Abstract
Objective:
Observational studies using routinely collected data indicate that pain management programmes (PMPs) based on cognitive-behavioural principles are associated with clinically meaningful improvements for individuals with chronic pain. This study evaluated change across functional measures in a sample of chronic pain patients attending a 4-week residential PMP between 2006 and 2010. The findings were directly compared with published outcomes from an earlier period (1989–1998) at the same service.
Methods:
Participants included 760 consecutive completers of a multidisciplinary PMP. Data were collected at pre-PMP, post-PMP (1-month post-discharge) and at a 9-month follow-up session. Group-based treatment effects and the reliability and clinical significance of change across functional measures were calculated and compared across cohorts.
Results:
Effect sizes for the recent cohort ranged from small to medium (.43–.67) for pain and physical functioning outcomes to large (.90–1.12) for psychological outcomes at post-treatment (n = 654), and from small (.30–.51) to medium (.58–.71) at 9-month follow-up (n = 493). Clinically significant gains on pain and psychological measures were achieved by 19–55% of patients at post-treatment and 17–44% at follow-up. Comparisons with the earlier cohort showed significantly stronger post-treatment outcomes but differences at follow-up were less marked.
Discussion:
These results add to the evidence base supporting the effectiveness of cognitive-behavioural therapy (CBT)-based pain management interventions. There were significantly larger gains in patient functioning in the recent dataset, suggesting improved programme delivery. But effects were less marked in the longer term, indicating a need for improvements in therapeutic models and related methods to promote meaningful and lasting changes.
Keywords: Chronic pain, interdisciplinary, pain management programme, clinical significance, reliable change
Introduction
Chronic pain (CP) can be a complex problem that is highly disabling and relatively unresponsive to biomedical-based treatments.1 Pain management programmes (PMPs) can be an appropriate way of addressing this complexity. These programmes take a broad interdisciplinary approach focussed directly on psychosocial aspects and on disability. They typically employ cognitive-behavioural therapy (CBT) techniques and self-management strategies to produce their effects.2 Both systematic reviews and meta-analyses of randomised controlled trials (RCTs) show that interdisciplinary approaches based on CBT principles are efficacious treatments for CP, producing mostly small but reliable improvements in pain experience, emotional distress, disability, pain behaviour and coping.3–6
Treatment effects from reviewed trials tend to be variable, a likely consequence of the marked heterogeneity of key study parameters, including patient selection and recruitment, outcome measures employed, intervention type/dose, staff expertise and experience, adherence to and delivery of therapeutic protocol and follow-up timing and frequency.5–7 This appears to reflect the complex reality of CBT-based treatment for the management of CP. This inconsistency is also reflected in the fact that while these treatments appear efficacious in research trials, they are not necessarily effective in actual practice, and the extent to which treatments provide patients with measurable benefits when implemented in actual practice remains uncertain.8,9 Furthermore, while the use of group-based statistics (most obviously, the effect size statistic) offers a common metric across studies and measures within studies to establish efficacious interventions, they are less informative about whether PMP interventions result in clinically important improvements at an individual level.8,10
An increasingly popular approach to determine the effectiveness of PMP interventions has been to administer large-scale observational studies using data generated in routine clinical practice to estimate the proportion of patients who make reliable and clinically significant improvements over the course of treatment and in the months beyond.8–11 In one of the first studies to apply this methodology, Morley et al.9 examined the outcome data of more than 800 patients who attended a 4-week residential PMP based in the United Kingdom (INPUT) from 1989–1998. Although all observed effect sizes showed mean change values reliably greater than the point of no change, only one-third to one-fifth of PMP patients achieved clinically significant gains on measures of pain, emotional distress and self-efficacy, and only 1 in 17 did so on a measure of physical functioning, the 5-minute walk test.
The impact of the findings from Morley and colleagues is limited to some extent by the age of the data and service changes within the 10-year period of study of the INPUT programme which likely impacted PMP delivery. For example, as the programme became established, the service expanded. It began as a small group of seven clinical staff delivering group-based programmes for approximately 150 patients per year and grew to include up to 20 clinical staff providing similar treatments for more than 350 patients per year. Significant staff turnover occurred during this time also, and this was shown to affect outcomes.12
The purpose of this study was to add to the evidence base for the effectiveness of multidisciplinary PMPs by examining the outcomes in a large recently treated cohort in relation to another large cohort treated earlier in the same centre. The more recent period allowed investigation of outcomes from an already well-established service, with staff and patient numbers that were relatively stable throughout. The basic treatment and assessment protocol had remained largely unchanged since the service was launched in 1989; however, thus offering a unique opportunity to investigate whether short- and long-term outcomes in pain, psychological and physical functioning had changed over the course of 20 years. The study employed the same methodology as Morley et al.,9 with an emphasis on clinically significant change, so as to allow direct comparisons of the outcomes from the 2006–2010 cohort with that of the 1989–1998 cohort.
Methods
Data were derived from consecutive patients attending a 4-week publicly funded (UK National Health Service) PMP based at INPUT, St Thomas’ Hospital from August 2006 to April 2010. Over this period, 792 patients participated in the programme as a result of referrals from general practice or other specialist services. All had been formally assessed to determine their suitability for a residential group-based programme by a multidisciplinary team (MDT). Inclusion criteria for the PMP are listed in Table 1.
Table 1.
Inclusion and exclusion criteria for the pain management programme.
| Inclusion criteria |
|---|
| 18 years of age or older |
| Pain present for longer than 6 months |
| A pattern of failures of surgical or medical treatments with no further pain interventions planned |
| Pain having a significant impact on quality of life (affecting two or more of the following): |
| (i) work impaired by pain |
| (ii) non-work activity impaired by pain |
| (iii) habitual overactivity/underactivity cycles |
| (iv) significant distress attributable to pain |
| (v) overuse of analgesic or psychotropic drugs for pain |
| (vi) overuse of aids |
| High levels of reported or observed pain behaviour |
| Willingness to function within a group setting |
| Ability to meet the demands of a residential programme (e.g. able to self-care) |
| Basic standard of reading and understanding in English |
| Exclusion criteria |
| Current major psychiatric disorder (e.g. active psychosis, severe depression with high risk of suicidal behaviour) |
| Brain injury or disease that would be expected to interfere with learning and/or programme participation |
Participants attended the programme as a standard treatment rather than as a research study. Nevertheless, all participants signed an informed consent form providing permission for their anonymised data to be used for research purposes and the study had been approved by the local Trust Research and Development Committee. Ethical approval for the research database was provided by the Oxfordshire Research Ethics Committee (REC number 12/SC/0451).
Participants
The demographic and pain profile data for the 792 PMP participants are displayed in Table 2. Most participants were female and almost 60% were unemployed; the majority of these (463; 58.5%) reported that they were unable to work because of their pain. Almost 90% of patients had experienced pain for more than 3 years. Data on primary pain site, sourced from patients’ clinical notes, indicated a range of pain complaints and sites, although just over half reported that pain was predominantly located in their lower back and/or buttocks. Most were also taking one or more types of medication for their pain at admission.
Table 2.
Socio-demographic data and pain profile for study sample at PMP entry (n = 792). Please note: values represent frequency (percentage) unless otherwise stated.
| Age (mean) | 46.5 (SD = 11.6 years; range: 18–85) |
| Male/female | 241 (30.4%)/551 (69.6%) |
| Marital status | |
| Single | 169 (21.3%) |
| Wife/husband or partner | 497 (62.8%) |
| Separated or divorced | 112 (14.1%) |
| Widowed | 14 (1.8%) |
| Employment status | |
| Employed/student | 237 (30.0%) |
| Unemployed | 472 (59.7% |
| Retired/home duties | 82 (10.4%) |
| Chronicity (mean number of years) | 11.8 (SD = 9.9 years; range: 1–63) |
| Pain for more than 3 years | 707 (89.3%) |
| Main area of pain | |
| Head or face | 31 (3.9%) |
| Neck | 116 (14.6%) |
| Shoulders, hands or arms | 73 (9.2% |
| Chest or throat | 12 (1.5%) |
| Low back or buttocks | 397 (50.1%) |
| Hips, legs or feet | 92 (11.6%) |
| Abdomen | 27 (3.4%) |
| Pelvis | 12 (1.5%) |
| Rectum/vagina | 11 (1.4%) |
| Widespread (no primary site) | 21 (2.7%) |
| How pain began | |
| Accident (not at work) | 186 (23.8%) |
| Accident at work | 78 (10.0%) |
| At work, but not accident | 42 (5.4%) |
| Illness | 56 (7.2%) |
| Following surgery | 61 (7.8%) |
| No precipitating cause | 307 (39.4%) |
| Other (e.g. childbirth, assault) | 50 (6.4%) |
| Medication at admission | 701 (88.5%) |
| Two or more classes | 539 (68.1%) |
| Opioids | 501 (63.3%) |
| Antidepressants | 407 (51.4%) |
| NSAIDs | 380 (48.0%) |
| Tranquilizers | 113 (14.3%) |
| Other pain medication | 331 (42.2%) |
NSAIDs: non-steroidal anti-inflammatory drugs.
Information on employment status was not available for one patient; information on how pain began was not available for 12 patients; information about medication was not available or not recorded at pre-treatment for nine patients; all percentages were calculated from samples that included only patients for which data were available.
Measures and computation of reliable change indices and clinically significant criteria
As part of routine assessment procedures, PMP participants completed standardised questionnaires relating to pain experience, psychological and physical functioning at pre-treatment, programme discharge, 1 month after treatment end (post-treatment) and a 9-month follow-up. Methods to establish clinically meaningful change at an individual level closely followed those of Morley et al.9 To summarise, for each outcome variable, reliable change indices (RCI) were computed using established measures of internal consistency (e.g. Cronbach’s alpha, intra-correlation coefficient (ICC)), or, in the case of single-item measures, test–retest reliability estimates.9 A normative criterion determined by reference to established change percentage was employed for judging clinically significant change on measures of pain experience. For all other measures, Jacobsen’s clinical significance analysis13,14 was adopted to calculate clinically significant thresholds or cut scores.
Pain experience (intensity, distress and interference)
Average pain intensity, distress and interference attributable to pain over the last 7 days were measured on 11-point numerical rating scales (NRS).15 The validity of the NRS and its sensitivity to treatment effects have been well documented.16,17 There were little data available specifically relating to test–retest reliability of (seven-day) average pain measures. In a sample of CP patients undergoing multidisciplinary treatment, Jensen et al.16 reported a test–retest stability coefficient of .82 and .65 of (2-week) average pain intensity scores for 2 weeks after treatment to 1-month post-discharge and 1-month and 2-months follow-up periods, respectively. However, stability coefficients for singular measures of distress and/or interference could not be found. As such, the more conservative of the two estimates in the Jensen et al.16 study (.65) was adopted for reliability of intensity, distress and interference, which yielded RCIs of 2.8, 3.6 and 3.9, respectively. The adopted criterion for clinically significant change on all three NRS scales was 30% change from baseline.18–20
Psychological and physical functioning
Catastrophising about pain was assessed using the Pain Catastrophising Scale (PCS).21 This is a 13-item measure of the tendency to attend to pain stimuli, to overestimate their threat value and to underestimate the ability to handle that threat. Each statement is rated on a scale ranging from 0 (not at all) to 4 (all the time), and total scores range from 0 to 52. An RCI of 12.3 was calculated using the original estimate of internal consistency (Cronbach’s alpha = .87)21 as the adopted reliability coefficient. Norming data from a community sample completing the PCS,22 in addition to baseline data in the present CP sample, was used to establish a clinically significant threshold of 20.8.
Depression was measured using the Beck Depression Inventory (BDI),23 a 21-item test which measures presence and degree of depression in adults consistent with the Diagnostic and Statistical Manual of Mental Disorders (DSM-IV).24 The BDI includes emotional, behavioural and somatic symptoms. Each answer is scored on a scale value of 0–3 and the sum score ranges from 0 to 63. The measure’s psychometric properties are well established. A review of the measure reported good internal consistency (Cronbach’s alpha = .92).25 This yielded a RCI criterion in the present study of 11.4. The adopted clinically significant cut score was 19.7, the same as in the Morley et al.9 study. This was derived from data reported in a study by Geisser et al.,26 in which the BDI was administered to a sample of CP patients with and without depression (as defined by DSM-IV criteria) that were comparable with the current sample.
The Pain Self Efficacy Questionnaire (PSEQ)27 was administered to measure patients’ beliefs about their ability to carry out activities despite their pain. The PSEQ comprises 10 self-report items which ask patients to rate how confident they are on a seven-point scale (0 = not at all confident; 6 = completely confident), for example, ‘I can still accomplish most of my goals in life, despite the pain’. A total score can be calculated, ranging from 0–60, with higher scores indicating greater self-efficacy beliefs. Reported internal consistency is very high (Cronbach’s alpha = .92)28 producing an RCI criterion of 9.4. Data from the distribution of baseline scores from the current sample were used to establish a clinically significant cut score criterion of 46.7.
Physical functioning was assessed using the 5-minute walk described by Harding et al.29 This measure was chosen from a battery of behavioural tests and involves recording the distance covered when walking up and down a corridor over a 5-minute period. The reliability of the 10-minute version (ICC = .94) was used to determine an RCI of 71.1 m, justified by the high correlation between 5- and 10-minute versions (r = .99).29 A clinically significant threshold of 454.6 m was established using the distribution of baseline scores from the current sample.
Programme description
The INPUT PMP is a multidisciplinary treatment programme administered by a specialist team of health care professionals.2 Delivered in a group setting, the 4-week (16-day) residential programme runs on a continuous basis throughout the year, typically with 10 patients per programme. Treatment consists of intensive cognitive and behavioural pain management, intended to provide participants with a better understanding of pain and its influences and to equip patients with skills and management strategies to improve their function. Specific aims of the PMP include restoring optimal physical functioning, improving mood and coping strategies and reducing the use of pain-related drugs. To meet these aims, the PMP involved interactive group sessions on psychological skills (such as anxiety management and methods to improve low mood), exercise routines, education on CP, relaxation techniques, goal setting and medication reviews. On each programme, there were 20–22 sessions of psychology, 22–23 sessions of physiotherapy, 17 sessions of occupational therapy, 7 sessions of nursing, 3 sessions with a pain consultant and additional time for individual sessions, practise time and measurement completion.
Staffing of the PMP
Staff numbers were relatively consistent throughout the examined period; in whole-time equivalents there was 0.3 pain consultant, between 4.0–5.8 clinical psychologists, 5 physiotherapists, between 2.1–2.6 occupational therapists and between 1.5–2.5 specialist nurses. Within each profession, there was a turnover (a total of 12 staff left the service while 10 new staff joined), but each profession retained at least two experienced members of staff who were consistent over the entire study period, except for occupational therapists and pain consultants where one member of staff was retained only. Leavers were replaced by new members of staff with similar experience and qualifications.
Data analysis
For all patients completing the programme, group means and standard deviations for treatment outcome measures at pre-treatment, post-treatment (1-month post-discharge) and follow-up (9 months) were calculated. Treatment outcome data were checked for non-normal distributions; all variables met requirements for univariate normality using skewness and kurtosis estimates (−1.5 through +1.5). Initially, to check for potential bias, those who completed treatment and provided data were compared to those who did not on demographic characteristics, baseline measures of pain and functioning and change from pre-programme to discharge (in the form of residualised change scores (RCS)). Chi square tests were used for categorical variables and independent group t-tests for continuous variables (with the exception of pain duration, where a Mann-Whitney U test was employed).
Improvements through treatment were analysed using repeated measures t-tests to test for statistical differences between pre-treatment and post-treatment/follow-up. The magnitude of change at each stage was expressed with effect size estimates and their 95% confidence intervals9,30,31 using the formula for computing effect size and its variance for repeated measures design, as advocated by Dunlap et al.32 The proportion of patients showing reliable and clinically significant improvements on PMP outcome measures was calculated via Jacobson et al.’s13 RCI and clinically significant change criteria, used to develop categorical outcomes from the continuous scales. Observed effect sizes and proportions of patients achieving reliable and clinically significant changes were explicitly compared to those reported for the cohort of patients attending INPUT from 1989–1998 using z and χ2 tests, respectively. The criterion for statistical significance was set at p < .05 with multiple comparisons in analyses controlled for using the False Discovery Rate Controlling Procedure.33 All statistical analyses were completed with the Statistical Package for the Social Sciences, Release 19.0.
Results
PMP completion and follow-up attendance
Seven hundred and sixty (96.0%) patients completed the entirety of the programme and associated measures at both pre-treatment and programme discharge. Twelve patients completed the programme and associated measures but did not provide consent for their outcome data to be used for audit/research purposes, so were excluded from all further analyses. Six hundred and fifty four consenting patients attended a 1-month post-treatment session, while 493 consenting patients attended a 9-month follow-up. Post-treatment data were available for between 623 and 639 (78.7% and 80.7%) patients depending on the measure, while at 9-month follow-up, outcome data across measures were recorded for between 464 and 480 (58.6% and 60.6%) patients.
There were no significant differences with respect to socio-demographic variables (e.g. gender, age and duration of CP condition) between PMP completers with valid data and those patients who dropped out and/or did not complete measures at discharge (for all variables, p > .090). Patients who dropped out of the PMP did tend to evidence higher average pain intensity at pre-treatment (mean = 7.98, SD = 1.44) than did PMP completers (mean = 7.29, SD = 1.68, p = .039). But there were no significant differences in average pain distress, pain disruptiveness and in psychological functioning (BDI, PCS and PSEQ; for all comparisons, p > .078). At both post-treatment (1-month after treatment end) and 9-month follow-up, attendees and non-attendees were comparable with respect to socio-demographic variables (p > .091). However, compared to post-treatment and 9-month attendees, non-attendees for the same period showed more severe levels of baseline pain intensity, distress and disruptiveness, in addition to elevated scores on pre-treatment measures of psychological dysfunction (for all comparisons, p < .040). Notably, change scores across the programme (RCS on pain variables and psychological measures for pre-treatment to PMP discharge) did not differ between attendees and non-attendees at post-treatment or 9-month follow-up (for all comparisons, p > .072).
Programme outcome and effect sizes
Table 3 shows the mean and standard deviation values on pain, psychological and physical measures at pre-treatment, post-treatment and 9-month follow-up. The reported values for the pre-treatment data are based on the sample sizes used in the pre- to post-treatment comparisons and these scores were not significantly different to the values obtained when the (reduced) sample attending the 9-month follow-up was considered. It is clear from the table that patients benefitted from the PMP across all measured domains, and all differences between pre- and post-treatment scores and between pre-treatment and follow-up scores were highly significant (t > 5.67, p < .001). Across measures, there was no treatment advantage according to patient gender (for all comparisons of gains at post-PMP and follow-up, p > .155) or pain site (for all comparisons of patients with pain predominantly in their neck, lower back/buttocks, shoulders/arms/hands hips/legs/feet or any other location, p > .080). The few significant associations between patient age and benefit in function across the PMP tended to be small in magnitude (pain distress at follow-up, r = .10, p = .024; 5-min walk at post-PMP, r = −.08, p = .040; 5-min walk at follow-up, r = −.17, p < .001). Treatment gains in pain distress at post-PMP tended to be larger for employed or retired patients compared with unemployed patients (p = .030) but comparisons across other measures yielded nonsignificant findings (p > .065).
Table 3.
Means (SDs) and effect sizes (95% CI) for the 2006–2010 sample at pre-treatment, post-treatment (1-month post-discharge) and 9-month follow-up.
|
Pre-treatment
|
Post-treatment
|
Follow-up
|
Pre- to post-treatment
|
Pre-treatment to follow-up
|
|||
|---|---|---|---|---|---|---|---|
| Mean (SD) | Mean (SD) | Mean (SD) | N | ES (95% CI) | N | ES (95% CI) | |
| Pain (0–10) | |||||||
| Intensity | 7.21 (1.71) | 6.32 (2.29) | 6.49 (2.31) | 637 | .43 (.34,.51) | 479 | .30 (.20,.41) |
| Distress | 6.77 (2.21) | 5.34 (2.76) | 5.72 (2.83) | 639 | .57 (.48,.66) | 480 | .36 (.25,.46) |
| Disruption | 6.72 (2.40) | 5.12 (2.94) | 5.47 (3.05) | 637 | .59 (.50,.69) | 479 | .42 (.32,.52) |
| BDI (0–63) | 21.26 (9.39) | 12.61 (9.79) | 15.12 (10.97) | 632 | .90 (.81,.98) | 467 | .58 (.49,.67) |
| PCS (0–52) | 27.65 (12.30) | 14.05 (11.78) | 17.72 (13.63) | 625 | 1.12 (1.03,1.22) | 464 | .71 (.62,.81) |
| PSEQ (0–60) | 22.63 (12.03) | 34.85 (14.20) | 32.41 (15.12) | 634 | .92 (.83,1.01) | 478 | .67 (.57,.77) |
| 5-minute walk (m) | 245.13 (104.74) | 318.76 (113.67) | 306.20 (119.49) | 623 | .67 (.60,.73) | 464 | .51 (.44,.58) |
BDI: Beck Depression Inventory; PCS: Pain Catastrophising Scale; PSEQ: Pain Self Efficacy Questionnaire; SD: standard deviation; N: number of observations at time-points shown; ES: effect size controlled for within-participant correlation; CI: confidence interval.
Intensity, rating of how intense pain was on average in previous seven days; distress, rating of how distressing pain was on average in previous 7 days; interference, rating of disruptiveness of pain on normal activities in previous 7 days.
Estimates of effect sizes (for correlated data) and their 95% confidence intervals for pre- to post-treatment and pre-treatment to follow-up comparisons are also shown in Table 3. Effect sizes were largest for improvements in psychological functioning with less pronounced (but still marked) effects for pain (7-day average intensity, distress and interference). The magnitude of functional change tended to be greater at post-treatment, with effect sizes ranging from .43 for pain intensity to 1.12 for catastrophising. The corresponding values for pre-treatment to follow-up comparisons were .30 for pain intensity and .71 for catastrophising.
Reliable and clinically significant changes in patient functioning
The combination of RCI and clinically significant thresholds was used to classify individual patients into one of eight outcome groups at post-treatment and follow-up (Table 4). For each measure, patients were (initially) separated into individuals who were above and below the defined thresholds at pre-treatment.9 The exception to this was the pain measures, for which the method employed to establish clinically significant cut scores meant that no individual was below criterion. It is notable that a significant proportion of patients were in fact below the threshold (reflecting that they were functioning well in these domains) on measures of depression (44.3%) and/or catastrophising (30.7%) before treatment. However, very few individuals were below criterion for self-efficacy to cope with pain (3.8%) and/or physical functioning (1.3%).
Table 4.
Frequency (percentage) of changes in different outcome categories at post-treatment and follow-up.
| Above criterion pre-treatment |
Below criterion pre-treatment |
|||||||
|---|---|---|---|---|---|---|---|---|
| Reliable deterioration | No change | Reliable improvement | Clinically significant improvement | Clinically significant deterioration | Reliable deterioration | No change | Reliable improvement | |
| Pre- to post-treatment | ||||||||
| Pain | ||||||||
| Intensity | 24 (4) | 482 (76) | 8 (1) | 123 (19) | ||||
| Distress | 18 (3) | 487 (76) | 134 (21) | |||||
| Interference | 22 (3) | 462 (73) | 153 (24) | |||||
| BDI | 3 (1) | 177 (28) | 16 (3) | 156 (25) | 1 (1) | 1 (1) | 237 (38) | 41 (6) |
| PCS | 1 (1) | 151 (24) | 43 (7) | 238 (38) | 2 (1) | 156 (25) | 34 (5) | |
| PSEQ | 24 (4) | 219 (35) | 232 (37) | 135 (21) | 7 (1) | 15 (2) | 2 (1) | |
| 5-minute walk | 9 (1) | 307 (49) | 264 (42) | 35 (6) | 2 (1) | 5 (1) | 1 (1) | |
| Pre-treatment to follow-up | ||||||||
| Pain | ||||||||
| Intensity | 38 (8) | 356 (74) | 2 (1) | 83 (17) | ||||
| Distress | 25 (5) | 372 (78) | 83 (17) | |||||
| Interference | 22 (5) | 361 (75) | 96 (20) | |||||
| BDI | 10 (2) | 149 (32) | 8 (2) | 84 (18) | 8 (2) | 180 (39) | 28 (6) | |
| PCS | 9 (2) | 146 (31) | 18 (4) | 137 (30) | 9 (2) | 126 (27) | 17 (4) | |
| PSEQ | 35 (7) | 194 (41) | 143 (30) | 87 (18) | 6 (1) | 12 (3) | 1 (1) | |
| 5-minute walk | 17 (4) | 260 (56) | 153 (33) | 30 (6) | 1 (1) | 1 (1) | 2 (1) | |
BDI: Beck Depression Inventory; PCS: Pain Catastrophising Scale; PSEQ: Pain Self Efficacy Questionnaire.
Intensity, rating of how intense pain was on average in previous 7 days; Distress, rating of how distressing pain was on average in previous 7 days; Interference, rating of disruptiveness of pain on normal activities in previous 7 days.
Patients above the clinically significant threshold were classified into one of four pre-treatment function classifications: reliable deterioration, no change, reliable (but not clinically significant) improvement, and clinically significant (and reliable) change. Conversely, for patients whose pre-treatment scores were below established criterion, the four possible outcomes were clinically significant (and reliable) deterioration, reliable (but not clinically significant) deterioration, no change, and reliable improvement. Notably, all reliable changes on the NRS pain distress and interference measures were also clinically significant, a consequence of the fact that determined RCI values for the NRS pain distress (3.6) and interference (3.9) were always greater than the corresponding clinically significant criterion (30% change).
Reliable improvements
The percentage of individuals demonstrating reliable improvements in measures of psychological and physical functioning ranged from 34% (BDI) to 59% (PSEQ) at post-treatment and from 26% (BDI) to 49% (PSEQ) at follow-up. The vast majority of patients demonstrated reliable change in at least one outcome measure at post-treatment (82%) and at follow-up (74%), while significant percentages (42% and 19%, respectively) showed reliable improvements on three or more measures.
Clinically significant improvements
Overall, 63% at post-treatment and 53% at follow-up achieved clinically significant change in at least one measure, and 24% and 21% (respectively) evidenced change on three or more measures. About one-fifth to a quarter of all patients at post-treatment evidenced clinically meaningful gains on the pain NRS, depression and self-efficacy measures. This decreased to slightly less than a fifth (17–20%) across these measures at follow-up. The percentage of patients making clinically significant changes was highest for catastrophising and lowest for physical functioning, both at post-treatment and follow-up. When only those whose scores fell above a clinically significant threshold at pre-treatment were included, the percentage at post-treatment and follow-up increased for depression (44% and 33%) and catastrophising (55% and 44%) but was largely unchanged for self-efficacy (22% and 19%) and physical functioning (6% and 7%).
Comparisons of effect size and reliable and clinically significant change between PMP cohorts
There were some notable differences between the 1989–1998 and 2006–2020 PMP cohorts with respect to their socio-demographic and clinical profiles. Although the mean age of the recent cohort (mean = 45.7, SD = 11.7) was highly comparable with that of the earlier cohort (mean = 46.5, SD = 11.6; p = .553), the recent cohort had a higher proportion of women (69.6% vs 61.8%; p < .001) and a lower proportion of patients who were unemployed or permanently sick/disabled (59.7% vs 66.6%; p < .001). However, duration of CP was longer in the 2006–2010 cohort (mean = 141.3, SD = 119.1 vs mean = 113.2, SD = 108.3; p < .001), although the proportion taking medication for pain was significantly less (88.5% vs 95.6%; p < .001). The distribution of primary pain sites was similar across both cohorts, save for lower back/buttocks, which was less prevalent in the recent cohort (50.1% vs 62.7%; p < .001), and neck, which was more prevalent (14.6% vs 6.5%; p < .001).
The cohorts were nevertheless generally well matched with respect to baseline values on corresponding PMP outcome measures. Pain intensity levels were comparable between 2006–2010 (mean = 7.21, SD = 1.71 on 11-point NRS) and 1989–1998 (mean = 72.62, SD = 19.48 on 101-point NRS) cohorts. Psychological functioning also appeared similar with the 2006–2010 cohort evidencing an almost identical mean PSEQ score (mean = 22.66, SD = 10.56) to that of the earlier sample (mean = 22.63, SD = 12.03; p = .960) and the mean BDI score was only slightly elevated in the recent cohort (2006–2010, mean = 21.26, SD = 9.39 vs 1989–1998, mean = 19.24, SD = 8.93; p < .001). The recent cohort did, however, evidence markedly better physical functioning at pre-treatment than the earlier sample (mean 5-minute walk = 245.13 m, SD = 104.74 vs mean = 196.8, SD = 104.46; p < .001).
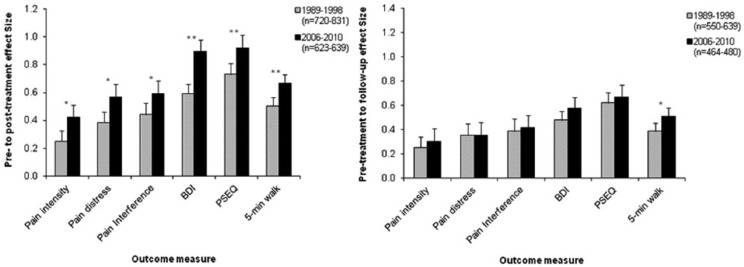
Comparisons between the 1989–1998 and 2006–2010 PMP cohorts with respect to key indices (effect sizes and proportions of individuals making reliable and clinically significant changes) were conducted across outcome measures (Figure 1). Most measures employed in the periods were identical (i.e. BDI, PSEQ, 5-minute walk) allowing like-for-like comparisons. Average pain intensity, distress and interference attributed to pain were measured on a 0–100 (101-point) NRS from 1989–1998 and on a 0–10 (11-point) scale from 2006–2010 – but as internal reliability estimates for these NRS (.65 and .69, respectively) are close, and the clinical cut-off criteria were determined in an identical manner (i.e. improvement of 30%), cohort comparisons on the NRS were performed. Comparisons on catastrophising outcomes were not administered, however, as the adopted internal reliability estimate of the Coping Strategies Questionnaire (CSQ)34 measure of catastrophising employed in 1989–1998 (0.71) was markedly less than the PCS (0.87).
Figure 1.
Effect sizes of pre-to-post-treatment (left) and pre-treatment to follow-up (right) differences for PMP patient cohorts 1989–1998 and 2006–2010. Note: Intensity, rating of how intense pain was on average in previous 7 days; Distress, rating of how distressing pain was on average in previous 7 days; Interference, rating of disruptiveness of pain on normal activities in previous 7 days; BDI, Beck Depression Inventory; PSEQ, Pain Self Efficacy Questionnaire; Error bars represent the 95% confidence interval.
*Indicates significant differences between cohorts (*p < .01; **p < .001).
Across all measures at pre- to post-treatment, effect sizes were significantly larger in the more recent cohort. However, although the 2006–2010 programme gains at follow-up tended to be numerically greater, only 5-minute walk effect sizes were significantly larger in the recent cohort. A similar pattern emerged when considering the proportion of patients above the pre-treatment criterion making reliable changes (Table 5). Across all (comparable) measures at follow-up, patients in 2006–2010 were more likely to demonstrate reliable change, although differences were only significant for pain intensity, pain self-efficacy and 5-minute walk. At follow-up, the proportion of patients in each cohort showing reliable change were more similar, with no (significant) differences after correction. Interestingly, patient numbers evidencing clinically significant changes were more closely aligned between cohorts, particularly at follow-up. Only the post-treatment difference in proportions of patients achieving clinically significant change in pain intensity was statistically significant (after correction).
Table 5.
Percentage (frequency) of patients above pre-treatment criterion demonstrating reliable improvement and clinically significant change at post-treatment and follow-up according to treatment period (1989–1998 vs 2006–2010).
|
Reliable improvement only
|
Clinically significant change
|
|||||
|---|---|---|---|---|---|---|
| 1989–1998 | 2006–2010 | 1989–1998 | 2006–2010 | |||
| Pre- to post-treatment | % (Freq.) | % (Freq.) | p | % (Freq.) | % (Freq.) | p |
| Pain | ||||||
| Intensity | 13 (109) | 21 (131) | <.001 | 13 (109) | 19 (123) | .002 |
| Distress | 19 (153) | 21 (134) | .270 | 19 (153) | 21 (134) | .270 |
| Interference | 22 (185) | 24 (153) | .513 | 22 (185) | 24 (153) | .513 |
| BDI | 42 (150) | 49 (172) | .059 | 37 (132) | 44 (156) | .042 |
| PSEQ | 50 (406) | 60 (367) | <.001 | 17 (139) | 22 (135) | .024 |
| 5-minute walk | 41 (324) | 49 (299) | .007 | 6 (51) | 6 (35) | .614 |
| Pre-treatment to follow-up | % (Freq.) | % (Freq.) | p | % (Freq.) | % (Freq.) | p |
| Pain | ||||||
| Intensity | 13 (76) | 18 (85) | .027 | 13 (76) | 17 (83) | .041 |
| Distress | 20 (119) | 17 (83) | .312 | 20 (119) | 17 (83) | .312 |
| Interference | 22 (129) | 20 (96) | .562 | 22 (129) | 20 (96) | .562 |
| BDI | 36 (88) | 37 (92) | .994 | 33 (79) | 33 (84) | .896 |
| PSEQ | 46 (271) | 50 (230) | .209 | 18 (106) | 19 (87) | .752 |
| 5-minute walk | 35 (188) | 40 (183) | .115 | 6 (31) | 7 (30) | .697 |
BDI: Beck Depression Inventory; PSEQ: Pain Self Efficacy Questionnaire.
Intensity, rating of how intense pain was on average in previous seven days; Distress, rating of how distressing pain was on average in previous 7 days; Interference, rating of disruptiveness of pain on normal activities in previous 7 days. All χ2 tests administered using Yates correction; Measures on which the significant differences between cohorts (after correction) are highlighted in bold.
Discussion
The purpose of this study was to add to the evidence base for multidisciplinary PMPs, analysing routine clinical outcome data from a large sample of CP patients attending a 4-week residential PMP between 2006 and 2010. The key finding is that all post-treatment effect sizes in this sample – which were in the medium range (.40–.70) for pain and physical function outcomes and in the large range (above .80) for psychological measures – were significantly larger than those reported for the 1989–1998 cohort. The proportion of patients at post-treatment meeting the more stringent criteria of reliable and clinically significant change also tended to be greater, although differences were significant only in the cases of pain intensity, self-efficacy and 5-minute walk for reliable change and pain intensity for clinically significant change. Post-treatment changes in catastrophising and self-efficacy – cognitive process variables known to predict disability and pain experience in patients with CP35–37 – were highly pronounced in the recent cohort, with reliable improvements made by more than 65% and 60% of patients above clinically significant thresholds at pre-treatment, respectively, and clinically meaningful gains achieved by 55% and 22% of patients, respectively.
Considering that the baseline pain levels and psychosocial functioning of the two cohorts were generally comparable, the marked improvement in post-treatment outcomes relative to those of the older cohort suggests an improvement in delivery of CBT-based pain management rather than an altered patient referral and/or pre-programme selection procedure. Improved delivery may reflect, at least in part, increased competence levels of PMP staff, a likely consequence of more experienced core staff, better developed training protocols for specialist clinical roles in PMPs and/or improved supervision techniques, factors intrinsically linked with the continued development of multidisciplinary pain management approaches in recent decades.38 More specifically, as previously noted, the unit experienced great organisational change over the course of its first 10 years, most obviously the growth of the clinical staff group and the increasing number of patients undergoing a variety of group-based programmes. The service underwent a consultation in 2006, but the following period was one where the size of the clinical team and the number of patients participating in a 4-week residential PMP on an annual basis were relatively stable. Furthermore, Williams and Potts12 highlighted that staff turnover can negatively affect outcomes in group-based treatment for CP. While there was significant staff turnover between 2006 and 2010, it was less than that during the (longer) earlier period (despite similar overall patient numbers) and somewhat less as a proportion of overall staffing levels (considering the small staff numbers in the initial 5 years; 1989–1994). The latter is likely to be especially relevant assuming that a change of staff was less noticeable when the staff complement was larger which is likely to be relevant.12
While the data indicate better post-treatment outcomes for 2006–2010 compared to 1989–1998 PMP patients, differences at 9-month follow-up were less marked. This suggests that, if indeed treatment is improving in some way, these improvements are not translating into better results in the longer term, or these improved results are less durable than desired, suggesting possibly the role of differing mediators at these separate time-points. Follow-up data from CBT-based pain management interventions frequently indicate that effect sizes are smaller compared to post-treatment.5,7,39 While improvements evidenced by the (recent) cohort under study here did lessen at follow-up, the fact that most patients at follow-up demonstrated reliable change in at least one outcome measure (74%) and effect sizes were still in the medium range (.60–.75) for key psychological domains, suggests that meaningful change occurring over the course of treatment did extend to the months beyond treatment.
Nevertheless, the findings highlight a major challenge for PMP approaches to CP management, namely the long-term maintenance of benefits. Within the context of tailored, individualised pain management, regular follow-up sessions post-treatment appear to help CP patients maintain initial benefits,40 but a similar approach in group-based therapy may not be feasible, at least within the public health system. Furthermore, the aim is to introduce participants to skills that enable them to manage more independently and empower them to be less reliant on health services. It is unclear if practise of learnt self-management strategies after programme completion significantly impacts subsequent function.35,41 But a model solely focussed on adherence is likely to fail to adequately address why some patients lose functional gains achieved over the course of PMP treatment. There is some evidence for the long-term effectiveness of Group-based Acceptance and Commitment Therapy (ACT).31,42 ACT focuses on psychological flexibility and acceptance, encouraging patients to make changes that go beyond efforts to control and manage pain, and it is possible that treatments specifically designed to target these aspects of CP experience lead to consistent improvements in long-term function. Future studies focussed on obstacles subsequently encountered by patients after PMP completion and identifying the specific cognitive and behavioural variables that mediate long-term benefits of pain management interventions are required.43
There are a number of limitations in the current study. First, there were potentially important differences between the 1989–1998 and 2006–2010 cohorts that could not be controlled. For example, the cohorts are both composed of large sample sizes, but the two timeframes are not consecutive or equal in length. Also, compared to the earlier cohort, the more recent cohort showed a greater proportion of women (69% vs 61%), lower pre-PMP unemployment levels (60% vs 67%) and less patients with predominant lower back pain (50% vs 62%). Percentage of women and employment status can significantly influence the outcome of pain management interventions.44–46 All appeared to have little impact on the magnitude of treatment gains in the (recent) cohort here, however, although primary pain site was established via clinical notes rather than PMP patient report (as in the earlier cohort) and vocational status was not assessed at follow-up. Additionally, the most profound changes observed in the recent cohort were in pain catastrophising, a key functional domain targeted by PMPs, yet the change of measure between cohorts did not allow for direct comparisons.
Second, although post-treatment data were (potentially) available from a high proportion (87%) of consenting PMP completers, the percentage attending follow-up dropped to 66%. While this appears better than the rate reported by Morley et al.,9 and only slightly less than the 70% cut-point recommended following a UK national survey of multidisciplinary PMPs,47 it was notable that follow-up attendees in the recent cohort were more functional at baseline than non-attendees across a range of domains. But no bias attributable to attrition was found when considering the magnitude of pain-related and psychological benefit gained by the PMP, suggesting it is unlikely the overall pattern of patient change results would have differed if follow-up attendance rates had been higher.
Third, as the design was not a RCT and no control, comparison groups or random assignment of patients were included, it cannot be assumed that the observed changes are due to treatment effects. However, such is the magnitude of observed effects across multiple domains of functioning in a large number of patients in this study that the pattern of findings would seem highly unlikely in the absence of some type of treatment effect. Also, previous RCTs conducted in the same setting did show that treatment was superior to a wait list control, at least in the short-term.2,48
Finally, as in the previous cohort, the current data identified a small number of patients who reliably deteriorated and the percentage of individuals in this group is slightly higher at 9-month follow-up. This phenomenon has been identified in general psychotherapy and in other pain management interventions.10,35,49 McCracken and Turk3 reported that highly distressed patients who see their pain as an uncontrollable and highly negative life event derive less benefit from PMPs than other patients. Selecting suitable patients for treatment, identifying non-responders early on in the process and preventing adverse effects are some of the issues needing further attention.
Conclusion
Using the methodology applied by Morley et al.9 to estimate clinical effectiveness of a residential multidisciplinary PMP, data from a large sample of participants recently attending the same service yielded post-treatment outcomes that were superior to those of the previous cohort across a range of functional domains. Long-term follow-up results were similar for both groups, however. The data confirm the clinical effectiveness of residential PMPs, but also highlight some challenges in the area of long-term maintenance of gains. Furthermore, the group of patients who did not change reliably or at a clinically significant level remains sizable and efforts need to be directed at making PMPs more impactful for a larger number of participants.
Acknowledgments
We thank Amanda Wall and Anne-Marie Ahern for their help with collating data.
Footnotes
Declaration of Conflicting Interests: The author declared no potential conflicts of interest with respect to the research, authorship and/or publication of this article.
Funding: The authors disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: We thank the Guy’s and St Thomas’ Charity for funding the Research Fellow post of the first author.
References
- 1. Turk DC, Dworkin RH, Revicki D, et al. Identifying important outcome domains for chronic pain clinical trials: an IMMPACT survey of people with pain. Pain 2008; 137: 276–285. [DOI] [PubMed] [Google Scholar]
- 2. Williams ACdC, Richardson PH, Nicholas MK, et al. Inpatient vs. outpatient pain management: results of a randomised controlled trial. Pain 1996; 66: 13–22. [DOI] [PubMed] [Google Scholar]
- 3. McCracken LM, Turk DC. Behavioral and cognitive-behavioral treatment for chronic pain: outcome, predictors of outcome, and treatment process. Spine 2002; 27: 2564–2573. [DOI] [PubMed] [Google Scholar]
- 4. Gatchel RJ, Okifuji A. Evidence-based scientific data documenting the treatment and cost-effectiveness of comprehensive pain programs for chronic nonmalignant pain. J Pain 2006; 7: 779–793. [DOI] [PubMed] [Google Scholar]
- 5. Williams ACdC, Eccleston C, Morley S. Psychological therapies for the management of chronic pain (excluding headache) in adults. Cochrane Database Syst Rev 2012; 11: CD007407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Scascighini L, Toma V, Dober-Spielmann S, et al. Multidisciplinary treatment for chronic pain: a systematic review of interventions and outcomes. Rheumatology 2008; 47: 670–678. [DOI] [PubMed] [Google Scholar]
- 7. Hoffman BM, Papas RK, Chatkoff DK, et al. Meta-analysis of psychological interventions for chronic low back pain. Health Psychol 2007; 26: 1–9. [DOI] [PubMed] [Google Scholar]
- 8. Morley S. Efficacy and effectiveness of cognitive behaviour therapy for chronic pain: progress and some challenges. Pain 2011; 152: S99–S106. [DOI] [PubMed] [Google Scholar]
- 9. Morley S, Williams ACdC, Hussain S. Estimating the clinical effectiveness of cognitive behavioural therapy in the clinic: evaluation of a CBT informed pain management programme. Pain 2008; 137: 670–680. [DOI] [PubMed] [Google Scholar]
- 10. Fedoroff IC, Blackwell E, Speed B. Evaluation of Group and Individual Change in a Multidisciplinary Pain Management Program. Clin J Pain 2013. [DOI] [PubMed] [Google Scholar]
- 11. Fullen B, Blake C, Horan S, et al. Ulysses: the effectiveness of a multidisciplinary cognitive behavioural pain management programme – an 8-year review. Ir J Med Sci 2014; 183: 265–275. [DOI] [PubMed] [Google Scholar]
- 12. Williams ACdC, Potts HWW. Group membership and staff turnover affect outcomes in group CBT for persistent pain. Pain 2010; 148: 481–486. [DOI] [PubMed] [Google Scholar]
- 13. Jacobson NS, Roberts LJ, Berns SB, et al. Methods for defining and determining the clinical significance of treatment effects: description, application, and alternatives. J Consult Clin Psychol 1999; 67: 300–307. [DOI] [PubMed] [Google Scholar]
- 14. Jacobson NS, Truax P. Clinical significance: a statistical approach to defining meaningful change in psychotherapy research. J Consult Clin Psychol 1991; 59: 12–19. [DOI] [PubMed] [Google Scholar]
- 15. Jensen MP, Karoly P, Braver S. The measurement of clinical pain intensity: a comparison of six methods. Pain 1986; 27: 117–126. [DOI] [PubMed] [Google Scholar]
- 16. Jensen MP, Turner JA, Romano JM, et al. Comparative reliability and validity of chronic pain intensity measures. Pain 1999; 83: 157–162. [DOI] [PubMed] [Google Scholar]
- 17. Jensen MP, Karoly P. Self-report scales and procedures for assessing pain in adults. In: Turk DC, Melzack R. (eds) Handbook for pain assessment. New York: Guilford Press, 1992, pp. 135–151. [Google Scholar]
- 18. Dworkin RH, Turk DC, Wyrwich KW, et al. Interpreting the clinical importance of treatment outcomes in chronic pain clinical trials: IMMPACT recommendations. J Pain 2008; 9: 105–121. [DOI] [PubMed] [Google Scholar]
- 19. Farrar JT. What is clinically meaningful: outcome measures in pain clinical trials. Clin J Pain 2000; 16: S106–S112. [DOI] [PubMed] [Google Scholar]
- 20. Farrar JT, Young JP, Jr, LaMoreaux L, et al. Clinical importance of changes in chronic pain intensity measured on an 11-point numerical pain rating scale. Pain 2001; 94: 149–158. [DOI] [PubMed] [Google Scholar]
- 21. Sullivan MJL, Bishop SR, Pivik J. The Pain Catastrophizing Scale: development and validation. Psychol Assessment 1995; 7: 524–532. [Google Scholar]
- 22. Osman A, Barrios FX, Gutierrez PM, et al. The Pain Catastrophizing Scale: further psychometric evaluation with adult samples. J Behav Med 2000; 23: 351–365. [DOI] [PubMed] [Google Scholar]
- 23. Beck AT, Ward CH, Mendelson M, et al. An inventory for measuring depression. Arch Gen Psychiatry 1961; 4: 561–571. [DOI] [PubMed] [Google Scholar]
- 24. American Psychiatric Association. Diagnostic and statistical manual of mental disorders, 4th edn. Washington, DC: American Psychiatric Association, 1994. [Google Scholar]
- 25. Beck AT, Steer RA, Carbin MG. Psychometric properties of the Beck Depression Inventory: twenty-five years of evaluation. Clin Psychol Rev 1988; 8: 77–100. [Google Scholar]
- 26. Geisser ME, Roth RS, Robinson ME. Assessing depression among persons with chronic pain using the Center for Epidemiological Studies-Depression Scale and the Beck Depression Inventory: a comparative analysis. Clin J Pain 1997; 13: 163–170. [DOI] [PubMed] [Google Scholar]
- 27. Nicholas MK. The pain self-efficacy questionnaire: taking pain into account. Eur J Pain 2007; 11: 153–163. [DOI] [PubMed] [Google Scholar]
- 28. Asghari A, Nicholas MK. Pain self-efficacy beliefs and pain behaviour. A prospective study. Pain 2001; 94: 85–100. [DOI] [PubMed] [Google Scholar]
- 29. Harding VR, Williams ACdC, Richardson PH, et al. The development of a battery of measures for assessing physical functioning of chronic pain patients. Pain 1994; 58: 367–375. [DOI] [PubMed] [Google Scholar]
- 30. Vowles KE, Witkiewitz K, Sowden G, et al. Acceptance and commitment therapy for chronic pain: evidence of mediation and clinically significant change following an abbreviated interdisciplinary program of rehabilitation. J Pain 2014; 15: 101–113. [DOI] [PubMed] [Google Scholar]
- 31. Vowles KE, McCracken LM, O’Brien JZ. Acceptance and values-based action in chronic pain: a three-year follow-up analysis of treatment effectiveness and process. Behav Res Ther 2011; 49: 748–755. [DOI] [PubMed] [Google Scholar]
- 32. Dunlap WP, Cortina JM, Vaslow JB, et al. Meta-analysis of experiments with matched groups or repeated measures designs. Psychol Methods 1996; 1: 170. [Google Scholar]
- 33. Curran-Everett D. Multiple comparisons: philosophies and illustrations. Am J Physiol Regul Integr Comp Physiol 2000; 279: R1–R8. [DOI] [PubMed] [Google Scholar]
- 34. Rosenstiel AK, Keefe FJ. The use of coping strategies in chronic low back pain patients: relationship to patient characteristics and current adjustment. Pain 1983; 17: 33–44. [DOI] [PubMed] [Google Scholar]
- 35. Nicholas MK, Asghari A, Corbett M, et al. Is adherence to pain self-management strategies associated with improved pain, depression and disability in those with disabling chronic pain? Eur J Pain 2012; 16: 93–104. [DOI] [PubMed] [Google Scholar]
- 36. Sullivan MJL, Lynch ME, Clark AJ. Dimensions of catastrophic thinking associated with pain experience and disability in patients with neuropathic pain conditions. Pain 2005; 113: 310–315. [DOI] [PubMed] [Google Scholar]
- 37. Turner JA, Dworkin SF, Mancl L, et al. The roles of beliefs, catastrophizing, and coping in the functioning of patients with temporomandibular disorders. Pain 2001; 92: 41–51. [DOI] [PubMed] [Google Scholar]
- 38. Gatchel RJ, McGeary DD, McGeary CA, et al. Interdisciplinary chronic pain management: past, present, and future. Am Psychol 2014; 69: 119–130. [DOI] [PubMed] [Google Scholar]
- 39. Oslund S, Robinson RC, Clark TC, et al. Long-term effectiveness of a comprehensive pain management program: strengthening the case for interdisciplinary care. Proc 2009; 22: 211–214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Monticone M, Ferrante S, Rocca B, et al. Effect of a long-lasting multidisciplinary program on disability and fear-avoidance behaviors in patients with chronic low back pain: results of a randomized controlled trial. Clin J Pain 2013; 29: 929–938. [DOI] [PubMed] [Google Scholar]
- 41. Curran C, Williams ACdC, Potts HW. Cognitive-behavioral therapy for persistent pain: does adherence after treatment affect outcome? Eur J Pain 2009; 13: 178–188. [DOI] [PubMed] [Google Scholar]
- 42. Hayes SC, Strosahl K, Wilson KG. Acceptance and Commitment Therapy: an experiential approach to behavior change. New York: Guilford Press, 1999. [Google Scholar]
- 43. Ehde DM, Dillworth TM, Turner JA. Cognitive-behavioral therapy for individuals with chronic pain: efficacy, innovations, and directions for research. Am Psychol 2014; 69: 153–166. [DOI] [PubMed] [Google Scholar]
- 44. Pieh C, Altmeppen J, Neumeier S, et al. Gender differences in outcomes of a multimodal pain management program. Pain 2012; 153: 197–202. [DOI] [PubMed] [Google Scholar]
- 45. Van Hooff ML, Spruit M, O’Dowd JK, et al. Predictive factors for successful clinical outcome 1 year after an intensive combined physical and psychological programme for chronic low back pain. Eur Spine J 2014; 23: 102–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Waterschoot FP, Dijkstra PU, Hollak N, et al. Dose or content? Effectiveness of pain rehabilitation programs for patients with chronic low back pain: a systematic review. Pain 2014; 155: 179–189. [DOI] [PubMed] [Google Scholar]
- 47. Peat GM, Moores L, Goldingay S, et al. Pain management program follow-ups. A national survey of current practice in the United Kingdom. J Pain Symptom Manage 2001; 21: 218–226. [DOI] [PubMed] [Google Scholar]
- 48. Williams ACdC, Nicholas MK, Richardson PH, et al. Generalizing from a controlled trial: the effects of patient preference versus randomization on the outcome of inpatient versus outpatient chronic pain management. Pain 1999; 83: 57–65. [DOI] [PubMed] [Google Scholar]
- 49. Mohr DC. Negative outcome in psychotherapy: a critical review. Clin Psychol: Sci Pr 1995; 2: 1–27. [Google Scholar]