrows) of both parotid glands.
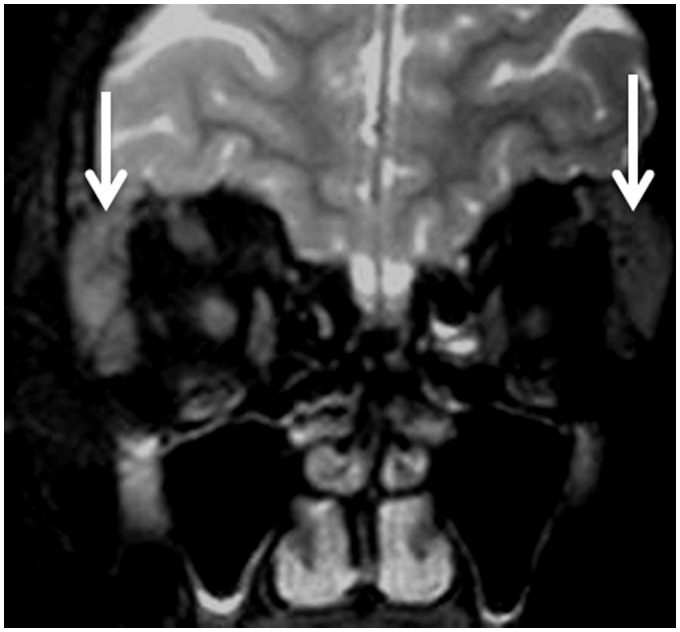
Figure 2.
Sjögren’s syndrome: Coronal T2-weighted image shows bilateral enlarged lacrimal glands with bilateral dacryoadenitis (arrows).
Rheumatoid arthritis
Rheumatoid arthritis is a generalized, chronic, inflammatory, polyarticular connective tissue disease. The average age at the time of rheumatoid arthritis onset in adults is 40 years, with a female/male ratio of 3:1. Rheumatoid arthritis affects the larynx, the temporo-mandibular joint and the cervical spine. Patients are usually asymptomatic (75%) but may present with hoarseness and foreign body sensation (25%).1–3 It may be associated with paraneoplastic syndrome in long-standing lesions such as acute myeloblastic lymphoma and oropharyngeal carcinoma.6
The prevalence of rheumatoid arthritis of the larynx varies between 54% and 72% on CT scans. There is crico-arytenoid thickening with sclerosis in the early stages of the disease and erosions of laryngeal cartilage with subluxation of the joints in the late stages of the disease. In addition, laryngeal nodules have been reported in some patients and may be aggressive, simulating malignancy.21 Imaging shows erosions, flattening, osteophyte, sclerosis and even complete destruction of the mandibular condyle (Figure 3). MR imaging and ultrasound can detect associated synovial proliferation of the temporo-mandibular joints.22,23 The cervical spine is frequently involved in rheumatoid arthritis, and atlanto-axial subluxation is the most common type of instability. Atlanto-axial subluxation occurs when rheumatoid changes affect the synovial joints between the dens and atlas anteriorly and the dens and transverse ligament posteriorly.24 Retropharyngeal abscess is reported in some patients25 (Figure 4).
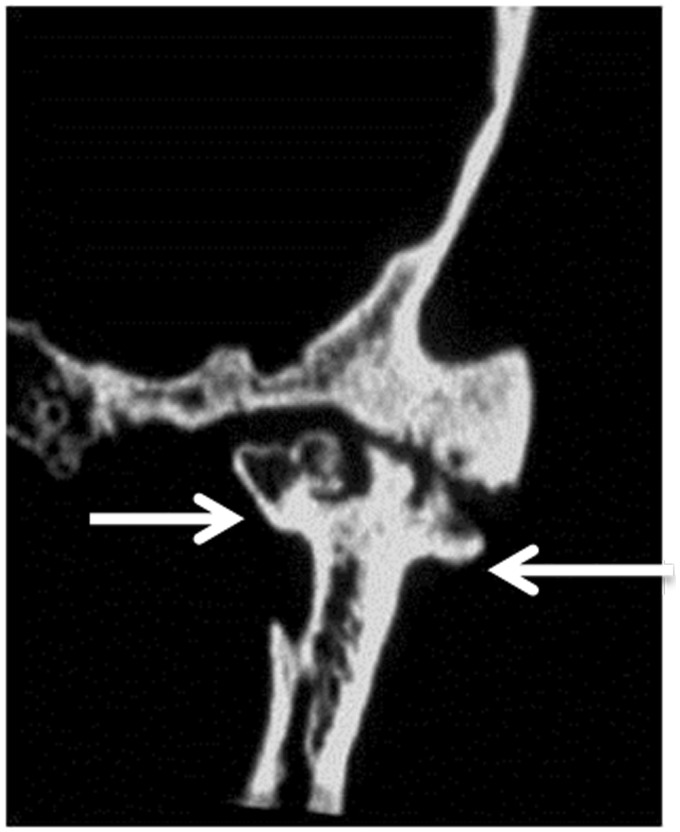
Figure 3.
Rheumatoid arthritis: Coronal CT scan shows destruction of mandibular condyle with subarticular erosions and cysts (arrows) on both sides of the temporo-mandibular joint space.
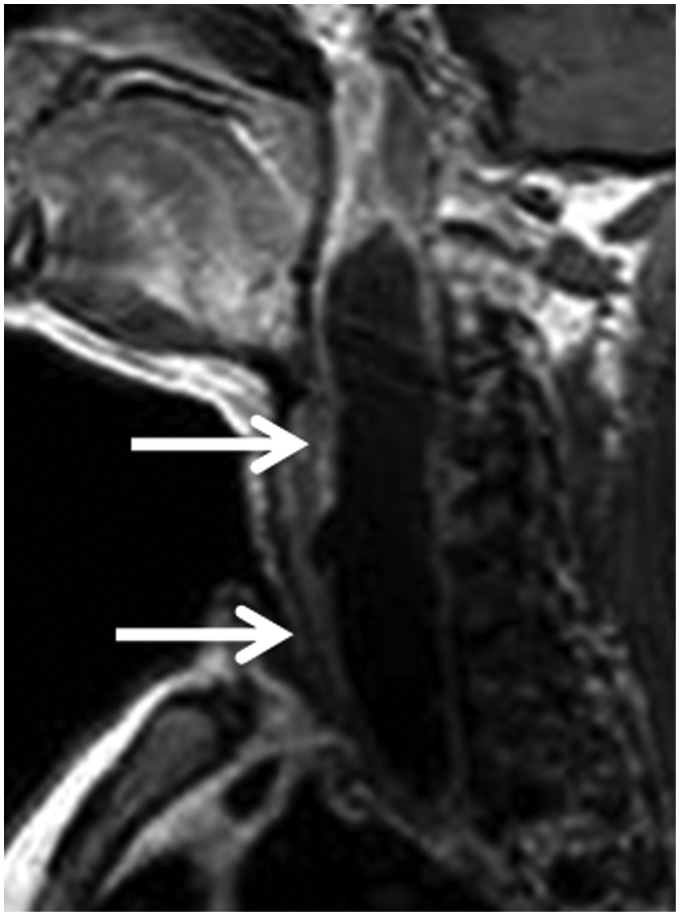
Figure 4.
Rheumatoid arthritis: Sagittal contrast T1-weighted image of the neck shows large marginally enhanced retropharyngeal abscess (arrows) in patient with rheumatoid arthritis.
Systemic lupus erythematosus
Systemic lupus erythematosus is an autoimmune disorder characterized by inflammation, immune complex deposition, vasculitis and vasculopathy. This disorder usually affects women in the third and fourth decades of life. Peripheral blood lymphocytes from patients with systemic lupus erythematosus often contain T cells that exhibit signs of activation.26 These patients are more likely to develop associated tumors such as lymphoma and hairy leukemia, and other malignancies of the head and neck. There is a 2.16-fold higher risk of malignancy in patients with systemic lupus erythematosus compared with the risk of first malignancy in the age-matched controls.1,27
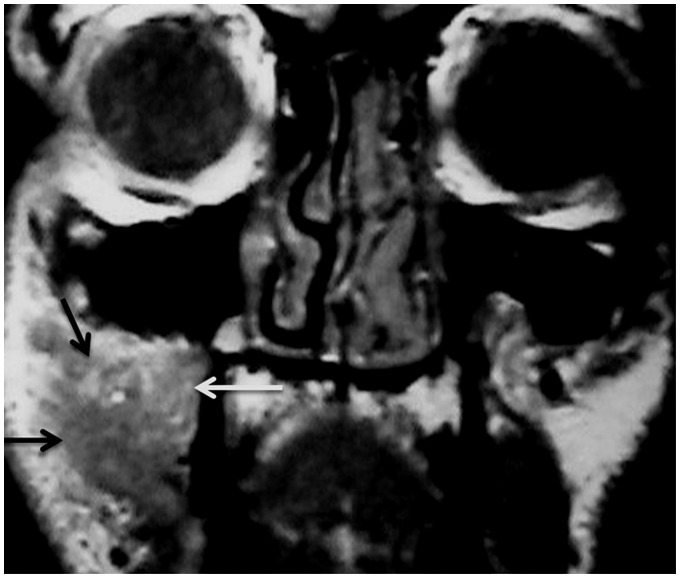
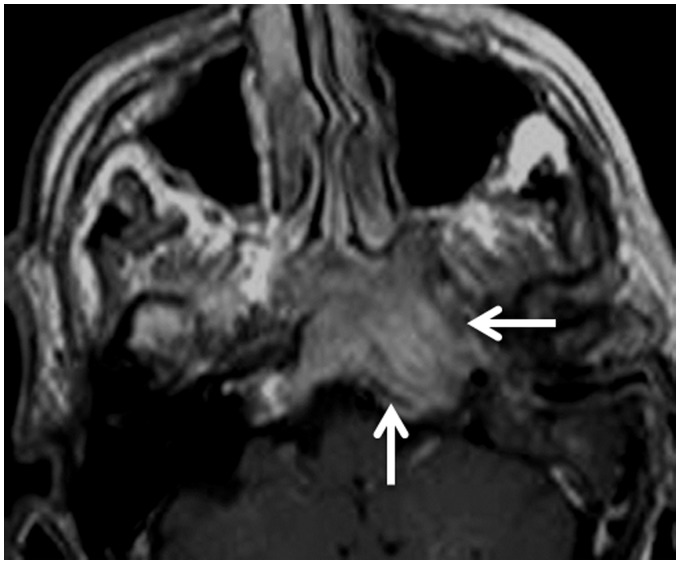
Lupus panniculitis appears as a diffuse thickening of the skin and the underlying subcutaneous soft tissue of the forehead and cheek at CT28 (Figure 5). Discoid lupus erythematosus causes mono-ocular diplopia with progressive, intra-orbital soft-tissue inflammation.29 Parotid necrosis appears as a necrotic region within the parotid gland.26 Bamboo nodules are reported in the true vocal cord in patients with systemic lupus erythromatosus.30 Invasive fungal infection of the mastoid air cells, larynx, cavernous sinus and paranasal sinuses are reported in these patients because they are immune-compromised, with low immunity31 (Figure 6).
Figure 5.
Systemic lupus erythematosus: Coronal contrast T1-weighted MR image shows that enhancing lesion (arrows) is seen involving the subcutaneous soft tissue of the cheek, denoting lupus panniculitis.
Figure 6.
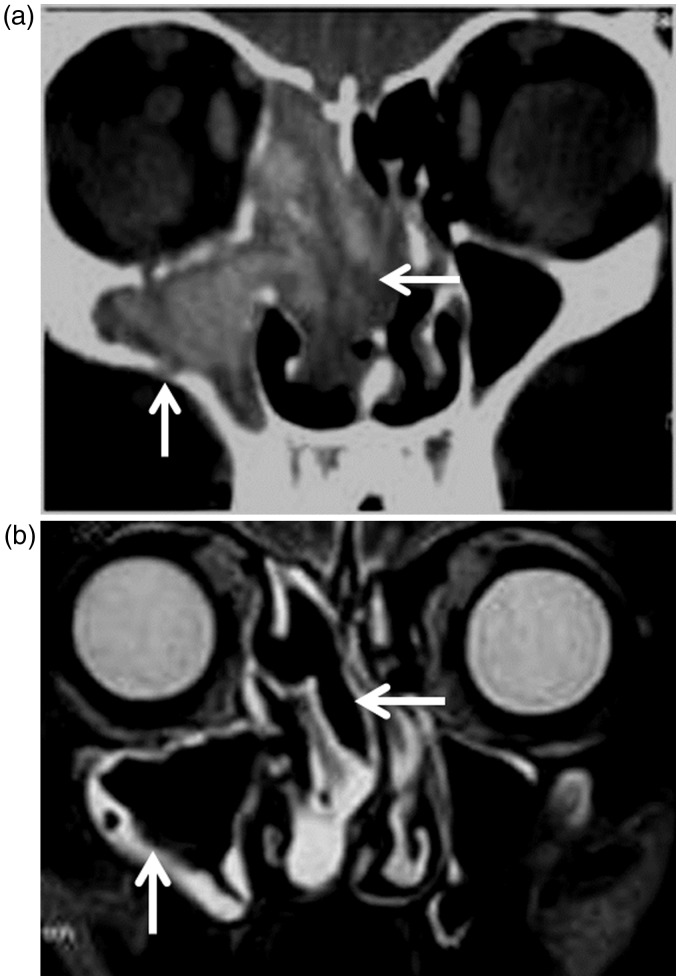
Systemic lupus erythematosus: (a) Coronal CT scan shows that hyperintense lesions (arrows) are seen in the right maxillary sinus, ethmoid air cells and nasal cavity on both sides coping with fungal infection in a patient with systemic lupus erythematosus; (b) Coronal T2-weighted image of the same patient shows signal void regions (arrows) of invasive fungal infection with hyperintense peripheral rim of mucosal thickening.
Relapsing polychondritis
Relapsing polychondritis is a rare autoimmune disease of unknown etiology, characterized by recurrent inflammation and destruction of the cartilage in the auricles, nose, trachea and joints. Relapsing polychondritis is generally observed in the fourth and fifth decade of life and occurs with equal frequency in both sexes.32 Clinical signs of relapsing polychondritis include bilateral auricular chondritis, nasal chondritis, respiratory tract chondritis, cochlear or vestibular dysfunction and non-erosive, sero-negative polyarthritis.3,32 Repeated attacks of inflammation of cartilage from relapsing polychondritis often lead to permanent destruction of the involved tissues and causes disability. The destruction of nose and ear cartilage results in deformity and can impair breathing when the trachea is affected.1 Recurrent inflammation of the larynx can result in permanent laryngomalacia or stenosis of the larynx with inspiratory dyspnea or obstructive sleep apnea syndrome.33–36 The association of relapsing polychondritis with hematological malignancies, particularly with myelodysplastic syndrome, has been described.6
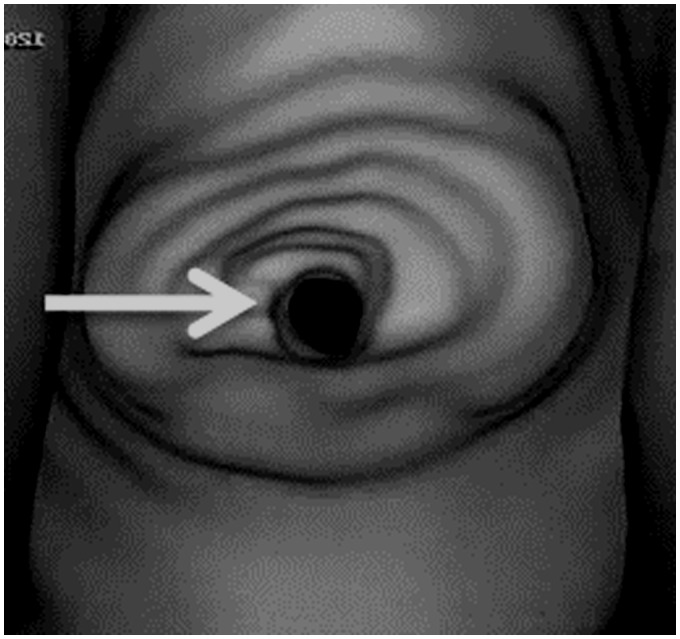
External auditory canal calcification should suggest the diagnosis of relapsing polychondritis. Diffusion-weighted MR imaging shows the characteristic finding of auricular signal hyperintensity, known as ‘prominent ear sign’.34 Destruction of the nasal cartilage with nasal cartilage collapse in patients with relapsing polychondritis results in a classic ‘saddle’ deformity of the nasal bridge.35 CT is very useful for an accurate and rapid assessment of laryngo-tracheo-bronchial involvement and the typical finding is lumen narrowing by wall thickening and collapse of the supporting cartilaginous structures33 (Figure 7). There is thickening and train-track calcifications of the posterior cricoid cartilage. The advanced calcification of the remaining laryngeal cartilages and spotty tracheobronchial calcifications helped to confirm diagnosis. This disorder may be associated with myelodysplastic syndromes.35
Figure 7.
Relapsing polychondritis: virtual laryngoscopy shows narrowed lumen (arrow) of the larynx.
Systemic sclerosis (scleroderma)
Systemic sclerosis (scleroderma) is an uncommon connective tissue disorder characterized by excessive collagen deposition of the skin, blood vessels and other organs. It has an approximately 3:1 female predilection. Diffuse and limited forms of systemic sclerosis refer to the extent of cutaneous involvement.37 CREST syndrome is a variant of systemic sclerosis with its own acronym defined as calcinosis, Raynaud’s phenomenon, esophageal dysmotility, sclerodactyly and telangiectasia.1–5 The association of this disease with malignancyis well established.2–5 There is smooth muscle atrophy and fibrosis of the esophagus with reduced or absent peristaltic contractions. This disorder may be associated with organ malignancy.6
Areas of osteolysis occur in the angle, condyle, ascending ramus and the coronoid process of the mandible has been reported in patients with systemic sclerosis due to abnormal collagen deposition in the adjacent oral facial tissue. The patients without osteolysis showed stronger T2 signals of the masseter muscle than did the normal individuals.37–39 Patients with systemic sclerosis presented with atrophy, fat replacement and rectification of the masseter muscle. The T2 signal is significantly stronger in patients with systemic sclerosis without osteolysis than patients with systemic sclerosis and osteolysis. There may be fibrosis within the intrinsic and extrinsic muscles of the tongue that exhibits low signal intensity on T2-weighted images.1 High-frequency ultrasound shows increased echogenicity of the oral mucosa due to fibrotic deposition. Optic and trigeminal neuropathy and brachial plexopathy is also reported.40 A uniform widening of the periodontal ligament space due to fibrotic thickening has been described in patients with systemic sclerosis.37 There is also calcinosis with the deposition of calcium in the skin and subcutaneous soft tissue simulating calcification in patients with renal osteodystrophy.41,42
Dermatomyositis/polymyositis
Dermatomyositis/polymyositis is a systemic immune disorder – a primary inflammatory myopathy with autoimmune pathogenesis. It is associated with substantial muscle weakening in its course, especially proximally in the extremities. Both polymyositis and dermatomyositis share the diagnostic criteria of symmetrical proximal muscle weakness, raised serum muscle enzymes and muscle biopsy, and electromyography results are consistent with myositis. In addition, dermatomyositis is associated with the presence of a skin rash. Women are affected twice more than men. The first skin signs can appear during childhood as well as in adults.43 In 7%–66% of patients with dermatomyositis, malignant tumors can develop. The associated tumors include nasopharyngeal carcinoma (Figure 8), undifferentiated squamous cell carcinoma, tonsillar papillary carcinoma, thyroid papillary carcinoma and chondrosarcoma.44,45
Figure 8.
Dermatomyositis/polymyositis: Axial contrast T1-weighted image shows an enhancing nasopharyngeal mass (arrows) that extends into the clivus, proved to be nasopharyngeal carcinoma in a patient with dermatomyositis/polymyositis.
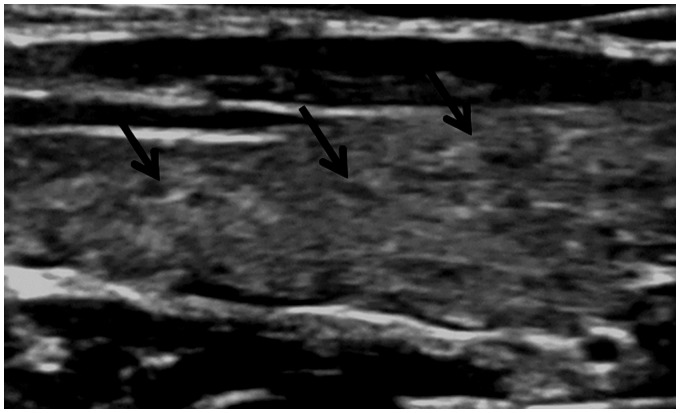
Patients with dermaomyositis/polymyositis may be presented with bilateral condylar resorption of the mandible.1 Autoimmune thyroiditis appears as mixed echo pattern of the thyroid gland, with hypoechoic regions scattered within the glands at ultrasound examination (Figure 9). Orbital infiltration with proptosis, commonly mistaken as myositis, is reported as proximal muscle weakness due to myositis.4,46
Figure 9.
Dermatomyositis/polymyositis: Thyroid ultrasound shows enlarged thyroid lobe with a few small, scattered hypoechoic regions (arrows) denoting thyroiditis.
Antiphospholipid antibody syndrome
Antiphospholipid syndrome is an autoimmune disorder characterized by thrombosis and/or pregnancy morbidity, associated with antiphospholipid antibodies. These thrombotic complications may affect different organ systems involving arterial, venous and microvascular structures. This disorder can exist as an isolated syndrome or may be associated with other connective tissue disorders such as systemic lupus erythematosus. Antiphospholipid syndrome is associated with a wide variety of neoplasms, including solid tumors (mainly renal cell carcinoma) and hematological neoplasms (B-cell lymphoma).6,47
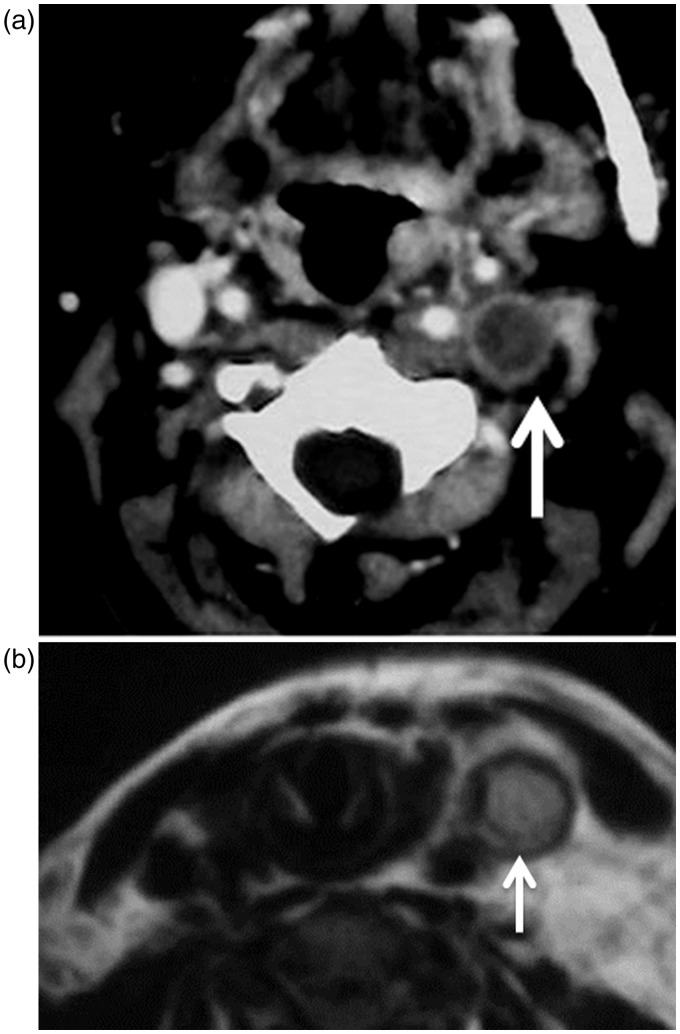
This disorder may be associated with arterial or venous thrombosis. CT and MR imaging and angiography are excellent imaging modalities used for the diagnosis of arterial and venous thrombosis (Figure 10). Lemierre’s syndrome, characterized by anaerobic septicemia, thrombophlebitis of the internal jugular vein and septic emboli, is associated with infections of the head and neck.34–37 At CT, the thrombus appears as non-enhanced within the lumen of the vein, with marginal wall enhancement. The thrombus appears as a hyper-intense signal on T1-weighted images and less hyper-intense on T2-weighted images.48–50
Figure 10.
Antiphospholipid antibody syndrome: (a) Axial CT shows thrombosis of the lumen of the left jugular vein (arrow) with enhancement of the wall of the vein. (b) Axial T2-weighted MR image shows hyperintense thrombus (arrow) is seen in the left jugular vein.
Adult-onset Still’s disease
Adult-onset Still’s disease is characterized by a combination of spiking fever, arthritis, cutaneous rash, sore throat, lymph node enlargement, neutrophilic leukocytosis and disturbed liver metabolism. Lymphadenopathy and splenomegaly are due to activated lymphocytes. Patients presenting with characteristic splenomegaly, cervical lymphadenopathy and non-suppurative pharyngitis is frequent.51 This disorder may be associated with hemophagocytic syndrome, papillary carcinoma of the thyroid gland and lymphoma. Reactive hemophagocytic syndrome is a hematological disorder characterized by the activation of differentiated macrophages to be involved in the phagocytosis of hematopoietic cells.3–6
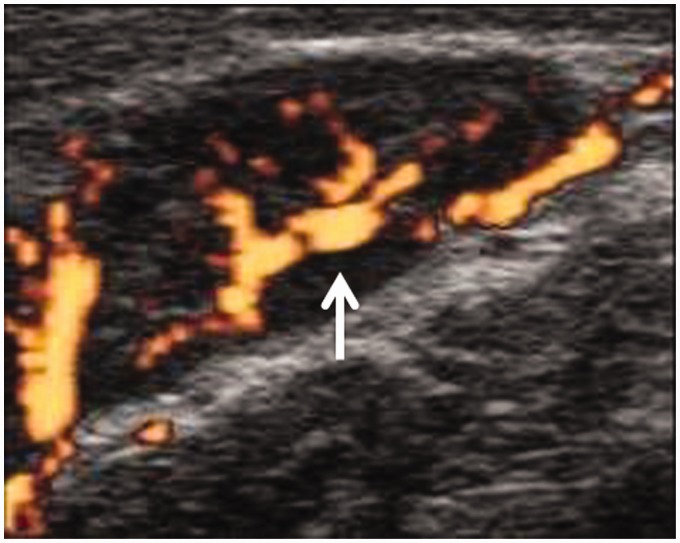
In the head and neck, this disorder shows enlarged cervical lymphadenopathy. Nodes have an oblong appearance with a central hyperechogenic hilum that is associated with a central flow pattern, seen via color duplex examination (Figure 11). Diffusion-weighted MR imaging helps in the characterization of cervical lymphadenopathy.52 Cranial nerve neuritis appears as diffusely enlarged and intense enhanced nerves.53 Thyroid autoimmune disease appears as both thyroid lobes enlarged, with small hypoechoic regions scattered within the thyroid parenchyma at ultrasound examination.54
Figure 11.
Adult-onset Still’s disease: Power duplex ultrasound shows enlarged upper deep cervical lymph nodes with central flow pattern (arrow).
Mixed connective tissue disease
Mixed connective tissue disease has been defined based on its features of four overlapping disease entities, including systemic lupus erythematosus, dermatomyositis/polymyositis, scleroderma or rheumatoid arthritis, plus the presence of high titers of the antibody to RNase-sensitive ribonuclear protein complex.55 Mixed connective tissue disease as a distinct disease entity is still a controversial. It may involve both children and adults, with predominance in women and prevalence in the third decade of life. This disorder may be associated with Castleman’s disease and malignancies such as thyroid carcinoma.3–6
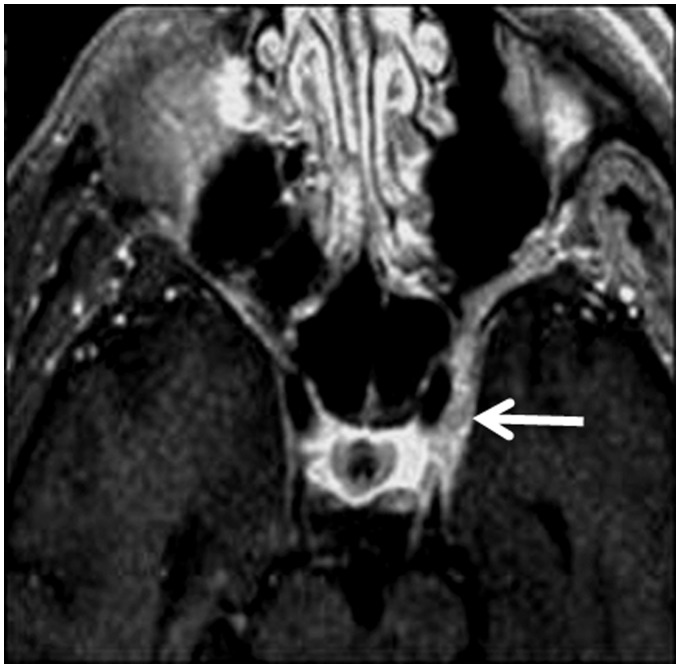
Optic or trigeminal neuritis appears as diffuse enlargement and contrast enhancement of the nerve in patients with connective tissue disease56 (Figure 12). Thyroid autoimmune disease has been reported in patients with mixed connective tissue disorder that appears as multiple small hypoechoic regions within the thyroid glands. Also, laryngeal nodes are reported in some patients.57
Figure 12.
Mixed connective tissue disorder: Axial contrast T1-weighted image shows enhanced and enlarged left trigeminal nerve (arrow) in the left cavernous sinus in patient with trigeminal neuritis.
Conclusion
We concluded that imaging is important for assessment of head and neck findings in patients with connective tissue disorders. Correlation of the imaging findings with the clinical features and laboratory findings is important for the diagnosis of connective tissue diseases and associated malignancies.
Funding
This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest
The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
References
- 1.Famorca L, O’Neill J. Connective tissue disease. In: O’Neill J. (ed). Essential imaging in rheumatology, New York: Springer, 2015, pp. 181–211. [Google Scholar]
- 2.Rao V, Bowman S. Latest advances in connective tissue disorders. Ther Adv Musculoskelet Dis 2013; 5: 234–249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Knopf A, Bas M, Chaker A, et al. Rheumatic disorders affecting the head and neck: underestimated diseases. Rheumatology 2011; 50: 2029–2034. [DOI] [PubMed] [Google Scholar]
- 4.Gera C, Kumar N. Otolaryngologic manifestations of various rheumatic diseases: awareness and practice among otolaryngologists. Indian J Otolaryngol Head Neck Surg 2015; 67: 366–369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Parra-García GD, Callejas-Rubio JL, Ríos-Fernández R, et al. Otolaryngologic manifestations of systemic vasculitis. Acta Otorrinolaringol Esp 2012; 63: 303–310. [DOI] [PubMed] [Google Scholar]
- 6.Azar L, Khasnis A. Paraneoplastic rheumatologic syndromes. Curr Opin Rheumatol 2013; 25: 44–49. [DOI] [PubMed] [Google Scholar]
- 7.Razek AA, Castillo M. Imaging appearance of granulomatous lesions of head and neck. Eur J Radiol 2010; 76: 52–60. [DOI] [PubMed] [Google Scholar]
- 8.Delle Sedie A, Riente L. Ultrasound in connective tissue diseases. Clin Exp Rheumatol 2014; 32: S53–S60. [PubMed] [Google Scholar]
- 9.Razek AA. Diffusion-weighted magnetic resonance imaging of head and neck. J Comput Assist Tomogr 2010; 34: 808–815. [DOI] [PubMed] [Google Scholar]
- 10.Razek AA, Elsorogy LG, Soliman NY, et al. Dynamic susceptibility contrast perfusion MR imaging in distinguishing malignant from benign head and neck tumors: a pilot study. Eur J Radiol 2011; 77: 73–79. [DOI] [PubMed] [Google Scholar]
- 11.Abdel Razek AA, Poptani H. MR spectroscopy of head and neck cancer. Eur J Radiol 2013; 82: 982–989. [DOI] [PubMed] [Google Scholar]
- 12.Tawfik AM, Razek AA, Kerl JM, et al. Comparison of dual-energy CT-derived iodine content and iodine overlay of normal, inflammatory and metastatic squamous cell carcinoma cervical lymph nodes. Eur Radiol 2014; 24: 574–580. [DOI] [PubMed] [Google Scholar]
- 13.Razek AA, Tawfik AM, Elsorogy LG, et al. Perfusion CT of head and neck cancer. Eur J Radiol 2014; 83: 537–544. [DOI] [PubMed] [Google Scholar]
- 14.Nishiyama Y, Yamamoto Y, Dobashi H, et al. Clinical value of 18F-fluorodeoxyglucose positron emission tomography in patients with connective tissue disease. Jpn J Radiol 2010; 28: 405–413. [DOI] [PubMed] [Google Scholar]
- 15.Goules AV, Tzioufas AG, Moutsopoulos HM. Classification criteria of Sjögren’s syndrome. J Autoimmun 2014; 48–49: 42–45. [DOI] [PubMed] [Google Scholar]
- 16.Cornec D, Jousse-Joulin S, Pers JO, et al. Contribution of salivary gland ultrasonography to the diagnosis of Sjögren’s syndrome: toward new diagnostic criteria? Arthritis Rheum 2013; 65: 216–225. [DOI] [PubMed] [Google Scholar]
- 17.Abdel Razek AA, Ashmalla GA, Gaballa G, et al. Pilot study of Ultrasound Parotid Imaging Reporting and Data System (PIRADS): inter-observer agreement. Eur J Radiol 2015; 85: 2533–2538. [DOI] [PubMed] [Google Scholar]
- 18.Takagi Y, Sumi M, Nakamura T. MR microscopy of the parotid glands in patients with Sjögren’s syndrome: quantitative MR diagnostic criteria. AJNR Am J Neuroradiol 2005; 26: 1207–1214. [PMC free article] [PubMed] [Google Scholar]
- 19.Regier M, Ries T, Arndt C, et al. Sjögren’s syndrome of the parotid gland: value of diffusion-weighted echo-planar MRI for diagnosis at an early stage based on MR sialography grading in comparison with healthy volunteers. Rofo 2009; 181: 242–248. [DOI] [PubMed] [Google Scholar]
- 20.Kawai Y, Sumi M, Kitamori H, et al. Diffusion-weighted MR microimaging of the lacrimal glands in patients with Sjögren’s syndrome. AJR Am J Roentgenol 2005; 184: 1320–1325. [DOI] [PubMed] [Google Scholar]
- 21.Chen J, Branstetter IVB, Myers E. Cricoarytenoid rheumatoid arthritis: an important consideration in aggressive lesions of the larynx. AJNR Am J Neuroradiol 2005; 26: 970–972. [PMC free article] [PubMed] [Google Scholar]
- 22.Kretapirom K, Okochi K, Nakamura S, et al. MRI characteristics of rheumatoid arthritis in the temporomandibular joint. Dentomaxillofac Radiol 2013; 42: 31627230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Abdel Razek AA, Al Belasy F, Ahmed W, et al. Assessment of articular disc displacement of temporomandibular joint with ultrasound. J Ultrasound 2015; 18: 159–163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ito K, Sakai K, Yako T, et al. Atlantoaxial dislocation associated with a mass in the extradural craniovertebral junction unrelated to rheumatoid arthritis. Case report. Neurol Med Chir 2007; 47: 182–185. [DOI] [PubMed] [Google Scholar]
- 25.Kakarala K, Durand ML, Emerick KS. Retropharyngeal abscess in the setting of immune modulation for rheumatoid arthritis. Laryngoscope 2010; 120: S131. [DOI] [PubMed] [Google Scholar]
- 26.Yu C, Gershwin ME, Chang C. Diagnostic criteria for systemic lupus erythematosus: a critical review. J Autoimmun 2014; 48–49: 10–13. [DOI] [PubMed] [Google Scholar]
- 27.Zaky MR, Shaat RM, Elbassiony SR, et al. Magnetic resonance imaging (MRI) brain abnormalities of neuropsychiatric systemic lupus erythematosus patients in Mansoura city: relation to disease activity. Egy Rheumatol 2015; 37: S7–S11. [Google Scholar]
- 28.Vattoth S, Curé JK. CT imaging of head and neck lupus panniculitis. AJNR Am J Neuroradiol 2009; 30: 1131–1133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hegde V, Schmalfuss IM. Discoid lupus—an unusual cause of diplopia. Neurographics 2015; 5: 85–87. [Google Scholar]
- 30.Malinvaud D, Mukundan S, Crevier-Buchman L, et al. Glottic bamboo nodules from systemic lupus erythematosus. Ann Otol Rhinol Laryngol 2013; 122: 496–499. [DOI] [PubMed] [Google Scholar]
- 31.Razek AA, Huang BY. Lesions of the petrous apex: classification and findings at CT and MR imaging. Radiographics 2012; 32: 151–173. [DOI] [PubMed] [Google Scholar]
- 32.Vitale A, Sota J, Rigante D, et al. Relapsing polychondritis: an update on pathogenesis, clinical features, diagnostic tools, and therapeutic perspectives. Curr Rheumatol Rep 2016; 18: 3. [DOI] [PubMed] [Google Scholar]
- 33.Thaiss WM, Nikolaou K, Spengler W, et al. Imaging diagnosis in relapsing polychondritis and correlation with clinical and serological data. Skeletal Radiol 2016; 45: 339–346. [DOI] [PubMed] [Google Scholar]
- 34.Kuwabara M, Shimono T, Toyomasu M, et al. “Prominent ear sign” on diffusion-weighted magnetic resonance imaging in relapsing polychondritis. Radiat Med 2008; 26: 438–241. [DOI] [PubMed] [Google Scholar]
- 35.Faix L, Branstetter B. Uncommon CT findings in relapsing polychondritis. AJNR Am J Neuroradiol 2005; 26: 2134–2136. [PMC free article] [PubMed] [Google Scholar]
- 36.Abdel Razek AA. Diagnostic role of magnetic resonance imaging in obstructive sleep apnea syndrome. J Comput Assist Tomogr 2015; 39: 565–571. [DOI] [PubMed] [Google Scholar]
- 37.Chapin R, Hant FN. Imaging of scleroderma. Rheum Dis Clin North Am 2013; 39: 515–546. [DOI] [PubMed] [Google Scholar]
- 38.Marcucci M, Abdala N. Clinical and radiographic study of orofacial alterations in patients with systemic sclerosis. Braz Oral Res 2009; 23: 82–88. [DOI] [PubMed] [Google Scholar]
- 39.Abdel Razek AA. Computed tomography and magnetic resonance imaging of lesions at masticator space. Jpn J Radiol 2014; 32: 123–137. [DOI] [PubMed] [Google Scholar]
- 40.Marcucci M, Abdala N. Analysis of the masseter muscle in patients with systemic sclerosis: a study by magnetic resonance imaging. Dentomaxillofacial Radiol 2009; 38: 524–530. [DOI] [PubMed] [Google Scholar]
- 41.Freire V, Becce F, Feydy A, et al. MDCT imaging of calcinosis in systemic sclerosis. Clin Radiol 2013; 68: 302–309. [DOI] [PubMed] [Google Scholar]
- 42.Abdel Razek AA. Computed tomography and magnetic resonance imaging of maxillofacial lesions in renal osteodystrophy. J Craniofac Surg 2014; 25: 1354–1357. [DOI] [PubMed] [Google Scholar]
- 43.Milisenda JC, Selva-O’Callaghan A, Grau JM. The diagnosis and classification of polymyositis. J Autoimmun 2014; 48–49: 118–121. [DOI] [PubMed] [Google Scholar]
- 44.Razek AA, Huang BY. Soft tissue tumors of the head and neck: imaging-based review of the WHO classification. Radiographics 2011; 31: 1923–1954. [DOI] [PubMed] [Google Scholar]
- 45.Maldonado-Romero LV, Sifuentes Giraldo WA, Larena-Grijalba C, et al. Follicular non-Hodgkin lymphoma-associated dermatomyositis. Rev Clin Esp 2014; 214: 108–109. [DOI] [PubMed] [Google Scholar]
- 46.Abdel Khalek Abdel Razek A, King A. MRI and CT of nasopharyngeal carcinoma. AJR Am J Roentgenol 2012; 198: 11–18. [DOI] [PubMed] [Google Scholar]
- 47.Gómez-Puerta JA, Cervera R. Diagnosis and classification of the antiphospholipid syndrome. J Autoimmun 2014; 48–49: 20–25. [DOI] [PubMed] [Google Scholar]
- 48.Johannesen K, Bødtger U, Heltberg O. Lemierre’s syndrome: the forgotten disease. J Thromb Thrombolysis 2014; 37: 246–248. [DOI] [PubMed] [Google Scholar]
- 49.Abdel Razek AA, Alvarez H, Bagg S, et al. Imaging spectrum of CNS vasculitis. Radiographics 2014; 34: 873–894. [DOI] [PubMed] [Google Scholar]
- 50.Razek AA, Gaballa G, Megahed AS, et al. Time resolved imaging of contrast kinetics (TRICKS) MR angiography of arteriovenous malformations of head and neck. Eur J Radiol 2013; 82: 1885–1891. [DOI] [PubMed] [Google Scholar]
- 51.Díez Morrondo C, Pantoja Zarza L. Current issues of adult-onset Still’s disease. Med Clin 2014; 142: 29–32. [DOI] [PubMed] [Google Scholar]
- 52.Abdel Razek AA, Soliman NY, Elkhamary S, et al. Role of diffusion-weighted MR imaging in cervical lymphadenopathy. Eur Radio 2006; 16: 1468–1477. [DOI] [PubMed] [Google Scholar]
- 53.Razek AA, Castillo M. Imaging lesions of the cavernous sinus. AJNR Am J Neuroradiol. 2009; 30: 444–452. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Ulas T, Dal MS, Bes C, et al. An adult-onset Still’s disease with autoimmune thyroiditis. Mymensingh Med J 2012; 21: 570–572. [PubMed] [Google Scholar]
- 55.Tani C, Carli L, Vagnani S, et al. The diagnosis and classification of mixed connective tissue disease. J Autoimmun 2014; 48–49: 46–49. [DOI] [PubMed] [Google Scholar]
- 56.Hamoir B, Giroux M, Outteryck O, et al. Mixed connective tissue disease presenting as trigeminal neuropathy. Acta Neurol Belg 2014; 114: 245–246. [DOI] [PubMed] [Google Scholar]
- 57.Kim DH, Kim HS. Recurrent orbital myositis in undifferentiated connective tissue disease. Joint Bone Spine 2013; 80: 110–111. [DOI] [PubMed] [Google Scholar]