Abstract
Exposure to high Ca concentrations may influence the development of low–turnover bone disease and coronary artery calcification (CAC) in patients on hemodialysis (HD). In this randomized, controlled study, we investigated the effects of lowering dialysate Ca level on progression of CAC and histologic bone abnormalities in patients on HD. Patients on HD with intact parathyroid hormone levels ≤300 pg/ml receiving dialysate containing 1.75 or 1.50 mmol/L Ca (n=425) were randomized to the 1.25-mmol/L Ca (1.25 Ca; n=212) or the 1.75-mmol/L Ca (1.75 Ca; n=213) dialysate arm. Primary outcome was a change in CAC score measured by multislice computerized tomography; main secondary outcome was a change in bone histomorphometric parameters determined by analysis of bone biopsy specimens. CAC scores increased from 452±869 (mean±SD) in the 1.25 Ca group and 500±909 in the 1.75 Ca group (P=0.68) at baseline to 616±1086 and 803±1412, respectively, at 24 months (P=0.25). Progression rate was significantly lower in the 1.25 Ca group than in the 1.75 Ca group (P=0.03). The prevalence of histologically diagnosed low bone turnover decreased from 85.0% to 41.8% in the 1.25 Ca group (P=0.001) and did not change in the 1.75 Ca group. At 24 months, bone formation rate, trabecular thickness, and bone volume were higher in the 1.25 Ca group than in the 1.75 Ca group. Thus, lowering dialysate Ca levels slowed the progression of CAC and improved bone turnover in patients on HD with baseline intact parathyroid hormone levels ≤300 pg/ml.
Keywords: hemodialysis, mineral metabolism, vascular calcification
Vascular calcifications (VCs), which occur more frequently and progress more rapidly in patients on hemodialysis (HD)1,2 compared with the general population, are associated with a high rate of cardiovascular events.3–5 Medial VC, frequently encountered in uremic patients, is associated with arterial stiffness, left ventricular hypertrophy and altered coronary perfusion, heart failure, and poor survival.6,7 In addition to the traditional risk factors, disturbances of mineral metabolism are probably among the key risk factors in the development and progression of VC in patients on HD.8 These patients may have positive calcium (Ca) balance secondary to use of Ca–based phosphate binders, high doses of vitamin D analogs, and high dialysate Ca concentrations. In turn, high Ca load is associated with more VC.9–11
Low–turnover bone disease has been found to be a frequent type of renal osteodystrophy in patients on dialysis.12 Positive Ca balance may lead to oversuppression of parathyroid gland and thus, contribute to the pathogenesis of low–turnover bone disease13 with the associated VC.14 Low–turnover bone disease is associated with low capacity of Ca incorporation into bone and an inability to buffer extra Ca load,15 which could explain the association between low–turnover bone disease and VC.14,16–18
The effects of lowering Ca load on bone disease and coronary artery calcification (CAC) in patients on HD by the use of non–Ca–based phosphate binders have been evaluated in numerous studies.3,10,19 However, the effect of lowering Ca exposure through dialysate20 on CAC and renal osteodystrophy is largely unknown. Available recommendations21 regarding use of dialysate Ca are not validated by studies evaluating effects on CAC and bone.
This study tests the hypothesis that lowering Ca exposure through dialysate reduces progression of CAC and increases bone turnover in patients on HD with baseline parathyroid hormone (PTH) levels ≤300 pg/ml treated with 1.75 or 1.50 mmol/L dialysate Ca.
Results
Patient Selection—Study Flow
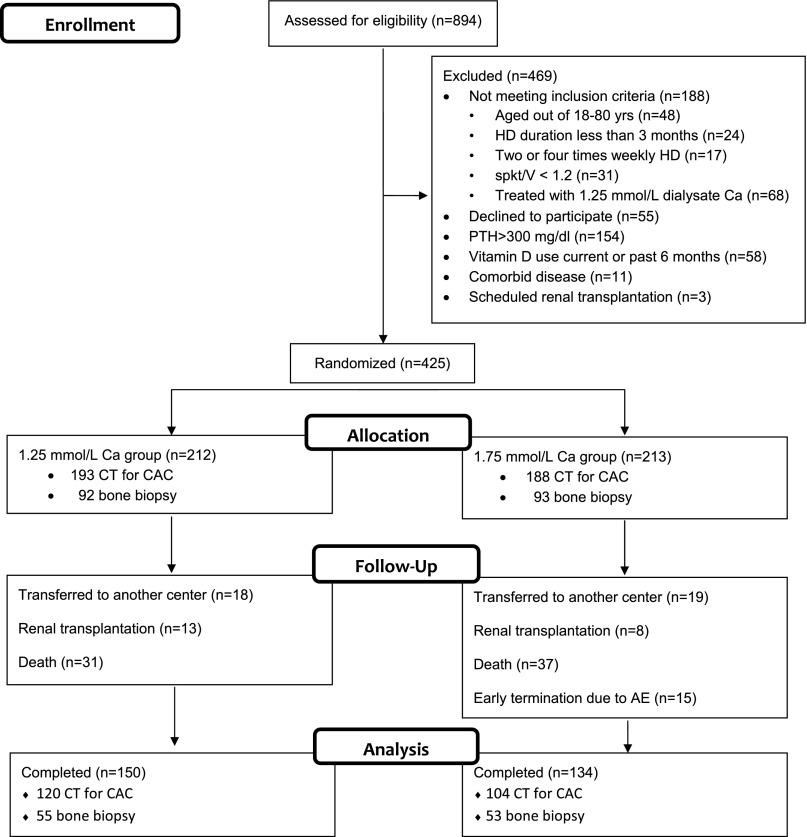
All 894 patients treated in eight dialysis clinics were assessed for eligibility (Figure 1). In total, 469 patients were excluded because of the following reasons: (1) not meeting inclusion criteria (n=188), (2) declined to participate (n=55), (3) PTH values >300 pg/ml (n=154), (4) vitamin D use current or during 6 months before randomization (n=58), (5) expecting living donor renal transplantation (n=3), or (6) serious life–limiting comorbid conditions, including active malignancy, chronic infection, and end stage cardiac, pulmonary, or hepatic disease (n=11). In total, 425 subjects met the selection criteria and were assigned in a 1:1 ratio to either 1.25- or 1.75-mmol/L dialysate Ca groups (1.25 and 1.75 Ca groups).
Figure 1.
Disposition of patients. Among 894 patients treated in eight dialysis clinics, 425 subjects were randomized to either 1.25 or 1.75 mmol/L dialysate Ca groups. After 24 months of follow-up period, 284 patients completed the study (150 in the 1.25 and 134 in the 1.75 Ca groups). AE, adverse event; CT, computerized tomography; spKt/V, single pool Kt/V.
During 24 months of follow-up, 68 patients died (28 from cardiovascular causes). Overall mortality was not different between the groups (7.86 and 9.84 per 100 patient-years in the 1.25 and 1.75 Ca groups, respectively; P=0.29); 21 patients (13 in the 1.25 and 8 in the 1.75 Ca groups) had renal transplantation, and 37 (18 in the 1.25 and 19 in the 1.75 Ca groups) moved to nonparticipating centers. In the 1.75 Ca group, the study was terminated prematurely in 15 patients (7%) because of hypercalcemia. No patient dropped out of the study because of hyper- or hypocalcemia in the 1.25 Ca group; 284 patients completed the study (150 in the 1.25 and 134 in the 1.75 Ca groups).
Baseline Characteristics
Patients in the two groups were matched with respect to baseline characteristics and concomitant therapy (Table 1). At baseline, 11.8% of the patients in the 1.25 Ca group and 12.7% of the patients in the 1.75 Ca group had hyperphosphatemia (>5.5 mg/dl; P=0.84). There was no difference between the 1.25 and 1.75 Ca groups in the percentage of patients with PTH levels <150 pg/ml (84.2% and 82.9%, respectively; P=0.78). Daily urine volume was <250 ml in >95% of the patients, and none of them were treated with diuretics.
Table 1.
Baseline patient characteristics and medication data (mean±SD)
| Variables | 1.25 Ca Group (n=212) | 1.75 Ca Group (n=213) | P Value |
|---|---|---|---|
| Age, yr | 59.3±13.8 | 59.9±13.5 | 0.63 |
| Women, % | 41 | 46 | 0.30 |
| Diabetes, % | 24.0 | 27.2 | 0.45 |
| Time on HD, mo | 51.2±38.9 | 50.5±44.1 | 0.86 |
| Cardiovascular disease in history, % | 21.1 | 21.9 | 0.85 |
| Smoking, % | 28 | 30 | 0.78 |
| Causes of ESRD, % | |||
| Diabetic nephropathy | 24.0 | 27.2 | |
| Glomerulonephritis | 8.8 | 6.6 | |
| Hypertension | 13.8 | 14.2 | |
| Other | 33.3 | 36.4 | |
| Body mass index, kg/m2 | 24.1±4.0 | 24.2±4.2 | 0.78 |
| Interdialytic weight gain, kg | 2.2±0.9 | 2.3±0.9 | 0.26 |
| Hypotension episodes, n/1000 sessions | 63.4 | 64.1 | 0.84 |
| Systolic BP, mmHg | 124±15 | 126±16 | 0.25 |
| Diastolic BP, mmHg | 74±9 | 75±9 | 0.18 |
| Biochemical parameters | |||
| Predialysis urea, mg/dl | 133±28 | 134±25 | 0.52 |
| Predialysis creatinine, mg/dl | 8.5±1.8 | 8.3±1.8 | 0.14 |
| Potassium, mEq/L | 4.9±0.6 | 4.9±0.6 | 0.85 |
| Ca, mg/dl | 9.18±0.63 | 9.12±0.62 | 0.29 |
| P, mg/dl | 4.31±1.02 | 4.40±0.97 | 0.31 |
| Ca-P product, mg2/dl2 | 39.6±9.8 | 40.1±9.1 | 0.60 |
| Alkaline phosphatase, U/L | 104±53 | 103±59 | 0.81 |
| PTH, pg/ml | 84±71 | 86±71 | 0.72 |
| Equilibrated Kt/V | 1.42±0.23 | 1.44±0.23 | 0.78 |
| Albumin, g/dl | 3.91±0.27 | 3.92±0.26 | 0.72 |
| Total cholesterol, mg/dl | 182±44 | 181±42 | 0.83 |
| Triglyceride, mg/dl | 165±98 | 164±113 | 0.88 |
| HDL-cholesterol, mg/dl | 40.9±11.5 | 41.7±12.0 | 0.66 |
| LDL-cholesterol, mg/dl | 108±35 | 107±34 | 0.94 |
| Hemoglobin, g/dl | 11.0±1.1 | 11.0±1.3 | 0.59 |
| Bicarbonate, mEq/L | 24.5±3.6 | 23.9±3.3 | 0.28 |
| C-reactive protein, mg/dl | 1.32±1.95 | 1.52±2.14 | 0.83 |
| Dialysate Mg, mmol/L | 1.0 | 1.0 | NA |
| Dialysate Ca before the study, % | 0.78 | ||
| 1.75 mmol/L | 64.4 | 63.5 | |
| 1.50 mmol/L | 35.6 | 36.5 | |
| Use of P binder, % | 58 | 60 | 0.73 |
| Dose of P binder (elementary Ca), mg/d | 535±685 | 598±797 | 0.38 |
| CAC score,a Agatston | 587±1369 | 551±967 | 0.77 |
| CAC score,a volume | 466±1084 | 444±755 | 0.82 |
| Bone formation rate,b mm3/cm2 per yr | 1.14±1.39 | 1.25±1.50 | 0.60 |
| Bone volume/tissue volume,b % | 18.2±7.7 | 19.3±6.3 | 0.31 |
| Mineralization lag time,b d | 156±196 | 137±213 | 0.61 |
| Low bone turnover,b % | 79.6 | 78.3 | 0.82 |
P, phosphorus; Mg, magnesium; NA, not applicable; P binder, phosphate binder.
n=193 in the 1.25 Ca group and n=188 in the 1.75 Ca group.
n=93 in the 1.25 Ca group and n=92 in the 1.75 Ca group.
Changes in Clinical, Biochemical, and Medication Data
Time–averaged clinical and biochemical parameters and medication data are shown in Table 2. There was no difference in BP and the frequency of intradialytic hypotension episodes between the groups. Two bone fractures occurred during 2 years (one in each group). Biochemical results showed that serum Ca levels were lower and that serum phosphorus, PTH, and alkaline phosphatase levels were higher in the 1.25 Ca group than in the 1.75 Ca group; episodes of hypercalcemia were more frequent in the 1.75 Ca group than in the 1.25 Ca group (11.3 versus 4.4 per 100 patient-months, respectively; P<0.001). The mean dose of elemental Ca given as a phosphate binder was similar in the two groups as well as the frequency of phosphate binder use (Supplemental Table 1). In the 1.25 Ca group, more patients received intravenous calcitriol compared with those in the 1.75 Ca group (38.7% and 13.0%, respectively; P<0.001); calcitriol use was more frequent in the 1.25 Ca group at all time points starting from the 12th month (Supplemental Table 1).
Table 2.
Time-averaged data during the 24-month studya (mean±SD)
| Variables | 1.25 Ca Group (n=212) | 1.75 Ca Group (n=213) | P Value |
|---|---|---|---|
| Clinical parameters | |||
| Body mass index, kg/m2 | 23.7±3.9 | 24.0±4.38 | 0.26 |
| Post–HD body weight, kg | 65.5±11.9 | 65.3±12.6 | 0.85 |
| Systolic BP, mmHg | 122.7±13.2 | 123.7±12.0 | 0.38 |
| Diastolic BP, mmHg | 74.7±7.1 | 75.0±6.2 | 0.54 |
| Hypotension episodes, n/1000 sessions | 64.4 | 69.1 | 0.66 |
| Biochemical parameters | |||
| Equilibrated Kt/V | 1.45±0.21 | 1.45±0.23 | 0.83 |
| Albumin, g/dl | 4.01±0.28 | 3.98±0.27 | 0.25 |
| Ca, mg/dl | 8.96±0.57 | 9.40±0.61 | <0.001 |
| P, mg/dl | 4.92±1.03 | 4.58±1.00 | 0.001 |
| Ca-P product, mg2/dl2 | 44.1±9.8 | 43.1±10.5 | 0.32 |
| Alkaline phosphatase, U/L | 139±72 | 101±52 | <0.001 |
| PTH, pg/ml | 297±228 | 141±120 | <0.001 |
| Bicarbonate, mmol/L | 22.2±1.5 | 21.7±1.7 | 0.001 |
| Total cholesterol, mg/dl | 161±39 | 164±41 | 0.45 |
| Triglyceride, mg/dl | 160±85 | 177±121 | 0.09 |
| HDL-cholesterol, mg/dl | 42.7±10.4 | 43.2±10.4 | 0.63 |
| LDL-cholesterol, mg/dl | 86.7±28.3 | 85.9±30.3 | 0.78 |
| C-reactive protein, mg/dl | 1.71±2.30 | 1.65±1.95 | 0.77 |
| Medication data | |||
| Use of phosphate binder at 24 mo, % | 58 | 52 | 0.30 |
| Dose of time–averaged elemental Ca, mg/d | 695±496 | 663±546 | 0.53 |
| Use of iv calcitriol vitamin D at 24 mo, % | 38.7 | 13 | <0.001 |
| Dose of time–averaged vitamin D, μg/wk | 0.30±0.46 | 0.09±0.30 | <0.001 |
| Mean erythropoietin dose, U/wk | 2373±2403 | 2789±2313 | 0.07 |
| Use of antihypertensive drug at 24 mo, % | 14.5 | 16.4 | 0.76 |
P, phosphorus.
Except frequencies of medication use at 24 months (n=150 in the 1.25 Ca group and n=134 in the 1.75 Ca group).
Changes in CAC Scores
CAC scores were measured in 89% of the patients at baseline. Baseline characteristics were not different between patients who underwent CAC score measurement (n=381) and those who did not (n=44; 19 patients unable to attend appointment, 13 withdrew consent, and 12 had cardiac arrhythmia) (Supplemental Table 2).
Of 381 patients who had initial CAC score measurement, 262 completed follow-up (death, 54; transfer to nonparticipating centers, 32; transplantation, 18; and dropout, 15). The second CAC score measurement could not be done in 38 of these 262 patients (11 patients unable to attend appointment, 26 withdrew consent for second measurement, and one had cardiac arrhythmia). Baseline characteristics were similar between patients who had two CAC score measurements (n=224) and those who only had the first CAC score measurement (n=157); only PTH and hemoglobin were different between these two groups (Supplemental Table 2). Baseline CAC score tended to be slightly higher in patients without final CAC measurement (P=0.08). Death was the major reason (34.4%) for not having a second CAC measurement. The 54 patients who died during the study had significantly higher mean baseline CAC scores compared with survivors (n=327; 1040±2067 and 491±951, respectively; P=0.02).
Progression of CAC score was evaluated in 224 patients who had two CAC score measurements (120 in the 1.25 Ca group and 104 in the 1.75 Ca group). There was no difference between the 1.25 and 1.75 Ca groups regarding baseline characteristics (Supplemental Table 3). Mean CAC scores were also similar between treatment groups at baseline (452±869 in the 1.25 Ca group and 500±909 in the 1.75 Ca group; P=0.68) (Table 3). Also, the frequency of patients with CAC score <100 was similar in the 1.25 and 1.75 Ca groups (53.3% and 47.1%, respectively; P=0.85). CAC score increased in both groups within 2 years (Table 3). Although CAC score at 24 months was lower in the 1.25 Ca group than in 1.75 Ca group, the difference was not statistically significant (616±1086 and 803±1412, respectively; P=0.25). However, the progression rate of CAC scores, which was the primary outcome measure, was significantly less in the 1.25 Ca group than in the 1.75 Ca group (160±299 versus 303±624, respectively) (Table 4). The mean absolute difference in progression of CAC scores between the 1.25 and 1.75 Ca groups assessed by Agatston method was −138 (P=0.03), and the mean absolute difference in progression of volume scores was −118 (P=0.01). The difference between changes in square root–transformed CAC volume scores in the two groups was also significant (P<0.01). Adjustment for baseline CAC score did not change the results.
Table 3.
CAC scores in treatment groups at baseline and at the end of study
| CAC score | 1.25 Calcium Group (n=120) | 1.75 Calcium Group (n=104) | ||||
|---|---|---|---|---|---|---|
| Baseline | Month 24 | P Value | Baseline | Month 24 | P Value | |
| CAC score—Agatston, mean±SD | 452±869 | 616±1086 | <0.001 | 500±909 | 803±1412 | <0.001 |
| CAC score—Agatston, median (interquartile range) | 63 (0–504) | 99 (0–661) | <0.001 | 135 (0–586) | 258 (10–945) | <0.001 |
| CAC score—volume, mean±SD | 351±679 | 466±821 | <0.001 | 383±698 | 617±1089 | <0.001 |
| CAC score—volume, median (interquartile range) | 46 (0–364) | 67 (0–530) | <0.001 | 116 (0–426) | 199 (8–728) | <0.001 |
Table 4.
Mean difference in changes of CAC scores between the groups (mean±SD)
| Changes in CAC Scores | 1.25 Calcium Group (n=120) | 1.75 Calcium Group (n=104) | Mean Difference between Groups (95% Confidence Interval) | P Value |
|---|---|---|---|---|
| Absolute difference | ||||
| ΔCAC score—Agatston | 160±299 | 303±624 | −138 (−265 to −12) | 0.03 |
| ΔCAC score—volume | 115±208 | 234±482 | −118 (−214 to −22) | 0.01 |
| Transformed difference | ||||
| ΔCAC score—Agatston | 3.01±3.94 | 4.79±6.22 | −1.77 (−3.13 to −0.42) | 0.01 |
| ΔCAC score—volume | 2.50±3.38 | 4.21±5.46 | −1.70 (−2.88 to −0.52) | <0.01 |
Univariate analysis revealed a positive association between progression of CAC score and baseline CAC score (Rho=0.507; P<0.001), age (Rho=0.194; P=0.003), and time-averaged levels of serum Ca (Rho=0.202; P=0.002) and phosphorus (Rho=0.133; P=0.04). There was an inverse correlation between CAC progression and alkaline phosphatase levels (Rho=−0.289; P<0.001). We found no association between the dose of Ca–based phosphate binder and CAC progression (P=0.52). In stepwise regression analysis for progression of CAC adjusted for demographics, medications, and biochemical data, the following factors were found to be significant predictors: baseline CAC score, Ca-phosphorus product, age, and dialysate Ca group (r2=0.43; P<0.001).
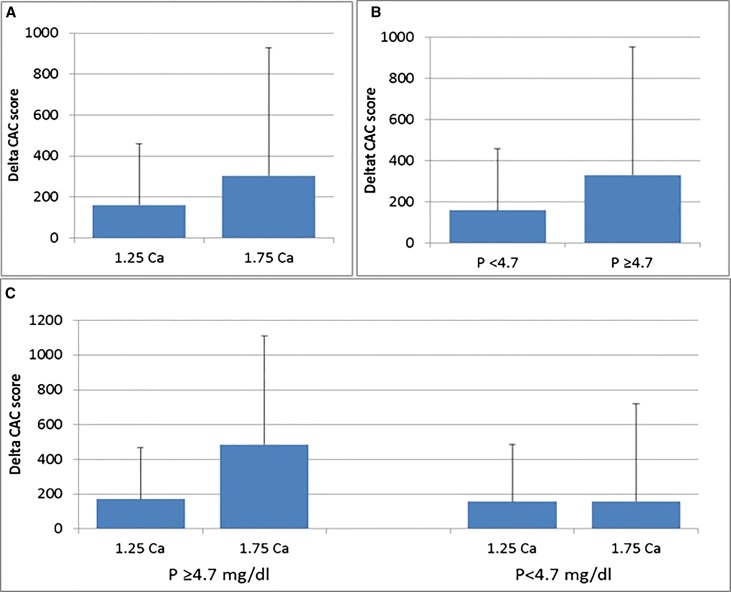
In post hoc analysis, we observed a significant interaction between time–averaged serum phosphorus level and treatment arm on the progression of CAC score outcome by the Agatston score (P interaction =0.001). We, therefore, dichotomized participants at the mean time–averaged phosphorus level (<4.7 mg/dl versus ≥4.7 mg/dl) and evaluated the effect of randomization in each strata. We observed that randomization to 1.75 mmol/L Ca concentration dialysate was associated with higher CAC progression rates in patients with time-averaged phosphate ≥4.7 mg/dl than in those randomized to 1.25 mmol/L Ca arm (ΔAgatston CAC scores of 171±324 in the 1.25 Ca group and 485±801 in the 1.75 Ca group; P<0.01), whereas progression rates were similar irrespective of dialysate Ca bath in those with lower time–averaged serum phosphate levels (ΔAgatston CAC score of 157±274 versus 158±386, respectively; P=0.98) (Figure 2).
Figure 2.
Higher CAC progression in the 1.75 Ca group than 1.25 Ca group (160±299 versus 303±624, P=0.03) (A); in the whole group, higher CAC progression in patients with serum phosphorus level ≥4.7 compared to <4.7 mg/dl (292±576 versus 160±342, P=0.03) (B); in patients with serum phosphorus level ≥4.7 mg/dl, higher CAC progression with 1.75 than 1.25 mmol/L dialysate Ca (485±801 versus 171±324, P=0.01 (C); in patients with serum phosphorus level <4.7 mg/dl, similar CAC progression in the 1.75 and 1.25 Ca groups (157±274 versus 158±386, P=0.98) (C).
Changes in Bone Histomorphometric Parameters
At baseline, bone biopsy was performed in 185 patients. Baseline characteristics were not different between patients who underwent bone biopsy (n=185) and those who did not (n=240) (Supplemental Table 4).
Of 185 patients who had a baseline biopsy, 137 completed the follow-up (death in 20, transplantation in 11, transfer to nonparticipating centers in 14, and dropout in three). Second bone biopsies were done in 108 of 137 patients, because 29 patients did not agree to a second biopsy.
Baseline characteristics were similar between patients who had two biopsies (n=108) and those who had only a first biopsy (n=77) (Supplemental Table 4).
Bone turnover, mineralization, and bone volume parameters were similar at the baseline in the two groups (Table 5). Other characteristics were also not different at the baseline between the groups, except for lower frequency of cardiovascular disease history in the 1.25 Ca group (Supplemental Table 5).
Table 5.
Bone histomorphometric parameters in treatment groups at baseline and the end of study (mean±SD)
| Bone Histomorphometry Parameters | 1.25 Calcium Group (n=55) | 1.75 Calcium Group (n=53) | ||||
|---|---|---|---|---|---|---|
| Baseline | Month 24 | P Value | Baseline | Month 24 | P Value | |
| Bone turnover | ||||||
| Bone formation rate/bone surface (normal: 1.80–3.80 mm3/cm2 per yr) | 1.03±1.33 | 3.24±2.77a | <0.001 | 1.41±1.61 | 1.93±1.37 | 0.07 |
| Activation frequency (normal: 0.49–0.72 yr−1) | 0.22±0.28 | 0.67±0.56a | <0.001 | 0.29±0.34 | 0.41±0.28 | 0.06 |
| Osteoblast no./bone perimeter (normal: 10–200/100 mm) | 61.5±87.2 | 144±199b | <0.01 | 103±180 | 66.7±124 | 0.12 |
| Osteoclast no./bone perimeter (normal: 1–53/100 mm) | 28.9±31.2 | 48.0±44.8 | <0.01 | 23.9±23.8 | 39.8±42.4 | 0.01 |
| Mineralization | ||||||
| Osteoid thickness (normal: <20 μm) | 10.1±2.9 | 13.1±5.5 | <0.001 | 11.1±4.8 | 11.0±6.1 | 0.87 |
| Mineralization lag time (normal: <100 d) | 165±178 | 126±127 | 0.09 | 109±95 | 83±73 | 0.17 |
| Osteoid maturation time (normal: <35 d) | 17.88±6.10 | 17.25±7.35 | <0.001 | 16.12±6.33 | 15.21±8.39 | <0.001 |
| Volume | ||||||
| Bone volume/tissue volume (normal: 16.8%–22.9%) | 18.6±8.0 | 21.3±6.6b | 0.02 | 18.9±6.1 | 18.0±7.1 | 0.43 |
| Trabecular thickness (normal: 99–142 μm) | 90.6±28.7 | 98.5±24.7c | 0.05 | 93.1±23.3 | 88.4±25.9 | 0.26 |
| Cortical thickness (normal: 0.52–1.65 μm) | 710±530 | 550±350 | 0.14 | 620±470 | 540±560 | 0.33 |
| Cortical porosity (normal: 1.9%–10%) | 2.47±2.22 | 14.78±7.34 | <0.001 | 2.69±2.75 | 10.88±6.27 | <0.001 |
P=0.03 (P value of the difference between 1.25 and 1.75 Ca groups at the end of the study).
P=0.01 (P value of the difference between 1.25 and 1.75 Ca groups at the end of the study).
P=0.04 (P value of the difference between 1.25 and 1.75 Ca groups at the end of the study).
Bone Turnover
Bone turnover is defined histomorphometrically by bone formation rate and activation frequency. Both of these parameters increased significantly in the 1.25 Ca group from baseline to month 24, whereas no changes were observed in the 1.75 Ca group (Table 5). The prevalence of histologically diagnosed low bone turnover decreased from 85.0% to 41.8% in the 1.25 Ca group (P=0.001) and did not change in the 1.75 Ca group (from 79.2% to 64.2%). There was a commensurate significant increase in the number of osteoblasts and osteoclasts in the 1.25 Ca group. In the 1.75 Ca group, the number of osteoclasts increased, with no change in osteoblast number.
Mineralization
Normal mineralization is defined by osteoid thickness <20 μm and mineralization lag time <100 days. Although mineralization lag times were long in both groups, osteoid thickness was normal (Table 5). Thus, overt osteomalacia was not found in any patients at baseline or the end of the study. No stainable bone aluminum was found at baseline or after 2 years of treatment. Osteoid thickness increased in the 1.25 Ca group but not to values above the normal range reflecting increased bone formation. In the 1.75 Ca group, there were no changes in osteoid thickness. Mineralization lag time did not change significantly in either group, whereas statistically but not physiologically relevant decreases (within the normal range) were observed for osteoid maturation time in both groups. Lack of abnormally prolonged mineralization lag time combined with no increase in osteoid maturation time and osteoid thickness document absence of osteomalacia.
Volume
Mean cancellous bone volume was within the normal range (16.8%–22.9% cancellous bone per total bone volume) at baseline and 24 months in the 1.25 and 1.75 Ca groups but increased significantly from low-normal to high-normal in the 1.25 Ca group but not the 1.75 Ca group (Table 5). Similarly, trabecular thickness increased in the 1.25 Ca group but not in the 1.75 Ca group. Cortical thickness did not change in either group, but cortical porosity increased in both groups.
Adherence to the Treatment and Adverse Events
All patients in the 1.25 Ca group received randomized treatment during follow-up. In the 1.75 Ca group, the study was terminated prematurely in 7% of the patients because of hypercalcemia. There were no cross-overs during follow-up. Adverse events occurred in 95 (44.8%) patients in the 1.25 Ca group and 117 (54.9%) patients in the 1.75 Ca group (P=0.04). Serious adverse events were reported in 79 (37.2%) patients in the 1.25 Ca group and 97 (45.5%) patients in the 1.75 Ca group (P=0.08) (Table 6).
Table 6.
Monitored adverse events during the study period between the groups
| Adverse Events | 1.25 Ca Group (n=212) No. of Patients (%) | 1.75 Ca Group (n=213) No. of Patients (%) | P Value |
|---|---|---|---|
| Any adverse event | 95 (44.8) | 117 (54.9) | 0.04 |
| Any serious adverse event | 79 (37.2) | 97 (45.5) | 0.08 |
| Requiring permanent discontinuationa | 0 | 15 (7.0) | NA |
| Death | 31 (14.6) | 37 (17.3) | 0.44 |
| Cardiovascular causes | 13 (6.1) | 15 (7.0) | 0.70 |
| Sudden death | 6 (2.8) | 4 (1.8) | 0.51 |
| Noncardiovascular causes | 18 (8.4) | 22 (10.3) | 0.51 |
| Infectionb | 7 (3.3) | 10 (4.6) | 0.46 |
| Otherc | 11 (5.1) | 12 (5.6) | 0.83 |
| Nonfatal cardiovascular eventsd | 3 (1.4) | 6 (2.8) | 0.31 |
| Infectione | 9 (4.2) | 11 (5.1) | 0.65 |
| Gastrointestinal disordersf | 6 (2.8) | 3 (1.4) | 0.35 |
| Hepatic disordersg | 2 (0.9) | 0 | NA |
| Hospitalizationh | 27 (12.7) | 24 (11.2) | 0.64 |
| Fracture | 1 (0.4) | 1 (0.4) | >0.99 |
NA, not applicable.
Only reason for permanent discontinuation was hypercalcemia in the 1.75 Ca group.
Pneumonia, diabetic foot infections, and catheter infections were the most frequent reasons.
Malignancy, pulmonary embolism, and gastrointestinal hemorrhage were most frequently reported.
Stroke, unstable angina pectoris requiring hospitalization, and acute myocardial infarction.
Pneumonia and diabetic foot infection were most frequently reported.
Gastrointestinal hemorrhage was the most frequent reason.
Two acute cholecystitis episodes.
Vascular access problems and infections were most frequently reported.
Femoral neck fracture in each group.
Discussion
This randomized clinical trial shows that lowering dialysate Ca levels slows down progression of CAC and improves bone turnover in patients on HD with baseline PTH levels ≤300 pg/ml while under treatment with ≥1.50 mmol/L Ca dialysate.
Although the CAC score in the 1.25 Ca group was not significantly different from that in the 1.75 Ca group at the end of the study, the progression rate of CAC was markedly lower in the 1.25 Ca group compared with the 1.75 Ca group. This confirms a role of high dialysate Ca exposure in the progression of CAC.
As a result of treatment with Ca–based phosphate binders, vitamin D, and 1.75 or 1.50 mmol/L Ca dialysate, many patients on HD may be in positive Ca balance. This may contribute to development of low bone turnover and CAC.9,22 Use of non–Ca–based phosphate binders to reduce Ca exposure has been claimed to stop progression of CAC in patients on prevalent HD and patients on incident HD.2,10 However, this was not confirmed in another study reporting equal progression rates in non–Ca– and Ca–based phosphate binders groups when similar LDL cholesterol levels were achieved with the use of statins.3 The effect of non–Ca–based phosphate binders on bone histomorphometry has been investigated only in small studies with inconclusive results.19,23–25
The effect of dialysate Ca, an important source of Ca exposure, on VC and bone disease in patients on HD has not been investigated before in a randomized, controlled trial. Dialysate Ca levels of 1.50–1.75 mmol/L may result in transfer of Ca and thus, increasing serum Ca levels. A mean accumulation of 879 mg Ca per HD session was found when patients with a predialysis serum Ca level of 2.27 mmol/L (9.1 mg/dl) were dialyzed with a dialysate containing 1.75 mmol/L Ca.20 In the same study, dialysate containing 1.25 mmol/L Ca did not cause net Ca flux, although another study reported a negative Ca balance with 1.25 mmol/L dialysate Ca.26 Because serum Ca affects PTH secretion, Ca transfer from the dialysate containing 1.75 or 1.50 mmol/L Ca can be expected to suppress PTH levels and may result in lowering of bone turnover. Although low–turnover bone disease is often asymptomatic, it may increase bone fracture incidence, cause poor healing of fractures, and predispose to extraosseous calcification because of diminished buffering capacity of bone.15,16,27
In the 1960s, when HD became available, a dialysate Ca concentration of 1.25 mmol/L was chosen to match physiologic blood Ca levels. In the era when vitamin D was not available and phosphate binders were aluminum based, the use of 1.25 mmol/L dialysate Ca resulted in an elevation of serum PTH levels. Therefore, dialysate Ca concentration was increased to 1.75 mmol/L. After the introduction of vitamin D treatment and replacement of aluminum-containing binders with Ca–based phosphate binders, dialysate Ca concentration was reduced back to 1.25 mmol/L, especially in the United States. In 2003, Kidney Disease Outcomes Quality Initiative (KDOQI) guidelines recommended use of a 1.25 mmol/L dialysate Ca concentration on the basis of clinical experience rather than outcome data.21 Because of the lack of randomized studies proving superiority and safety of 1.25 mmol/L dialysate Ca concentration, the use of 1.50 or even 1.75 mmol/L dialysate Ca concentrations continued in many countries. In the early 2000s, the frequency of ≤1.25 mmol/L dialysate Ca use was 64.1% in the United States, 23.9% in Europe, and 19.8% in Japan.28 In 2009, Kidney Disease Improving Global Outcomes guidelines advised a dialysate Ca concentration of 1.25 or 1.50 mmol/L, but again, this was not evidence based.29
This randomized clinical trial produced evidence to favor reduction of dialysate Ca level from 1.75 or 1.50 mmol/L to 1.25 mmol/L in patients on HD with PTH levels ≤300 pg/ml. Reduced Ca exposure through dialysate was associated with less progression of CAC and improved bone turnover without a decrease in bone volume within 2 years. Although this study was not designed to evaluate hard end points, such as death rate and incidence of bone fracture, CAC, the primary outcome measure of this study, is a well known independent risk factor for mortality.6 Also, low–turnover bone disease, the secondary outcome measure, is known to be associated with abnormal Ca homeostasis15 and increased incidence of bone fractures.27
This study showed significantly lower serum Ca levels and lower frequency of hypercalcemic episodes in the 1.25 Ca group compared with the 1.75 Ca group. PTH and alkaline phosphatase levels were higher in the 1.25 Ca group. Higher phosphorus levels in the 1.25 Ca group may be related to increased release of phosphorus from bone because of higher turnover and/or increased intestinal phosphorus absorption resulting from more vitamin D use30; however, compliance with phosphate binders may be an additional factor affecting serum phosphate levels. Along with a rise in PTH levels, all bone turnover parameters dramatically increased in the 1.25 Ca group; thus, prevalence of low–turnover bone disease decreased from 85% to 41.8%. We do not have a clear explanation for the increase in osteoclasts and the slight rise in PTH observed in the 1.75 Ca group, which was not accompanied by changes in alkaline phosphatase levels. It can be speculated that there might be an inappropriate overuse of vitamin D before initiation that is screening in the study. The long mineralization lag times observed with normal osteoid maturation time and normal osteoid thickness in all study groups and the absence of changes in these parameters indicate that there was no osteomalacia present at baseline or the end of the study in all patients. This prolongation of mineralization lag time reflects low bone formation. The differences in osteoid maturation time between baseline and the end of the study are statistically significant, but they are so small that they are unlikely to be biologically relevant. In the 1.25 Ca group, we documented increases in cancellous bone volume and trabecular thickness. Higher cancellous bone volume has been reported to be associated with lower CAC scores.31 The rise in cortical porosity in both groups can be explained by higher concentrations of serum PTH. The effects of PTH levels on cancellous and cortical bone are well established in the literature.32–34 This study confirms these observations in a prospective manner.
Haris et al.35 investigated the effect of lowering dialysate Ca levels from 1.62 to 1.00 mmol/L during 16 months in 51 patients with adynamic bone disease on peritoneal dialysis (24 patients in the 1.0 mmol/L dialysate Ca groups and 27 patients in 1.62 mmol/L dialysate Ca group). The results of this partially randomized small trial (only 14 patients on low Ca completed the study) clearly showed improved bone turnover, increased PTH levels, and fewer hypercalcemic episodes. This randomized, controlled study confirms these results but differs, because it was performed in patients on HD, was of longer duration, and investigated not only bone histomorphometry but also, VC.
Considering significant interaction between serum phosphorus and dialysate Ca level on progression of CAC score in covariance analysis, additional post hoc analyses revealed that high Ca exposure increases progression of CAC mainly in the presence of hyperphosphatemia. In patients with time–averaged phosphorus levels <4.7 mg/dl, progression of CAC was much lower compared with that in those patients with phosphorus levels ≥4.7 mg/dl. More importantly, progression of CAC was not different in the 1.25 and 1.75 Ca groups in the absence of hyperphosphatemia. This indicates a key role of phosphate in CAC as shown in in vitro studies.36 However, it should be noted that this is a post hoc analysis, and it does not prove that higher Ca exposure is safe in the absence of hyperphosphatemia in terms of CAC progression.
It has been described that the corrected QT interval is prolonged by use of 1.25 mmol/L dialysate Ca concentration and that dialysate Ca level <1.25 mmol/L is associated with increased risk of sudden death.37,38 Although the study was underpowered to detect differences in death outcomes, we found no difference between the groups regarding death, sudden death, cardiovascular death, and nonfatal cardiovascular events. Adverse events were more frequent (P=0.04), and serious adverse events tended to be higher (P=0.08) in the 1.75 Ca group, mainly because of hypercalcemia requiring permanent discontinuation.
Dialysate Ca of 1.25 mmol/L is used worldwide without supporting evidence. The strength of the study is the randomized, controlled, prospective approach to explore the effects of lowering the dialysate Ca level from 1.75 or 1.50 mmol/L to 1.25 mmol/L on VC and bone disease in patients with PTH levels ≤300 pg/ml.
This study has some limitations. First, the statistical power of the study was lower than hypothesized because of higher dropout rates and the significant increase in CAC score also observed in the 1.25 group, whereas no progression was assumed. Therefore, the difference between CAC scores between the two groups did not reach statistical significance at the end of the study. However, progression rate of CAC score was significantly lower in the 1.25 Ca group. Second, in this study, reduction of dialysate Ca level from 1.75 or 1.50 mmol/L to 1.25 mmol/L was restricted to patients with PTH levels ≤300 pg/ml. The study population had quite low PTH levels (mean =85±71 pg/ml), and the majority of them had low–turnover bone disease. Therefore, the favorable results with the reduction of dialysate Ca level from 1.75 or 1.50 mmol/L to 1.25 mmol/L may not be applicable to the entire patient population with HD, and we cannot claim that such a reduction in dialysate Ca level produces better outcomes in patients with PTH levels >300 pg/ml and those without low bone turnover disease. Although dialysate containing 1.25 mmol/L Ca is currently widely used, especially in the United States, an earlier study reported worsening of hyperparathyroid bone disease with reduction of dialysate Ca level from 1.75 to 1.25 mmol/L, despite concomitant use of Ca–based phosphate binders and vitamin D.39 We, therefore, did not enroll patients with PTH levels >300 pg/ml. Although patients with PTH levels ≤300 pg/ml constituted the majority of our dialysis population at the time that this study was designed (use of 1.75–1.50 mmol/L dialysate Ca in >95% of the patients and PTH levels ≤300 pg/ml in 88% of the patients), we cannot claim that the study population represents the general HD population worldwide. In fact, it may not be reasonable to recommend the same dialysate Ca concentration for all patients. Third, we did not measure other serum markers, including fibroblast growth factor 23 level, which has been shown to be associated with VC. Both PTH levels and thus, vitamin D use were higher in the 1.25 Ca group. More vitamin D use in the 1.25 Ca group may increase fibroblast growth factor 23 level and contribute to VC in this group.40 However, low doses of vitamin D have been shown to be protective against aortic calcification.41 As a matter of fact, both high and low levels of calcitriol may be associated with VC.42 Fourth, physicians following the enrolled patients in clinics were not blinded; however, investigators who evaluated CAC score and bone histomorphometry, which are the major outcome measures, were blinded.
In conclusion, lowering Ca exposure through dialysate attenuates progression of CAC and improves low bone turnover. A dialysate Ca concentration of 1.25 mmol/L is preferred over 1.75 mmol/L in patients on HD with baseline PTH levels of ≤300 pg/ml.
Concise Methods
Study Design and Patients
The Dialysate Calcium Study was a randomized, controlled trial (Clinical Trials ID no. NCT00297219) to evaluate the effects of lowering dialysate Ca concentration from 1.75 or 1.50 mmol/L to 1.25 mmol/L on the progression of CAC and the change in bone turnover, mineralization, and bone volume in patients on HD (Figure 1).
All patients treated in eight dialysis clinics were screened (n=894). At the time of screening, >95% of those patients were treated with a dialysate containing Ca ≥1.50 mmol/L; 88% of them had PTH levels ≤300 pg/ml, and 11% were treated with active vitamin D. Inclusion criteria were use of a dialysate containing 1.75 or 1.50 mmol/L Ca, age between 18 and 80 years old, conventional HD treatment three times per week for at least 3 months, single pool Kt/V>1.2, and written informed consent. Exclusion criteria were hypercalcemia (Ca>10.2 mg/dl), PTH values >300 pg/ml, vitamin D treatment within 6 months before randomization, expecting living donor renal transplantation, pregnancy or nursing, mental incompetence, and serious life–limiting comorbid conditions, including active malignancy, chronic infection, and end stage cardiac, pulmonary, or hepatic disease.
In total, 425 subjects met the selection criteria (Figure 1) and were assigned in a 1:1 ratio to either the 1.25- or 1.75-mmol/L dialysate Ca group (1.25 and 1.75 Ca groups, respectively). The study was Institutional Review Board approved and conducted in accordance with the Declaration of Helsinki.
Outcomes
The primary outcome was progression of CAC, and the secondary outcome was changes in histomorphometric parameters of bone turnover, mineralization, and volume. Although the planned follow-up period was 18 months, it was extended to 24 months because of a technical problem in the device used for CAC measurement. CAC measurements and bone biopsies were performed at baseline and the end of the study. Changes in CAC and bone parameters were compared between the 1.25 and 1.75 Ca groups.
All blood samples were taken before a midweek dialysis session and analyzed in a central laboratory. Routine biochemical parameters were measured monthly by an autoanalyzer (Architect c-8000; Abbott Diagnostics, Chicago, IL). Alkaline phosphatase, high–sensitivity C-reactive protein, lipid parameters, and PTH were evaluated quarterly. Intact PTH was determined by the electrochemiluminescence Elecsys PTH Assay (Roche Diagnostics, Indianapolis, IN). The intra- and interassay coefficients of variations were 3.8% and 4.2%, respectively.
Except Ca concentration, composition of dialysate was the same and unchanged in the study groups. Reversed osmosis was used in all dialysis clinics. Only Ca–based phosphate binders (Ca acetate or carbonate) were used (a non–Ca–based phosphate binder was not available in the country before the study). Intravenous calcitriol or alfacalcidol was prescribed to control PTH. Treatment strategies were standardized according to KDOQI guidelines.21 No calcimimetics were used before or during the study. Subjects were removed from the study if hypercalcemia (>10.2 mg/dl) was found on three sequential measurements.
CAC Scores
CAC scores were measured in all subjects, except those with cardiac arrhythmia or pacemakers, using the same device (Dual Score 64-Slice Multislice Computed Tomography Scanner; Aquilion 16; Toshiba, Tokyo, Japan) and a Ca-scoring program (TeraRecon 3.4.2.11; TeraRecon, Foster City, CA) as described elsewhere.14 Reported CAC scores were calculated as the sum of scores obtained from the left coronary artery and the right coronary artery. The CAC score was evaluated by two radiologists without knowledge of the study group according to the method by Agatston et al.43 Inter- and intraobserver variabilities were 1.2% and 2.5%, respectively. In addition, volume scores were measured, and square roots of the volumes were calculated.44
Mineralized Bone Histology and Bone Histomorphometry
Iliac crest bone biopsies were performed after two courses of oral tetracycline hydrochloride.45 Bone samples were processed, and histomorphometry was done as published before.12,46 Renal osteodystrophy was blindly assessed by evaluating bone turnover, mineralization, and volume.46,47
Statistical Analyses
Sample size was estimated to compare changes in CAC scores in the two groups. We assumed that CAC scores might progress with a rate of 1.27±1.88 per day in the 1.75 Ca group within 18 months as reported previously48 and that the use of 1.25 mmol/L dialysate Ca concentration might stop the progression of CAC scores. We estimated that 322 patients would provide 90% power with a two–sided α-error rate of 5% for detecting a significant difference between the treatment arms. Additionally, progression rates of CAC scores in the two groups were compared. The planned follow-up was 18 months. Considering an estimated dropout rate of 30%, 425 patients were enrolled in the study.
A power analysis for the bone biopsy part of the study was not performed, because there were no data on this subject in the literature. Considering the result of a preliminary questionnaire, it was estimated that only one third of study patients would be willing to undergo bone biopsies.
Data were expressed as means±SDs unless otherwise indicated. Baseline and time-averaged parameters (mean of all measurements during follow-up) were compared between the treatment groups by using the t test or the Wilcoxon rank sum test on the basis of whether the data were normally distributed and the chi-squared test for categorical variables. Changes in CAC scores and bone parameters from baseline to end of study were compared by paired t test. Univariate (Spearman correlation) analyses were done to determine predictors of change in CAC. The absolute difference between the second and first CAC scores (Agatston and volume) and the square root of the volume were assessed.44 Sensitivity analyses were also performed using analysis of covariance, with the change in CAC and bone parameters as the dependent variables. Statistical significance was defined as P<0.05. All analyses were done using SPSS (version 13.0; IBM SPSS, Chicago, IL). SAS software (version 9.2; SAS Institute Inc., Cary, NC) was used for covariance analysis to test the interaction between serum phosphorus and dialysate Ca level on progression of CAC score.
Disclosures
E.O. is a member of the scientific advisory board of Fresenius Medical Care, Turkey. G.A., S.B., H.T., M.O., M.Y., F.K., E.S.O., N.C., S.D., M.C., M.-C.M.-F., and H.H.M. declare that they have no conflicts of interest.
Supplementary Material
Acknowledgments
We thank Dr. Evert J. Dorhout Mees for support. Kristina Stasko provided valuable administrative assistance. We thank Dr. Kivanc Yuksel for support in statistical analyses.
Kentucky Nephrology Research Trust provided support for histologic processing and analysis of bone samples. The study was supported by Fresenius Medical Care, Turkey with an unrestricted grant.
The sponsor had no role in study design and conduction; data management, collection, and analysis; or preparation and submission of the manuscript.
The following investigators participated in the Dialysate Calcium Study: Ali Basci, Meltem Sezis Demirci, Cenk Demirci, Devrim Bozkurt, Hamad Dheir, Savas Sipahi, Ender Hur, (Ege University School of Medicine); Can Boydak, Taskin Colak, (Ege Nefroloj Dialysis Clinic); Burcu Ozmalkoc, (Nefron Dialysis Clinic); Yuksel Yucadag, (Buca Dialysis Clinic); Dilhan Yucedag, (Nefron Dialysis Clinic); Mehmet Sonbahar, (Buca Dialysis Clinic); Banu Kinay, Erdal Karaca, (Sevgi Dialysis Clinic); Ali İlaslan, Müjgan Sifil, (Nasir Dialysis Clinic); Ertan Bertan, Sermin Odabasioglu, Erdal Sevinc, (Hatay Dialysis Clinic); Sinan Erten, Nurdan Isık, (Narlıdere Dialysis Clinic); Yesim Peker, Erhan Lale, and Pinar Uretmen (Gaziemir Dialysis Clinic).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015030268/-/DCSupplemental.
References
- 1.Malluche HH, Blomquist G, Monier-Faugere MC, Cantor TL, Davenport DL: High parathyroid hormone level and osteoporosis predict progression of coronary artery calcification in patients in dialysis. J Am Soc Nephrol 26: 2534–2544, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Block GA, Spiegel DM, Ehrlich J, Mehta R, Lindbergh J, Dreisbach A, Raggi P: Effects of sevelamer and calcium on coronary artery calcification in patients new to hemodialysis. Kidney Int 68: 1815–1824, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Qunibi W, Moustafa M, Muenz LR, He DY, Kessler PD, Diaz-Buxo JA, Budoff M CARE-2 Investigators : A 1-year randomized trial of calcium acetate versus sevelamer on progression of coronary artery calcification in hemodialysis patients with comparable lipid control: The Calcium Acetate Renagel Evaluation-2 (CARE-2) study. Am J Kidney Dis 51: 952–965, 2008 [DOI] [PubMed] [Google Scholar]
- 4.Criqui MH, Denenberg JO, Ix JH, McClelland RL, Wassel CL, Rifkin DE, Carr JJ, Budoff MJ, Allison MA: Calcium density of coronary artery plaque and risk of incident cardiovascular events. JAMA 311: 271–278, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Block GA, Raggi P, Bellasi A, Kooienga L, Spiegel DM: Mortality effect of coronary calcification and phosphate binder choice in incident hemodialysis patients. Kidney Int 71: 438–441, 2007 [DOI] [PubMed] [Google Scholar]
- 6.London GM, Guérin AP, Marchais SJ, Métivier F, Pannier B, Adda H: Arterial media calcification in end-stage renal disease: Impact on all-cause and cardiovascular mortality. Nephrol Dial Transplant 18: 1731–1740, 2003 [DOI] [PubMed] [Google Scholar]
- 7.Blacher J, Guérin AP, Pannier B, Marchais SJ, London GM: Arterial calcifications, arterial stiffness, and cardiovascular risk in end-stage renal disease. Hypertension 38: 938–942, 2001 [DOI] [PubMed] [Google Scholar]
- 8.Floege J, Ketteler M: Vascular calcification in patients with end-stage renal disease. Nephrol Dial Transplant 19[Suppl 5]: V59–V66, 2004 [DOI] [PubMed] [Google Scholar]
- 9.Goodman WG, Goldin J, Kuizon BD, Yoon C, Gales B, Sider D, Wang Y, Chung J, Emerick A, Greaser L, Elashoff RM, Salusky IB: Coronary-artery calcification in young adults with end-stage renal disease who are undergoing dialysis. N Engl J Med 342: 1478–1483, 2000 [DOI] [PubMed] [Google Scholar]
- 10.Chertow GM, Burke SK, Raggi P Treat to Goal Working Group : Sevelamer attenuates the progression of coronary and aortic calcification in hemodialysis patients. Kidney Int 62: 245–252, 2002 [DOI] [PubMed] [Google Scholar]
- 11.Yamada K, Fujimoto S, Nishiura R, Komatsu H, Tatsumoto M, Sato Y, Hara S, Hisanaga S, Ochiai H, Nakao H, Eto T: Risk factors of the progression of abdominal aortic calcification in patients on chronic haemodialysis. Nephrol Dial Transplant 22: 2032–2037, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Malluche HH, Mawad HW, Monier-Faugere MC: Renal osteodystrophy in the first decade of the new millennium: Analysis of 630 bone biopsies in black and white patients. J Bone Miner Res 26: 1368–1376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hercz G, Pei Y, Greenwood C, Manuel A, Saiphoo C, Goodman WG, Segre GV, Fenton S, Sherrard DJ: Aplastic osteodystrophy without aluminum: The role of “suppressed” parathyroid function. Kidney Int 44: 860–866, 1993 [DOI] [PubMed] [Google Scholar]
- 14.Asci G, Ok E, Savas R, Ozkahya M, Duman S, Toz H, Kayikcioglu M, Branscum AJ, Monier-Faugere MC, Herberth J, Malluche HH: The link between bone and coronary calcifications in CKD-5 patients on haemodialysis. Nephrol Dial Transplant 26: 1010–1015, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kurz P, Monier-Faugere MC, Bognar B, Werner E, Roth P, Vlachojannis J, Malluche HH: Evidence for abnormal calcium homeostasis in patients with adynamic bone disease. Kidney Int 46: 855–861, 1994 [DOI] [PubMed] [Google Scholar]
- 16.London GM, Marty C, Marchais SJ, Guerin AP, Metivier F, de Vernejoul MC: Arterial calcifications and bone histomorphometry in end-stage renal disease. J Am Soc Nephrol 15: 1943–1951, 2004 [DOI] [PubMed] [Google Scholar]
- 17.London GM, Marchais SJ, Guérin AP, Boutouyrie P, Métivier F, de Vernejoul MC: Association of bone activity, calcium load, aortic stiffness, and calcifications in ESRD. J Am Soc Nephrol 19: 1827–1835, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barreto DV, Barreto FC, Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, Moyses RM, Neves KR, Jorgetti V, Miname M, Santos RD, Canziani ME: Association of changes in bone remodeling and coronary calcification in hemodialysis patients: A prospective study. Am J Kidney Dis 52: 1139–1150, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Ferreira A, Frazão JM, Monier-Faugere MC, Gil C, Galvao J, Oliveira C, Baldaia J, Rodrigues I, Santos C, Ribeiro S, Hoenger RM, Duggal A, Malluche HH Sevelamer Study Group : Effects of sevelamer hydrochloride and calcium carbonate on renal osteodystrophy in hemodialysis patients. J Am Soc Nephrol 19: 405–412, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hou SH, Zhao J, Ellman CF, Hu J, Griffin Z, Spiegel DM, Bourdeau JE: Calcium and phosphorus fluxes during hemodialysis with low calcium dialysate. Am J Kidney Dis 18: 217–224, 1991 [DOI] [PubMed] [Google Scholar]
- 21.National Kidney Foundation : K/DOQI clinical practice guidelines for bone metabolism and disease in chronic kidney disease. Am J Kidney Dis 42[Suppl 3]: S1–S201, 2003 [PubMed] [Google Scholar]
- 22.Malluche HH, Monier-Faugere MC: Risk of adynamic bone disease in dialyzed patients. Kidney Int Suppl 38: S62–S67, 1992 [PubMed] [Google Scholar]
- 23.Barreto DV, Barreto FC, de Carvalho AB, Cuppari L, Draibe SA, Dalboni MA, Moyses RM, Neves KR, Jorgetti V, Miname M, Santos RD, Canziani ME: Phosphate binder impact on bone remodeling and coronary calcification--results from the BRiC study. Nephron Clin Pract 110: c273–c283, 2008 [DOI] [PubMed] [Google Scholar]
- 24.Salusky IB, Goodman WG, Sahney S, Gales B, Perilloux A, Wang HJ, Elashoff RM, Jüppner H: Sevelamer controls parathyroid hormone-induced bone disease as efficiently as calcium carbonate without increasing serum calcium levels during therapy with active vitamin D sterols. J Am Soc Nephrol 16: 2501–2508, 2005 [DOI] [PubMed] [Google Scholar]
- 25.Freemont AJ, Hoyland JA, Denton J Lanthanum Carbonate SPD405-303 Study Group : The effects of lanthanum carbonate and calcium carbonate on bone abnormalities in patients with end-stage renal disease. Clin Nephrol 64: 428–437, 2005 [PubMed] [Google Scholar]
- 26.Sigrist M, McIntyre CW: Calcium exposure and removal in chronic hemodialysis patients. J Ren Nutr 16: 41–46, 2006 [DOI] [PubMed] [Google Scholar]
- 27.Coco M, Rush H: Increased incidence of hip fractures in dialysis patients with low serum parathyroid hormone. Am J Kidney Dis 36: 1115–1121, 2000 [DOI] [PubMed] [Google Scholar]
- 28.Young EW, Albert JM, Satayathum S, Goodkin DA, Pisoni RL, Akiba T, Akizawa T, Kurokawa K, Bommer J, Piera L, Port FK: Predictors and consequences of altered mineral metabolism: The Dialysis Outcomes and Practice Patterns Study. Kidney Int 67: 1179–1187, 2005 [DOI] [PubMed] [Google Scholar]
- 29.Kidney Disease: Improving Global Outcomes (KDIGO) CKD-MBD Work Group : KDIGO clinical practice guideline for the diagnosis, evaluation, prevention, and treatment of Chronic Kidney Disease-Mineral and Bone Disorder (CKD-MBD). Kidney Int Suppl 113: S1–S130, 2009 [DOI] [PubMed] [Google Scholar]
- 30.Fujimori A, Yorifuji M, Sakai M, Oyama M, Nakao N, Tokuyama M, Fukagawa M: Low-calcium dialysate improves mineral metabolism in hemodialysis patients. Clin Nephrol 67: 20–24, 2007 [DOI] [PubMed] [Google Scholar]
- 31.Adragao T, Herberth J, Monier-Faugere MC, Branscum AJ, Ferreira A, Frazao JM, Dias Curto J, Malluche HH: Low bone volume--a risk factor for coronary calcifications in hemodialysis patients. Clin J Am Soc Nephrol 4: 450–455, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ritz E, Krempien B, Mehls O, Malluche H: Skeletal abnormalities in chronic renal insufficiency before and during maintenance hemodialysis. Kidney Int 4: 116–127, 1973 [DOI] [PubMed] [Google Scholar]
- 33.Selye H: On the stimulation of new bone formation with parathyroid extract and irradiated ergosterol. Endocrinology 16: 547–558, 1932 [Google Scholar]
- 34.Standbury SW: Bone complication of renal disease. In: Renal Disease, edited by Dak B, Philadelphia, Davis, 1968, pp 665–713 [Google Scholar]
- 35.Haris A, Sherrard DJ, Hercz G: Reversal of adynamic bone disease by lowering of dialysate calcium. Kidney Int 70: 931–937, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Reynolds JL, Joannides AJ, Skepper JN, McNair R, Schurgers LJ, Proudfoot D, Jahnen-Dechent W, Weissberg PL, Shanahan CM: Human vascular smooth muscle cells undergo vesicle-mediated calcification in response to changes in extracellular calcium and phosphate concentrations: A potential mechanism for accelerated vascular calcification in ESRD. J Am Soc Nephrol 15: 2857–2867, 2004 [DOI] [PubMed] [Google Scholar]
- 37.Pun PH, Horton JR, Middleton JP: Dialysate calcium concentration and the risk of sudden cardiac arrest in hemodialysis patients. Clin J Am Soc Nephrol 8: 797–803, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Di Iorio B, Torraca S, Piscopo C, Sirico ML, Di Micco L, Pota A, Tartaglia D, Berardino L, Morrone LF, Russo D: Dialysate bath and QTc interval in patients on chronic maintenance hemodialysis: Pilot study of single dialysis effects. J Nephrol 25: 653–660, 2012 [DOI] [PubMed] [Google Scholar]
- 39.Fernández E, Borràs M, Pais B, Montoliu J: Low-calcium dialysate stimulates parathormone secretion and its long-term use worsens secondary hyperparathyroidism. J Am Soc Nephrol 6: 132–135, 1995 [DOI] [PubMed] [Google Scholar]
- 40.Nishi H, Nii-Kono T, Nakanishi S, Yamazaki Y, Yamashita T, Fukumoto S, Ikeda K, Fujimori A, Fukagawa M: Intravenous calcitriol therapy increases serum concentrations of fibroblast growth factor-23 in dialysis patients with secondary hyperparathyroidism. Nephron Clin Pract 101: c94–c99, 2005 [DOI] [PubMed] [Google Scholar]
- 41.Mathew S, Lund RJ, Chaudhary LR, Geurs T, Hruska KA: Vitamin D receptor activators can protect against vascular calcification. J Am Soc Nephrol 19: 1509–1519, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Shroff R, Egerton M, Bridel M, Shah V, Donald AE, Cole TJ, Hiorns MP, Deanfield JE, Rees L: A bimodal association of vitamin D levels and vascular disease in children on dialysis. J Am Soc Nephrol 19: 1239–1246, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Agatston AS, Janowitz WR, Hildner FJ, Zusmer NR, Viamonte M Jr., Detrano R: Quantification of coronary artery calcium using ultrafast computed tomography. J Am Coll Cardiol 15: 827–832, 1990 [DOI] [PubMed] [Google Scholar]
- 44.Budoff MJ, Hokanson JE, Nasir K, Shaw LJ, Kinney GL, Chow D, Demoss D, Nuguri V, Nabavi V, Ratakonda R, Berman DS, Raggi P: Progression of coronary artery calcium predicts all-cause mortality. JACC Cardiovasc Imaging 3: 1229–1236, 2010 [DOI] [PubMed] [Google Scholar]
- 45.Malluche HH, Monier-Faugere MC: Atlas of Mineralized Bone Histology, New York, Karger, 1986 [Google Scholar]
- 46.Malluche HH, Monier-Faugere MC: Renal osteodystrophy: What’s in a name? Presentation of a clinically useful new model to interpret bone histologic findings. Clin Nephrol 65: 235–242, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Moe S, Drüeke T, Cunningham J, Goodman W, Martin K, Olgaard K, Ott S, Sprague S, Lameire N, Eknoyan G Kidney Disease: Improving Global Outcomes (KDIGO) : Definition, evaluation, and classification of renal osteodystrophy: A position statement from Kidney Disease: Improving Global Outcomes (KDIGO). Kidney Int 69: 1945–1953, 2006 [DOI] [PubMed] [Google Scholar]
- 48.Moe SM, O’Neill KD, Reslerova M, Fineberg N, Persohn S, Meyer CA: Natural history of vascular calcification in dialysis and transplant patients. Nephrol Dial Transplant 19: 2387–2393, 2004 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.