Abstract
Objective
The objectives were: 1) measure polycyclic aromatic hydrocarbons (PAHs), polychlorinated dibenzo-p-dioxins (PCDDs) and dibenzofurans (PCDFs) in 100 μL of human serum and 2) assess PAH and PCDD/PCDF as markers of burn pit exposures during military deployments.
Methods
PAHs and PCDDs/PCDFs were analyzed in 100μL serum samples collected pre- and post-deployment from 200 persons deployed to Iraq or Afghanistan (CASE); 200 persons not deployed (CONTROL) with GC/MS.
Results
Naphthalene was found in ~83% of the samples and was statistically different between post-deployment CASE personnel and pre-deployment. 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin, Octachlorodibenzo-p-dioxin, 1,2,3,7,8,9-Hexachlorodibenzofuran, and 1,2,3,4,6,7,8-Heptachlorodibenzofuran were found in ~38% of samples. Concentrations were significantly different between CASE and CONTROL and between pre- and post-deployment samples.
Conclusions
PAH and PCDD/PCDF in serum can serve as exposure markers and measurements in small volumes is feasible for quantifying exposure to burn pits.
Deployment of personnel to forward operating bases leads to a variety of exposures to environmental hazards that may produce post-deployment chronic illnesses. With recent deployments to Iraq and Afghanistan, one of the major hazards has been the use of burn pits to eliminate solid wastes produced on the base. A prior review by the Institute of Medicine1 suggested that the Department of Defense had not provide sufficient exposure assessments for their deployed personnel as would be needed to perform applicable health effects studies. As further described by Mallon et al,2 there is a need to determine the exposure of deployed military service personnel to environmental hazards such as burn pits. It is possible to use ambient measurements to estimate the concentrations to which personnel were exposed if they served in areas where measurements were performed.3 If time/activity patterns were available, exposures could then be estimated. However, it is generally difficult to obtain detailed time/activity relationships given the diversity of personnel and activities on forward deployment bases. Thus, it is necessary to develop biomarkers to determine exposure to specific hazards and to determine if these chemical marker species could be quantitatively measured in readily obtained biological samples. In the case of personnel deployed to Iraq and/or Afghanistan, blood samples were routinely collected by the Department of Defense before and after personnel deployment from which approximately 5 ml serum samples were obtained and stored in the DoD Serum Repository (DoDSR).4
Two potential classes of marker species for environmental exposure to burn pits are polycyclic aromatic hydrocarbon (PAH) compounds and polychlorinated dibenzo-p-dioxin and dibenzofurans (PCDDs/PCDFs). These compounds are produced through incomplete combustion.5,6 In the case of PCDDs/PCDFs, incomplete combustion in the presence of chlorine gives rise to their formation.7 Given the diversity of materials that are incinerated in burn pits and the nature of the combustion process, both of these compound classes are expected to be produced. The ambient measurements presented by Masiol et al.3 confirm that measurable ambient concentrations were observed and thus, personnel were likely to be exposed. The body burden of PAHs is generally assessed using hydroxy-PAHs (phase-1 metabolites) in urine. The commonly measured species is 1-hydroxy-pyrene.8,9 Unfortunately, urine samples were not obtained since collecting such samples at a forward operating base and shipping them to the United States for analysis would be extremely difficult. In their recommendations for future exposure assessments, the IOM report1 did not suggest the collection of exposure biomarker samples in the field. Thus, the serum samples were collected outside of the combat zones and were the only material in which exposure biomarkers could be sought.
PCDDs and PCDFs are not easily metabolized and have long half-lives in the body ranging from a year to a decade.10 In contrast, PAHs are readily metabolized and excreted leading to half-lives of the order of hours to tens of hours.11,12 Since PAHs have relatively short half-lives, they do not accumulate in the body. Fat tends to contain more PAHs than other tissues.13 However, PAH contents in fat and lung tissue do not correlate well. 14
Prior work on PCDD/PCDF concentrations in serum samples15 did not provided useful information. The serum dioxin measurements were severely compromised by high detection limits. However, improved instrumentation and analytical methods provide the possibility of more sensitive and accurate measurements. Thus, the objectives of this study were to determine if PAHs or PCDDs/PCDFs could be detected in the serum samples, and if so, were there statistically significant differences in the concentrations among the four groups of samples.
This study had 3 objectives: The first objective was to determine if polycyclic aromatic hydrocarbons (PAHs) and/or dioxins/dibenzofurans could be detected in very small serum samples. Since only very small volumes of serum (0.1 mL per analysis) were available for the analyses and the standard approaches have called for milliliter quantities of serum, improved methods are needed to prepare such small samples for analysis. Our second objective was to determine if these concentrations of PAHs and dioxins and dibenzofurans in human serum from military personnel could serve as markers of exposure to environmental hazards such as burn pits and motor vehicle exhaust. A third objective was to provide exposure data for the comparison with the analyses of the metabolites and biological response markers to be presented in subsequent papers.
Methods
Samples
Archival samples were selected from the Department of Defense Serum Repository based on the criteria described by Mallon.2 The samples were stored by the DoDSR at −20°C until thawing, aliquoting, and shipping for analysis. Woeller et al.16 report that these samples are of sufficient quality that a variety of biomarker species including microRNAs and cytokines can be successfully measured in these samples. Although DoD does have demographic information and other relevant data such as smoking prevalence and previous deployments, this information was not provided and thus, it is not possible to account for factors like smoking in the analyses presented in the present work.
Extraction
Because of the small sample size, it was not possible to use standard liquid/liquid extraction methods that employ 5 mL of sample.17 Sirimanne et al.18 employed cloud-point extraction to preconcentrate, extract, and clean-up PAHs and PCDDs/PCDFs from 0.5 and 5.0 mL samples of human serum. Thus, we reasoned that this approach could be employed to extract even these smaller 0.1 mL samples. In this approach, a non-ionic surfactant, Triton X-100, is added to the sample and a phase transition is induced by the addition of sodium chloride and then raising its temperature to what is termed its cloud point. The non-polar species will concentrate in the solid material and can then be extracted into a solvent such as hexane. It was found that different proportions of salt and surfactant were required for the two compound classes. For the PAH analysis, we transfer 0.1 mL of human serum sample into a centrifuge tube. It is spiked with 50 μl of internal standard (anthracene-d10). Triton X-100 is added to yield an 8% (v/v) solution. Sodium chloride is added to yield a 5.5 M NaCl solution. The mixture was incubated in a thermostatic shaking water bath for 15 min at 50°C. A picture of centrifuge tubes with the surfactant layer is shown in Supplemental Figure S1. We then add 0.5 mL of hexane, mixed well for another minute using a vortex mixer after which it was centrifuged for 5 min at 15000 rpm. The top layer is transferred to a GC vial, 20 μL nonane is added and the sample was concentrated to 20 μL using a Turbo Vap II. The ability to effectively extract this smaller sample was tested by examining the recovery of spikes in 0.1 mL human serum collected at the University of Rochester Medical Center from healthy non-smoking donors, processed, and frozen in aliquots at −80. Donors gave informed written consent and the protocol was under the supervision of the University of Rochester Research Subjects Review Board.
For the PCDDs/PCDFs, different proportions of surfactant and salt were used. Triton X-100 was added to yield a 12% (v/v) solution and the NaCl concentration was adjusted to be 4.5 M. Each sample was spiked with 20 μL of EPA Method 1613 (Wellington EPA-1613LCS) mixture of deuterated surrogates containing 6 dioxins and 9 furans. Only hexane was used for the extraction and the samples were blown to dryness under dry nitrogen. Again, recovery studies were performed using the Rochester serum sample to ensure that these species could be efficiently recovered from 0.1 mL samples.
PAH Analyses
The sample extracts were then analyzed by Thermo TraceGC coupled to a Thermo DSQ mass spectrometer. The separations were done using an Rxi®-XLB fused silica column with a low polarity proprietary phase. The column dimension was 30 mm x 0.25 mm ID x 0.25 μm df. It exhibited extremely low bleed, ideal for PAHs analysis. The inlet temperature was 220°C. Samples are injected with splitless injection. High purity He was used as the carrier gas and kept constant at 1.2 ml/min. The GC oven initial temperature was 60°C and held for one minute, then the temperature was increased to 210°C at 12°C /min, then to 320°C at 8°C /min, and the final temperature was held for 10 minutes. The specific conditions for the MS were as follow: EI with SIM positive mode. The filament emission current was set at 70 μA. MS transfer line temperature was set at 300°C and ion source temperature at 200°C. Five-point calibration PAH standard mixtures were run to determine the instrument response factors. The resulting chromatograms and mass spectra were inspected to ensure proper peak integration and the peak areas were the converted to concentrations using the response factors and the known surrogate recoveries. The PAH concentration results were then reported as ng of each detected PAH per mL of serum.
PCDD/PCDF Analyses
The dioxin/furan analyses were performed using high resolution GC-high resolution MS (HRGC/HRMS) utilizing an Agilent 7890A GC with a Micromass Autospec Premier HRMS. The system is capable of performing selected ion monitoring at a resolving power of at least 10,000 in electron ionization mode. The samples for dioxin/furan analyses were reconstituted with 20 μL of nonane, with a 1 μL aliquot injected into the HRGC/HRMS. The column used to determine dioxin/furan compounds is the DB-5MSUI (60 m long, 0.25 mm inner diameter, 0.25 μm film thickness). Ultrahigh purity helium was used as the carrier gas. The interface temperature is at 300°C. Lock mass ion descriptors for each analyte are based on EPA Method 1613B. The retention times of the native compounds are based on the retention times of the corresponding Carbon-13 isotopically labeled standards which helps ensure accurate identification of the dioxin and furan compounds. Once the required spectral and retention time identification criteria are met, the detector responses for target ions are used to calculate concentrations. Peak identification requirements and representative data calculations are those found in EPA Method 1613B. A five-point calibration curve is employed using five calibration solutions where each point in the calibration must meet specific signal to noise and ion abundance ratio measures in order to pass quality control criteria defined by the reference method. PCDD/PCDF compounds are reported in concentrations of pg/L (picograms per liter extracted) or ppq (parts per quadrillion).
Statistical Analyses
The samples were divided into 4 groups, CONTROL pre- and post- and CASE pre- and post-. Review of the data showed that they uniformly failed tests for normality (Shapiro-Wilk) and thus, only non-parametric statistical tests were employed. Thus, initial analyses were made using a 2-factor ANOVA on ranks (pre-/post-, CASE/CONTROL) using Dunn’s test.19
Within the CONTROL and CASE groups, we have paired pre- and post-deployment samples for each subject. These two groups of paired samples have been analyzed using a Wilcoxon Signed Rank Test.20 The CASE and CONTROL pre-deployment and the CASE and CONTROL post-deployment are unpaired samples and have been compared using the Mann-Whitney Rank Sum Test.21
Results
PAHs
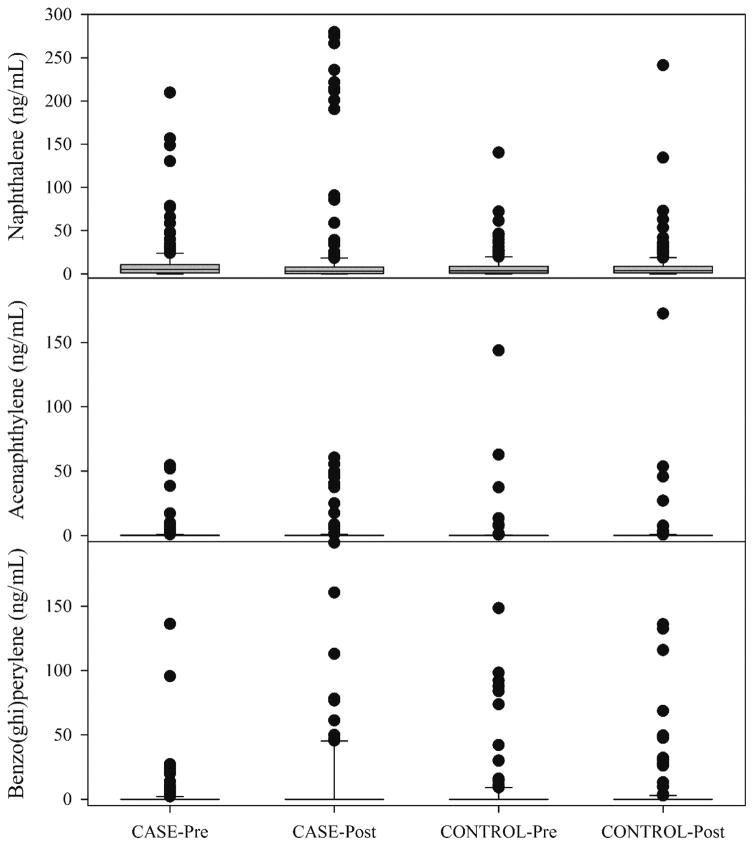
The PAH compound most commonly found in the serum samples was naphthalene. Table 1 provides a listing of the compounds sought in the analysis, the number of samples in which the given analyte was found, and the summary statistics for each group of samples. Naphthalene is the only compound that was measured in a majority of the samples. The other observed species, acenaphthylene (216), acenaphthene (258), fluorene (266), phenanthrene (196), anthracene (240), fluoranthene (234), pyrene (181), benzo(a)pyrene (161), dibenz(a,h)anthracene (145), and benzo(ghi)perylene (170) were found in only 20 to 30% of the samples. The distributions of the concentrations of naphthalene, acenaphthylene, and benzo(ghi)perylene for the 4 groups are compared in Figure 1. The complete data set is provided in the supplemental materials files.
Table 1.
Summary statistics for PAH compounds in the human serum samples (ng/mL).
| Compound | CASE-Pre | CASE-Post | ||||||
|---|---|---|---|---|---|---|---|---|
| Detects | Median | Mean | Max | Detects | Median | Mean | Max | |
| Naphthalene | 162 | 4.94 | 11.60 | 209.6 | 158 | 3.15 | 16.28 | 279.6 |
| acenaphthylene | 56 | 0.00 | 1.13 | 54.5 | 57 | 0.00 | 2.12 | 60.5 |
| acenaphthene | 69 | 0.00 | 1.59 | 58.0 | 60 | 0.00 | 1.74 | 49.4 |
| fluorene | 71 | 0.00 | 0.83 | 29.0 | 73 | 0.00 | 0.82 | 21.9 |
| phenanthrene | 56 | 0.00 | 1.30 | 47.5 | 47 | 0.00 | 2.31 | 176.0 |
| anthracene | 55 | 0.00 | 1.15 | 115.4 | 61 | 0.00 | 1.28 | 128.8 |
| Fluoranthene | 61 | 0.00 | 1.51 | 81.4 | 58 | 0.00 | 1.67 | 95.3 |
| pyrene | 50 | 0.00 | 0.27 | 22.4 | 52 | 0.00 | 0.30 | 8.5 |
| Benz(a)anthracene | 19 | 0.00 | 0.09 | 3.3 | 29 | 0.00 | 0.13 | 8.1 |
| chrysene | 19 | 0.00 | 0.08 | 2.4 | 21 | 0.00 | 0.09 | 3.3 |
| Benzo(b)fluoranthene | 10 | 0.00 | 0.30 | 52.1 | 7 | 0.00 | 0.20 | 34.9 |
| Benzo(k)fluoranthene | 10 | 0.00 | 0.30 | 50.2 | 8 | 0.00 | 0.20 | 33.6 |
| Benzo(a)pyrene | 40 | 0.00 | 3.39 | 177.5 | 50 | 0.00 | 3.37 | 104.1 |
| Dibenz(a,h)anthracene | 35 | 0.00 | 7.71 | 517.9 | 39 | 0.00 | 19.07 | 1436.7 |
| Benzo(ghi)perylene | 38 | 0.00 | 3.79 | 298.0 | 48 | 0.00 | 24.29 | 775.5 |
| Indeno(123cd)pyrene | 1 | 0.00 | 0.00 | 1.0 | 4 | 0.00 | 0.09 | 8.2 |
| Compound | CONTROL-Pre | CONTROL-Post | ||||||
|---|---|---|---|---|---|---|---|---|
| Detects | Median | Mean | Max | Detects | Median | Mean | Max | |
| Naphthalene | 159 | 3.44 | 7.66 | 140.3 | 165 | 3.55 | 8.83 | 241.3 |
| acenaphthylene | 50 | 0.00 | 1.44 | 143.6 | 53 | 0.00 | 1.69 | 172.3 |
| acenaphthene | 67 | 0.00 | 2.31 | 60.8 | 62 | 0.00 | 3.23 | 230.6 |
| fluorene | 57 | 0.00 | 0.53 | 24.1 | 65 | 0.00 | 0.84 | 50.2 |
| phenanthrene | 46 | 0.00 | 1.60 | 75.7 | 47 | 0.00 | 0.97 | 39.4 |
| anthracene | 63 | 0.00 | 3.09 | 126.4 | 61 | 0.00 | 12.40 | 2015.5 |
| Fluoranthene | 56 | 0.00 | 1.60 | 84.6 | 59 | 0.00 | 1.53 | 77.2 |
| pyrene | 40 | 0.00 | 0.35 | 26.4 | 39 | 0.00 | 0.45 | 53.8 |
| Benz(a)anthracene | 20 | 0.00 | 0.25 | 27.3 | 21 | 0.00 | 0.37 | 31.8 |
| chrysene | 13 | 0.00 | 0.12 | 12.4 | 15 | 0.00 | 0.18 | 15.0 |
| Benzo(b)fluoranthene | 4 | 0.00 | 0.14 | 21.5 | 6 | 0.00 | 0.15 | 15.6 |
| Benzo(k)fluoranthene | 5 | 0.00 | 0.63 | 99.5 | 7 | 0.00 | 0.16 | 14.5 |
| Benzo(a)pyrene | 32 | 0.00 | 5.56 | 226.6 | 39 | 0.00 | 3.49 | 159.6 |
| Dibenz(a,h)anthracene | 41 | 0.00 | 13.41 | 445.1 | 30 | 0.00 | 12.85 | 1085.8 |
| Benzo(ghi)perylene | 46 | 0.00 | 11.56 | 384.7 | 38 | 0.00 | 6.60 | 316.7 |
| Indeno(123cd) pyrene | 4 | 0.00 | 0.12 | 15.7 | 4 | 0.00 | 0.10 | 10.0 |
Figure 1.
Box and whisker plot showing the distributions of naphthalene, acenaphthylene, and benzo(ghi)perylene in the serum samples. Boxes represent the distribution from the 25th to the 75th percentiles and the whiskers represent the 10th and 90th percentiles.
There are limited reports on the concentrations of PAHs in serum. Pleil et al.22 measured 22 PAHs in human plasma and found mean cohort values ranging from 0.050 to 0.380 ng/ml (mean= 0.200 ng/ml). Singh et al.23 report values of 1.05 to 160.6 ppb (~ng/mL) representing the 25th percentile to 75th percentile values in the distributions of PAH concentrations in the blood of children in Lucknow, India. Kamal et al.24 measured naphthalene in highly exposed workers in Pakistan. They found naphthalene in 32 of 60 samples with a median value of 155.9 ng/mL. They reported substantial increases (factors of 4 to 6) in naphthalene concentrations in smokers compared to non-smokers for the same occupational classes. Thus, smoking behavior appears to have a substantial influence on blood naphthalene concentrations.
The 2-factor ANOVA on ranks for all of the compounds found no statistically significant differences among the four groups. The results of the signed rank tests on the paired data are presented in Table 2. They confirm what can be observed in Figure 1. The CASE-pre values are statistically higher than the paired CASE-post values for naphthalene (p=0.003) and acenaphthylene (p=0.021) while the benzo(ghi)perylene (p=0.045) are higher in the CASE-post than in the CASE-pre samples. None of the paired CONTROL pre- and post- values are statistically different.
Table 2.
Results of the statistical analyses of the PAH data.
| Compound | Signed Rank Test
|
|||
|---|---|---|---|---|
| CASE | CONTROL | |||
|
| ||||
| Z-statistic | p | Z-statistic | P | |
| Naphthalene | −2.997* | 0.003 | 0.699 | 0.485 |
| Acenaphthylene | −2.317 | 0.021 | 0.351 | 0.727 |
| Acenaphthene | −1.436 | 0.151 | −0.562 | 0.575 |
| Fluorene | −1.357 | 0.175 | 1.005 | 0.316 |
| Phenanthrene | −1.492 | 0.136 | −0.244 | 0.809 |
| Anthracene | −0.589 | 0.558 | −0.235 | 0.816 |
| Fluoranthene | −1.130 | 0.259 | −0.778 | 0.438 |
| Pyrene | 1.035 | 0.302 | −0.568 | 0.572 |
| Benzo(a)pyrene | 1.341 | 0.181 | 0.011 | 0.994 |
| Dibenz(a,h)anthracene | 0.801 | 0.425 | −1.109 | 0.269 |
| Benzo(ghi)perylene | 2.009 | 0.045 | −1.442 | 0.150 |
| Compound | Rank Sum Test
|
|||
|---|---|---|---|---|
| Pre | Post | |||
|
| ||||
| Mann-Whitney U | p | Mann-Whitney U | P | |
| Naphthalene | 17573.5 | 0.035 | 18993.0 | 0.382 |
| Acenaphthylene | 19059.0 | 0.295 | 19546.0 | 0.618 |
| Benzo(ghi)perylene | 19007.5 | 0.228 | 18888.0 | 0.181 |
Bolded entries are statistically significant.
The pre-deployment samples were statistically different between the CASE and CONTROL samples for naphthalene (p=0.035), but not for either acenaphthylene or benzo(ghi)perylene. The post-deployment samples were not statistically different between the CASE and CONTROL groups for any of the other measured PAH compounds. It is not clear why post-CASE naphthalene and acenaphthylene values would be lower than the pre-CASE values. However, we can hypothesize that the major source of these compounds is smoking tobacco since they are highest multiple ring compound concentrations in tobacco smoke.25,26 As noted previously, the smoking histories of these individuals has not been made available for this study and future work will explore the relationships between these compounds and smoking. Benzo(ghi)perylene is emitted in relatively small quantities in tobacco smoke and is more likely to reflect diesel emissions.27,28 The lower ring number compounds could be cleared during the process of ending the deployment. At the end of a deployment, there is a long airplane flight following by a variety of post-deployment activities including blood sample collection. Thus, given PAHs are rapidly cleared from the body (e.g. naphthalene has a median half-life of 4.3 hr12) and during the return and processing there would be no opportunity to smoke, most of the small PAHs could be cleared from the body. There are no reports of the biological lifetime of benzo(ghi)perylene, but it’s much larger structure (6 rings) would make it much more lipophilic and thus, likely to be retained longer than the 2- and 3-ringed species. Thus, it may represent a marker of diesel exposure for the deployed personnel.
PCDDs/PCDFs
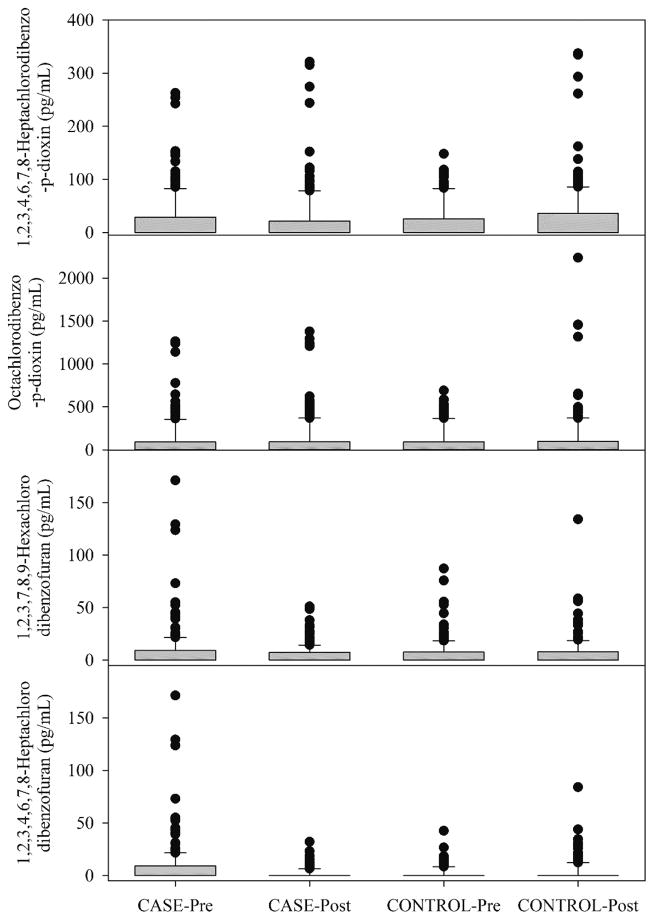
For the PCDDs/PCDFs, compounds were identified in a smaller percentage of the samples as shown in Table 3. It can be seen that only four compounds, 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin, Octachlorodibenzo-p-dioxin, 1,2,3,7,8,9-Hexachlorodibenzofuran, and 1,2,3,4,6,7,8-Heptachlorodibenzofuran, were present in more than 25% of the samples. The distributions for these compounds are shown in Figure 2. In this study, despite the very small sample volume, we were able to measure the presence of these compounds in a much larger fraction of samples than in a prior pilot study of dioxins and furans in repository serum samples for 25 persons pre- and post-deployment (50 samples in total).15 In the Thomas et al. study, 1 mL of serum was analyzed for the 21 congeners including the species listed in Table 2. However, values for most of the samples were below detection limits. Of the 21 congeners, only 7 showed above the detection limit values in any sample. Even among these 7 congeners, many results were below the detection limit (64 percent). The above detection limit values were more often found in the pre-deployment specimen, rather than in the post-deployment specimen. Thus, exposure during deployment did not appear to lead to a rise in congener levels. When both pre- and post- deployment results were above the detection limit, there were about an equal number of individuals for which the pre-deployment results were higher and for which the post-deployment were higher. The observed values were above the typical range of values observed in the general population as given by the National Health and Nutrition Examination Survey (NHANES) 2003–04 values.29
Table 3.
PCDDs/PCDFs compounds detected in the serum samples (pg/mL).
| Compound | Detects | Mean Value | NHANES29 03–04 Geo. Mean |
|---|---|---|---|
| 2,3,7,8-Tetrachlorodibenzo-p-dioxin (TCDD) | 8 | 0.2 | <0.0038 |
| 1,2,3,7,8-Pentachlorodibenzo-p-dioxin (PeCDD) | 21 | 1.0 | <0.0045 |
| 1,2,3,4,7,8-Hexachlorodibenzo-p-dioxin (HxCDD) | 53 | 1.4 | <0.012 |
| 1,2,3,6,7,8-Hexachlorodibenzo-p-dioxin (HxCDD) | 64 | 1.5 | 0.105 |
| 1,2,3,7,8,9-Hexachlorodibenzo-p-dioxin (HxCDD) | 62 | 1.5 | <0.012 |
| 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin (HpCDD) | 291 | 23.5 | 0.155 |
| Octachlorodibenzo-p-dioxin (OCDD) | 330 | 104.2 | 2.23* |
| 2,3,7,8-Tetrachlorodibenzofuran (TCDF) | 13 | 0.2 | <0.0060 |
| 1,2,3,7,8-Pentachlorodibenzofuran (PeCDF) | 31 | 1.1 | <0.0071 |
| 2,3,4,7,8-Pentachlorodibenzofuran (PeCDF) | 23 | 0.9 | <0.0068 |
| 1,2,3,4,7,8-Hexachlorodibenzofuran (HxCDF) | 131 | 2.5 | 0.0074 |
| 1,2,3,6,7,8-Hexachlorodibenzofuran (HxCDF) | 142 | 3.0 | <0.0079 |
| 1,2,3,7,8,9-Hexachlorodibenzofuran (HxCDF) | 259 | 6.3 | <0.0083 |
| 2,3,4,6,7,8-Hexachlorodibenzofuran (HxCDF) | 98 | 2.6 | <0.0082 |
| 1,2,3,4,6,7,8-Heptachlorodibenzofuran (HpCDF) | 265 | 9.0 | 0.0622* |
| 1,2,3,4,7,8,9-Heptachlorodibenzofuran (HpCDF) | 126 | 3.8 | <0.0086 |
| Octachlorodibenzofuran (OCDF) | 83 | 12.9 | <0.0120 |
From 01–02 survey. Not reported for 03–04 because of a high proportion of values below LOD
Figure 2.
Box and whisker plot showing the distributions of 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin (HpCDD), Octachlorodibenzo-p-dioxin (OCDD), 1,2,3,7,8,9-Hexachlorodibenzofuran (HxCDF), and 1,2,3,4,6,7,8-Heptachlorodibenzofuran in the serum samples. Boxes represent the distribution from the 25th to the 75th percentiles and the whiskers represent the 10th and 90th percentiles.
The 2-factor ANOVA analyses of 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin (HpCDD), Octachlorodibenzo-p-dioxin (OCDD), 1,2,3,7,8,9-Hexachlorodibenzofuran (HxCDF), and 1,2,3,4,6,7,8-Heptachlorodibenzofuran showed no significant differences between any of the groups. However, as shown in Table 4, the signed rank test showed that the CASE-pre and CASE-post were statistically different for all four of these compounds. The concentrations of 1,2,3,4,6,7,8-Heptachlorodibenzofuran in the Pre- and post-CONTROL samples were also statistically different. Table 5 compares the mean values for the four compounds for the four groups. In Figure 2 and Table 5, it can again be seen that there were higher concentrations for the pre-deployment samples for 3 of the 4 species. It is not clear why we would observe higher concentrations in the individuals prior to deployment. However, some of these personnel may have had multiple deployments. Given the relatively long half-lives of these species in the body, there may be residual concentrations arising from the multiple deployments leading to an individual having higher concentrations pre-deployment than post deployment. Future work will explore this possibility when the demographic and deployment histories of the specific personnel become available.
Table 4.
Results of the statistical analyses of the PCDD/PCDF concentrations.
| Compound | Signed Rank Test
|
|||
|---|---|---|---|---|
| CASE | CONTROL | |||
|
| ||||
| z-statistic | p | z-statistic | p | |
| 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin | 6.95* | <0.001 | 1.538 | 0.125 |
| Octachlorodibenzo-p-dioxin | −7.961 | <0.001 | 0.682 | 0.496 |
| 1,2,3,7,8,9-Hexachlorodibenzofuran | −5.915 | <0.001 | 0.105 | 0.918 |
| 1,2,3,4,6,7,8-Heptachlorodibenzofuran | 6.95 | <0.001 | 2.794 | 0.005 |
| Compound | Rank Sum Test
|
|||
|---|---|---|---|---|
| Pre- | Post- | |||
|
| ||||
| Mann-Whitney U | p | Mann-Whitney U | p | |
| 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin | 19038 | 0.567 | 15646 | <0.001 |
| Octachlorodibenzo-p-dioxin | 19125 | 0.640 | 18725 | 0.702 |
| 1,2,3,7,8,9-Hexachlorodibenzofuran | 19034 | 0.557 | 18710 | 0.664 |
| 1,2,3,4,6,7,8-Heptachlorodibenzofuran | 18040 | 0.102 | 17808 | 0.168 |
Bolded entries are statistically significant.
Table 5.
Mean values of the four most frequently measured PCDDs/PCDFs for each of the four subject groups.
| Compound | Case-Pre | Case-Post | Control-Pre | Control-Post |
|---|---|---|---|---|
| 1,2,3,4,6,7,8-Heptachlorodibenzo-p-dioxin (HpCDD) | 24.7 | 21.2 | 20.8 | 27.4 |
| Octachlorodibenzo-p-dioxin (OCDD) | 103.8 | 104.6 | 90.7 | 117.7 |
| 1,2,3,7,8,9-Hexachlorodibenzofuran (HxCDF) | 8.4 | 4.4 | 5.9 | 6.3 |
| 1,2,3,4,6,7,8-Heptachlorodibenzofuran (HpCDF) | 11.2 | 7.9 | 6.6 | 10.2 |
The rank sum tests showed that the CASE-post samples were higher than the CONTROL-post for 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin while all of the other pre- and post-deployment samples did not show statistically significant differences for the PCDDs or the PCDFs.
Conclusions
The cloud point extraction procedure followed by GC/MS analysis permitted the measurement of PAHs and PCDDs/PCDFs in 100 μL samples of human serum. Based on the analyses of 800 samples of serum from 400 military personnel, 200 of which were deployed to Iraq or Afghanistan, a limited number of statistically significant differences were observed using the appropriate non-parametric tests. For the PAHs, naphthalene and acenaphthylene were higher in pre-CASE personnel than post-CASE personnel suggesting a possible change in smoking habits while deployed or the excretion of these compounds during post-deployment procedures where smoking would be prohibited. Benzo(ghi)perylene was higher in the post-CASE compared to the pre-CASE suggesting the possibility that it is a marker of exposure to diesel exhaust. Also CASE naphthalene values were higher than the CONTROL values suggesting differences in smoking behavior between these groups. The current lack of smoking data precludes the testing of this hypothesis.
For the PCDD/PCDF results, 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin, octachlorodibenzo-p-dioxin, 1,2,3,7,8,9-hexachlorodibenzofuran, and 1,2,3,4,6,7,8-heptachlorodibenzofuran were the only compounds found in many samples (~38%). Only octachlorodibenzo-p-dioxin (OCDD) was higher in the post-deployment CASE personnel. In addition, CONTROL-post subjects had significantly higher concentrations of 1,2,3,4,6,7,8-Heptachlorodibenzofuran (HpCDF) (p=0.005). CONTROL-post subjects had higher concentrations of 1,2,3,4,6,7,8-heptachlorodibenzo-p-dioxin (HpCDD) (p<0.001) than CASE-post personnel. These differences will be the subject of future studies when the additional data on the individuals is made available. These concentration data have been made available to the rest of the project team to compare with the analyses of metabolites and biological response markers and these results are presented in other papers in this issue.
We can conclude that it is possible to measure PAH and PCDD/PCDF compounds in very small quantities of human serum and these measurements appear to provide the potential for environmental hazard exposure assessments for personnel deployed to forward operating bases.
Supplementary Material
Acknowledgments
This work was supported by The Henry M. Jackson Foundation for the Advancement of Military Medicine, Inc. grant number HT9404-13-1-0030, and the National Institute of Environmental Health Sciences Grant # P30-ES01247.
Footnotes
There are no conflicts of interest.
References
- 1.Institute of Medicine (IOM) Long-term Health Consequences of Exposure to Burn Pits in Iraq and Afghanistan. The National Academies Press; Washington, DC: 2011. [Google Scholar]
- 2.Mallon TM. Introduction to DoD research on burn-pit biomarkers and health outcomes. J Occup Environ Med. 2016 doi: 10.1097/JOM.0000000000000775. this issue. [DOI] [PubMed] [Google Scholar]
- 3.Masiol M, Mallon TM, Haynes KM, Utell MJ, Hopke PK. Airborne Dioxins, Furans and Polycyclic Aromatic Hydrocarbons Exposure to Military Personnel in Iraq. J Occup Environ Med. 2016 doi: 10.1097/JOM.0000000000000771. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Perdue CL, Eich Cost AA, Rubertone MV, Lindler LE, Ludwig SL. Description and Utilization of the United States Department of Defense Serum Repository: A Review of Published Studies, 1985–2012; PLOS ONE. 2015;10(2):e0114857. doi: 10.1371/journal.pone.0114857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Baek SO, Field RA, Goldstone ME, Kirk PW, Lester JN, Perry R. A review of atmospheric polycyclic aromatic hydrocarbons: Sources, fate and behavior. Water, Air, Soil Pollut. 1991;60:279–300. [Google Scholar]
- 6.Simoneit BRT. Biomass burning — a review of organic tracers for smoke from incomplete combustion, Appl. Geochem. 2002;17:129–162. [Google Scholar]
- 7.McKay G. Dioxin characterisation, formation and minimisation during municipal solid waste (MSW) incineration: review. Chemical Engineering Journal. 2002;86:343–368. [Google Scholar]
- 8.Castaño-Vinyals G, D’Errico A, Malats N, Kogevinas M. Biomarkers of exposure to polycyclic aromatic hydrocarbons from environmental air pollution. Occup Environ Med. 2004;61:e12. doi: 10.1136/oem.2003.008375. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hansen AM, Mathiesen L, Pedersen M, Knudsen LE. Urinary 1-hydroxypyrene (1-HP) in environmental and occupational studies--a review. Int J Hyg Environ Health. 2008;211:471–503. doi: 10.1016/j.ijheh.2007.09.012. [DOI] [PubMed] [Google Scholar]
- 10.Milbraith MO, Wenger Y, Chang CW, Emond C, Garabrant D, Gillespie BW, Jolliet O. Apparent Half-Lives of Dioxins, Furans, and Polychlorinated Biphenyls as a Function of Age, Body Fat, Smoking Status, and Breast-Feeding. Environ Health Perspect. 2009;117:417–425. doi: 10.1289/ehp.11781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Brzeznicki S, Jakubowski M, Czweaki B. Elimination of 1-hydroxypyrene after human volunteer exposure to polycyclic aromatic hydrocarbons. Int Arch Occup Environ Health. 1997;70:257–260. doi: 10.1007/s004200050216. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Romanoff L, Bartell S, Pittman EN, Trinidad DA, McClean M, Webster TF, Sjödin A. Excretion Profiles and Half-Lives of Ten Urinary Polycyclic Aromatic Hydrocarbon Metabolites after Dietary Exposure, Chem Res. Toxicol. 2012;25:1452–1461. doi: 10.1021/tx300108e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Agency for Toxic Substances and Disease Registry. Public Health Statement; Polycyclic Aromatic Hydrocarbons (PAHs) Atlanta, GA: Available at http://www.atsdr.cdc.gov/ToxProfiles/tp69-c1-b.pdf. [PubMed] [Google Scholar]
- 14.Goldman R, Enewold L, Pellizzari E, Beach JE, Bowman ED, Krishnan SS, Shields PG. Smoking Increases Carcinogenic Polycyclic Aromatic Hydrocarbons in Human Lung Tissue. Cancer Res. 2001;61:6367–6371. [PubMed] [Google Scholar]
- 15.Taylor G, Rush V, Deck A, Vietas JA. Aberdeen Proving. Ground, MD: Army Center for Health Promotion and Preventive Medicine; 2008. Screening Health Risk Assessment: Burn Pit Exposures Balad Air Base, Iraq and Addendum Report. Available at http://oai.dtic.mil/oai/oai?verb=getRecord&metadataPrefix=html&identifier=ADA493142. [Google Scholar]
- 16.Woeller CF, Thatcher T, Van Twisk D, Thakar J, Croadsdell A, Kim N, Hopke PK, Xia X, Mallon TM, Utell MJ, Phipps RP. Detection of serum microRNAs from Department of Defense Serum Repository: correlation with cotinine, cytokine and polycyclic aromatic hydrocarbon levels. J Occup Environ Med. doi: 10.1097/JOM.0000000000000742. this issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Patterson DG, Jr, Hampton L, Lapeza CR, Jr, Belser WT, Green V, Alexander L, Needham LL. High-resolution gas chromatographic/high-resolution mass spectrometric analysis of human serum on a whole-weight lipid basis for 2, 3, 7, 8- tetrachlorodibenzo-p-dioxin. Anal Chem. 1987;59:2000–2005. doi: 10.1021/ac00142a023. [DOI] [PubMed] [Google Scholar]
- 18.Sirimanne SR, Barr JR, Patterson DG., Jr Quantification of Polycyclic Aromatic Hydrocarbons and Polychlorinated Dibenzo-p-dioxins in Human Serum by Combined Micelle-Mediated Extraction (Cloud-Point Extraction) and HPLC. Anal Chem. 1996;68:1556–1568. doi: 10.1021/ac951028+. [DOI] [PubMed] [Google Scholar]
- 19.Dunn OJ. Multiple comparisons using rank sums. Technometrics. 1964;6:241–252. [Google Scholar]
- 20.Wilcoxon F. Individual comparisons by ranking methods. Biometrics Bulletin. 1945;1:80–83. [Google Scholar]
- 21.Mann HB, Whitney DR. On a test of whether one of two random variables is stochastically larger than the other. Ann Math Stat. 1947;18:50–60. [Google Scholar]
- 22.Pleil JD, Stiegel MA, Sobus JR, Tabucchi S, Ghio AJ, Madden MC. Cumulative exposure assessment for trace-level polycyclic aromatic hydrocarbons (PAHs) using human blood and plasma analysis. Journal of Chromatography B: Analytical Technologies in the Biomedical and Life Sciences. 2010;878:1753–1760. doi: 10.1016/j.jchromb.2010.04.035. [DOI] [PubMed] [Google Scholar]
- 23.Singh VK, Patel DK, Ram S, Mathur N, Siddiqui MKJ, Behari JR. Blood levels of polycyclic aromatic hydrocarbons in children of Lucknow. India Arch Environ Contam Toxicol. 2008;54:348–354. doi: 10.1007/s00244-007-9015-3. [DOI] [PubMed] [Google Scholar]
- 24.Kamal A, Qayyum M, Cheema IU, Rashid A. Levels as a Marker of Occupational Exposure to PAHs among Auto-Mechanics and Spray Painters in Rawalpindi. BMC Public Health. 2011;11:467. doi: 10.1186/1471-2458-11-467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Pullman A, Pullman B. Electronic structure and carcinogenic activity of aromatic molecules. New developments; Adv Cancer Res. 1955;3:117–169. doi: 10.1016/s0065-230x(08)60919-7. [DOI] [PubMed] [Google Scholar]
- 26.Rodgman A, Perfetti TA. The composition of cigarette smoke: a catalogue of the polycyclic aromatic hydrocarbons. Beiträge zur Tabakforschung International. 2006;22:13–69. [Google Scholar]
- 27.Zielinska B, Sagebiel J, Arnott WP, Rogers CF, Kelly KE, Wagner DA, Lighty JS, Sarofim AF, Palmer G. Phase and size distribution of polycyclic aromatic hydrocarbons in diesel and gasoline vehicle emissions. Environ Sci Technol. 2004;38:2557–2567. doi: 10.1021/es030518d. [DOI] [PubMed] [Google Scholar]
- 28.Mabilia R, Cecinato A, Tomasi Scianò MC, Di Palo V, Possanzini M. Characterization of polycyclic aromatic hydrocarbons and carbonyl compounds in diesel exhaust emissions. Annali di Chimica. 2004;94:733–740. doi: 10.1002/adic.200490091. [DOI] [PubMed] [Google Scholar]
- 29.Centers for Disease Control and Prevention. Fourth National Report on Human Exposure to Environmental Chemicals. Centers for Disease Control and Prevention; Atlanta, GA: Available at: http://www.cdc.gov/exposurereport/pdf/fourthreport.pdf. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.