Abstract
Brown adipose tissue (BAT) is predominantly regulated by the sympathetic nervous system (SNS) and the adrenergic receptor signaling pathway. Knowing that a mouse with triple β-receptor knockout (KO) is cold intolerant and obese, we evaluated the independent role played by the β1 isoform in energy homeostasis. First, the 30 min i.v. infusion of norepinephrine (NE) or the β1 selective agonist dobutamine (DB) resulted in similar interscapular BAT (iBAT) thermal response in WT mice. Secondly, mice with targeted disruption of the β1 gene (KO of β1 adrenergic receptor (β1KO)) developed hypothermia during cold exposure and exhibited decreased iBAT thermal response to NE or DB infusion. Thirdly, when placed on a high-fat diet (HFD; 40% fat) for 5 weeks, β1KO mice were more susceptible to obesity than WT controls and failed to develop diet-induced thermogenesis as assessed by BAT Ucp1 mRNA levels and oxygen consumption. Furthermore, β1KO mice exhibited fasting hyperglycemia and more intense glucose intolerance, hypercholesterolemia, and hypertriglyceridemia when placed on the HFD, developing marked non-alcoholic steatohepatitis. In conclusion, the β1 signaling pathway mediates most of the SNS stimulation of adaptive thermogenesis.
Introduction
The brown adipose tissue (BAT) is a highly specialized organ capable of heat production in response to a drop in environmental temperature or feeding on a high-caloric diet (Silva 1995). While BAT’s role in small and in hibernating animals is well established (Silva 1995), more recently, its presence in adult humans has pointed to a possible metabolic role and therapeutic targeting for obesity (Virtanen et al. 2009). BAT thermogenesis is activated by the sympathetic nervous system (SNS) with the release of norepinephrine (NE) and stimulation of α and β adrenergic receptors (Bachman et al. 2002). The latter are G-protein-coupled receptors that include the β1, β2, and β3 isoforms, all of which are expressed in BAT (Collins & Surwit 2001). The critical role played by these receptors in BAT function and overall energy homeostasis is illustrated by the fact that mice with combined targeted disruption of the three β1, β2, and β3 adrenergic receptors (TKO mice) have increased susceptibility to cold-induced hypothermia as well as diet-induced obesity (Bachman et al. 2002, Jimenez et al. 2002). However, the relative contribution of each β adrenergic receptor isoform to these processes is less understood (Collins & Surwit 2001, Lowell & Bachman 2003, Masuo & Lambert 2011).
BAT β1, β2, and β3 adrenergic receptor mRNA transcripts are found at a ratio of 3:1:150 in mice acclimated at thermoneutrality, respectively, with the β3 receptor exhibiting a much lower affinity for catecholamines than β1/β2 (Collins et al. 1994). In isolated murine BAT membrane preparations, it has been estimated that about 23% of maximally stimulated adenylate cyclase activity is mediated by the β1/β2 receptors, while ~77% is mediated by the β3 receptors (Collins et al. 1994). By contrast, in preparations of rat BAT membranes, about 70% of the maximally stimulated adenylate cyclase activity is via the β1/β2 receptors and only about 30% is via the β3 receptor (Granneman 1992).
Studies performed on isolated brown adipocytes indicate that the β3 adrenergic receptor plays the leading role in BAT activation (Zhao et al. 2011). However, mice with global β3 receptor knockout (β3KO) have no significant defect in basal metabolic rate, adaptive response to cold exposure (Susulic et al. 1995), or metabolic response to diet-induced obesity (Rohrer 1998). These animals do exhibit upregulation of β1 (not β2) adrenergic receptors, suggesting that either the β3 receptor is not critical for thermogenesis or that its absence is compensated by an increase in β1 receptor expression (Atgie et al. 1997, Rohrer 1998). At the same time, mice with β1 adrenergic receptor overexpression are resistant to diet-induced obesity (Soloveva et al. 1997). Studies on humans confirm the relative role played by β1 and β2 adrenergic receptors in the determination of the basal metabolic rate, with β1 being the most relevant isoform (Jansky 1995, Jansky et al. 2008).
Here, we report that infusion of the β1 adrenergic receptor selective agonist dobutamine (DB) triggers a similar interscapular BAT (iBAT) thermal response as NE. Mice with targeted disruption of the β1 gene (β1KO) developed cold-induced hypothermia and exhibit decreased catecholamine-stimulated iBAT thermal response. They also fail to activate energy expenditure when placed on a high-fat diet (HFD) and thus are more susceptible to diet-induced obesity, indicating that the β1 signaling pathway mediates most of the SNS stimulation of adaptive thermogenesis.
Materials and Methods
Animals
Ninety-day-old male β1KO mice in C57BL6/J background were evaluated. This investigation conforms to the guidelines of the committee on animal research at the Center of Biological Sciences and Health, University Presbyterian Mackenzie, and was approved by the Institutional Animal Welfare Committee. Each experiment was repeated two or three times on different sets of animals. In one set of experiments, mice were fed with chow diet and cold-induced thermogenesis was evaluated. In another set of experiments, mice were fed a HFD and diet-induced thermogenesis was evaluated.
Core body temperature during cold exposure
For cold exposure, conscious mice were housed individually in cages with no bedding in a cold room and maintained at 4 °C (Eletrolab, São Paulo, SP, Brazil) for up to 3 h with central body temperature measurements every hour. This parameter was measured using an electronic thermistor equipped with a rectal probe of 1 mm diameter (Y4000, Yellow Springs Instrument Co., Yellow Springs, OH, USA) as described previously (de Jesus et al. 2001).
Interscapular brown fat thermal response to NE and DB infusion in anesthetized mice
The measurements of these parameters were performed under anesthesia as described (Ribeiro et al. 2001). Briefly, all animals were anesthetized with urethane (560 mg/kg, i.p.) and chloralose (38 mg/kg, i.p.) on the morning of the experiment and a polyethylene (P-50) cannula was inserted into the left jugular vein. For measurement of iBAT temperatures (°C), a precalibrated thermistor probe (YSI 427; Yellow Springs Instrument Co.) was surgically placed under the brown fat pad. The iBAT temperature was measured for ~10 min to obtain a stable baseline and then NE (2 mg/ml) or DB (600 mg/ml) was infused into the left jugular vein via infusion pump (model 2274, Harvard Apparatus, Holliston, MA, USA) at a rate of 0·643 µl/min for 30 min. After the experiment, animals were killed.
Treatment
To evaluate diet-induced thermogenesis, β1KO male mice were placed on a HFD (Rhoster, Sao Paulo, Brazil) for 5 weeks. HFD presents 5·44 cal/g (40% lipid) while chow diet presents 1·8 cal/g. Food intake and body weight (BW) were measured daily.
Oxygen consumption
Resting oxygen consumption (VO2) was measured in an open circuit respirometer system (O2-10, Sable System, Las Vegas, NV, USA) as described previously (17, 18). For cold exposure, mice were exposed to different temperatures (5, 15, 25, 30, 32, 34, and 36 °C) and the O2 consumption was measured for 1 h at each temperature. The experiments begin in the late morning and finished by the end of the day (1100–1900 h). The data were collected and analyzed by the Sable Systems Software. The results are expressed as milliliters of O2/min per g BW. For experiments performed at room temperature (25 °C), measurements were obtained over 30 min, in the afternoon (1400–1800 h) in animals fed ad libitum.
mRNA analysis
Total RNA of iBAT was extracted using TRIzol (Life Technologies, Inc.), according to the manufacturer’s instructions, and quantified by spectrophotometry. For the reverse transcriptase reaction, 1·0 µg total RNA was used in the ImProm-II Reverse Transcription System for real-time PCR (RT-PCR; Promega) on a Robocycler thermocycler (Stratagene, La Jolla, CA, USA). Based on the reaction efficiency, about 120 ng cDNA was used for amplification. Quantitative RT-PCR was performed using IQ SYBR Green PCR kit (Bio-Rad) on an iCycler thermal cycler (Bio-Rad). The housekeeping gene cyclophilin A was used as the internal reference. Primer sequences are available upon request. The cycle conditions were 5 min at 94 °C, 30 s at 94 °C, 30 s at 58 °C, and 45 s at 72 °C for 50 cycles followed by the melting curve protocol to verify the specificity of amplicon generation. Gene expression was determined by the ΔΔCt method, as previously described by Livak (Christoffolete et al. 2004).
Western blot
iBAT was rapidly removed and processed for mitochondrial isolation. Uncoupling protein-1 (UCP1; Santa Cruz Biotechnology) was quantified after mitochondrial proteins were size-fractionated by 12% SDS–PAGE and identified by western blotting.
Intraperitoneal glucose tolerance test
Animals were fasted overnight and glucose (2 g/kg) was administered by i.p. injection between 0900 and 1000 h. Blood samples were collected from the tail at various times after the glucose load, as indicated, and glycemia was immediately determined on a glucose analyzer (LifeScan, Inc., Milipitas, CA, USA).
Intraperitoneal insulin tolerance test
Food was removed 6 h before the experiment was carried out between 1400 and 1500 h. Blood samples were collected from the tail at various times after insulin (0·5 U/kg) was administered by i.p. injection, as indicated, and glycemia was immediately determined on the glucose analyzer.
Blood chemistry
Total cholesterol and triglycerides were assessed via enzymatic method using a commercial kit (Roche Molecular Biochemicals) in a supernatant aliquot.
Histology
After careful dissection, tissues were immersed in buffered formol solution and fixed for 24 h. Paraffin-embedded tissues were sectioned and processed. Analyses were performed after hematoxylin–eosin or Masson staining. The area of the white adipocytes was estimated by analyzing pictures taken at 200× magnification. Picture printouts were cut, and the area of at least 40 adipocytes per animal was estimated.
Statistical analysis
Statistical analyses were done by ANOVA followed by Student’s and Newman–Keuls post-test when P<0·05.
Results
β1 adrenergic receptors are key to cold-stimulated adaptive thermogenesis
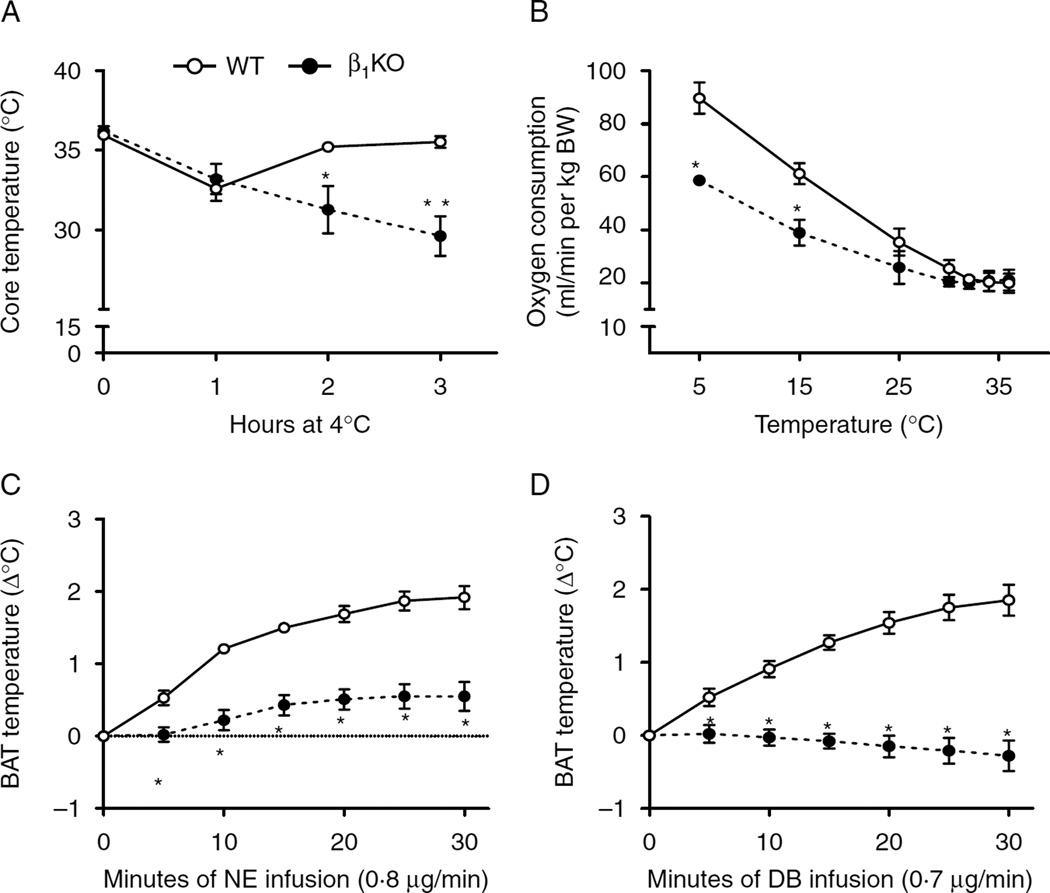
To evaluate the role of β1 adrenergic receptor in cold-stimulated adaptive thermogenesis, mice were housed individually with no bedding at 4 °C for up to 3 h and core body temperature measured hourly. All animals experienced a slight decrease in core temperature 1 h into the cold exposure, with the wild-type (WT) controls returning to baseline values at the end of the experiment (Fig. 1A). However, β1KO animals experienced a more prolonged and intense hypothermia, with a substantial drop in core temperature by the end of the experiment (−6·2±0·7 °C). A similar contrasting response between the two groups of animals was observed while monitoring VO2 during cold exposure, with WT animals exhibiting a much more pronounced acceleration of VO2 when compared with β1KO mice (Fig. 1B).
Figure 1.
Lack of β1 adrenergic receptor on cold-induced thermogenesis. (A) Central body temperature during cold exposure (4 °C) for 3 h of C57 (WT) and β1KO; *P<0·05 and **P<0·001 vs WT; (B) oxygen consumption during decreasing temperature exposure of C57 (WT) and β1KO mice; *P<0·001 vs WT; (C) BAT thermogenic response to NE infusion of C57 (WT) and β1KO mice; *P<0·004 vs WT; (D) BAT thermogenic response during infusion of DB of C57 (WT) and β1KO mice; *P<0·004 vs WT; entries are mean±s.e.m. of six animals per group.
To test the hypothesis that β1KO animals have defective BAT adaptive thermogenesis, we tested their iBAT thermal response to a 30-min infusion with NE. While WT animals exhibited an increase in ~2 °C in iBAT temperature, β1KO mice exhibited a much reduced response (only about 0·55 °C; Fig. 1C). The relevance of the β1 adrenergic signaling pathway in this process was further explored during infusion with the β1 receptor selective agonist DB (Aikawa et al. 1996, Huang et al. 1998). In WT, the increase in iBAT temperature in response to DB infusion was not different from the response to NE, supporting the major role played by the β1 adrenergic signaling pathway (Fig. 1D). By contrast, there was no response to DB infusion in β1KO animals (Fig. 1D). As a whole, these data indicate that the β1 adrenergic signaling pathway mediates most of the iBAT response to NE infusion and is critical in the acute BAT activation during cold exposure.
Role of β1 adrenergic receptor in diet-induced thermogenesis
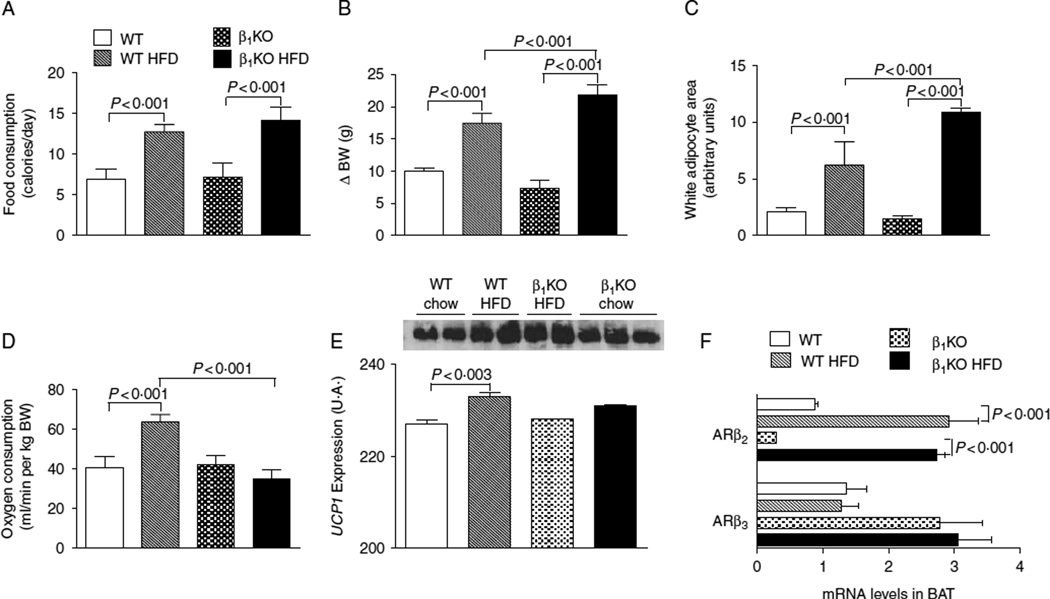
To further evaluate the role of the β1 adrenergic receptor in energy homeostasis, WT and β1KO mice were fed with a HFD for 5 weeks. The integrated caloric intake was approximately two times higher in animals fed with a HFD (Fig. 2A), resulting in a greater weight gain in both WT and β1KO mice when compared with the respective controls kept on chow diet (Fig. 2B). However, in response to high-fat feeding, β1KO animals gained significantly more BW (Fig. 2B) and exhibited increased white adipocyte area (Fig. 2C) when compared with WT animals placed on the same diet. Remarkably, whereas in the WT animals fed a HFD resulted in an ~50% increase in VO2, this induction in VO2 was blunted in the β1KO mice (Fig. 2D), explaining their increased susceptibility to diet-induced obesity. This is further supported by the observation that the HFD-induced increase in BAT UCP1 levels was blunted in the β1KO animals (Fig. 2E). Notably, no compensatory changes in the expression of the other two BAT β adrenergic receptor isoforms were observed in the β1KO animals (Fig. 2F) and the HF-induced increase in BAT β2 adrenergic receptor mRNA was similar in both the groups of animals (Fig. 2F).
Figure 2.
Lack of β1 adrenergic receptor on diet-induced obesity of mice placed on a HFD. WT and β1KO mice were followed for 5weeks while kept on a chow diet or on a HFD; (A) caloric intake was calculated based on daily food consumption; By (B) variances of BW (ΔBW) are shown; (C) estimated individual epididymal adipocytes area; 40 cells for each group were analyzed; (D) VO2 as assessed during 30 min at the end of the experiment; (E) UCP1 expression performed by western blot; (F) gene expression of β2 and β3 adrenergic receptors in mice kept on a chow or HFD in BAT.
Glucose homeostasis
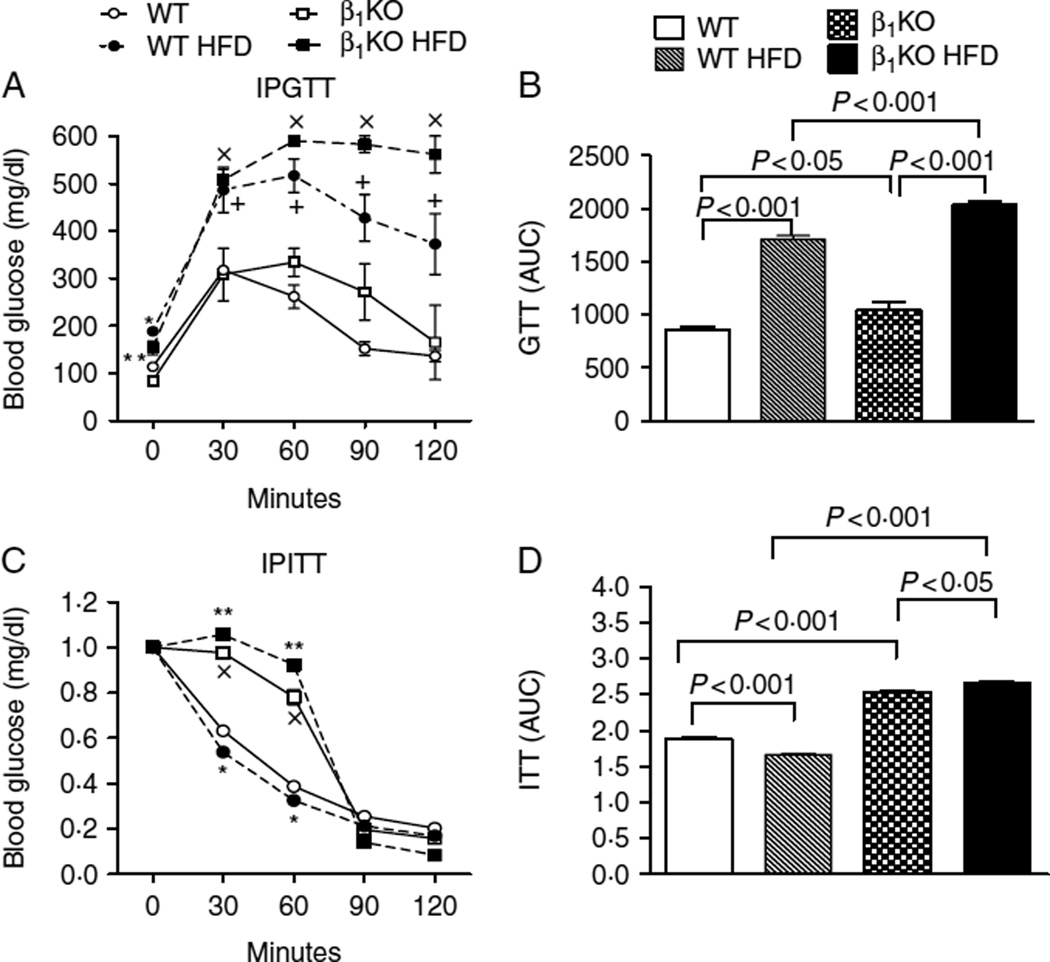
Fasting blood glucose levels and tolerance to glucose administration were similar in WT and β1KO animals kept on a chow diet (Fig. 3A and B). At the same time, feeding on a HFD promoted fasting hyperglycemia and glucose intolerance in both strains of animals, with much more pronounced changes observed in the β1KO mice (Fig. 3A and B). Sensitivity to insulin was significantly decreased in B1KO mice as compared to WT animals. Feeding on an HFD only minimally affected this parameter (Fig. 3C and D).
Figure 3.
Lack of β1 adrenergic receptor on glucose, insulin tolerance of mice kept on a chow diet or on a HFD. (A and B) Blood glucose levels before and after i.p. administration of 2 g/kg glucose (GTT) or (C and D) after i.p. injection of 0·5 U/kg insulin (ITT) at the indicated time points in WT and β1KO animals kept on chow or high-fat diet; A) *P<0·05 vs WT, **P<0·05 vs β1KO,+P<0·001 vs WT, ×P<0·001 vs β1KO; C) *P<0·01 vs. WT; **P<0·01 vs β1KO; ×P<0·01 vs WT.
Cholesterol and triglyceride plasma levels of β1KO mice
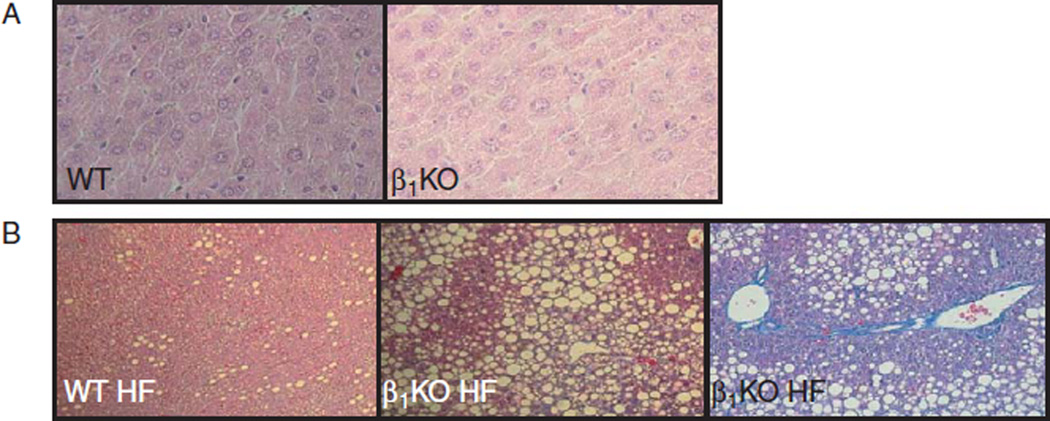
Cholesterol and triglycerides plasma levels were assessed to evaluate the effect of β1KO on lipid metabolism during high-fat feeding (Table 1). β1KO mice did not exhibit changes in plasma cholesterol or triglyceride levels when kept on a chow diet. However, when placed on HFD, the β1KO mice exhibited a much higher elevation in plasma cholesterol and triglycerides levels compared with WT mice (Table 1). Also, high-fat feeding caused a much more dramatic increase in liver fat deposition in β1KO when compared with WT animals, leading to the development of nonalcoholic steatohepatitis (Fig. 4).
Table 1.
Lack of β1 adrenergic receptor on lipid metabolism of mice placed on chow diet or high-fat diet. Values are mean±s.e.m. of six animals per group
| WT | WT HFD | β1KO | β1KO HFD | |
|---|---|---|---|---|
| Cholesterol | 91·5±9·8 | 158·9±37·8a | 82·3±15·7 | 185·7±48·9b |
| Triglycerides | 100·1±8·1 | 163·7±5·1a | 107·2±9·1 | 203·9±12·1b,c |
P<0·001 vs WT.
P<0·01 vs β1KO.
P<0·001 vs WT HF.
Figure 4.
Liver histology. Sections of liver stained by H&E (A) or Masson’s trichrome (B); magnification is 180×.
Discussion
It is well accepted that β3 adrenergic receptors play the lead role in thermoregulation and energy homeostasis through their mediation in sympathetic stimulation of BAT adaptive thermogenesis (Silva 2005). However, the present data challenge this paradigm by demonstrating that a major fraction of the sympathetic stimulation of BAT thermogenesis in response to cold exposure and high-fat feeding is mediated through β1 adrenergic receptors. Targeted inactivation of the β1 adrenergic receptor gene promoted hypothermia, limited acceleration of energy expenditure in response to cold exposure or HFD, poor thermal iBAT response to NE and DOB infusion, increased adiposity and obesity during high-fat feeding, as well as glucose intolerance, hypercholesterolemia, and hypertriglyceridemia. These findings are particularly relevant in view of the recent observation that functionally responsive BAT is present in adult individuals (Cypess et al. 2009, Virtanen et al. 2009). It is conceivable that a disruption in β1 adrenergic receptor could play a role in thermoregulation and energy homeostasis if a similar role for the β1 adrenergic receptor pathway is found in humans.
Our data suggest that the β1 adrenergic receptor is fundamental for the activation of thermogenesis during cold exposure, as evidenced by the finding that β1KO mice do not maintain their body temperature at 4 °C. In fact, the BAT of β1KO mice is thermogenically inactive as NE or DB infusion failed to increase its temperature and histology showed a pale BAT, although the amount of BAT tissue and UCP1 levels in β1KO are normal when compared with WT. These data confirm the previous study showing that the increase in cAMP levels is fundamental to activate BAT to produce heat, despite high levels of UCP1 (Ribeiro et al. 2001). Several other studies performed on mice with β1KO have shown that compensatory mechanisms may be found in KO models (Atgie et al. 1997, Rohrer 1998). In fact, there was a tendency to an increase in β3 adrenergic receptor mRNA in β1KO BAT, although it is not sufficient to compensate the absence of β1 as the mice developed severe hypothermia when exposed to 4 °C. This study showed that β1 adrenergic receptor is the main isoform that mediates BAT activation during cold-induced thermogenesis.
The increase in oxygen consumption induced by lower temperatures is severely impaired in β1KO mice but not entirely abolished. The small increase in oxygen consumption observed in cold-exposed β1KO mice may be due to shivering thermogenesis, the first line of defense against cold environments in mammals. Other mechanisms of BAT-independent cold-induced thermogenesis should be considered for the oxygen consumption increase observed in β1KO. UCP1-ablated mice clearly present a UCP1-independent thermogenesis when stimulated by NE, a situation similar to cold stimulation (Enerback et al. 1997, Feldmann et al. 2009). Nonetheless, these mechanisms are not sufficient to compensate for the lack of BAT thermogenesis in order to maintain body temperature, as β1KO mice maintained at 4 °Chave a significant drop in body temperature and die if not warmed. These data are consistent with the concept that BAT is the main site mediating body temperature regulation by the SNS in mice and also that this physiological response is mediated by the β1 adrenergic receptor.
Given that BAT is the main site for cold-induced thermogenesis and that it is logical to assume that as the β1KO mice are cold sensitive, they should be more prone to develop obesity when fed with a HFD. In agreement with our data, a previous study showed that transgenic mice over-expressing the β1 adrenergic receptor in adipose tissue are resistant to obesity (Soloveva et al. 1997).
The present data showed that the absence of the β1 adrenergic receptor leads to a more severe metabolic syndrome induced by HFD. The animals showed significant alterations of metabolic parameters such as hyperglycemia, hypertriglyceridemia, and hypercholesterolemia. Also, β1KO mice presented an increase in liver fat deposition and the development of nonalcoholic steatohepatitis.
Although hyperglycemia is commonly related to obesity, it is possible that the hyperglycemia observed in β1KO mice may be due to a direct effect of the absence of the β1 adrenergic receptor. Previous work with NE-induced activation of glucose uptake in brown adipocytes has implicated the β1 and β3 adrenergic receptors in SNS-mediated glucose uptake in brown adipocytes, allowing for synergy between SNS and insulin signaling (Nikami et al. 1996, Chernogubova et al. 2004). In conclusion, our data give strong support to the idea that β1 adrenergic receptors are the main mediators of the facultative thermogenesis activated by SNS and have a fundamental role in regulating energy expenditure.
Acknowledgments
Funding
This work was supported by grants from Fundacão de Amparo à Pesquisa do Estado de São Paulo (FAPESP; M O R and C B U) and MackPesquisa (M O R), Brazil.
Footnotes
Declaration of interest
The authors declare that there is no conflict of interest that could be perceived as prejudicing the impartiality of the research reported.
References
- Aikawa J, Fukazawa M, Ishikawa M, Moroi M, Namiki A, Yamaguchi T. Vascular smooth muscle relaxation by α-adrenoceptor blocking action of dobutamine in isolated rabbit aorta. Journal of Cardiovascular Pharmacology. 1996;27:33–36. doi: 10.1097/00005344-199601000-00006. [DOI] [PubMed] [Google Scholar]
- Atgie C, D’Allaire F, Bukowiecki LJ, et al. Role of β1- and β3-adrenoceptors in the regulation of lipolysis and thermogenesis in rat brown adipocytes. American Journal of Physiology. 1997;273:C1136–C1142. doi: 10.1152/ajpcell.1997.273.4.C1136. [DOI] [PubMed] [Google Scholar]
- Bachman ES, Dhillon H, Zhang C-Y, Cinti S, Bianco AC, Kobilka B, Lowell BB. βAR signaling required for diet-induced thermogenesis and obesity resistance. Science. 2002;297:843–845. doi: 10.1126/science.1073160. [DOI] [PubMed] [Google Scholar]
- Chernogubova E, Cannon B, Bengtsson T, et al. Norepinephrine increases glucose transport in brown adipocytes via β3-adrenoceptors through a cAMP, PKA, and PI3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology. 2004;145:269–280. doi: 10.1210/en.2003-0857. [DOI] [PubMed] [Google Scholar]
- Christoffolete MA, Linardi CC, de Jesus L, Ebina KN, Carvalho SD, Ribeiro MO, Rabelo R, Curcio C, Martins L, Kimura ET, et al. Mice with targeted disruption of the Dio2 gene have cold-induced overexpression of the uncouspling protein 1 gene but fail to increase brown adipose tissue lipogenesis and adaptive thermogenesis. Diabetes. 2004;53:577–584. doi: 10.2337/diabetes.53.3.577. [DOI] [PubMed] [Google Scholar]
- Collins S, Surwit RS. The β-adrenergic receptors and the control of adipose tissue metabolism and thermogenesis. Recent Progress in Hormone Research. 2001;56:309–328. doi: 10.1210/rp.56.1.309. [DOI] [PubMed] [Google Scholar]
- Collins S, Daniel KW, Rohlfs EM, Ramkumar V, Taylor IL, Gettys TW. Impaired expression and functional activity of the β3- and β1-adrenergic receptors in adipose tissue of congenitally obese (C57BL/6J ob/ob) mice. Molecular Endocrinology. 1994;8:518–527. doi: 10.1210/mend.8.4.7914350. [DOI] [PubMed] [Google Scholar]
- Cypess AM, Lehman S, Williams G, Tal I, Rodman D, Goldfine AB, Kuo FC, Palmer EL, Tseng YH, Doria A, et al. Identification and importance of brown adipose tissue in adult humans. New England Journal of Medicine. 2009;360:1509–1517. doi: 10.1056/NEJMoa0810780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Enerback S, Jacobsson A, Simpson EM, Guerra C, Yamashita H, Harper ME, Kozak LP. Mice lacking mitochondrial uncoupling protein are cold-sensitive but not obese. Nature. 1997;387:90–94. doi: 10.1038/387090a0. [DOI] [PubMed] [Google Scholar]
- Feldmann HM, Golozoubova V, Cannon B, Nedergaard J. UCP1 ablation induces obesity and abolishes diet-induced thermogenesis in mice exempt from thermal stress by living at thermoneutrality. Cell Metabolism. 2009;9:203–209. doi: 10.1016/j.cmet.2008.12.014. [DOI] [PubMed] [Google Scholar]
- Granneman JG. Effects of agonist exposure on the coupling of β1 and β3 adrenergic receptors to adenylyl cyclase in isolated adipocytes. Journal of Pharmacology and Experimental Therapeutics. 1992;261:638–642. [PubMed] [Google Scholar]
- Huang Y, Kwok KH, Chan NW, Lau CW, Chen ZY. Role of endothelium and K+ channels in dobutamine-induced relaxation in rat mesenteric artery. Clinical and Experimental Pharmacology & Physiology. 1998;25:405–411. doi: 10.1111/j.1440-1681.1998.tb02223.x. [DOI] [PubMed] [Google Scholar]
- Jansky L. Humoral thermogenesis and its role in maintaining energy balance. Physiological Reviews. 1995;75:237–259. doi: 10.1152/physrev.1995.75.2.237. [DOI] [PubMed] [Google Scholar]
- Jansky L, Vybiral S, Trubacova M, Okrouhlik J. Modulation of adrenergic receptors and adrenergic functions in cold adapted humans. European Journal of Applied Physiology. 2008;104:131–135. doi: 10.1007/s00421-007-0627-0. [DOI] [PubMed] [Google Scholar]
- de Jesus LA, Carvalho SD, Ribeiro MO, Schneider M, Kim SW, Harney JW, Larsen PR, Bianco AC. The type 2 iodothyronine deiodinase is essential for adaptive thermogenesis in brown adipose tissue. Journal of Clinical Investigation. 2001;108:1379–1385. doi: 10.1172/JCI13803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jimenez M, Leger B, Canola K, Lehr L, Arboit P, Seydoux J, Russell AP, Giacobino JP, Muzzin P, Preitner F. β(1)/β(2)/β(3)-Adrenoceptor knockout mice are obese and cold-sensitive but have normal lipolytic responses to fasting. FEBS Letters. 2002;530:37–40. doi: 10.1016/s0014-5793(02)03387-2. [DOI] [PubMed] [Google Scholar]
- Lowell BB, Bachman ES. β-Adrenergic receptors, diet-induced thermogenesis, and obesity. Journal of Biological Chemistry. 2003;278:29385–29388. doi: 10.1074/jbc.R300011200. [DOI] [PubMed] [Google Scholar]
- Masuo K, Lambert GW. Relationships of adrenoceptor polymorphisms with obesity. Journal of Obesity. 2011;2011:609485. doi: 10.1155/2011/609485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nikami H, Shimizu Y, Sumida M, Minokoshi Y, Yoshida T, Saito M, Shimazu T. Expression of β3-adrenoceptor and stimulation of glucose transport by β3-agonists in brown adipocyte primary culture. Journal of Biochemistry. 1996;119:120–125. doi: 10.1093/oxfordjournals.jbchem.a021196. [DOI] [PubMed] [Google Scholar]
- Ribeiro MO, Carvalho SD, Schultz JJ, Chiellini G, Scanlan TS, Bianco AC, Brent GA. Thyroid hormone – sympathetic interaction and adaptive thermogenesis are thyroid hormone receptor isoform – specific. Journal of Clinical Investigation. 2001;108:97–105. doi: 10.1172/JCI12584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rohrer DK. Physiological consequences of β-adrenergic receptor disruption. Journal of Molecular Medicine. 1998;76:764–772. doi: 10.1007/s001090050278. [DOI] [PubMed] [Google Scholar]
- Silva JE. Thyroid hormone control of thermogenesis and energy balance. Thyroid. 1995;5:481–492. doi: 10.1089/thy.1995.5.481. [DOI] [PubMed] [Google Scholar]
- Silva JE. Thyroid hormone and the energetic cost of keeping body temperature. Bioscience Reports. 2005;25:129–148. doi: 10.1007/s10540-005-2882-9. [DOI] [PubMed] [Google Scholar]
- Soloveva V, Graves RA, Rasenick MM, Spiegelman BM, Ross SR. Transgenic mice overexpressing the β1-adrenergic receptor in adipose tissue are resistant to obesity. Molecular Endocrinology. 1997;11:27–38. doi: 10.1210/mend.11.1.9870. [DOI] [PubMed] [Google Scholar]
- Susulic VS, Frederich RC, Lawitts J, Tozzo E, Kahn BB, Harper ME, Himms-Hagen J, Flier JS, Lowell BB. Targeted disruption of the β3-adrenergic receptor gene. Journal of Biological Chemistry. 1995;270:29483–29492. doi: 10.1074/jbc.270.49.29483. [DOI] [PubMed] [Google Scholar]
- Virtanen KA, Lidell ME, Orava J, Heglind M, Westergren R, Niemi T, Taittonen M, Laine J, Savisto NJ, Enerback S, et al. Functional brown adipose tissue in healthy adults. New England Journal of Medicine. 2009;360:1518–1525. doi: 10.1056/NEJMoa0808949. [DOI] [PubMed] [Google Scholar]
- Zhao J, Cannon B, Nedergaard J. Thermogenesis is β3- but not β1-adrenergically mediated in rat brown fat cells, even after cold acclimation. American Journal of Physiology. 2011;275:R2002–R2011. doi: 10.1152/ajpregu.1998.275.6.R2002. [DOI] [PubMed] [Google Scholar]