Abstract
Background:
This study was performed to effects of garlic and lemon juice mixture on lipid profile and some cardiovascular risk factors in people 30–60 years old with moderate hyperlipidemia.
Methods:
In a parallel-designed randomized controlled clinical trial, a total of 112 hyperlipidemic patients 30–60 years, were recruited from Isfahan Cardiovascular Research Center. People were selected and randomly divided into four groups. Control blood samples were taken and height, weight, and blood pressure were recorded. (1) Received 20 g of garlic daily, plus 1 tablespoon lemon juice, (2) received 20 g garlic daily, (3) received 1 tablespoon of lemon juice daily, and (4) did not receive garlic or lemon juice. A study technician was done the random allocations using a random numbers table. All participants presented 3 days of dietary records and 3 days of physical activity records during 8 weeks. Blood samples were obtained at study baseline and after 8 weeks of intervention.
Results:
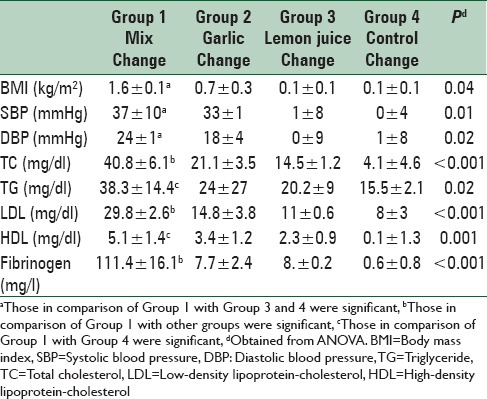
Results showed a significant decrease in total cholesterol (changes from baseline: 40.8 ± 6.1, P < 0.001), low-density lipoprotein-cholesterol (29.8 ± 2.6, P < 0.001), and fibrinogen (111.4 ± 16.1, P < 0.001) in the Group 1, in comparison with other groups. A greater reduction in systolic and diastolic blood pressure was observed in Group 1 compared with the Groups 3 and 4 (37 ± 10, P = 0.01) (24 ± 1, P = 0.02); respectively. Furthermore, a great reduction in body mass index was observed in the mixed group compared with the lemon juice and control groups (1.6 ± 0.1, P = 0.04).
Conclusions:
Administration of garlic plus lemon juice resulted in an improvement in lipid levels, fibrinogen and blood pressure of patients with hyperlipidemia.
Keywords: Cardiovascular disease, garlic, hypercholesterolemic patient, lemon juice
INTRODUCTION
Cardiovascular diseases (CVDs) are multifactorial disorders which are characterized by multiple metabolic dysfunctions. Epidemiologic studies indicate that increased serum lipids profiles, elevated plasma fibrinogen, and coagulation factors play critical roles.[1] CVDs are the major cause of mortality and morbidity in the world. Unfortunately, they lead to 17 million deaths every year. It has been estimated that this number will reach 24.8 million people in 2030 worldwide.[2] CVDs are considered to be the most common cause of death (25–45% mortality) and the fifth most common cause of disability.[3] According to the World Health Organization statistics, the most important risk factors include tobacco usage, high blood pressure, alcoholism, high cholesterol, low fruit, and vegetable consumption, lack of appropriate physical activity and obesity.[1] Iran is the fifth country, throughout the world in terms of having high blood pressure related diseases.[4] High blood pressure and risk factors in CVDs are the main causes of death and mortality in Iran and according to the report of Iranian Health Profile Survey in 1999, the prevalence of blood pressure in adults over 30 years increased by 12–45%.[5] Previous studies showed that 46% of the total deaths in 18 provinces of Iran were caused by CVD.[6] Approximately, 6.6 million people between the ages of 25–64 have high blood pressure, and it is estimated that 12 million persons between 25 and 46 years of age are at increased risk of CVD.[7] Oxidative stress has a pathological role in CVD, particularly its effects on low-density lipoprotein-cholesterol (LDL-C) oxidation, and eventually leads to inflammation status.[8] Dietary factors show a key role in the management of CVD. Epidemiologic studies noticed that diets rich in vegetables and fruits are affiliated with a lower risk of mortality, particularly cardiovascular diseases related deaths.[9,10] It has also been indicated that the benefits of vegetable and fruit intake appear to be related to CVD.[11] These food groups contain different phytochemicals which can demonstrate anti-inflammatory properties. One of the most well-known sources of anti-CVD phytochemicals is garlic, which plays an effective role in the suppression and treatment of CVDs.[12] Even though pharmacological interventions cause a significant reduction in high blood pressure and dyslipidemia, lifestyle modification and correcting the dietary regime can be a key step in the management of CVD.[13] Among foods which are effective in reducing inflammation status and ultimately cardiovascular parameters, the use of garlic and lemon juice is common. Garlic is used in various forms such as raw garlic, powdered garlic tablets, or extracted oil. Lipid levels, blood pressure, reactive oxygen species, and other cardiovascular risk factors affected by garlic will decline that has been confirmed by several studies.[14] Epidemiological studies have demonstrated that increased consumption of polyphenols such as flavonoids and phenylpropanoids phytochemicals that are present in fruits and vegetables are associated with reduced risk of CVD. Lemon juice is high in erycosytryn and hesperidin flavones. Animal studies showed that erycosytryn and hesperidin have antioxidant properties, and they can decrease oxidative stress.[12] Several studies have been conducted concerning the relationship between garlic and lemon juice in reducing inflammatory biomarkers and lipid levels in patients with CVD, separately. However, the effects of garlic and lemon juice mixture on the indicators of CVDs have not yet been analyzed. Regarding the increasing prevalence of these diseases, we examined the effects of garlic and lemon juice mixture on some CVD risk factors such as total cholesterol (TC), LDL-C, systolic blood pressure (SBP), and diastolic blood pressure (DBP) and fibrinogen in people 30–60 years old with moderate hyperlipidemia.
METHODS
Participants
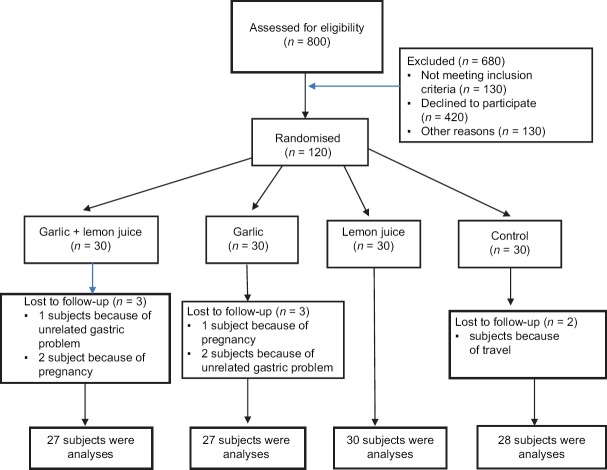
In a parallel-designed randomized controlled clinical trial, hyperlipidemic patients 30–60 years were recruited from Isfahan Cardiovascular Research Center (ICRC) from November to December 2013. Considering the proposed formula for parallel-design randomized trials, given the Type I error of 5%, the study power of 90% considering TC as the key variable. The sample size calculation was determined,[15] we needed 120 patients to be enrolled. Subjects were eligible if they had a fasting TC and LDL-C levels between 200–240 mg/dl and 100–160 mg/dl, respectively. Participants were excluded if they were suffering from any other chronic diseases, including heart diseases, kidney failure, lung dysfunction, thyroid disorders, gastrointestinal disorders, rheumatoid arthritis, and other liver diseases such as hepatitis or fatty liver. Participants that were pregnant lactating, or that smoked were also excluded. In addition, participants’ body weight should not have changed in the last 2 months. Subjects were excluded if they were on medication which affected blood sugar, lipid profile, blood pressure, or anti-inflammatory markers. Participants should not have had any food allergy or sensitivity to garlic or lemon juice consumption. Individuals with a history of following any special diet (vegetarian/vegan, etc.) were excluded as well. From the revision of the medical records available in ICRC, 800 patients were chosen. These subjects were invited for screening as they met nearly all of the inclusion criteria. After the screening process, 120 eligible volunteers enrolled into the study and were randomly allocated to four groups: (1) Mixed with garlic and lemon juice group, (2) garlic group, (3) lemon juice group and (4) control group. Of the 120 subjects randomly assigned to treatment, two subjects in the control group in the beginning of intervention were dropped out from the study because of their busy schedule and over the duration of the study, two subjects in the mixed group because of pregnancy and three subjects because of the gastric problem were eliminated from this study. One subject in the garlic group because of pregnancy and two subjects because of travel were eliminated. Finally, 112 subjects continued until the end of the study. The participant flow diagram is shown in Figure 1. All participants provided informed written consent. This trial was approved by the Isfahan University of Medical Sciences (392436) and was registered in clinical trials center's website address (www.irct.ir) (Code: IRCT2015020720986N1).
Figure 1.
Study flow diagram
Study design
In a parallel-designed randomized controlled clinical trial, professor of biostatistics did the random allocations. In this study, group blinding was not possible because the intervention involved taking or not taking garlic or lemon juice. However, laboratory personnel analyzing results were blinded. During of the intervention, subjects were allowed to continue performing daily diet and activities. Group 1 received 20 g of raw garlic daily plus 1 tablespoon of lemon juice (n = 30), Group 2 received 20 g garlic daily (n = 30), Group 3 received 1 tablespoon of lemon juice daily, 2 h after dinner (n = 30), and Group 4 did not receive garlic, or lemon juice (n = 30) for 8 weeks. Participants were asked to use garlic and lemon juice for 8 weeks. When the participants were given packets of garlic and bottle of lemon juice, we were wanted not to change their lifestyle, physical activity, or diet during the study. Participants were asked to bring back the empty packets and bottles at the final session to help assure compliance.
Variables assessment
They were instructed to avoid change in diet and physical activity, so their diet and physical activity were similar, and it was checked by researchers and their records. All participants provided 3 physical activity records (3 days) and 3 dietary records (3 days) during the intervention at 0, 4, and 8 weeks. They provided 3 days of physical activity records and 3 days of dietary records (1 weekend day and 2 weekdays) to certify they preserved their usual diet and activity levels during the intervention. The presented portion sizes in the dietary records were changed to grams. The average amount (g) of food intake data, based on 3, 3 days of dietary records, was joined with Nutritionist IV software (N-Squared Computing, Cincinnati, OH, USA) to conclude nutrient intake data. The nutrient database of Nutritionist IV software was based on the United States Department of Agriculture food composition table, reclaimed for Iranian foods. Physical activity was declared as metabolic equivalents per hour per day. To quantify measures of blood sampling and metabolic profiles were carried out at study baseline and after 8 weeks of intervention.
After overnight fasting, blood samples were collected from patients between 7 and 10 am. After centrifuging at 4°C at 500 × g for 10 min, tubes were frozen at −80°C until analysis.). Fibrinogen was determined by latex immunoturbidimetric assay (Bionic, Iran). TC, triglyceride (TG), high-density lipoprotein (HDL) were measured by photometric enzyme assay (Pars Azmoon, Iran) and LDL were measured. Both intra- and inter-assay coefficients of variation were <5% for all of the used measurement kits. Biochemical parameters were measured at baseline and after 8 weeks of the intervention. When participants were in the comfortable seat position, after 10 min rest, blood pressure was measured on the left arm using electronic blood monitor. Body weight and height were measured with light clothing and without shoes to the nearest 0.1 kg and 0.1 cm, respectively and body mass index (BMI) was calculated. Except height, other physical characteristics and blood pressure were measured 2 times, in study week 0, 8, respectively.
Statistical analysis
Data were analyzed using SPSS (version 18, SPSS, Chicago, IL, USA), and a two-sided P < 0.05 was considered to be significant. To quantify measures of metabolic profiles, blood sampling was done at the beginning of the study to establish a baseline and after 8 weeks of intervention. To ensure the normal distribution of variables, we applied the Kolmogorov–Smirnov test. For main effect analysis, the difference between the four groups based on outcome variables such as dietary intake, physical activity, and metabolic profile were compared using ANCOVA test controlling for a baseline of these variables and age/gender. Baseline general characteristics were examined using Chi-square for categorical variables and one-way ANOVA for continuous variables. We used Tukey's post hoc comparisons to identify pairwise differences when we reached a significant finding in ANOVA.
RESULTS
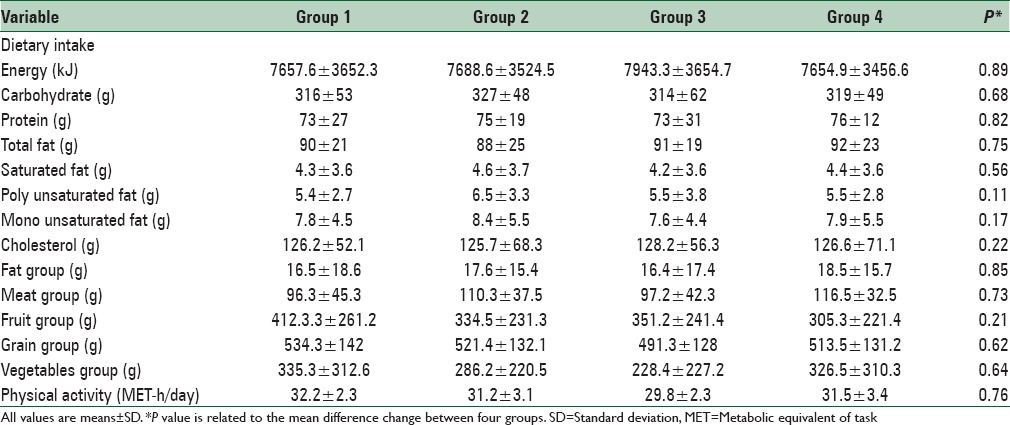
From the review of the medical records available in ICRC, 800 patients were selected. These subjects were called for screening as they met almost all of the inclusion criteria. After the screening process, 120 eligible volunteers enrolled into the study. After randomization, 8 person withdrew due to travel (n = 2), gastric problem (n = 3), and pregnancy (n = 3). Thus, 112 subjects completed the study. The mean age of the participants was 42.46 ± 8.11 years. None of them were consume any nutritional supplement and no one on specific medication. The distribution of participants in terms of sex, weight, BMI was not significantly different between the four intervention groups. The baseline characteristics of the 112 subjects are listed in Table 1. Comparison of baseline metabolic profiles such as serum TC, LDL-C, HDL-C, triglyceride and fibrinogen levels, SBP and DBP revealed no significant difference between the groups. No significant differences were found between groups regarding food groups, carbohydrates, saturated fatty acids, or the majority of micronutrients as derived from the food record. Participants’ physical activity level was generally moderate and did not have any strenuous exercise. They were instructed to avoid change in their physical activity and were checked by researchers. We did not find any significant difference in the physical activity levels between the four intervention groups. Energy, nutrient intake, food groups, and physical activity during the intervention period were shown in Table 2.
Table 1.
Baseline characteristics of study participants
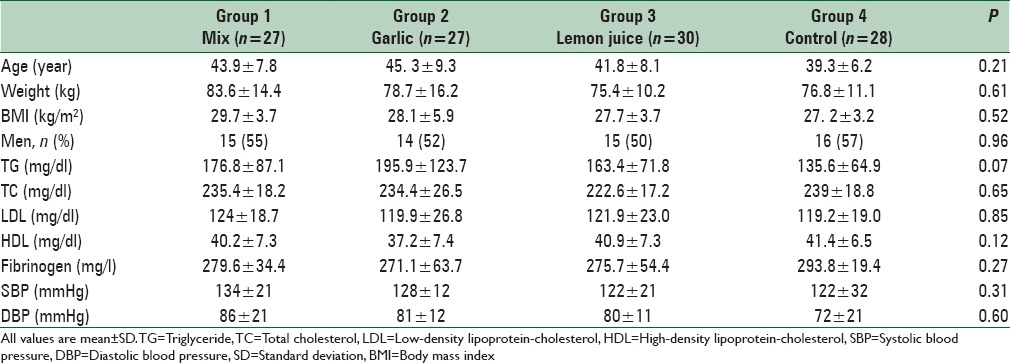
Table 2.
Nutrient intake and physical activity of study participants throughout the study
Changes of all variables within mixed and garlic groups were significant before and after intervention (P < 0.001). The effects of intervention within groups on general characteristics and metabolic profiles are shown in Table 3. We found a significant decrease in TC, LDL-C and fibrinogen in the mixed group in comparison with other groups (P < 0.001). No significant changes were observed regarding serum HDL and TG concentration in the mixed group in comparison with garlic and lemon juice groups (P = 0.08) but this change was significant in mixed group compared with control group (P = 0.02) (P = 0.001); respectively. A greater reduction in systolic and diastolic blood pressure was observed in the mixed group compared with the lemon juice and control groups (P = 0.01) (P = 0.02); respectively. Also a great reduction in BMI was observed in the mixed group compared with the lemon juice and control groups (P = 0.04). No significant differences in BMI, systolic and diastolic blood pressure between mixed and garlic groups were observed. The effects of a mixture of garlic and lemon juice, garlic and lemon juice on general characteristics and metabolic profiles are shown in Table 4.
Table 3.
The effect of intervention on metabolic profiles and anthropometric measurements within groups
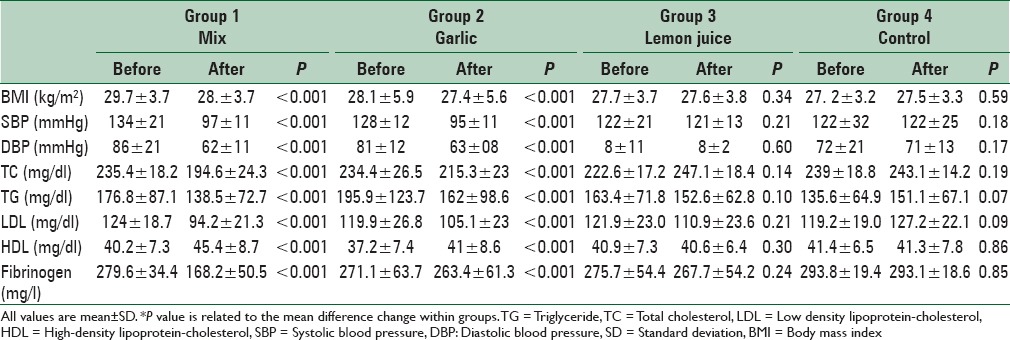
Table 4.
The comparison on changes of metabolic profiles and anthropometric measurements between four groups
DISCUSSION
Much research has concentrated on garlic for preventing atherosclerosis. Multiple useful cardiovascular effects have been discovered including enhancement of fibrinolytic activity, lowering of blood pressure, reduction in cholesterol, and triglyceride.[14] The results showed that combination of garlic and lemon juice significantly reduced serum TC, LDL-C, and blood pressure. Garlic, such as many other food additives, gained substantial interest due to its effects on lipid levels.[16,17,18] Moderate garlic intake causes few adverse effects. Allergic contact dermatitis can happen.[19] Many human and animal studies have investigated the effects of garlic on lipid levels, fasting blood sugar, BMI, fibrinogen, and SBP and DBP but no studies have yet been performed on the effects of a combination of garlic and lemon juice consumption on these biomarkers. Since 1993, 25 clinical trials have been published that have investigated the hypolipidemic effects of garlic. Eleven of the studies showed that garlic reduced serum cholesterol levels, but fourteen studies showed no effect on lowering cholesterol.[10] In addition, different extracts of garlic alone have been demonstrated to lower serum cholesterol, triglycerides, and LDL in rodents and humans.[20,21] Maha and Khalil showed that adding 8% raw garlic along with 2% cholesterol to the diet of rats decreased plasma TC and LDL-C.[22] A number of human studies have shown that raw garlic favorably affects important risk factors for CVD. Consumption has been shown to decrease total and LDL-C and triglyceride levels. An intake of the half to one clove of garlic per day lowers cholesterol levels approximately 10%.[23,24] Mechanisms that explain the observed effects of garlic include a decrease in cholesterol absorption, cholesterol, and fatty acid synthesis.[25] However, there is some evidence that garlic powder does not lower cholesterol levels, which may reflect a loss of active compound(s) during processing.[26] The formation of these active compounds is impressed by crushing garlic, a period of the drying process, the temperature at which garlic is dried, and humidity.[27] Therefore, we used raw garlic in this study. Direct measurements of enzyme activity have demonstrated that garlic and various constituents prohibit human 3-hydroxy-3-methylglutaryl-coenzyme A (HMG-CoA) reductase and squalene monooxygenase, enzymes required in cholesterol biosynthesis.[28,29,30,31,32] This prohibition of HMG-CoA reductase by garlic has also been supported in a recent study.[33] In addition, LDL-C reduction by garlic extract may be owing to decreases of hepatic 3-hydroxy-3-methylglutaryl-CoA reductase, cholesterol 7α-hydroxylase, pentose-phosphate pathway activities,[34] cholesteryl ester transfer protein activity,[35] microsomal triglyceride transfer protein,[36] enhanced bile acid excretion,[37] and prohibition of hepatic fatty acid synthesis[38] by allicin and/or other components.[28] It should also be emphasized that certain garlic preparations (e.g., garlic oils) do not indicate the degree of cholesterol lowering seen with specific powdered formulations.[39] In this study, we did not observe significant changes in HDL-C levels between groups. Aouadi showed that adding 10% fresh crushed garlic and 2% cholesterol to diet led to significant reduction in LDL-C levels, and increased HDL-C levels in rats.[40] The present study showed that fasting blood glucose showed no significant change due to consumption of a combination of garlic and lemon juice. However, in the lemon juice group after the intervention, we observed a significant increase in fasting blood glucose compared with other groups. Mohammadi and Abbas showed that fasting blood glucose levels decreased in garlic group compared with hypercholesterolemia group, but this decrease was not significant.[41] Ali et al.[42] reported that garlic administration has no effect on blood glucose, similar to this study. In our study, there was a significant reduction in mean SBP and DBPs in the garlic plus lemon juice group when compared with control and lemon juice groups. Since 1993, some studies have been published on the effects of garlic on blood pressure.[43,44,45,46] In one study has been reported to be moderately reduced in blood pressure by garlic alone.[47] In contrast of this, a meta-analysis published in 2001 represented that garlic has no considerable effects on blood pressure.[48] The moderate subside of blood pressure with garlic may be because of increased nitric oxide production and a more vasodilators state.[49] G-glutamylcysteines are compounds discovered in garlic, and these may lower blood pressure as illustrated by their ability to prohibit angiotension-converting enzyme in vitro.[50] In this study, we observed a significant reduction in triglycerides in the mixed group compared with control group. However, no significant changes were observed in the mixed group compared with the garlic and lemon juice groups. In our study, we observed a significant reduction in fibrinogen in the mixed group compared with other groups. In some studies, it has been reported that garlic causes a significant reduction in fibrinogen.[51,52] Contrarily, a study published in 1998 concluded that garlic has no effects on fibrinogen.[53] Previous studies regarding the effect of garlic alone have focused on lipid levels. One important difference between our study and these other studies was the combination of garlic and lemon juice. Even though the subjects were instructed to consume only 20 g garlic and 1 tablespoon of lemon juice, some significant effects were seen for the mentioned variables. In the present study, we provided garlic and lemon juice for participants and did not recommend a specific diet. This limitation should be considered in interpreting the findings of this research. We analyzed patient food records. The present study has some other potential limitations; it cannot suggest appropriate doses of garlic and lemon juice for people with hyperlipidemia. To establish this, other studies examining the effects of different doses of garlic and lemon juice are required.
CONCLUSIONS
In conclusion, a combination of garlic and lemon juice resulted in an improvement in lipid levels and blood pressure of people with hyperlipidemia. Further studies to determine the appropriate doses of garlic and lemon juice for these patients are warranted. In addition, the effect of a combination of garlic and lemon juice might be different based on the degree of hyperlipidemia. Therefore, studies are needed to examine the effects of garlic plus lemon juice according to the degree of hyperlipidemia.
Financial support and sponsorship
This study was provided by Department of Clinical Nutrition, School of Nutrition and Food Sciences, Isfahan, Iran, Grant Number: 392435.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
The writers appreciate the cooperation of Isfahan Cardiovascular Research Center laboratory for providing facilities to do the biochemical experiments. We also would like to thank all people who participated in the present study. This study was extracted from MSc dissertation which was approved by School of Nutrition and Food Sciences, Isfahan University of Medical Sciences (392436).
REFERENCES
- 1.Wood D. Joint European Societies Task Force. Established and emerging cardiovascular risk factors. Am Heart J. 2001;141(2 Suppl):S49–57. doi: 10.1067/mhj.2001.109951. [DOI] [PubMed] [Google Scholar]
- 2.Estruch R, Ros E, Salas-Salvadó J, Covas MI, Corella D, Arós F, et al. PREDIMED Study Investigators. Primary prevention of cardiovascular disease with a Mediterranean diet. N Engl J Med. 2013;368:1279–90. doi: 10.1056/NEJMc1806491. doi: http://dx.doi.org/10.1056/ NEJMoa1200303 PMID: 23432189. [DOI] [PubMed] [Google Scholar]
- 3.Mobasseri M, Fakhrzade H, Pourebrahim R, Nouri M, Shoushtarizade P, Pajuhi M. Evaluation of lipid abnormalities in urban population 25-64 years-old in Tehran University of Medical Science (population lab region) 2003. Iran J Diabetes Lipid Dis (Suppl) 2003;1:53–62. [Google Scholar]
- 4.Esteghamati A, Abbasi M, Alikhani S, Gouya MM, Delavari A, Shishehbor MH, et al. Prevalence, awareness, treatment, and risk factors associated with hypertension in the Iranian population: The national survey of risk factors for noncommunicable diseases of Iran. Am J Hypertens. 2008;21:620–6. doi: 10.1038/ajh.2008.154. [DOI] [PubMed] [Google Scholar]
- 5.Rahmanian K, Shojaie M. The prevalence of pre-hypertension and its association to established cardiovascular risk factors in South of Iran. BMC Res Notes. 2012;5:386. doi: 10.1186/1756-0500-5-386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.World Health Organization. 2006. [Last accessed on 2007 Jun 15]. Available from: http://www.who.int/ncd_surveillance/infobase/web/InfoBasePolicyMaker/reports/ReporterFullView.aspx?id=5.About 4 pages .
- 7.Kapil V, Milsom AB, Okorie M, Maleki-Toyserkani S, Akram F, Rehman F, et al. Inorganic nitrate supplementation lowers blood pressure in humans: Role for nitrite-derived NO. Hypertension. 2010;56:274–81. doi: 10.1161/HYPERTENSIONAHA.110.153536. [DOI] [PubMed] [Google Scholar]
- 8.Khosravi A, Akhavan Tabib A, Golshadi I, Dana Siadat Z, Bahonar A, Zarfeshani S, et al. The relationship between weight and CVD risk factors in a sample population from Central Iran (Based on IHHP) ARYA Atheroscler. 2012;8:82–9. [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman K. Historical perspective on garlic and cardiovascular disease. J Nutr. 2001;131:977S–9S. doi: 10.1093/jn/131.3.977S. [DOI] [PubMed] [Google Scholar]
- 10.Genkinger JM, Platz EA, Hoffman SC, Comstock GW, Helzlsouer KJ. Fruit, vegetable, and antioxidant intake and all-cause, cancer, and cardiovascular disease mortality in a community-dwelling population in Washington County, Maryland. Am J Epidemiol. 2004;160:1223–33. doi: 10.1093/aje/kwh339. [DOI] [PubMed] [Google Scholar]
- 11.Hung HC, Joshipura KJ, Jiang R, Hu FB, Hunter D, Smith-Warner SA, et al. Fruit and vegetable intake and risk of major chronic disease. J Natl Cancer Inst. 2004;96:1577–84. doi: 10.1093/jnci/djh296. [DOI] [PubMed] [Google Scholar]
- 12.Rahman K, Lowe GM. Garlic and cardiovascular disease: A critical review. J Nutr. 2006;136(3 Suppl):736S–40S. doi: 10.1093/jn/136.3.736S. [DOI] [PubMed] [Google Scholar]
- 13.Miyake Y, Mochizuki M, Okada M, Hiramitsu M, Morimitsu Y, Osawa T. Isolation of antioxidative phenolic glucosides from lemon juice and their suppressive effect on the expression of blood adhesion molecules. Biosci Biotechnol Biochem. 2007;71:1911–9. doi: 10.1271/bbb.70115. [DOI] [PubMed] [Google Scholar]
- 14.Batsis JA, Lopez-Jimenez F. Cardiovascular risk assessment – From individual risk prediction to estimation of global risk and change in risk in the population. BMC Med. 2010;8:29. doi: 10.1186/1741-7015-8-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Adler AJ, Holub BJ. Effect of garlic and fish-oil supplementation on serum lipid and lipoprotein concentrations in hypercholesterolemic men. Am J Clin Nutr. 1997;65:445–50. doi: 10.1093/ajcn/65.2.445. [DOI] [PubMed] [Google Scholar]
- 16.Augusti KT. Therapeutic values of onion (Allium cepa L.) and garlic (Allium sativum L.) Indian J Exp Biol. 1996;34:634–40. [PubMed] [Google Scholar]
- 17.Bhalla K, Hwang BJ, Dewi RE, Twaddel W, Goloubeva OG, Wong KK, et al. Metformin prevents liver tumorigenesis by inhibiting pathways driving hepatic lipogenesis. Cancer Prev Res (Phila) 2012;5:544–52. doi: 10.1158/1940-6207.CAPR-11-0228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Pinsky PF, Prorok PC. Glycemic control in youth with type 2 diabetes. N Engl J Med. 2012;367:1066–7. doi: 10.1056/NEJMc1208408. [DOI] [PubMed] [Google Scholar]
- 19.Delaney TA, Donnelly AM. Garlic dermatitis. Australas J Dermatol. 1996;37:109–10. doi: 10.1111/j.1440-0960.1996.tb01019.x. [DOI] [PubMed] [Google Scholar]
- 20.Rahman K. Garlic and aging: New insights into an old remedy. Ageing Res Rev. 2003;2:39–56. doi: 10.1016/s1568-1637(02)00049-1. [DOI] [PubMed] [Google Scholar]
- 21.Budoff MJ, Ahmadi N, Gul KM, Liu ST, Flores FR, Tiano J, et al. Aged garlic extract supplemented with B Vitamins, folic acid and L-arginine retards the progression of subclinical atherosclerosis: A randomized clinical trial. Prev Med. 2009;49:101–7. doi: 10.1016/j.ypmed.2009.06.018. [DOI] [PubMed] [Google Scholar]
- 22.Maha EB, Khalil MH. The effect of fresh crushed garlic bulbs (Allium sativum) on plasma lipids in hypercholesterolemic rats. Res J Anim Vet Sci. 2008;3:15–9. [Google Scholar]
- 23.Gore JM, Dalen JE. Cardiovascular disease. JAMA. 1994;271:1660–1. [PubMed] [Google Scholar]
- 24.Warshafsky S, Kamer RS, Sivak SL. Effect of garlic on total serum cholesterol. A meta-analysis. Ann Intern Med. 1993;119(7 Pt 1):599–605. doi: 10.7326/0003-4819-119-7_part_1-199310010-00009. [DOI] [PubMed] [Google Scholar]
- 25.Matsuura H. Saponins in garlic as modifiers of the risk of cardiovascular disease. J Nutr. 2001;131:1000S–5S. doi: 10.1093/jn/131.3.1000S. [DOI] [PubMed] [Google Scholar]
- 26.Isaacsohn JL, Moser M, Stein EA, Dudley K, Davey JA, Liskov E, et al. Garlic powder and plasma lipids and lipoproteins: A multicenter, randomized, placebo-controlled trial. Arch Intern Med. 1998;158:1189–94. doi: 10.1001/archinte.158.11.1189. [DOI] [PubMed] [Google Scholar]
- 27.Pentz R, Siegers CP. Garlic preparations: Methods for qualitative and quantitative assessment of their ingredients. In: Lawson LD, Koch HP, editors. Garlic: The Science and Therapeutic Application of Allium sativum L. and Related Species. 2nd ed. Baltimore: Williams and Wilkins; 1996. [Google Scholar]
- 28.Gebhardt R. Multiple inhibitory effects of garlic extracts on cholesterol biosynthesis in hepatocytes. Lipids. 1993;28:613–9. doi: 10.1007/BF02536055. [DOI] [PubMed] [Google Scholar]
- 29.Liu L, Yeh YY. Water-soluble organosulfur compounds of garlic inhibit fatty acid and triglyceride syntheses in cultured rat hepatocytes. Lipids. 2001;36:395–400. doi: 10.1007/s11745-001-0734-4. [DOI] [PubMed] [Google Scholar]
- 30.Yeh YY, Liu L. Cholesterol-lowering effect of garlic extracts and organosulfur compounds: Human and animal studies. J Nutr. 2001;131:989S–93S. doi: 10.1093/jn/131.3.989S. [DOI] [PubMed] [Google Scholar]
- 31.Yeh YY, Yeh SM. Garlic reduces plasma lipids by inhibiting hepatic cholesterol and triglyceride synthesis. Lipids. 1994;29:189–93. doi: 10.1007/BF02536728. [DOI] [PubMed] [Google Scholar]
- 32.Gupta N, Porter TD. Garlic and garlic derived compounds inhibit human squalene monooxygenase. J Nutr. 2001;131:1662–7. doi: 10.1093/jn/131.6.1662. [DOI] [PubMed] [Google Scholar]
- 33.Augusti KT, Chackery J, Jacob J, Kuriakose S, George S, Nair SS. Beneficial effects of a polar fraction of garlic (Allium sativum Linn) oil in rats fed with two different high fat diets. Indian J Exp Biol. 2005;43:76–83. [PubMed] [Google Scholar]
- 34.Qureshi AA, Din ZZ, Abuirmeileh N, Burger WC, Ahmad Y, Elson CE. Suppression of avian hepatic lipid metabolism by solvent extracts of garlic: impact on serum lipids. J Nutr. 1983;113:1746–55. doi: 10.1093/jn/113.9.1746. [DOI] [PubMed] [Google Scholar]
- 35.Kwon MJ, Song YS, Choi MS, Park SJ, Jeong KS, Song YO. Cholesteryl ester transfer protein activity and atherogenic parameters in rabbits supplemented with cholesterol and garlic powder. Life Sci. 2003;72:2953–64. doi: 10.1016/s0024-3205(03)00234-0. [DOI] [PubMed] [Google Scholar]
- 36.Lin MC, Wang EJ, Lee C, Chin KT, Liu D, Chiu JF, et al. Garlic inhibits microsomal triglyceride transfer protein gene expression in human liver and intestinal cell lines and in rat intestine. J Nutr. 2002;132:1165–8. doi: 10.1093/jn/132.6.1165. [DOI] [PubMed] [Google Scholar]
- 37.Stephen W, Russell SK, Sivak SL. Effect of garlic on total serum cholesterol. J Nutr. 1993;119:599–605. doi: 10.7326/0003-4819-119-7_part_1-199310010-00009. [DOI] [PubMed] [Google Scholar]
- 38.Chang MLW, Johnson MA. Effect of garlic on carbohydrate metabolism and lipid synthesis in rats. J Nutr. 1980;110:931–6. doi: 10.1093/jn/110.5.931. [DOI] [PubMed] [Google Scholar]
- 39.Dc A Santos OS, Johns RA. Effects of garlic powder and garlic oil preparations on blood lipids, blood pressure and well-being. Br J Clin Res l. 995;:91–l00. [Google Scholar]
- 40.Aouadi R. Effect of fresh garlic (Allium sativum) on lipid metabolism in male rats. Nutr Res. 2000;20:273–80. [Google Scholar]
- 41.Mohammadi A, Oshaghi EA. Effect of garlic on lipid profile and expression of LXR alpha in intestine and liver of hypercholesterolemic mice. J Diabetes Metab Disord. 2014;13:20. doi: 10.1186/2251-6581-13-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ali M, Al-Qattan KK, Al-Enezi F, Khanafer RM, Mustafa T. Effect of allicin from garlic powder on serum lipids and blood pressure in rats fed with a high cholesterol diet. Prostaglandins Leukot Essent Fatty Acids. 2000;62:253–9. doi: 10.1054/plef.2000.0152. [DOI] [PubMed] [Google Scholar]
- 43.Banerjee SK, Maulik SK. Effect of garlic on cardiovascular disorders: A review. Nutr J. 2002;1:4. doi: 10.1186/1475-2891-1-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Turner B, Mølgaard C, Marckmann P. Effect of garlic (Allium sativum) powder tablets on serum lipids, blood pressure and arterial stiffness in normo-lipidaemic volunteers: A randomised, double-blind, placebo-controlled trial. Br J Nutr. 2004;92:701–6. doi: 10.1079/bjn20041255. [DOI] [PubMed] [Google Scholar]
- 45.Dhawan V, Jain S. Effect of garlic supplementation on oxidized low density lipoproteins and lipid peroxidation in patients of essential hypertension. Mol Cell Biochem. 2004;266:109–15. doi: 10.1023/b:mcbi.0000049146.89059.53. [DOI] [PubMed] [Google Scholar]
- 46.Durak I, Kavutcu M, Aytaç B, Avci A, Devrim E, Ozbek H, et al. Effects of garlic extract consumption on blood lipid and oxidant/antioxidant parameters in humans with high blood cholesterol. J Nutr Biochem. 2004;15:373–7. doi: 10.1016/j.jnutbio.2004.01.005. [DOI] [PubMed] [Google Scholar]
- 47.Silagy CA, Neil HA. A meta-analysis of the effect of garlic on blood pressure. J Hypertens. 1994;12:463–8. [PubMed] [Google Scholar]
- 48.Ackermann RT, Mulrow CD, Ramirez G, Gardner CD, Morbidoni L, Lawrence VA. Garlic shows promise for improving some cardiovascular risk factors. Arch Intern Med. 2001;161:813–24. doi: 10.1001/archinte.161.6.813. [DOI] [PubMed] [Google Scholar]
- 49.Das I, Khan NS, Sooranna SR. Nitric oxide synthase activation is a unique mechanism of garlic action. Biochem Soc Trans. 1995;23:136S. doi: 10.1042/bst023136s. [DOI] [PubMed] [Google Scholar]
- 50.Sendl A, Elbl G, Steinke B, Redl K, Breu W, Wagner H. Comparative pharmacological investigations of Allium ursinum and Allium sativum. Planta Med. 1992;58:1–7. doi: 10.1055/s-2006-961378. [DOI] [PubMed] [Google Scholar]
- 51.Bordia A, Bansal HC, Arora SK, Singh SV. Effect of the essential oils of garlic and onion on alimentary hyperlipemia. Atherosclerosis. 1975;21:15–9. doi: 10.1016/0021-9150(75)90091-x. [DOI] [PubMed] [Google Scholar]
- 52.Harenberg J, Giese C, Zimmermann R. Effect of dried garlic on blood coagulation, fibrinolysis, platelet aggregation and serum cholesterol levels in patients with hyperlipoproteinemia. Atherosclerosis. 1988;74:247–9. doi: 10.1016/0021-9150(88)90244-4. [DOI] [PubMed] [Google Scholar]
- 53.Bordia A, Verma SK, Srivastava KC. Effect of garlic (Allium sativum) on blood lipids, blood sugar, fibrinogen and fibrinolytic activity in patients with coronary artery disease. Prostaglandins Leukot Essent Fatty Acids. 1998;58:257–63. doi: 10.1016/s0952-3278(98)90034-5. [DOI] [PubMed] [Google Scholar]