Abstract
Purpose
To identify lateral lymph node (LN) characteristics predictive of outcome in papillary thyroid cancer patients with clinically evident nodal disease.
Methods
A total of 438 patients with lateral neck metastases from papillary thyroid cancer were identified from an institutional database of 3,664 differentiated thyroid cancers. The number of positive LNs, size of the largest LN, number of positive LNs to total number of LNs removed (LN burden), and presence of extranodal spread (ENS) were recorded. Cutoffs for continuous variables were determined by receiver operating characteristic curves. LN variables predictive of recurrence free survival and disease-specific survival (DSS) were identified by the Kaplan–Meier method and the Cox proportional hazard model.
Results
The median age was 41 years (range 5–86 years). The median follow-up was 65 months (range 1– 332 months). Fifty-nine patients developed disease recurrence; these were local in five, regional in 40, and distant in 30 patients. Fifteen patients died of disease. Receiver operating characteristic cutoffs were>10 positive LNs and a LN burden >17 %. No lateral LN characteristics were predictive of DSS. In patients <45 years old, univariate predictors of recurrence were >10 positive nodes (p = 0.049) and LN burden >17 % (p < 0.001). In patients ≥45 years old, >10 positive nodes, LN burden >17 %, and presence of ENS were predictive of recurrence (p = 0.019, p = 0.019, and p = 0.029, respectively).
Conclusions
LN burden >17 % (1 positive LN in 6 LNs removed) in the lateral neck is predictive for recurrence in patients of all ages, whereas ENS is also prognostic for recurrence in older patients.
Previously published retrospective studies have suggested that regional lymph node (LN) metastases do not affect survival in patients with papillary thyroid cancer (PTC).1–3 As such, prognostic scoring systems such as GAMES from our institution, as well as MACIS and AMES, based on multivariate analyses, did not find nodal disease to be a predictor of outcome.1–3 However, more recent literature shows that nodal disease is important, particularly in patients over 45 years of age; this is now reflected in the American Joint Committee on Cancer (AJCC) staging system for thyroid cancer.4 In patients aged ≥45 years, N1a status is considered stage III disease, and N1b status is considered stage IV disease. This staging system, however, considers only the location of the metastatic nodes and does not take into consideration other characteristics of the metastatic nodes. Several recent publications have suggested that increasing number, size, and ratio of positive LNs in the neck predicts poorer outcome in patients with PTC.5–10 The objective of this study was to define which lateral LN characteristics have prognostic significance in patients with clinical or radiologic evidence of metastatic LNs in the lateral neck.
METHODS
After receipt of approval by the institutional review board, the records of 3,664 consecutive patients treated surgically for PTC between 1986 and 2010 were identified from an institutional database. Of these, 438 patients had pathologic confirmation of palpable or radiologically demonstrated lateral neck metastases after a therapeutic neck dissection. Patients who were treated at an outside facility before referral, who did not have pN1b disease, who presented with distant metastasis, or who were discovered to have distant metastasis on postoperative radioactive iodine (RAI) scan (within 6 months) were excluded from analysis. Figure 1 demonstrates the inclusion cohort. Our institutional policy is not to perform prophylactic neck dissections. Therefore, these patients had macroscopic LNs either on clinical or radiologic examination.
FIG. 1.
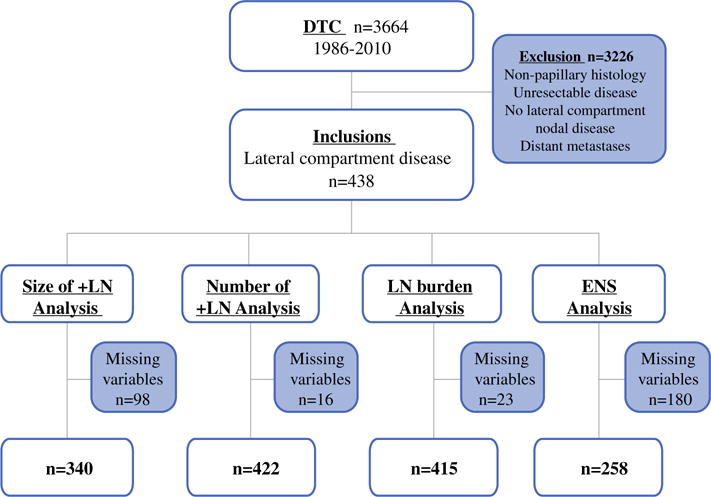
Flowchart indicating study inclusion and exclusion criteria
Patient demographic information, surgical details, and histopathologic details, including number of positive lateral LNs, total number of LNs removed, size of largest LN, and presence of extranodal spread (ENS), were recorded. Details of postoperative use of RAI were recorded. Postoperative thyroid-stimulating hormone suppression was practiced according to recurrence risk for all patients.
Because this study spans patients with pathology reports between 1986 through 2010, the desired LN features were not reported in some patients from earlier years. Figure 1 shows the LN data available for analysis. Patients with one or more missing LN features were censored for the analysis of that LN variable.
Disease outcomes of interest were disease-specific survival (DSS) and recurrence-free survival (RFS). DSS was calculated using the date of last follow-up with a Memorial Sloan Kettering Cancer Center physician from the thyroid cancer multidisciplinary team. Details of death were determined from the Social Security Death Index and hospital records. All patients who had evidence of structural disease at the time of last follow-up and died during follow-up were considered to have died of disease. Local and regional recurrences were determined by clinical examination supplemented with imaging and fine-needle aspiration. Distant recurrence was determined by imaging studies including ultrasound, RAI scan, computer tomography, and positron emission tomography scan. Confirmation of disease by cytology and histopathology were recorded when available. Before 2000, postoperative serum thyroglobulin was not routinely used to detect recurrence. Serum thyroglobulin measurements and serial ultrasounds have become standard practice at our institution since 2005, and they now influence the further investigation of patients in this cohort. The median follow-up for the cohort was 65 months (range 1–332 months). Outcomes data were therefore calculated at 5 years.
Statistical analysis was carried out by SPSS software, version 21 (IBM, Armonk, NY). To determine categorical cutoffs for LN size, number, and burden of metastatic LNs that were predictive of DSS and RFS, we used cutoff values previously reported in the literature as being significant. In addition, we also plotted receiver operating curves for number of positive LNs as well as LN burden (LN burden was defined as the percentage of positive LN out of total number LNs resected) from the lateral compartment. Values were chosen to maximize sensitivity and specificity for RFS and DSS. Survival outcomes were analyzed by the Kaplan–Meier method. Univariate analysis was carried out by the log rank test, and hazard ratios were calculated by the Cox proportional hazard model. A p value of <0.05 was considered significant.
RESULTS
The median age was 41 years (range 5–86 years). A total of 188 patients (43 %) were ≥45 years old at the time of diagnosis. Extent of lateral neck dissection was determined by a combination of surgeon preference and patient risk profile. Of the 438 patients, the majority of the cohort (71 %) underwent an ipsilateral comprehensive neck dissection including at least levels 2, 3, 4, and 5. Twenty percent of patients had an ipsilateral selective neck dissection of neck levels at highest risk of harboring disease, and a further 9 % received bilateral neck dissection. An average of 33 LNs were removed per patient.
The majority (79 %) of this cohort received adjuvant RAI therapy within 6 months of thyroidectomy surgery. The median dose received was 150 mCi (range 29–478 mCi). Fifty-nine patients (13.5 %) had recurrent disease; this was local in 5, regional in 40, and distant in 30 patients (Supplementary Fig. 1). Receiver operating characteristic analysis showed that cutoffs that maximized sensitivity and specificity were number of positive nodes of >10 and a LN burden >17 %.
LN Characteristics Prognostic of DSS
A total of 15 patients died of disease. All but one patient was aged <45 years at the time of diagnosis. As such, predictors of DSS were limited to only patients ≥45 years of age. No LN characteristics—size, number, burden, or presence of ENS—were predictive of DSS (Supplementary Table 1).
LN Characteristics Predictive of RFS
Factors predictive of RFS in patients <45 years of age are shown in Table 1. Number of positive nodes >10 (5-year RFS 81.0 vs. 91.7 %, p = 0.05) and a LN burden >17 % (5-year RFS 81.2 vs. 99.1 %, p < 0.001) were predictive of recurrence on Kaplan–Meier analysis (Fig. 2a, b). Increasing LN size was associated with poorer RFS, but this was not statistically significant (5-year RFS 79.4 vs. 93.0 %, p = 0.20 for size >3 cm). LN ENS was not predictive of recurrence (Fig. 2c). Using the Cox proportional hazard model, we found that patients with >10 positive nodes were 2.2 times more likely to experience recurrence. Patients with a LN burden >17 % were 4.8 times more likely to experience recurrence compared to patients with a ≤17 % LN burden (p < 0.001).
TABLE 1.
LN characteristics predictors of 5-year recurrence-free survival in patients <45 years of age
| Characteristic | 5 year RFS (%) | p | HR (95 % CI) | p |
|---|---|---|---|---|
| No. of positive LNs | ||||
| ≤5 nodes | 92.0 | – | – | |
| >5 nodes | 87.1 | 0.46 | 1.3 (0.6–2.6) | 0.46 |
| ≤6 nodes | 91.8 | – | – | |
| >6 nodes | 86.1 | 0.26 | 1.5 (0.7–3.1) | 0.27 |
| ≤10 nodes | 91.7 | – | – | |
| >10 nodes | 81.1 | 0.05 | 2.2 (1.0–4.8) | 0.05 |
| Largest LN size (cm) | ||||
| ≤1 | 94.1 | – | – | |
| >1 | 89.4 | 0.54 | 0.7 (0.2–2.1) | 0.54 |
| ≤2 | 93.3 | – | – | |
| >2 | 87.1 | 0.68 | 1.19 (0.5–2.7) | 0.68 |
| ≤3 | 93.0 | – | – | |
| >3 | 79.4 | 0.22 | 1.7 (0.7–4.2) | 0.22 |
| LN burden (%) | ||||
| ≤17 | 99.1 | – | – | |
| >17 | 81.2 | <0.001 | 4.8 (2.0–11.9) | 0.001 |
| Extranodal spread | ||||
| None | 91.1 | – | – | |
| Yes | 92.7 | 0.99 | 1.0 (0.3–3.3) | 0.99 |
LN lymph node, HR hazard ratio, CI confidence interval
FIG. 2.
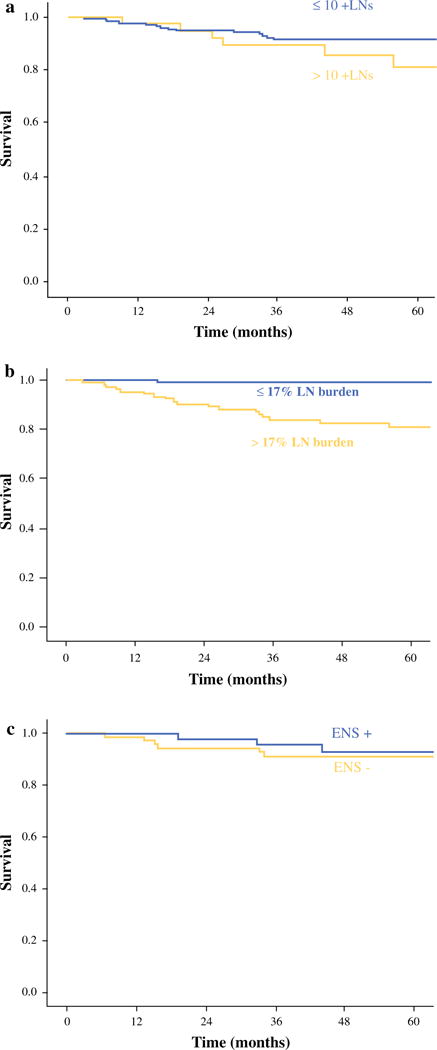
Kaplan–Meier graph for RFS for patients <45 years old based on a number of positive LNs (p = 0.05), b LN burden (p < 0.001), and c ENS (p = 0.99)
Table 2 shows factors predictive of recurrence in patients ≥45 years of age. Number of positive nodes >10 (5-year RFS 71.8 vs. 87.3 %, p = 0.02) and a LN burden >17 % (5-year RFS 77.7 vs. 90.5 %, p = 0.02) were also predictive of recurrence (Fig. 3a, b). Patients with >10 positive nodes were 3.1 times more likely to experience recurrence (p = 0.03). Patients with a LN burden >17 % were 2.7 times more likely to experience recurrence compared to patients with <17 % LN burden (p = 0.02). Increasing LN size was not significant. In addition, the presence of ENS was also significant in the older patients (5-year RFS 79.5 vs. 93.0 % p = 0.03) (Fig. 3c). Patients with ENS were 3.4 times more likely to experience recurrence compared to patients with no ENS (p = 0.04).
TABLE 2.
LN characteristics predictors of 5-year RFS in patients ≥45 years of age
| Characteristic | 5 year RFS (%) | p | HR (95 % CI) | p |
|---|---|---|---|---|
| No. of positive LNs | ||||
| ≤5 nodes | 87.7 | – | – | |
| >5 nodes | 81.2 | 0.16 | 1.81 (0.8–4.2) | 0.17 |
| ≤6 nodes | 87.5 | – | – | |
| >6 nodes | 80.8 | 0.19 | 1.77 (0.7–4.2) | 0.20 |
| ≤10 nodes | 87.4 | – | – | |
| >10 nodes | 71.8 | 0.02 | 3.10 (1.5–8.4) | 0.03 |
| Largest LN size (cm) | ||||
| ≤1 | 80.0 | – | – | |
| >1 | 86.5 | 0.84 | 1.2 (0.2–9.2) | 0.84 |
| ≤2 | 88.3 | – | – | |
| >2 | 84.6 | 0.72 | 1.2 (0.5–2.8) | 0.72 |
| ≤3 | 87.1 | – | – | |
| >3 | 83.1 | 0.75 | 0.8 (0.3–2.5) | 0.75 |
| LN burden (%) | ||||
| ≤17 | 90.5 | – | – | |
| >17 | 77.7 | 0.02 | 2.70 (1.1–6.4) | 0.02 |
| Extranodal spread | ||||
| None | 93.0 | – | – | |
| Yes | 74.2 | 0.03 | 3.38 (1.1–10.7) | 0.04 |
LN lymph node, RFS recurrence-free survival, HR hazard ratio, CI confidence interval
FIG. 3.
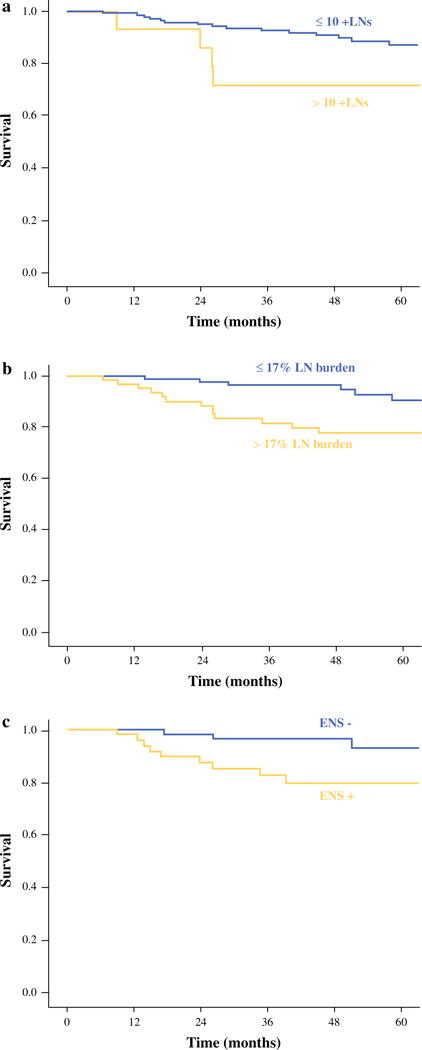
Kaplan–Meier graph for RFS for patients ≥45 years old based on a number of positive LNs (p = 0.02), b LN burden (p = 0.02), and c ENS (p = 0.03)
A total of 30 patients (6.8 %) developed distant metastases, 21 of whom were ≥45 years old at the time of initial diagnosis. Univariate predictors of distant recurrence were >10 positive lateral neck LNs (5-year distant RFS 87.2 vs. 94.5 %, p = 0.01) and LN burden >17 % (5-year distant RFS 89.8 vs. 96.4 %, p = 0.01). Presence of ENS was associated with a poorer distant RFS, but this was not statistically significant (5-year distant RFS 96.0 vs. 92.6 %, p = 0.08).
DISCUSSION
Metastatic disease in the lateral compartment LNs of the neck in patients with PTC is known to be predictive of poorer outcomes, particularly in older PTC patients, and as such, it is recognized by the current edition of the AJCC staging manual.4,11 Recent data suggest that in addition to LN location, LN characteristics such as maximal size, number, ratio, and ENS are also prognostic.5–10 Age at diagnosis is uniquely critical in the staging of differentiated thyroid cancer, and in this series of 438 PTC patients with lateral neck disease, we report LN characteristics that are predictive of outcome in two age cohorts of patients, those <45 years old and those ≥45 years old. In both age groups, both number of positive LNs >10 and LN burden >17 % (which equated to 1 positive node per 6 removed) were predictive for recurrence. In addition, the presence of ENS was predictive of recurrence in patients ≥45 years old. Size of LNs did not appear to be predictive in our cohort of patients.
Many studies have reported the size of the metastatic lateral neck LN to be prognostic in PTC. Studies have suggested that LN metastases >3 cm are associated with poorer prognosis.9,12 In a study of LN characteristics by Ito et al., N1b patients with metastatic nodes >3 cm were found to have a significantly poorer RFS compared to N1a patients.12 In a study of both central and lateral LNs by Sugitani et al., it was found that LN metastases >3 cm were predictive of DSS in patients aged ≥50 years but not in patients aged <50 years at the time of diagnosis.9 These results suggest that the size of lateral neck LN is likely to be prognostic in PTC, particularly in older patients. In the present study, no discrete size cutoff was statistically associated with poorer prognosis in either age cohort. However, the data suggest that larger LN metastases are associated with poorer RFS; patients with LNs measuring <1 cm had a 5-year RFS of 91.2 % compared to those with LN >3 cm, who had a 5-year RFS of just 80.9 %.
Several groups have also investigated the prognostic impact of the number of positive lateral compartment LNs. Studies have reported a wide range of LNs considered to be prognostic, ranging from 2 to >10 positive LNs, when looking at the number of LNs within the central and lateral compartments.7,8 When focusing only on LN metastases within the lateral compartment, Ito et al. reported that >5 positive lateral neck LNs affected RFS.12 Moreno et al. similarly focused on the lateral neck LNs features and observed that the number of abnormal neck compartments on ultrasound, as an indirect marker of the number of positive LNs, correlated with the risk of regional recurrence.13 In our own cohort, receiver operating characteristic analysis suggested that 10 positive nodes was the cutoff that maximized sensitivity and specificity. Indeed, >10 nodes was predictive of recurrence. Interestingly, the previously reported cutoffs of >5 positive nodes was not prognostic in our series.
LN burden is defined as the number of metastatic LNs divided by the total number of LNs resected, expressed as a percentage. Because LN burden is the percentage of positive to total LNs resected, the two are closely correlated. However, LN burden additionally takes into consideration the extent of surgical resection as well as the extent and aggressiveness of disease. LN burden has been found to be a significant predictor of outcome in other cancers, including oral and cutaneous squamous cell carcinoma of the head and neck.14–19 Within the thyroid cancer literature, the prognostic impact of lateral LN burden has not been well explored. Several publications have previously investigated the significance of LN burden in the central compartment alone and within the central and lateral compartments combined. Beal et al. analyzed data from the Surveillance, Epidemiology, and End Results database to identify 9,926 patients with primary, well-differentiated, M0 thyroid cancer.20 Increasing LN burden was associated with decreased survival on multivariate analysis in all primary thyroidectomy patients. Others have observed central compartment LN burden to be predictive of postoperative thyroglobulin levels as well as recurrence.5,6 Our study specifically focuses on the prognostic significance of lateral compartment LN characteristics. Our data show that younger patients with LN burden >17 % were 4.8 times (p < 0.001) and older patients 2.7 times (p < 0.02) more likely to develop disease recurrence.
With regard to ENS, we have noted that ENS was the LN characteristic most prognostic of nodal recurrence within the central neck compartment.21 In the current study on lateral neck LN metastases, we observed that ENS is predictive of RFS specifically in patients ≥45 years of age. ENS was similarly distributed between the older (41.3 %) and younger (39.4 %) cohorts (p = 0.755). However, in older patients, the presence of lateral nodal ENS was associated with higher T4 disease (p < 0.001) as well as ATA high-risk category (p < 0.001) (data not shown). Lee et al. similarly found ENS to be associated with large tumor size.22 Others have reported similar findings; Ito et al. concluded that the presence of lateral compartment ENS was independently prognostic of DSS in patients aged ≥55 years.12 ENS is a known indicator of aggressive tumor biology in other head and neck cancers.23,24 Its importance in relation to older age is a unique finding and requires further research.
Some limitations of this study warrant discussion. This is a retrospectively conducted study and therefore susceptible to limitations associated with such studies. First, our institutional policy is to perform lateral neck dissection only for patients with clinical or radiologic evidence of neck disease. This cohort therefore does not include subclinical disease. However, it can be argued that it is the higher-risk, clinically evident N1b patients who are of most relevance. Second, the extent of lateral neck dissection is surgeon dependent, and the identification of LN characteristics is pathologist dependent. Both of these physician factors may bias the reported LN characteristics. Last, only 224 (51.1 %) of our patients had all LN features available on histopathology for data collection and analysis. This could limit our analysis. To ensure there was no selection bias by excluding patients without all characteristics, we compared the clinical and tumor characteristics of patients with variables reported and those without. For LN size, number, and ENS, we found no significant differences in terms of age, T stage, adjuvant therapy, or duration of follow-up between groups with these variables reported and not reported (data not shown). Nevertheless, it would be desirable to have complete data on all these LN variables on all patients. As such, future studies with larger sample size and complete synoptic reporting are required to validate our findings.
In summary, our data suggest that the presence of LN burden >17 % is predictive for overall recurrence and development of distant metastases in both patient age cohorts, <45 and ≥45 years of age. In patients ≥45 years, the presence of ENS is also predictive of recurrence.
Supplementary Material
Footnotes
Electronic supplementary material The online version of this article (doi:10.1245/s10434-015-4398-2) contains supplementary material, which is available to authorized users.
DISCLOSURE The authors declare no conflict of interest.
References
- 1.Shaha AR, Loree TR, Shah JP. Intermediate-risk group for differentiated carcinoma of thyroid. Surgery. 1994;116:1036–40. [PubMed] [Google Scholar]
- 2.Hay ID, Bergstralh EJ, Goellner JR, Ebersold JR, Grant CS. Predicting outcome in papillary thyroid carcinoma: development of a reliable prognostic scoring system in a cohort of 1779 patients surgically treated at one institution during 1940 through 1989. Surgery. 1993;114:1050–7. [PubMed] [Google Scholar]
- 3.Cady B, Rossi R. An expanded view of risk-group definition in differentiated thyroid carcinoma. Surgery. 1988;104:947–53. [PubMed] [Google Scholar]
- 4.Sobin LH, Gospodarowicz MK, Wittekind CW. TNM classification of malignant tumours. 7th. New York: Wiley-Blackwell; 2009. [Google Scholar]
- 5.Lang BHH, Wong KP, Wan KY, Lo CY. Significance of metastatic lymph node ratio on stimulated thyroglobulin levels in papillary thyroid carcinoma after prophylactic unilateral central neck dissection. Ann Surg Oncol. 2012;19:1257–63. doi: 10.1245/s10434-011-2105-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Jeon M, Yoon J, Han J, et al. The prognostic value of the metastatic lymph node ratio and maximal metastatic tumor size in pathological N1a papillary thyroid carcinoma. Eur J Endocrinol. 2013;168:219–25. doi: 10.1530/EJE-12-0744. [DOI] [PubMed] [Google Scholar]
- 7.Leboulleux S, Rubino C, Baudin E, et al. Prognostic factors for persistent or recurrent disease of papillary thyroid carcinoma with neck lymph node metastases and/or tumor extension beyond the thyroid capsule at initial diagnosis. J Clin Endocrinol Metab. 2005;90:5723–9. doi: 10.1210/jc.2005-0285. [DOI] [PubMed] [Google Scholar]
- 8.Lee J, Song Y, Soh E. Prognostic significance of the number of metastatic lymph nodes to stratify the risk of recurrence. World J Surg. 2013;4:4. doi: 10.1007/s00268-013-2345-6. [DOI] [PubMed] [Google Scholar]
- 9.Sugitani I, Kasai N, Fujimoto Y, Yanagisawa A. A novel classification system for patients with PTC: addition of the new variables of large (3 cm or greater) nodal metastases and reclassification during the follow-up period. Surgery. 2004;135:139–48. doi: 10.1016/s0039-6060(03)00384-2. [DOI] [PubMed] [Google Scholar]
- 10.Ito Y, Jikuzono T, Higashiyama T, et al. Clinical significance of lymph node metastasis of thyroid papillary carcinoma located in one lobe. World J Surg. 2006;30:1821–8. doi: 10.1007/s00268-006-0211-5. [DOI] [PubMed] [Google Scholar]
- 11.Nixon I, Wang L, Palmer F, et al. The impact of nodal status on outcome in older patients with papillary thyroid cancer. Surgery. 2014;156:137–46. doi: 10.1016/j.surg.2014.03.027. [DOI] [PubMed] [Google Scholar]
- 12.Ito Y, Fukushima M, Tomoda C, et al. Prognosis of patients with papillary thyroid carcinoma having clinically apparent metastasis to the lateral compartment. Endocr J. 2009;56:759–66. doi: 10.1507/endocrj.k09e-025. [DOI] [PubMed] [Google Scholar]
- 13.Moreno MA, Agarwal G, de Luna R, et al. Preoperative lateral neck ultrasonography as a long-term outcome predictor in papillary thyroid cancer. Arch Otolaryngol Head Neck Surg. 2011;137:157–62. doi: 10.1001/archoto.2010.254. [DOI] [PubMed] [Google Scholar]
- 14.Marchet A, Mocellin S, Ambrosi A, et al. The ratio between metastatic and examined lymph nodes (N ratio) is an independent prognostic factor in gastric cancer regardless of the type of lymphadenectomy: results from an Italian multicentric study in 1853 patients. Ann Surg. 2007;245:543–52. doi: 10.1097/01.sla.0000250423.43436.e1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Dekker J, Peeters K, Putter H, Vahrmeijer A, van de Velde C. Metastatic lymph node ratio in stage III rectal cancer; prognostic significance in addition to the 7th edition of the TNM classification. Eur J Surg Oncol. 2010;36:1180–6. doi: 10.1016/j.ejso.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 16.Berger A, Watson J, Ross E, Hoffman J. The metastatic/examined lymph node ratio is an important prognostic factor after pancreaticoduodenectomy for pancreatic adenocarcinoma. Am Surg. 2004;70:235–40. [PubMed] [Google Scholar]
- 17.Woodward W, Vinh-Hung V, Ueno N, et al. Prognostic value of nodal ratios in node-positive breast cancer. J Clin Oncol. 2006;24:2910–6. doi: 10.1200/JCO.2005.03.1526. [DOI] [PubMed] [Google Scholar]
- 18.Gil Z, Carlson D, Boyle J, et al. Lymph node density is a significant predictor of outcome in patients with oral cancer. Cancer. 2009;115:5700–10. doi: 10.1002/cncr.24631. [DOI] [PubMed] [Google Scholar]
- 19.Mizrachi A, Hadar T, Rabinovics N, et al. Prognostic significance of nodal ratio in cutaneous squamous cell carcinoma of the head and neck. Eur Arch Otorhinolaryngol. 2013;270:647–53. doi: 10.1007/s00405-012-2050-3. [DOI] [PubMed] [Google Scholar]
- 20.Beal S, Chen S, Schneider P, Martinez S. An evaluation of lymph node yield and lymph node ratio in well-differentiated thyroid carcinoma. Am Surg. 2010;76:28–32. [PubMed] [Google Scholar]
- 21.Wang L, Palmer F, Nixon I, et al. Central lymph node characteristics predictive of outcome in patients with differentiated thyroid cancer. Thyroid. 2014;30:30. doi: 10.1089/thy.2014.0256. [DOI] [PubMed] [Google Scholar]
- 22.Lee Y, Lim Y, Lee J, et al. Nodal status of central lymph nodes as a negative prognostic factor for papillary thyroid carcinoma. J Surg Oncol. 2013;107:777–82. doi: 10.1002/jso.23308. [DOI] [PubMed] [Google Scholar]
- 23.Carter R, Bliss J, Soo K, O’Brien C. Radical neck dissections for squamous carcinomas: pathological findings and their clinical implications with particular reference to transcapsular spread. Int J Radiat Oncol Biol Phys. 1987;13:825–32. doi: 10.1016/0360-3016(87)90094-0. [DOI] [PubMed] [Google Scholar]
- 24.Johnson J, Myers E, Bedetti C, Barnes E, Schramm VJ, Thearle P. Cervical lymph node metastases. Incidence and implications of extracapsular carcinoma. Arch Otolaryngol. 1985;111:534–7. doi: 10.1001/archotol.1985.00800100082012. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


