Abstract
Evidence suggests that the glycogen synthase kinase 3 (GSK3)–dictated nuclear exclusion and degradation of Nrf2 is pivotal in switching off the self-protective antioxidant stress response after injury. Here, we examined the mechanisms underlying this regulation in glomerular disease. In primary podocytes, doxorubicin elicited cell death and actin cytoskeleton disorganization, concomitant with overactivation of GSK3β (the predominant GSK3 isoform expressed in glomerular podocytes) and minimal Nrf2 activation. SB216763, a highly selective small molecule inhibitor of GSK3, exerted a protective effect that depended on the potentiated Nrf2 antioxidant response, marked by increased Nrf2 expression and nuclear accumulation and augmented production of the Nrf2 target heme oxygenase-1. Ectopic expression of the kinase-dead mutant of GSK3β in cultured podocytes reinforced the doxorubicin-induced Nrf2 activation and prevented podocyte injury. Conversely, a constitutively active GSK3β mutant blunted the doxorubicin-induced Nrf2 response and exacerbated podocyte injury, which could be abolished by treatment with SB216763. In murine models of doxorubicin nephropathy or nephrotoxic serum nephritis, genetic targeting of GSK3β by doxycycline-inducible podocyte-specific knockout or pharmacologic targeting by SB216763 significantly attenuated albuminuria and ameliorated histologic signs of podocyte injury, including podocytopenia, loss of podocyte markers, podocyte de novo expression of desmin, and ultrastructural lesions of podocytopathy (such as foot process effacement). This beneficial outcome was likely attributable to an enhanced Nrf2 antioxidant response in glomerular podocytes because the selective Nrf2 antagonist trigonelline abolished the proteinuria-reducing and podocyte-protective effect. Collectively, our results suggest the GSK3β-regulated Nrf2 antioxidant response as a novel therapeutic target for protecting podocytes and treating proteinuric glomerulopathies.
Keywords: podocyte, reactive oxygen species, glomerulopathy, Nrf2 antioxidant, response, glycogen synthase kinase, conditional knockout
Podocytes are highly specialized epithelial cells with elaborate interdigitating foot processes that envelop the capillaries of the glomerulus in the kidney and prevent protein in the bloodstream from leaking into the urine.1 Through their constant motility, driven by an intricate cytoskeletal machinery, podocytes play a pivotal role in counteracting the pulsating hydrostatic pressure and maintaining the structural and functional integrity of the glomerular filtration barrier.2 In addition, podocytes are actively involved in other energy-intensive cellular events, including membrane trafficking and recycling and the transcytosis of the primary ultrafiltrate.3,4 Moreover, podocytes are instrumental in sustaining the fine porous molecular sieve formed by the slit diaphragm, secreting essential soluble factors to consolidate the homeostasis of other glomerular cells, and provide synthesis and maintenance of the glomerular basement membrane (GBM).4–6 All of these highly energy-consuming cellular processes are powered by ATP, which is produced mainly by aerobic cellular respiration that requires oxygen. Cellular metabolism of oxygen inevitably generates potentially deleterious free radicals and reactive oxygen species (ROS).7 Under normal conditions, the rate and magnitude of oxidant formation are balanced by the rate of oxidant elimination. However, an imbalance between pro-oxidants and antioxidants can lead to oxidative stress and cause glomerular injury and destruction.8,9
Upon the oxidative challenge of free radicals and ROS, mammalian cells leverage an adaptive antioxidant stress response to sustain redox homeostasis and cellular integrity.10 Central to this antioxidant response is NF-E2–related factor (Nrf2), a cap'n'collar basic-region leucine zipper nuclear transcription factor that dictates the primary cellular defense against the cytotoxic effects of oxidative stress.10,11 As the master regulator of antioxidant response and self-defense, Nrf2 transactivates a broad spectrum of molecules involved in antioxidation, detoxification, cell survival, anti-inflammatory response, and more; it has emerged as an attractive therapeutic target for disease of multiple organ systems, including the kidney.12–14
The Nrf2-dependent self-protective antioxidant response is a complex and highly orchestrated cellular process that is regulated by myriad signaling pathways. In its inactive state, Nrf2 is sequestered in the cytoplasm and associated with the ubiquitin E3 ligase adapter Kelch-like epichlorohydrin-associated protein 1 (Keap1), leading to ubiquitination and proteasome degradation.11,15,16 Upon its activation, triggered by oxidative stress, Nrf2 dissociates from Keap1 and subsequently translocates into the nucleus and binds to a conserved antioxidant response element to initiate the transcription of a battery of chemoprotective antioxidant genes,11 including those encoding antioxidant enzymes, such as heme oxygenase 1 (HO-1).17,18 Of the many regulatory pathways, the redox sensitive glycogen synthase kinase (GSK) 3 has emerged as the converging point.19 GSK3 is a ubiquitously expressed, constitutively active, proline-directed serine/threonine kinase involved in diverse cellular processes.20 Recent evidence points to GSK3β as a key player involved in switching off the Nrf2 pathway after injury21 and suggests that GSK3β regulation of Nrf2 is implicated in aging,22 type 2 diabetes,23 liver disease,24 and neurologic degeneration.25–27 Very little, however, is known about how GSK3β regulates Nrf2 antioxidant response in glomerular disease. This study examined the regulatory effect of GSK3β on podocyte Nrf2 antioxidant response in vitro and in murine models of doxorubicin nephropathy and nephrotoxic serum (NTS) nephritis.
Results
Inhibition of GSK3 by SB216763 Potentiates the Nrf2 Antioxidant Response and Confers Cytoprotection in Podocytes
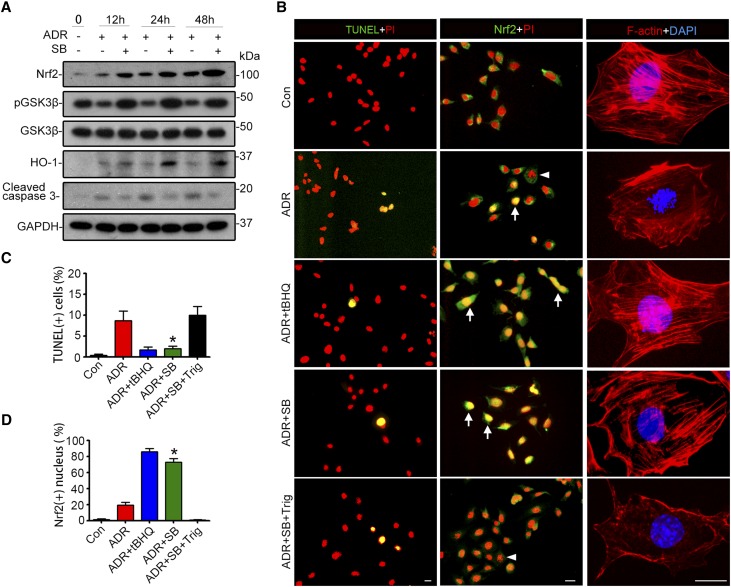
GSK3 regulates Nrf2 in other types of cells.24,28 To examine whether this regulation also occurs in podocytes, primary podocyte cultures were prepared and pretreated with vehicle or SB216763, a highly selective small molecule inhibitor of GSK3,29 before doxorubicin injury. Doxorubicin injury elicited podocyte apoptosis, shown by immunoblot analysis for cleaved caspase 3 (Figure 1A), terminal deoxynucleotidyl transferase–mediated digoxigenin-deoxyuridine nick-end labeling (TUNEL) and nuclear fragmentation (Figure 1B), and disrupted actin cytoskeleton integrity, detected by labeling filamentous actin (F-actin) with rhodamine phalloidin. This was concomitant with GSK3β overactivity, marked by diminished inhibitory phosphorylation of GSK3β (Figure 1A). SB216763 corrected GSK3β overactivity after doxorubicin injury, resulting in protection against actin cytoskeleton disorganization and podocyte apoptosis (Figure 1, A–C). This beneficial effect of SB216763 was associated with an elevated expression and activation of Nrf2, as estimated by immunoblot analysis (Figure 1A) and by an augmented Nrf2 nuclear accumulation on fluorescent immunocytochemistry staining (Figure 1B). Moreover, SB216763 treatment amplified the expression of the Nrf2 target gene HO-1 (Figure 1A), denoting an enhanced Nrf2 antioxidant response. The SB216763-potentiated Nrf2 response seems to be necessary and sufficient for its podocyte protective effect because the antiapoptotic and cytoskeleton protective effect of SB216763 was markedly abolished by trigonelline (Trig),24 a selective antagonist of Nrf2, but was highly mimicked by tertiary butylhydroquinone (tBHQ),30 a standard activator of Nrf2 (Figure 1, B–D).
Figure 1.
SB216763, a highly selective small molecule inhibitor of GSK3, reinforces the Nrf2 antioxidant response in podocytes upon doxorubicin injury, attenuates podocyte apoptosis, and preserves actin cytoskeleton integrity. (A) Primary podocytes were prepared from WT mice, pretreated with SB216763 (SB, 10 μmol/L) or vehicle for 30 minutes, and then stimulated with doxorubicin (ADR; 0.25 μg/mL) or an equal volume of vehicle. Cells were harvested at indicated time points, and cell lysates prepared for immunoblot analysis for indicated molecules. GAPDH served as a loading control. (B) Podocytes were pretreated with tBHQ, 20 μmol/L), a standard Nrf2 activator, SB216763 (SB; 10 μmol/L), Trig (30 μmol/L), or an equal volume of vehicle for 30 minutes and then injured with doxorubicin (0.25 μg/mL) for 24 hours. Cells were fixed and subjected to rhodamine phalloidin staining of F-actin (red), fluorescence immunocytochemistry staining for Nrf2 (green), or TUNEL staining (green) and counterstaining with propidium iodide (PI, red) or 4,6-diamidino-2-phenylindole (DAPI, blue). Arrows indicate Nrf2 nuclear accumulation. Arrowheads indicate nuclear fragmentation in cells without Nrf2 nuclear accumulation following doxorubicin exposure, suggestive of cellular apoptosis. Scale bar, 20 μm. (C) Absolute counting of TUNEL-positive podocytes expressed as percentage of the total number of podocyte nuclei per high-power field. The data are expressed as mean±SEM. *P<0.05 versus ADR- or ADR+SB+Trig–treated cells (n=3). (D) Absolute count of Nrf2-positive nuclei expressed as percentage of the total number of podocytes per high-power field. The data are expressed as mean±SEM. *P<0.05 versus ADR- or ADR+SB+Trig-treated cells (n=3). Con, control.
The β Rather Than the α Isoform of GSK3 Is Predominantly Expressed in Glomerular Podocytes In Vivo and In Vitro
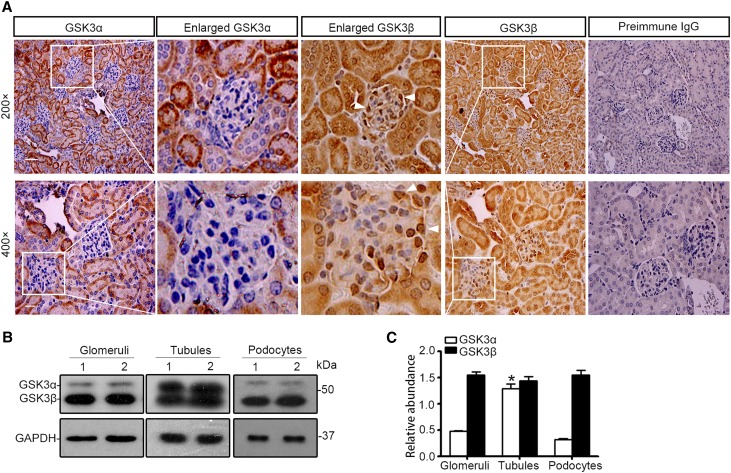
GSK3 is ubiquitously expressed throughout various organ systems20; nevertheless, its expression in the kidney, in particular in the glomerulus, has been barely examined before. Consecutive sections of normal mouse kidneys were processed for immunoperoxidase staining for GSK3α and GSK3β in parallel. Both GSK3α and GSK3β were basally detected to a similar magnitude in renal tubules (Figure 2A). In the glomerulus, the β isoform of GSK3 was weakly detected in glomerular capillaries and mesangium but intensely distributed in the periphery of glomerular tuft, consistent with podocyte localization. In stark contrast, the α isoform of GSK3 was very weakly stained in all glomeruli (Figure 2A). Immunoblot analysis using a monoclonal antibody against both α and β isoforms of GSK3 demonstrated that the β rather than the α isoform is predominantly expressed in isolated glomeruli and in cultured podocytes (Figure 2B). This contrasted sharply with the immunoblot of isolated proximal tubules, in which the α and β isoforms were similarly expressed. The specificity of the detection was confirmed by preincubation of the kidney sections and immunoblots with a control peptide or the blocking peptide specific for GSK3α or GSK3β, which abolished the immunoreactive staining and immunoblot bands (Supplemental Figure 1).
Figure 2.
The β isoform rather than the α isoform of GSK3 is predominantly expressed in glomerulus and intensely distributed in podocytes. (A) Consecutive sections of formalin-fixed, paraffin-embedded WT murine kidney specimens were subjected to peroxidase immunohistochemistry staining for the α and the β isoforms of GSK3 in parallel. Enlarged views demonstrate the staining of α and β isoforms of GSK3 in the same glomeruli. Arrowheads indicate GSK3β-positive podocytes (scale bar, 50 μm for original magnification of ×200; scale bar, 20 μm for original magnification of ×400). (B) Homogenates of glomeruli and proximal tubules isolated from WT mouse kidneys and lysates of primary podocytes prepared from WT mice were processed for immunoblot analysis for GSK3 by using a monoclonal antibody against both α and β isoforms of GSK3 (sc-7291, Santa Cruz Biotechnology). GAPDH served as a loading control. (C) Relative abundance of GSK3α and GSK3β expressed in murine glomeruli, proximal tubules, and primary podocytes determined by densitometric analyses of immunoblot data from experiments described in B. The data are expressed as mean±SEM. *P<0.05 versus GSK3α in glomeruli or podocytes (n=6).
GSK3β Inhibition Is Essential and Sufficient for Nrf2 Antioxidant Response in Podocytes upon Injury
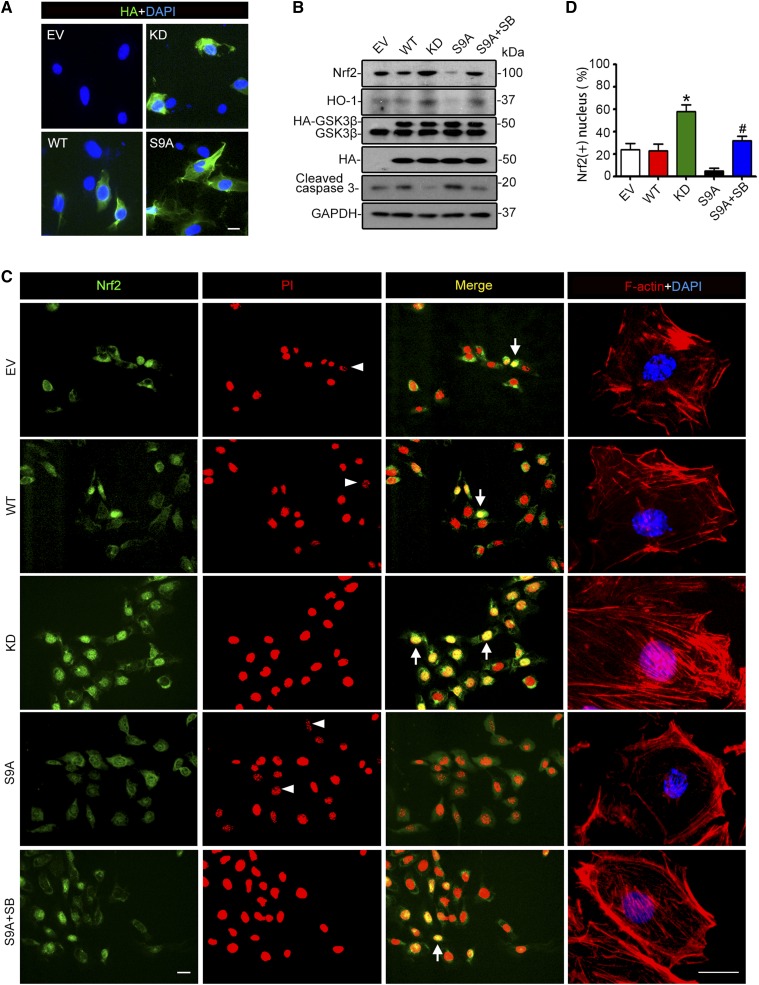
To validate a direct causal relationship between GSK3 signaling and Nrf2 antioxidant response, the activity of GSK3β, the predominant isoform of GSK3 in podocytes, was manipulated in primary podocytes by forced expression of an empty vector or vectors encoding the hemagglutinin (HA)-conjugated wild-type (WT) GSK3β, kinase dead (KD), or constitutively active (S9A) mutant of GSK3β, with a transfection efficiency of about 50%, as estimated by immunostaining (Figure 3A) and immunoblot analysis (Figure 3B) for GSK3β or HA. After doxorubicin injury, ectopic expression of the KD mutant of GSK3β diminished podocyte death, evidenced by a reduced caspase 3 cleavage (Figure 3B) and nuclear fragmentation (Figure 3C), and attenuated actin cytoskeleton disorganization (Figure 3C). This was paralleled by an elevated Nrf2 antioxidant response, marked by enhanced Nrf2 expression (Figure 3B) and nuclear accumulation (Figure 3, C and D) and increased production of HO-1 (Figure 3B), reminiscent of the effect of SB216763. In contrast, in S9A-expressing podocytes, the doxorubicin-elicited Nrf2 expression and nuclear accumulation, and HO-1 expression was markedly blunted, accompanied by exacerbated apoptosis and disruption of actin cytoskeleton integrity. This effect was largely counteracted by the GSK3 inhibitor SB216763 (Figure 3, B and C).
Figure 3.
Inhibition of GSK3β is necessary and sufficient for the induced Nrf2 antioxidant response and protection against actin cytoskeleton disorganization in podocytes upon doxorubicin injury. Primary podocytes were prepared from WT mice and transiently transfected with vectors encoding the empty vector (EV), the HA-conjugated WT, dominant negative KD, or constitutively active (S9A) GSK3β. (A) Representative micrographs of fluorescent immunocytochemistry staining for HA (green). Cells were counterstained with 4,6-diamidino-2-phenylindole (DAPI; blue). Scale bar, 20 μm. Transfection efficiency in the primarily cultured podocytes typically ranged from 30% to 60% and varied depending on the condition of the cultures. The batch of cultures with transfection efficiency >50% was used for subsequent experiments. (B) After transfection, cells were treated with 0.25 µg/ml of doxorubicin for 24 hours in the presence or absence of SB216763 (SB; 10 µmol/L). Cell lysates were collected and subjected to immunoblot analysis for Nrf2, HO-1, GSK3, HA, cleaved caspase 3, and GAPDH. (C) Cells, treated as stated in B, were fixed and subjected to immunofluorescence staining for Nrf2 (green) and rhodamine phalloidin labeling of F-actin (red) and counterstained with propidium iodide (PI, red) or DAPI (blue). Arrowheads indicate nuclear fragmentation in cells without Nrf2 nuclear accumulation after doxorubicin exposure, suggestive of cellular apoptosis. Arrows indicate Nrf2 nuclear accumulation. Scale bar, 20 μm. (D) Absolute count of Nrf2-positive nuclei expressed as percentage of the total number of podocytes per high-power field. The data are expressed as mean±SEM. *P<0.05 versus all other groups; #P<0.05 versus S9A (n=3).
Podocyte-Specific Deletion of GSK3β Ameliorates Podocytopathy and Proteinuria in Doxorubicin-Injured Mice via an Nrf2-Dependent Mechanism
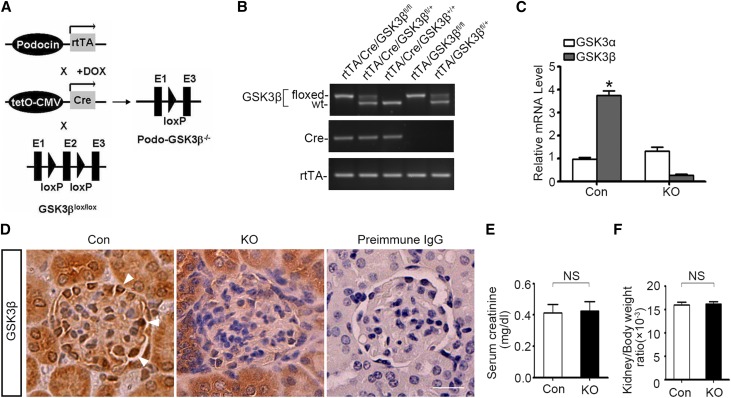
To determine whether GSK3 regulation of Nrf2 also occurs in podocytes in vivo, we opted to knock out the β isoform of GSK3 in mice. Systemic GSK3β knockout (KO) caused embryonic lethality.31 Thus, the doxycycline-inducible Cre-loxP site-specific gene targeting strategy (Figure 4A) was used to knock out GSK3β specifically in podocytes in adult mice. By crossing the GSK3β-floxed mice with transgenic mice expressing reverse tetracycline-controlled transcriptional activator (rtTA) under the control of podocin promoter and those expressing Cre driven by the tetracycline operator (tetO)-cytomegalovirus (CMV) promoter, progeny with the genotype (rtTA/Cre/GSK3βfl/fl) of doxycycline-inducible podocyte-specific GSK3β KO on a friend virus B (FVB) genetic background were successfully bred and confirmed by tail-snip genotyping (Figure 4B). All littermates lacking the Cre transgene were designated as control mice. After oral administration of doxycycline for 2 weeks, mRNA expression of GSK3β in glomerular podocytes was substantially ablated in KO mice but not affected in control mice, as shown by real-time RT-PCR analysis (Figure 4C). In contrast, mRNA expression of GSK3α was not blunted in KO mice (Figure 4C), denoting a β-isoform–specific ablation of GSK3. Immunoperoxidase staining of kidney specimens revealed that the expression of GSK3β was barely affected in tubulointerstitium or other glomerular structures in KO kidneys compared with control kidneys but was specifically ablated in the periphery of glomerular tufts, in accordance with podocyte localization (Figure 4D). Under physiologic conditions, KO mice, compared with control littermates, were viable and exhibited normal development and behavior as well as normal kidney physiology in terms of kidney function (Figure 4E), kidney weight (Figure 4F), urine excretion of albumin (Figure 5, A and B), and kidney histology (Figure 5, C–F).
Figure 4.
Mice with podocyte-specific somatic ablation of GSK3β are healthy and have normal kidney function and histology. (A) Schematic diagram depicts the doxycycline-inducible tissue specific Cre-loxP gene recombination strategy to target the GSK3β gene selectively in glomerular podocytes in adult mice. By crossing the GSK3β-floxed mice with transgenic mice expressing rtTA under the control of podocin promoter and those expressing Cre driven by the tetracycline operator (tetO)-cytomegalovirus (CMV) promoter, the KO mice were bred. These mice carry double transgenes (Cre and rtTA transgenes) and homozygous floxed-GSK3β. In KO mice, rtTA transgene is specifically expressed in the glomerular podocytes. Feeding the KO mice with doxycycline (DOX), a compound enabling rtTA binding to the tetO promoter, will switch on Cre transgene expression in podocytes, which will subsequently execute the podocyte-specific excision of floxed exon 2 (E2) of GSK3β gene. (B) PCR genotyping of tail DNA from representative mice for detecting the Cre and rtTA transgenes and the floxed or WT GSK3β. All littermates lacking the Cre transgene were designated as control mice (Con). (C) Primary podocytes were prepared from glomeruli isolated from KO and control kidneys. The mRNA expression levels of GSK3α and GSK3β in primary podocytes were determined by real-time RT-PCR assay of the RNA samples extracted from the primary podocytes and presented as relative mRNA levels normalized to the endogenous GAPDH mRNA levels. The data are expressed as mean±SEM. *P<0.05 versus GSK3β expression levels in KO group (n=6). (D) Formalin-fixed, paraffin-embedded kidney specimens from KO mice or control littermates (Con) were sectioned and subjected to peroxidase immunohistochemistry staining for the β isoform of GSK3. Note that the staining of GSK3β was substantially diminished in the KO kidney, specifically in the periphery of glomerular tufts, in concert with the podocyte localization. As negative control, the primary antibody was replaced with preimmune IgG and no staining occurred. Arrowheads indicate podocytes positive for GSK3β in Con mice. Scale bar, 20 μm. (E and F) KO mice were phenotypically normal with serum creatinine levels (E) and kidney-to-body ratios (F) similar to those of control littermates at age 12 weeks. The data are expressed as mean±SEM. P>0.99 in E and P=0.70 in F (n=5).
Figure 5.
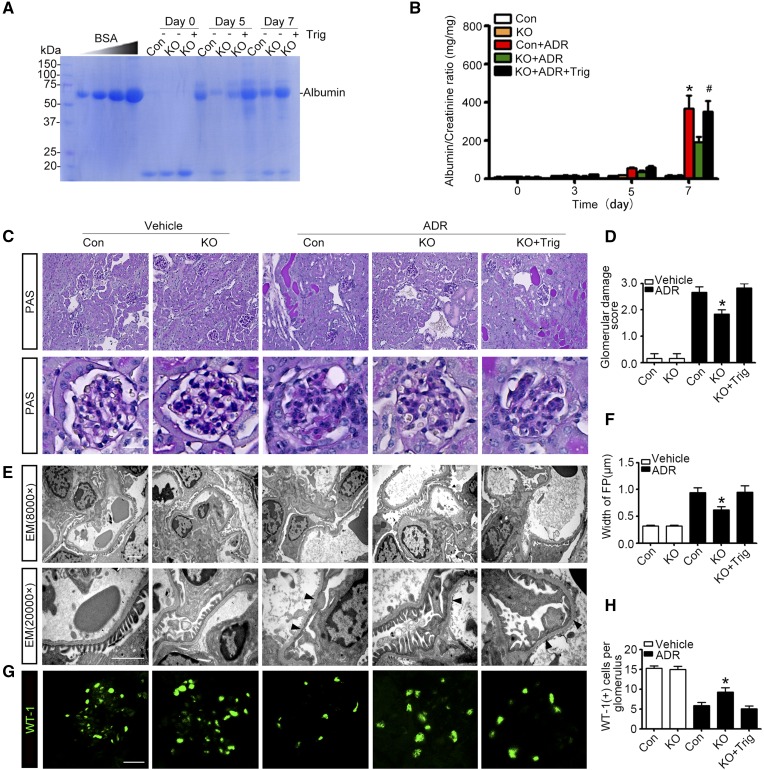
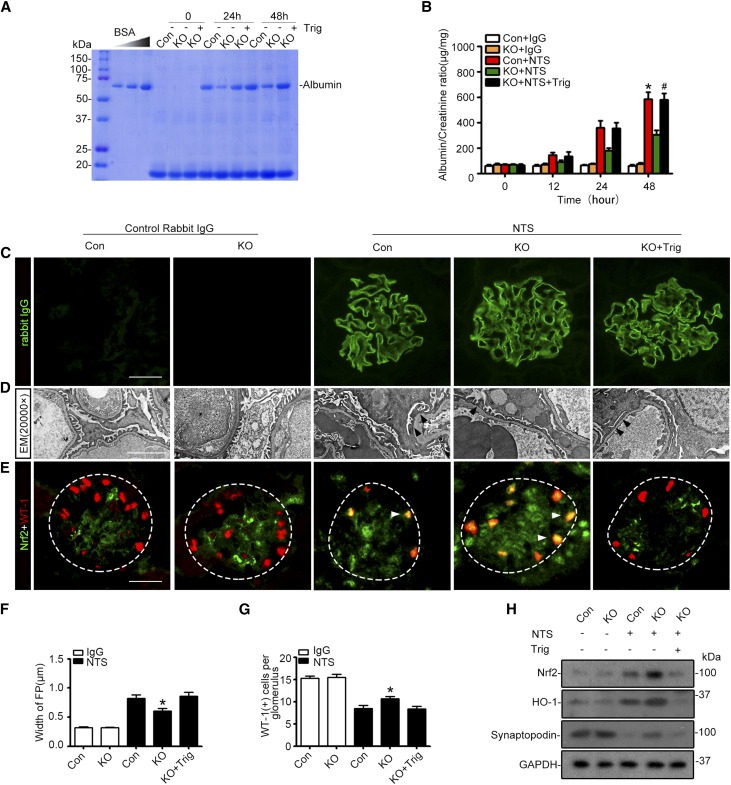
Podocyte-specific deletion of GSK3β ameliorates podocytopathy and proteinuria in experimental doxorubicin nephropathy. (A) KO and control (Con) male mice were injured with a single tail-vein injection of doxorubicin (ADR, 25 mg/kg) or vehicle 1 hour after an intraperitoneal injection of Trig (1 mg/kg) or vehicle. Mice were afterward treated with Trig (1 mg/kg) or vehicle via intraperitoneal injection every other day. Spot urine was collected at the indicated time points, and an aliquot (1.5 µl) was subjected to SDS-PAGE followed by Coomassie brilliant blue staining. BSA (5, 10, 20, and 40 µg) served as standard controls. (B) Quantification of urine albumin levels adjusted by urine creatinine concentrations. The data are expressed as mean±SEM and were subjected to logarithmic transformation and analyzed by repeated-measures ANOVA followed by post hoc Scheffé test. The test for a difference in urine albumin-to-creatinine ratios over time was significant (F[3, 75]=199.831; P<0.01). The test for equality of treatment means over time was also significant (F[4, 25]=34.788; P<0.01). *P<0.05 versus control, KO, or KO+ADR; #P<0.05 versus KO+ADR (n=6). (C) Representative micrographs demonstrate PAS staining of mouse kidneys. Scale bar, 20 μm. (D) Morphometric scoring of glomerular damage on PAS staining of kidney tissues procured from mice on day 7. *P<0.05 versus ADR+control or ADR+KO+Trig (n=6). (E) Electron microscopy (EM) of podocyte ultrastructural injuries in kidney tissues procured from mice on day 7. Arrowheads indicate the lesions of podocyte foot process effacement. Scale bar, 2 μm. (F) Measurement of the width of podocyte foot processes (FP) on electron microscopy in mice on day 7. *P<0.05 versus ADR+control or ADR+KO+Trig (n=6). (G) Representative micrographs of immunofluorescence staining of kidney specimens procured on day 7 for the podocyte-specific marker WT-1. (H) Absolute count of the number of WT-1–positive podocytes in each glomerulus in kidney specimens. *P<0.05 versus ADR+control or ADR+KO+Trig (n=6).
Next, we examined whether podocyte-specific ablation of GSK3β affects glomerular pathology. To this end, we adopted the murine model of doxorubicin nephropathy, which recapitulates key features of podocytopathy in humans, including podocyte injury, massive proteinuria, and progressive glomerulosclerosis.32,33 After a single tail-vein injection of doxorubicin, albuminuria started to develop in control mice on day 5 and peaked on day 7, as estimated by urine electrophoresis and Coomassie brilliant blue staining (Figure 5A) and quantitated by measuring the urine albumin-to-creatinine ratios (Figure 5B). This was associated with typical renal lesions of nephrotic glomerulopathy, featured by large protein casts in tubules, podocytic swelling and vacuolization, glomerular synechiae, glomerular capillary congestion, collapse and/or obliteration accompanied by hyaline material deposition, mesangial extracellular matrix accumulation, and mild glomerulosclerosis, shown by periodic acid-Schiff (PAS) staining (Figure 5C) and assessed by glomerular damage scores (Figure 5D). Ultrastructural lesions of podocytopathy, including podocyte microvillous transformation and foot process effacement, were noted on transmission electron microscopy (Figure 5E) and quantified by measurement of width of foot processes (Figure 5F), and podocytopenia was evidenced by Wilms tumor-1 (WT-1) staining (Figure 5G) and absolute count of WT-1–positive podocytes in each glomerulus (Figure 5H).
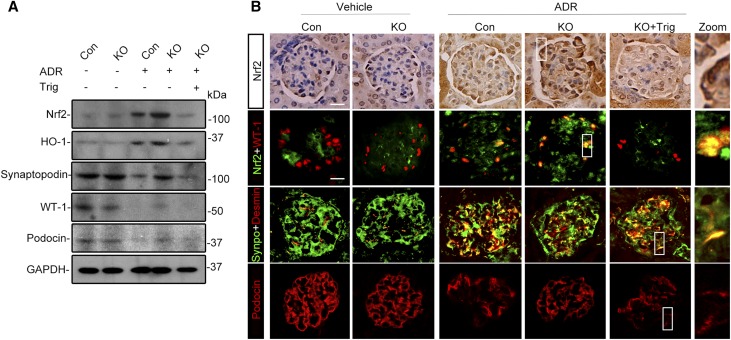
After doxorubicin injury, a mild level of Nrf2 induction and activation occurred in glomeruli, as estimated by immunoblot analysis of isolated glomeruli for Nrf2 and HO-1 (Figure 6A). The Nrf2 expression was located to the nuclei in the periphery of glomerular tufts, as shown by peroxidase immunohistochemistry staining (Figure 6B), and was further confirmed to partially colocalize with the podocyte nuclear marker WT-1 by dual color fluorescent immunohistochemistry staining, in agreement with an injury-induced Nrf2 nuclear accumulation and activation in glomerular podocytes. The specificity of the Nrf2 detection was confirmed by preincubation of the kidney sections and immunoblots with a control peptide or the blocking peptide specific for Nrf2, which abolished the immunoreactive staining and immunoblot bands (Supplemental Figure 2). Despite the mild level of spontaneous Nrf2 antioxidant response in glomeruli, podocytopathy evidently developed, characterized by the loss of podocyte markers in glomerulus, including synaptopodin, podocin, and WT-1, and by de novo expression of podocyte injury marker desmin, shown by coexpression of desmin with podocyte marker synaptopodin on dual color fluorescent immunohistochemistry staining (Figure 6B).
Figure 6.
The enhanced Nrf2 antioxidant response in glomerular podocytes is responsible for the ameliorated podocytopathy in mice with podocyte-specific KO of GSK3β upon doxorubicin injury. Control and KO mice were treated as described in Figure 5. (A) Kidneys were excised from animals on day 7, and glomeruli were isolated from kidneys by the magnetic beads–based approach. Glomerular homogenates were prepared for immunoblot analysis for Nrf2, HO-1, synaptopodin, WT-1, and podocin. GAPDH served as a loading control. (B) Formalin-fixed, paraffin-embedded kidney sections were subjected to peroxidase immunohistochemistry staining for Nrf2, and representative micrographs indicate that the doxorubicin (ADR)-elicited Nrf2 was enhanced in KO glomerulus and largely located to the nuclei in the periphery of glomerular tufts; cryosections of kidney tissues were processed for dual color immunofluorescence staining for Nrf2 (green) and WT-1 (red) or for synaptopodin (green) and podocyte injury marker desmin (red). Additional cryosections were subjected to immunofluorescence staining for the podocyte marker podocin (red). In the high-power views of the boxed areas, Nrf2 was detected in WT-1–positive podocyte nuclei and desmin was found to partially colocalize with synaptopodin. Nuclear accumulation of Nrf2 in glomerular podocytes was augmented in KO mice compared with control mice after doxorubicin injury, and this effect was abolished by Trig treatment. The loss of glomerular expression of podocyte marker podocin was attenuated, and de novo expressions of podocyte injury marker desmin in synaptopodin-positive podocytes was mitigated in kidneys from KO mice after doxorubicin injury. This beneficial effect was blunted by Trig treatment. Scale bar, 20 μm.
In stark contrast, KO mice excreted significantly less proteinuria on all observed days after doxorubicin injury (Figure 5, A and B) and presented attenuated glomerular injury (Figure 5, C and D) and podocytopathy, including podocyte foot process effacement (Figure 5, E and F) and podocytopenia (Figure 5, G and H). In addition, the loss of glomerular expression of podocyte marker proteins was attenuated (Figure 6, A and B) and podocyte de novo expressions of desmin (Figure 6B) was mitigated in KO mice after doxorubicin injury, associated with increased Nrf2 activation and HO-1 production in glomeruli (Figure 6A) and enhanced Nrf2 nuclear accumulation in podocytes (Figure 6B). The reinforced Nrf2 antioxidant response in glomerular podocytes seemingly accounts, at least in part, for the protection in KO mice because selective blockade of Nrf2 by Trig abolished the Nrf2 response in KO mice upon doxorubicin injury (Figure 6) and abrogated the protection against proteinuria (Figure 5, A and B), glomerular injury (Figure 5, C–F), and podocytopathy (Figures 5, E–H and 6).
Pharmacologic Targeting of GSK3β Reduces Proteinuria and Protects against Podocytopathy in Doxorubicin-Injured Mice through Potentiating the Nrf2 Antioxidant Response in Podocytes
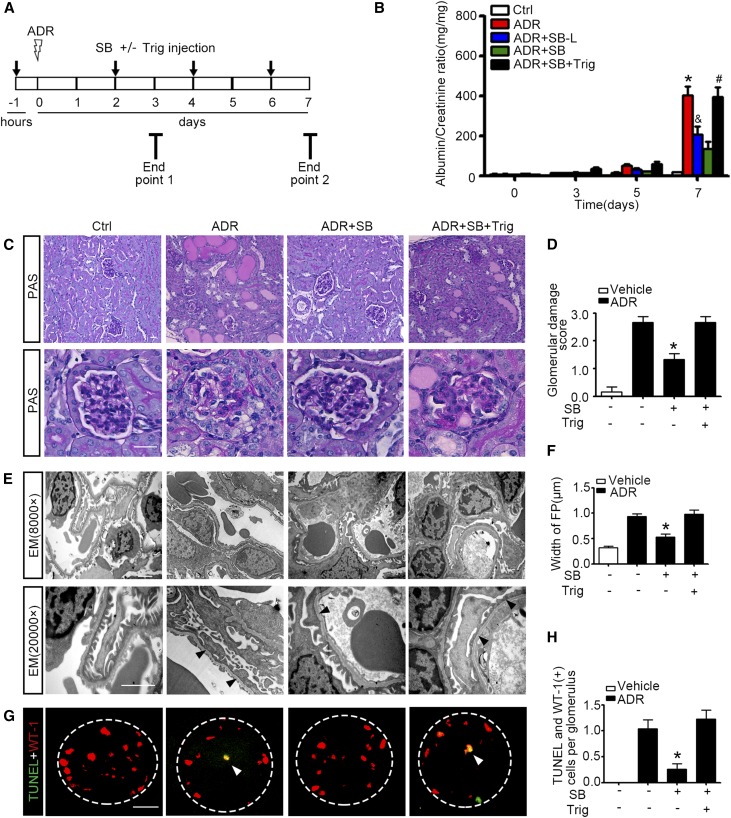
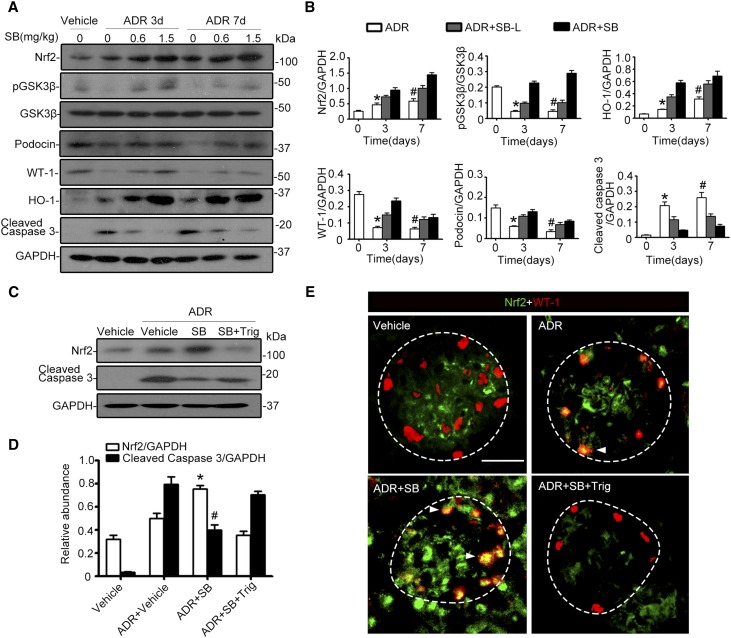
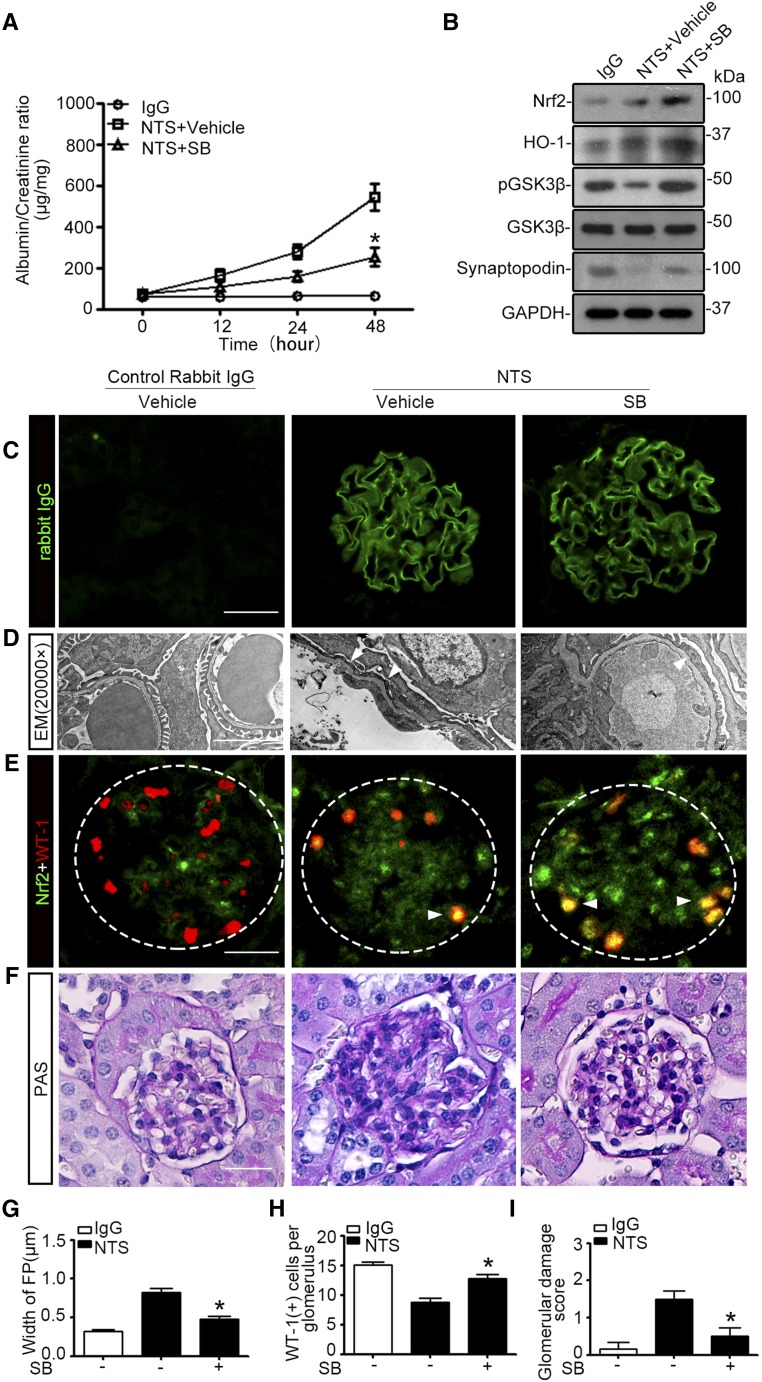
Numerous preclinical studies have shown that GSK3β is druggable in many organ systems using novel small molecule inhibitors, such as SB216763.29 To examine whether systemic administration of SB216763 could regulate the Nrf2 antioxidant response in glomerular podocytes and affect podocytopathy in vivo, SB216763 at a dose of 1.5 mg/kg (SB) or at a low dose (0.6 mg/kg, SB-L) was given to murine models of doxorubicin nephropathy (Figure 7A). SB216763 therapy markedly attenuated albuminuria in a dose-dependent fashion (Figure 7B). This antiproteinuric effect was associated with an improvement in glomerular histology, as assessed by morphometric glomerular damage scores on PAS staining (Figure 7, C and D), in parallel with amelioration of podocyte foot process effacement on electron microscopy (Figure 7, E and F). Moreover, SB216763 therapy markedly diminished podocyte apoptosis in doxorubicin-injured kidneys, as estimated by absolute count of cells positive for both WT-1 and TUNEL in each glomerulus (Figure 7, G and H).34 Immunoblot analysis of isolated glomeruli (Figure 8A) in conjunction with densitometry (Figure 8B) indicated that SB216763 therapy induced inhibitory phosphorylation of GSK3β and counteracted the doxorubicin-elicited GSK3β overactivity in a dose-dependent fashion, denoting a successful blockade of GSK3β in glomeruli in vivo. This was concomitant with enhanced glomerular expression of Nrf2 and HO-1, prevented loss of podocyte markers (such as podocin and WT-1) (Figure 8, A and B), and diminished glomerular cell apoptosis, detected by cleaved caspase 3 (Figure 8, C and D). Dual color fluorescent immunohistochemistry staining demonstrated that SB216763 treatment augmented Nrf2 colocalization with the podocyte nuclear marker WT-1, in agreement with a reinforced Nrf2 nuclear accumulation and activation in glomerular podocytes (Figure 8E). In contrast, after Trig treatment (Figure 7A), the SB216763-promoted glomerular Nrf2 expression and Nrf2 nuclear accumulation in podocytes was abolished (Figure 8, A, B, and E) and the beneficial effects of SB216763 on proteinuria (Figure 7B), glomerular injury, and podocytopathy (Figures 7, C–F, and 8, A and B), were largely blunted.
Figure 7.
Pharmacologic targeting of GSK3β by SB216763 attenuates proteinuria and podocytopathy in mice with doxorubicin nephropathy. WT FVB/NJ male mice aged 8 weeks were injured with a single tail-vein injection of doxorubicin (ADR, 25 mg/kg) or vehicle 1 hour after a subcutaneous injection of SB216763 at a dose of 1.5 mg/kg (SB) or a low dose of 0.6 mg/kg (SB-L) with or without intraperitoneal injection of Trig (1 mg/kg). The animals were afterward treated every other day with or without a subcutaneous injection of SB216763 at a dose of 1.5 mg/kg (SB) or at a low dose of 0.6 mg/kg (SB-L) or an intraperitoneal injection of Trig (1 mg/kg). (A) Schematic diagram depicts the animal study designed to test the effect of targeted inhibition of GSK3β by SB216763. (B) Spot urine was collected at the indicated time points and subjected to ELISA quantification of urine albumin levels adjusted by urine creatinine concentrations. The data are expressed as mean±SEM and were subjected to logarithmic transformation and analyzed by repeated-measures ANOVA followed by post hoc Scheffé test. The test for a difference in urine albumin-to-creatinine ratios over time was significant (F[3, 75]=236.348; P<0.01). The test for equality of treatment means over time was also significant (F[4, 25]=16.707; P<0.01). *P<0.05 versus control, ADR+SB-L, or ADR+SB; &P<0.05 versus ADR+SB, #P<0.05 versus ADR+SB (n=6). (C) Representative micrographs demonstrate PAS staining of kidneys procured from animals on day 7. Scale bar, 20 μm. (D) Morphometric scoring of glomerular damage on PAS staining of kidney tissues procured from mice on day 7. *P<0.05 versus ADR or ADR+SB+Trig (n=6). (E) Transmission electron microscopy (EM) of podocyte ultrastructural lesions after doxorubicin injury. Arrowheads indicate lesions of podocyte foot process effacement. Scale bar, 2 μm. (F) Measurement of the width of podocyte foot processes (FP) on electron microscopy in mice on day 7. *P<0.05 versus ADR or ADR+SB+Trig (n=6). (G) Frozen kidney sections were fixed and subjected to dual color fluorescent staining for TUNEL (green) and WT-1 (red). Arrowheads indicate representative glomerular podocytes positive for both TUNEL and WT-1 staining. Scale bar, 20 μm. (H) Absolute count of the number of apoptotic glomerular podocytes positive for both TUNEL and WT-1 staining in each glomerulus as the means of 50 glomeruli. *P<0.05 versus ADR or ADR+SB+Trig (n=6).
Figure 8.
Inhibition of GSK3β by the highly selective small molecule inhibitor SB216763 in murine models of doxorubicin (ADR) nephropathy protects against podocyte injury through reinforcing the Nrf2 antioxidant response. Mice were treated as described in Figure 7A. Kidneys were excised from animals on day 3 or 7, and glomeruli were isolated from mouse kidneys by the magnetic beads–based approach. (A) Glomerular homogenates were prepared for immunoblot analysis for Nrf2, pGSK3β, GSK3β, podocin, WT-1, HO-1, and cleaved caspase 3. GAPDH served as a loading control. (B) Densitometric analyses of the expression of Nrf2, pGSK3β, podocin, WT-1, HO-1, and cleaved caspase 3 in glomeruli, presented as relative levels normalized to GAPDH levels. *P<0.05 versus ADR+SB-L or ADR+SB on day 3. #P<0.05 versus ADR+SB-L or ADR+SB on day 7. (n=6). (C) Glomerular homogenates were prepared for immunoblot analysis for Nrf2 and cleaved caspase 3. GAPDH served as a loading control. (D) Densitometric analyses of the expression of Nrf2 and cleaved caspase 3 in glomeruli, presented as relative levels normalized to GAPDH levels. *P<0.05 versus ADR+vehicle or ADR+SB+Trig. #P<0.05 versus ADR+vehicle or ADR+SB+Trig. (n=6). (E) Kidney cryosections were prepared for dual color immunofluorescence staining for Nrf2 (green) and WT-1 (red) to demonstrate the nuclear accumulation of Nrf2 in glomerular podocytes (indicated by arrowheads), which was enhanced by SB216763 treatment and abolished by Trig treatment. Scale bar, 20 μm.
Genetic and Pharmacologic Targeting of GSK3β in Podocytes Protects against Podocyte Injury and Proteinuria in NTS Nephritis via an Nrf2-Dependent Mechanism
To test if the Nrf2-mediated podocyte protective effect of GSK3β inhibition could be generalized to other types of glomerular disease, the murine model of NTS-induced heterologous phase proteinuria and podocytopathy was used (Figures 9 and 10). After injection of the GBM-reactive rabbit anti-mouse glomerular lysate NTS, control transgenic mice (Figure 9) or WT mice (Figure 10) developed a drastic increase in proteinuria within 24 hours that persisted for more than 48 hours (Figures 9, A and B, and 10A), concomitant with prominent linear deposition of the GBM-reactive IgG along glomerular capillary loops (Figures 9C and 10C), whereas injection of control IgG had no apparent effect. The NTS-induced proteinuria was paralleled by evident podocytopathy, characterized by a variable degree of foot process effacement (Figures 9D and 10D) on electron microscopy, which was assessed by measurement of the width of foot processes (Figures 9F and 10G); podocytopenia, as evidenced by WT-1 staining (Figures 9E and 10E) and absolute count of WT-1–positive podocytes in each glomerulus (Figures 9G and 10H); and loss of glomerular expression of podocyte markers, such as synaptopodin, as determined by immunoblot analysis of isolated glomeruli (Figures 9H and 10B).
Figure 9.
Podocyte-specific ablation of GSK3β potentiates the Nrf2 antioxidant response and ameliorates proteinuria and podocyte injury in NTS nephritis. (A) KO and control (Con) male mice aged 10–12 weeks were treated with a tail-vein injection of control rabbit IgG or GBM-reactive rabbit anti-mouse glomerular lysate NTS at a dose of 10 µl/g body wt to induce the heterologous phase proteinuria and podocyte injury. KO mice received an intraperitoneal injection of Trig (1 mg/kg) or vehicle 1 hour before NTS injection and afterward every other day. Spot urine was collected at the indicated time points, and an aliquot (10 µl) was subjected to SDS-PAGE followed by Coomassie brilliant blue staining. BSA (1, 2, and 4 µg) served as standard controls. (B) Quantification of urine albumin levels adjusted by urine creatinine concentrations. The data are expressed as mean±SEM and were subjected to logarithmic transformation and analyzed by repeated-measures ANOVA followed by post hoc Scheffé test. The test for a difference in urine albumin-to-creatinine ratios over time was significant (F[3, 75]=76.797; P<0.01). The test for equality of treatment means over time was also significant (F[4, 25]=46.532; P<0.01). *P<0.05 versus control+IgG, KO+IgG, or KO+NTS; #P<0.05 versus KO+NTS (n=6). (C) Representative micrographs of immunofluorescence staining of kidney specimens procured on day 3 for the GBM-reactive heterologous rabbit IgG in glomeruli. Scale bar, 20 μm. (D) Electron microscopy of podocyte ultrastructural injury in kidney tissues procured from mice on day 3. Arrowheads indicate lesions of podocyte foot process effacement. Scale bar, 2 μm. (E) Cryosections of kidney specimens were prepared for dual color immunofluorescence staining for Nrf2 (green) and WT-1 (red) to demonstrate Nrf2 nuclear accumulation of in-glomerular podocytes (indicated by arrowheads). Scale bar, 20 μm. (F) Measurement of the width of podocyte foot processes (FP) on electron microscopy in mice 3 days after NTS injury. *P<0.05 versus control mice with NTS nephritis or Trig-treated KO mice with NTS nephritis (n=6). (G) Absolute counts of the number of WT-1–positive podocytes in each glomerulus in kidney specimens procured on day 3, as shown by fluorescent immunohistochemistry staining for WT-1 in E. *P<0.05 versus control mice with NTS nephritis or Trig-treated KO mice with NTS nephritis (n=6). (H) Kidneys were excised from animals on day 3, and glomeruli were isolated by the magnetic beads–based approach. Glomerular homogenates were prepared for immunoblot analysis for Nrf2, HO-1, and synaptopodin. GAPDH served as a loading control.
Figure 10.
Blockade of GSK3β by SB216763 in murine models of nephrotoxic serum nephritis reinforces the Nrf2 antioxidant response in podocytes and protects against podocytopathy and proteinuria. WT FVB/NJ male mice aged 8 weeks were treated with a tail-vein injection of control rabbit IgG or GBM-reactive rabbit anti-mouse glomerular lysate NTS at a dose of 10 µl/g body wt. Mice received a subcutaneous injection of SB216763 (1.5 mg/kg) or vehicle 1 hour before NTS or control IgG injection and afterward every the other day. (A) Quantification of urine albumin levels adjusted by urine creatinine concentrations. The data are expressed as mean±SEM and were subjected to logarithmic transformation and analyzed by repeated-measures ANOVA followed by post hoc Scheffé test. The test for a difference in urine albumin-to-creatinine ratios over time was significant (F[3, 45]=61.061; P<0.01). The test for equality of treatment means over time was also significant (F[2, 15]=45.082; P<0.01). *P<0.05 versus NTS+vehicle (n=6). (B) Kidneys were excised from animals on day 3, and glomeruli were isolated by the magnetic beads–based approach. Glomerular homogenates were prepared for immunoblot analysis for Nrf2, HO-1, pGSK3β, GSK3β, and synaptopodin. GAPDH served as a loading control. (C) Representative micrographs of immunofluorescence staining of kidney specimens procured on day 3 for the GBM-reactive heterologous rabbit IgG in glomeruli. Scale bar, 20 μm. (D) Electron microscopy (EM) of podocyte ultrastructural injury in kidney tissues procured from mice on day 3. Arrowheads indicate lesions of podocyte foot process effacement. Scale bar, 2 μm. (E) Cryosections of kidney specimens were prepared for dual color immunofluorescence staining for Nrf2 (green) and WT-1 (red) to demonstrate Nrf2 nuclear accumulation in glomerular podocytes (indicated by arrowheads). Scale bar, 20 μm. (F) Representative micrographs demonstrate PAS staining of mouse kidneys. Scale bar, 10 μm. (G) Measurement of the width of podocyte foot processes (FP) on electron microscopy in mice 3 days after NTS injury. *P<0.05 versus vehicle-treated mice with NTS nephritis (n=6). (H) Absolute counts of the number of WT-1–positive podocytes in each glomerulus in kidney specimens procured on day 3, as shown by fluorescent immunohistochemistry staining for WT-1 in E. *P<0.05 versus vehicle-treated mice with NTS nephritis (n=6). (I) Morphometric scoring of glomerular damage on PAS staining of kidney tissues procured from mice on day 3. *P<0.05 versus vehicle-treated mice with NTS nephritis (n=6).
The NTS injury triggered a mild level of Nrf2 induction and activation in glomeruli, as estimated by immunoblot analysis of isolated glomeruli for Nrf2 and HO-1 (Figures 9H and 10B). The induced Nrf2 expression partially colocalized with the podocyte nuclear marker WT-1, as revealed by dual color fluorescent immunohistochemistry staining (Figures 9E and 10E), denoting Nrf2 nuclear accumulation and activation in glomerular podocytes. In contrast, KO mice developed much less proteinuria (Figure 9, A and B) and exhibited an ameliorated podocytopathy upon NTS injury (Figure 9, D–G), despite equal amounts of glomerular deposition of GBM-reactive IgG (Figure 9C). This was associated with elevated glomerular expression of Nrf2 and HO-1 (Figure 9H) and an increased Nrf2 nuclear accumulation in podocytes (Figure 9E), which was seemingly responsible for the podocyte protective effect, because selective blockade of Nrf2 by Trig abolished the Nrf2 response in KO mice (Figure 9, E and H) and abrogated the protection against proteinuria (Figure 9, A and B) and podocytopathy (Figure 9, D–G). Similarly, in WT mice, SB216763 treatment restored GSK3β inhibitory phosphorylation after NTS injury (Figure 10B), attenuated proteinuria (Figure 10A), ameliorated podocytopathy (Figure 10, B, D–H) and improved glomerular injury, marked by hypercellularity of the glomeruli with segmental necrosis (Figure 10, F and I). These beneficial effects were concomitant with elevated glomerular expression of Nrf2 and HO-1 (Figure 10B) and an increased Nrf2 nuclear accumulation in podocytes (Figure 10E).
Discussion
Unlike other types of cells constituting the nephron, podocytes have a very limited capacity for cell division, self-renewal, and repopulation.35–37 Podocytes are terminally differentiated postmitotic cells similar to neurons; nevertheless, podocytes withstand constant oxidative stress incurred by numerous energy-intensive cellular processes.38 Thus, the fate of podocytes entirely relies on antistress measures for self-protection. Antioxidant response is a key antistress mechanism exploited by diverse mammalian cells and plays an important role in sustaining podocyte homeostasis. Indeed, free radicals and ROS are responsible for podocyte injury and cytoskeleton disorganization in various models of podocytopathy.9,39,40 Central to the antioxidant response is the transcription and production of a battery of antioxidant and detoxifying enzymes capable of eliminating free radicals and ROS.10 This process is universally driven by Nrf2, thus providing biologic plausibility for potentiating the endogenous antioxidant response for self-protection.10
The benefits of Nrf2 signaling in human kidney disease were initially recognized in patients with cancer treated with bardoxolone, a potent Nrf2 inducer.41 Subsequent clinical trials demonstrated that bardoxolone is effective in improving kidney function in patients with diabetes and advanced-stage CKD.12 However, this effect was lately found to be associated with elevated albuminuria, excess serious adverse effects, and mortality caused by heart failure and cardiovascular events, which prematurely terminated the phase 3 large-scale, long-term, randomized controlled trial of bardoxolone (Bardoxolone Methyl Evaluation in Patients with Chronic Kidney Disease and Type 2 Diabetes Mellitus: the Occurrence of Renal Events) in patients with diabetic kidney disease.42 Of note, the Nrf2 cellular defense pathway could be modulated via two mechanisms: the Keap1-dependent and the Keap1-independent regulations.43 Bardoxolone and many other synthetic Nrf2 agonists activate Nrf2 indirectly through inhibiting Keap1, the intrinsic Nrf2 repressor that sequesters Nrf2 in the cytoplasm and facilitates its ubiquitination and proteasomal degradation.44 The augmented constitutive activity of Nrf2 after Keap1 inhibition, however, seems detrimental. In support of this, Keap1-deficient mice do not survive >3 weeks postnatally.45 In Keap1-deficient cells, the inducibility of the Nrf2-driven cell defense genes was hugely blunted upon stress, despite an elevated Nrf2 activity and increased transcription of antioxidant genes under basal conditions.45 Moreover, in a murine model of podocyte depletion and FSGS, genetic knockdown of Keap1 promoted the constitutive Nrf2 activity, attenuated glomerulosclerosis, but failed to diminish proteinuria,46 recapitulating the drawbacks of the Keap1 inhibitor bardoxolone observed in humans.47 With these findings taken together, it seems that blocking Keap1 potentiates the basal rather than the stress-inducible Nrf2 activation and incurs considerable adverse effects. Thus, it is imperative to develop other strategies targeting the Keap1-independent instead of Keap1-dependent Nrf2 regulatory pathways to harness the Nrf2 antioxidant response and cellular self-defense.
The Keap1-independent Nrf2 regulation involves a multitude of cell signaling cascades, including PI3K-Akt pathway and mitogen-activated protein kinase–Erk.43 Among these regulatory signaling, GSK3β has emerged as the convergent point.43 Recent evidence suggests that Nrf2 protein encompasses multiple GSK3β phosphorylation consensus motifs, entailing Nrf2 as a cognate substrate of GSK3β.21,24 Indeed, GSK3β is able to directly catalyze Nrf2 phosphorylation in the Neh6 region, which in turn enables its nuclear exit, ubiquitination, and ensuing proteasomal degradation.21,48 Alternatively, GSK3β can phosphorylate Nrf2 indirectly through Fyn.49 After phosphorylation by GSK3β, Fyn could translocate into nuclei, catalyze the phosphorylation of nuclear Nrf2, and thus facilitate Nrf2 nuclear export and degradation to switch off the Nrf2-mediated antioxidant response.49 The GSK3β-directed regulation of Nrf2 represents a key mechanism of control of the magnitude and duration of the stress elicited Nrf2 antioxidant response at a delayed/late phase following insults50 and has become an appealing therapeutic target.43 Consistent with this, inhibition of GSK3β enhances the Nrf2 antioxidant response upon injury in multiple organ systems.22–27 For instance, patients with chronic hepatitis C who received long-term treatment with lithium carbonate, a typical inhibitor of GSK3β, primarily for concomitant psychiatric disorders, exhibited much less liver injury, associated with enhanced hepatic Nrf2 activity.24 In agreement, in this study, inhibition of GSK3β by genetic targeting or by a pharmacologic inhibitor potentiated the inducibility of the Nrf2 antioxidant response in podocytes upon doxorubicin or NTS injury, ameliorated podocytopathy and glomerular injury, and attenuated proteinuria. Whether targeting the Keap1-dependent Nrf2 regulatory pathway, by maneuvers such as bardoxolone or Keap1 knockdown, also protects against doxorubicin nephropathy and NTS nephritis merits further investigation. Of note, as a multitasking signaling transducer, GSK3β may target other pathways besides Nrf2. Indeed, other mechanisms, such as suppression of proinflammatory NFκB activation and desensitization of mitochondria permeability transition, have been implicated, at least in part, in the podocyte protective effect following GSK3β inhibition.32,51
In summary, targeted inhibition of GSK3β in podocytes via conditional KO or pharmacologic blockade reinforced the Nrf2 antioxidant response after doxorubicin or NTS insult, alleviated podocytopathy and glomerular injury and attenuated proteinuria. Our study suggests that the GSK3β-regulated Nrf2 cell defense pathway in glomerular podocytes might serve as a novel and feasible therapeutic target for proteinuric glomerulopathies.
Concise Methods
Animal Studies
Animal studies were approved by the institution’s Animal Care and Use Committee, and they conformed to the U.S. Department of Agriculture regulations and the National Institutes of Health's "Guide for Human Care and Use of Laboratory Animals."
Generation of Mice with Doxycycline Inducible Podocyte-Specific GSK3β KO and Genotyping
Mice with the floxed GSK3β gene (GSK3βfl/+) were kindly provided by Dr. James Woodgett (The Lunenfeld-Tanenbaum Research Institute of Mount Sinai Hospital, Toronto, ON) and had been generated by introducing loxP sites upstream and downstream of exons 2 on a genetic background of C57Bl/6.52,53 GSK3βfl/+ mice were backcrossed for ten generations into a congenic FVB/N background. tetO-Cre mice on a genetic background of FVB/N (Jackson Laboratory, Bar Harbor, ME) contain a Cre recombinase cassette under control of the tet-operator. NPHS2 [nephrosis 2, idiopathic, steroid-resistant (podocin)]-rtTA mice on a genetic background of FVB/N (Jackson Laboratory) produce the rtTA protein under control of an NPHS2 promoter fragment. To generate doxycycline-inducible podocyte-specific GSK3β KO mice (rtTA/Cre/GSK3βfl/fl), GSK3βfl/+ mice were crossed, respectively, with tetO-Cre mice and NPHS2-rtTA mice. Then, Cre/GSK3βfl/+ mice were crossed with rtTA/GSK3βfl/+ mice. All littermates lacking the Cre transgene were designated as control mice. A routine PCR protocol was used for genotyping tail DNA samples with the following primer pairs: rtTA transgene genotyping (sense: 5′-GAACAACGCC AAGTCATTCCG-3′; antisense: 5′-TACGCAGCCCAGTGTAAAGTG-G-3′, 196-bp fragment); Cre transgene genotyping (sense: 5′-AGGTGTAGAGAAGGCACTTAGC-3′; antisense: 5′-CTAATCGCCATCTTCCAGCAGG-3′, 411-bp fragment); GSK3β genotyping (sense: 5′-GGGGCAACCTTAA TTTCATT-3′; antisense: 5′-GTGTCTGTATAACTGACT TCCTGTGGC-3′, which yielded 685- and 585-bp bands, respectively, for the floxed and WT alleles. At 8 weeks old, mice received doxycycline hydrochloride (TCI, Tokyo, Japan) treatment via drinking water (2 mg/ml with 5% sucrose, protected from light) for 14 days to induce podocyte-specific GSK3β deletion.
Animal Models of Podocyte Injury and Proteinuria
Murine Models of Doxorubicin Nephropathy
The mouse model of doxorubicin nephropathy was established by a single tail-vein injection of doxorubicin. Different genetic backgrounds and strains of mice displayed significant divergences in response to doxorubicin injury. At the standard dose of doxorubicin (8–12 mg/kg body wt) that has been documented to induce podocytopathy and nephrotic range proteinuria in the doxorubicin-sensitive BALB/c mice, none of the transgenic or WT mice with FVB/N backgrounds developed massive proteinuria or glomerular injury. Subsequently, several doses were tested (15, 20, 25, and 30 mg/kg) before the dose of 25 mg/kg was chosen. From days 0–5, mice were given two daily intraperitoneal injections of 1 ml of a glucose-electrolyte solution to prevent weight loss due to low appetite.
KO and control male mice were randomly assigned to the following groups to receive different treatments: (1) control group: Control mice received saline (0.1 ml) treatment as a single tail-vein injection; (2) KO group: KO mice received saline (0.1 ml) treatment as a single tail-vein injection; (3) control+doxorubicin group: Control mice were treated with doxorubicin (25 mg/kg) via a single tail-vein injection; (4) KO+doxorubicin group: KO mice were treated with doxorubicin (25 mg/kg) via a single tail-vein injection; and (5) KO+doxorubicin+Trig group: One hour after an intraperitoneal injection of Trig (1 mg/kg), KO mice were treated with doxorubicin (25 mg/kg) via a single tail-vein injection and afterward received an intraperitoneal injection of Trig (1 mg/kg) every other day.
WT FVB/NJ male mice aged 8 weeks were randomly assigned to the following groups to receive different treatments: (1) Control group: Mice received saline (0.1 ml) treatment as a single tail-vein injection; (2) doxorubicin group: Mice were treated with doxorubicin (25 mg/kg) via a single tail-vein injection; (3) doxorubicin+SB group: One hour after a subcutaneous injection of SB216763 (1.5 mg/kg), mice were treated with doxorubicin (25 mg/kg) via a single tail-vein injection and afterward received a subcutaneous injection of SB216763 (1.5 mg/kg) every other day; (4) doxorubicin+SB-L group: One hour after a subcutaneous injection of low-dose SB216763 (0.6 mg/kg), mice were treated with doxorubicin (25 mg/kg) via a single tail-vein injection and afterward received a subcutaneous injection of low-dose SB216763 (0.6 mg/kg) every other day; and (5) doxorubicin+SB+Trig group: One hour after a subcutaneous injection of SB216763 (1.5 mg/kg) and an intraperitoneal injection of Trig (1 mg/kg), mice were treated with doxorubicin (25 mg/kg) via a single tail-vein injection and afterward received a subcutaneous injection of SB216763 (1.5 mg/kg) and an intraperitoneal injection of Trig (1 mg/kg) every other day. Spot urine was collected at indicated time. All mice were euthanized 3 or 7 days after doxorubicin injury. Six mice were randomly assigned to each group for each observed time point.
Murine Models of NTS Nephritis
The GBM-reactive rabbit anti-mouse glomerular lysate NTS, courtesy of Drs. Chandra Mohan and Yong Du at the University of Houston, was generated by Lampire Laboratories (Pipersville, PA) as elaborated elsewhere.54 NTS is known to have reactivity to podocyte antigen in addition to the GBM.55 KO and control male mice aged 10–12 weeks were treated with a tail-vein injection of control rabbit IgG (Sigma-Aldrich, St. Louis, MO) or NTS at a dose of 10 µl/g body wt to induce the heterologous phase proteinuria and podocyte injury. KO mice received an intraperitoneal injection of Trig (1 mg/kg) or vehicle 1 hour before NTS injection and afterward every other day. WT FVB/NJ male mice aged 8 weeks were treated with a tail-vein injection of NTS or control rabbit IgG at a dose of 10 µl/g body wt. Mice received a subcutaneous injection of SB216763 (1.5 mg/kg) or vehicle 1 hour before NTS or control IgG injection and afterward every other day. Spot urine was collected at indicated time. Six mice were randomly assigned to each group. All mice were euthanized 3 days after NTS or control IgG injection.
Glomerular Isolation
Glomerular isolation was carried out as described elsewhere.32 In brief, mice were anesthetized and perfused by infusing the abdominal artery with 5 ml of PBS containing 8×107 Dynabeads M-450 (Dynal Biotech ASA, Oslo, Norway). After perfusion, the kidneys were removed and cut into 1-mm3 pieces and digested in collagenase A (1 mg/ml, Sigma-Aldrich) at 37°C for 30 minutes with gentle shaking. The tissue was pressed gently through a 100-μm cell strainer (BD Falcon, Bedford, MA), and glomeruli containing Dynabeads were then gathered using a magnetic particle concentrator. An aliquot (1:1500) of the glomerular isolate was visualized under a microscope to ensure that the sample contained fewer than five tubular fragments per ×200 field. Most isolated glomeruli (80%) were decapsulated, which was similar to what had been reported previously.56 During the procedure, kidney tissues were kept at 4°C except for the collagenase digestion at 37°C.56,57
Primary Culture of Podocytes and Transient Transfection
The enriched glomeruli were plated on collagen type I–coated dishes at 37°C in RPMI 1640 medium (Life Technologies, Grand Island, NY) with 10% FBS (Life Technologies), 0.075% sodium pyruvate (Sigma-Aldrich), 100 U/ml penicillin, and 100 μg/ml streptomycin (Life Technologies) in a humidified incubator with 5% CO2. Subculture of primary podocytes was performed by detaching the glomerular cells with 0.25% trypsin-EDTA (Invitrogen, Carlsbad, CA), followed by sieving through a 40-μm cell strainer (BD Falcon) and culture on collagen type I–coated dishes as reported before.58 Podocytes of passages 1 or 2 were characterized by the expression of multiple podocyte-specific markers and used in all experiments. Confluent primary podocytes were pretreated with SB216763 (10 μmol/L, Tocris Bioscience, Bristol, United Kingdom), tBHQ (20 μmol/L, Sigma-Aldrich), Trig (30 μmol/L, Sigma-Aldrich) for 30 minutes, followed by doxorubicin (0.25 μg/ml, Sigma-Aldrich) injury for 12, 24, or 48 hours. As control, cells were treated with vehicle. The expression vectors encoding WT GSK3β, S9A, or KD mutants of GSK3β were provided by Dr. Johnson (Birmingham, AL). Podocyte transfection was carried out using Lipofectamine 2000 (Life Technologies) as previously described.32 In brief, primary podocytes were cultured at 50%–70% confluence in the absence of antibiotics. The vector–Lipofectamine 2000 complexes were prepared and applied to proliferating podocytes for transfection.59 The ratio of Lipofectamine 2000 to vectors was optimized by a series of pilot experiments for each study until the best transfection efficiency was achieved. After transfection, the cells were in normal growth medium for 48 hours before transfection efficiency was assessed by immunocytochemistry staining or immunoblot analysis for target molecules. Transfection efficiency in the primarily cultured podocytes typically ranged from 30% to 60% and varied between different experimental batches of primary podocytes depending on the condition of the cultures. The batch of cultures with transfection efficiency >50% was subsequently used for indicated treatments.
Isolation of Mouse Renal Proximal Tubules
Mouse kidney cortices were dissected visually in ice-cold PBS solution and minced into 1 mm3 pieces as described elsewhere.60,61 Tissues were transferred to collagenase IV solution (1 mg/ml, Sigma-Aldrich) at 37°C and digested for 30 minutes with gentle shaking. After digestion, the supernatant was sieved through two sterile cell strainers with pore sizes of 250 μm and 80 μm (BD Falcon). The 80-μm cell strainer yielded a large number of long proximal tubule fragments without substantial contamination of other nephron segments or glomeruli. The longer proximal tubule fragments retained in the 80-μm cell strainer were resuspended by flushing the sieve in the reverse direction with warm PBS containing 1% BSA, washed, and collected for immunoblot analysis.
Urine Analyses
To discern the protein compositions in urine, equal amounts of urine samples were subjected to SDS-PAGE followed by Coomassie blue (Sigma-Aldrich) staining. Urine albumin concentration was measured using a mouse albumin ELISA quantitation kit (Bethyl Laboratories Inc., Montgomery, TX). Urine creatinine concentration was measured by a creatinine assay kit (BioAssay Systems, Hayward, CA).
Western Immunoblot Analysis
Cultured cells were lysed and isolated renal glomeruli were homogenized in radioimmunoprecipitation assay buffer supplemented with protease inhibitors, and samples were processed for immunoblot analysis. The antibodies against Nrf2, phosphorylated glycogen synthase kinase 3β at serine 9 (pGSK3β), glycogen synthase kinase 3α/β (GSK3α/β), HO-1, WT-1, podocin, and glyceraldehyde-3-phosphate dehydrogenase (GAPDH) were purchased from Santa Cruz Biotechnology (Santa Cruz, CA), those against synaptopodin were purchased from Santa Cruz Biotechnology and Progen Biotechnik GmbH (Heidelberg, Germany), and those against GSK3β and cleaved caspase 3 were acquired from Cell Signaling Technology (Danvers, MA).
RNA Extraction and Real-Time RT-PCR
Total RNA was extracted from isolated glomeruli using TRIzol solution (Invitrogen, Carlsbad, CA) according to the instructions specified by the manufacturer. RNA was then diluted to 3 mg/ml in RNase-free distilled water. First-strand cDNA was prepared using RNA Superscript real-time RT (Invitrogen), and oligo (dT) primer was used according to the manufacturer’s instructions. Real-time PCR was performed in an Stratagene Mx4000 multiplex quantitative PCR system (Stratagene, La Jolla, CA) using the SYBR Green PCR master Mix kit. The amplification signals in each experimental sample were analyzed using Stratagene Mx4000 software and were normalized to the endogenous GAPDH mRNA levels. The sequences of primers are as follows: for murine GSK3α sense: 5′-GTGGACCAGCTTGTGGAGAT-3′, antisense: 5′-CCTTGAGGATG GCGTGT ACT-3′; for murine GSK3β, sense: 5′-GGCAGCAAGGTAACCACAGT-3′, antisense: 5′-GATGG CAACCAGTTCTCCAG-3′; and for murine GAPDH, sense: 5′-CGAGATCCCT CCAAAATCAA-3′, antisense: 5′-TTCACACCCATGACGAACAT-3′.62
Immunofluorescence Staining
Podocytes or cryosections of kidneys were fixed with 4% paraformaldehyde (Sigma-Aldrich), permeabilized, and stained with primary antibodies against Nrf2, GSK3β, podocin, desmin, WT-1, and synaptopodin, followed by Alexa Fluor–conjugated secondary antibody staining (Life Technologies). Finally, cells were counterstained with propidium iodide or 4,6-diamidino-2-phenylindole, mounted with Vectashield mounting medium (Vector Laboratories, Burlingame, CA), and visualized using a fluorescence microscope (BX43, Olympus, Tokyo, Japan) or a Zeiss LSM710 Meta confocal microscope (Carl Zeiss AG, Cologne, Germany). As a negative control, the primary antibody was replaced by preimmune serum from the same species and no staining was noted. F-actin in podocytes was stained by rhodamine phalloidin (Cytoskeleton Inc., Denver, CO).
Renal Morphology Assessment and Immunohistochemistry Analysis
The paraffin-embedded formalin-fixed kidney tissues were prepared into 3-µm sections. For general histology, sections were processed for PAS staining. A semiquantitative glomerular damage score was used to evaluate the degree of glomerular injury, which was featured by one or more of the following histologic signs in the doxorubicin nephropathy model: podocytic swelling and vacuolization, glomerular synechiae, glomerular capillary congestion, collapse and/or obliteration accompanied by hyaline material and/or mesangial matrix expansion, or by histologic signs in the NTS nephritis model (glomerular hypercellularity, swelling of glomerular endothelial cells, occluded and distended glomerular capillaries filled with PAS-positive fibrinous material, or fibrinoid necrosis of glomerular capillary tufts). The morphologic features of all sections were assessed by a single observer in a blinded manner with the assistance of a renal pathologist. The severity of glomerular injury was graded from 0 to 3 as follows: 0, no glomerular lesions; 1, <25% of the glomerular area affected; 2, 25%–50% of the glomerular area affected; and 3, >50% of the glomerular area affected. A whole-kidney mean glomerular injury score was obtained by averaging scores from all glomeruli on one section.63
For electron microscopy, kidney cortical tissues were cut into small pieces (1 mm3), fixed with 2.5% glutaraldehyde, and embedded in Epon 812 (Polysciences Inc., Warrington, PA). Transmission electron micrographs were obtained using an EM-10 microscope (Zeiss) operated at 60 kV. Formalin-fixed tissues were subject to antigen retrieval before immunohistochemical staining. Immunoperoxidase staining was performed with a Vectastain ABC kit (Vector Laboratories) using different primary antibodies against GSK3α (sc-5264, Santa Cruz Biotechnology), GSK3β (9315, Cell Signaling Technology), and Nrf2 (sc-722, sc-30915, Santa Cruz Biotechnology). As a negative control, the primary antibody was replaced by preimmune IgG from the same species and no specific staining was noted. The specificity of immunohistochemistry staining was confirmed by preincubation of the kidney sections with control peptides or the blocking peptides specific for the primary antibodies.
Measurement of Cell Apoptosis
Fixed cells or kidney cryosections were subjected to TUNEL staining using a cell apoptosis detection kit (Roche Applied Science, Indianapolis, IN) according to the manufacturer’s instructions.
Statistical Analyses
For immunoblot analysis, bands were scanned and the integrated pixel density was determined using a densitometer and the ImageJ analysis program, version 1.48 (National Institutes of Health, Bethesda, MD). All in vitro studies and immunoblot analyses were repeated three to six times. All data are expressed as mean±SEM or as otherwise indicated. For analyses of urine albumin-to-creatinine ratios over time, data were subjected to logarithmic transformation and then analyzed by repeated-measures ANOVA, followed by the post hoc Scheffé test. One-way ANOVA tests were performed for multiple comparisons of values, followed by the Student–Newman–Keuls test if there were three comparisons or by the Scheffé test if there were more than three comparisons. Data from two groups were compared by t test. P<0.05 was considered to represent a statistically significant difference.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors are grateful to Dr. Chandra Mohan for providing the nephrotoxic serum.
This work was supported in part by the US National Institutes of Health grant R01DK092485 and the Natural Science Foundation of China, grants 81070574 and 81270807.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050565/-/DCSupplemental.
References
- 1.Mundel P, Shankland SJ: Podocyte biology and response to injury. J Am Soc Nephrol 13: 3005–3015, 2002 [DOI] [PubMed] [Google Scholar]
- 2.Haraldsson B, Jeansson M: Glomerular filtration barrier. Curr Opin Nephrol Hypertens 18: 331–335, 2009 [DOI] [PubMed] [Google Scholar]
- 3.Swiatecka-Urban A: Membrane trafficking in podocyte health and disease. Pediatr Nephrol 28: 1723–1737, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Pavenstädt H, Kriz W, Kretzler M: Cell biology of the glomerular podocyte. Physiol Rev 83: 253–307, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Kretzler M: Regulation of adhesive interaction between podocytes and glomerular basement membrane. Microsc Res Tech 57: 247–253, 2002 [DOI] [PubMed] [Google Scholar]
- 6.Akilesh S, Huber TB, Wu H, Wang G, Hartleben B, Kopp JB, Miner JH, Roopenian DC, Unanue ER, Shaw AS: Podocytes use FcRn to clear IgG from the glomerular basement membrane. Proc Natl Acad Sci U S A 105: 967–972, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Finkel T: Oxygen radicals and signaling. Curr Opin Cell Biol 10: 248–253, 1998 [DOI] [PubMed] [Google Scholar]
- 8.Chen S, Meng XF, Zhang C: Role of NADPH oxidase-mediated reactive oxygen species in podocyte injury. BioMed Res Int 2013: 839761, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Spurney RF, Coffman TM: Stressed-out podocytes in diabetes? J Am Soc Nephrol 19: 2035–2037, 2008 [DOI] [PubMed] [Google Scholar]
- 10.Nguyen T, Nioi P, Pickett CB: The Nrf2-antioxidant response element signaling pathway and its activation by oxidative stress. J Biol Chem 284: 13291–13295, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Motohashi H, Yamamoto M: Nrf2-Keap1 defines a physiologically important stress response mechanism. Trends Mol Med 10: 549–557, 2004 [DOI] [PubMed] [Google Scholar]
- 12.Pergola PE, Raskin P, Toto RD, Meyer CJ, Huff JW, Grossman EB, Krauth M, Ruiz S, Audhya P, Christ-Schmidt H, Wittes J, Warnock DG BEAM Study Investigators : Bardoxolone methyl and kidney function in CKD with type 2 diabetes. N Engl J Med 365: 327–336, 2011 [DOI] [PubMed] [Google Scholar]
- 13.Aleksunes LM, Goedken MJ, Rockwell CE, Thomale J, Manautou JE, Klaassen CD: Transcriptional regulation of renal cytoprotective genes by Nrf2 and its potential use as a therapeutic target to mitigate cisplatin-induced nephrotoxicity. J Pharmacol Exp Ther 335: 2–12, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ruiz S, Pergola PE, Zager RA, Vaziri ND: Targeting the transcription factor Nrf2 to ameliorate oxidative stress and inflammation in chronic kidney disease. Kidney Int 83: 1029–1041, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Niture SK, Kaspar JW, Shen J, Jaiswal AK: Nrf2 signaling and cell survival. Toxicol Appl Pharmacol 244: 37–42, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Uruno A, Yagishita Y, Yamamoto M: The Keap1-Nrf2 system and diabetes mellitus. Arch Biochem Biophys 566: 76–84, 2015 [DOI] [PubMed] [Google Scholar]
- 17.Alam J, Stewart D, Touchard C, Boinapally S, Choi AM, Cook JL: Nrf2, a Cap’n’Collar transcription factor, regulates induction of the heme oxygenase-1 gene. J Biol Chem 274: 26071–26078, 1999 [DOI] [PubMed] [Google Scholar]
- 18.Lee SE, Jeong SI, Yang H, Park CS, Jin YH, Park YS: Fisetin induces Nrf2-mediated HO-1 expression through PKC-δ and p38 in human umbilical vein endothelial cells. J Cell Biochem 112: 2352–2360, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Brown J, Martin M: Glycogen synthase kinase 3: A point of convergence for the host inflammatory response. Cytokine 53: 130–140, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cohen P, Frame S: The renaissance of GSK3. Nat Rev Mol Cell Biol 2: 769–776, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Salazar M, Rojo AI, Velasco D, de Sagarra RM, Cuadrado A: Glycogen synthase kinase-3beta inhibits the xenobiotic and antioxidant cell response by direct phosphorylation and nuclear exclusion of the transcription factor Nrf2. J Biol Chem 281: 14841–14851, 2006 [DOI] [PubMed] [Google Scholar]
- 22.Tomobe K, Shinozuka T, Kuroiwa M, Nomura Y: Age-related changes of Nrf2 and phosphorylated GSK-3β in a mouse model of accelerated aging (SAMP8). Arch Gerontol Geriatr 54: e1–e7, 2012 [DOI] [PubMed] [Google Scholar]
- 23.Bitar MS, Al-Mulla F: A defect in Nrf2 signaling constitutes a mechanism for cellular stress hypersensitivity in a genetic rat model of type 2 diabetes. Am J Physiol Endocrinol Metab 301: E1119–E1129, 2011 [DOI] [PubMed] [Google Scholar]
- 24.Jiang Y, Bao H, Ge Y, Tang W, Cheng D, Luo K, Gong G, Gong R: Therapeutic targeting of GSK3β enhances the Nrf2 antioxidant response and confers hepatic cytoprotection in hepatitis C. Gut 64: 168–179, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rojo AI, Rada P, Egea J, Rosa AO, López MG, Cuadrado A: Functional interference between glycogen synthase kinase-3 beta and the transcription factor Nrf2 in protection against kainate-induced hippocampal cell death. Mol Cell Neurosci 39: 125–132, 2008 [DOI] [PubMed] [Google Scholar]
- 26.Espada S, Rojo AI, Salinas M, Cuadrado A: The muscarinic M1 receptor activates Nrf2 through a signaling cascade that involves protein kinase C and inhibition of GSK-3beta: Connecting neurotransmission with neuroprotection. J Neurochem 110: 1107–1119, 2009 [DOI] [PubMed] [Google Scholar]
- 27.Correa F, Mallard C, Nilsson M, Sandberg M: Activated microglia decrease histone acetylation and Nrf2-inducible anti-oxidant defence in astrocytes: Restoring effects of inhibitors of HDACs, p38 MAPK and GSK3β. Neurobiol Dis 44: 142–151, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Rojo AI, Sagarra MR, Cuadrado A: GSK-3β down-regulates the transcription factor Nrf2 after oxidant damage: Relevance to exposure of neuronal cells to oxidative stress. J Neurochem 105: 192–202, 2008 [DOI] [PubMed] [Google Scholar]
- 29.Coghlan MP, Culbert AA, Cross DA, Corcoran SL, Yates JW, Pearce NJ, Rausch OL, Murphy GJ, Carter PS, Roxbee Cox L, Mills D, Brown MJ, Haigh D, Ward RW, Smith DG, Murray KJ, Reith AD, Holder JC: Selective small molecule inhibitors of glycogen synthase kinase-3 modulate glycogen metabolism and gene transcription. Chem Biol 7: 793–803, 2000 [DOI] [PubMed] [Google Scholar]
- 30.Shih AY, Johnson DA, Wong G, Kraft AD, Jiang L, Erb H, Johnson JA, Murphy TH: Coordinate regulation of glutathione biosynthesis and release by Nrf2-expressing glia potently protects neurons from oxidative stress. J Neurosci 23: 3394–3406, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Hoeflich KP, Luo J, Rubie EA, Tsao MS, Jin O, Woodgett JR: Requirement for glycogen synthase kinase-3beta in cell survival and NF-kappaB activation. Nature 406: 86–90, 2000 [DOI] [PubMed] [Google Scholar]
- 32.Bao H, Ge Y, Peng A, Gong R: Fine-tuning of NFκB by glycogen synthase kinase 3β directs the fate of glomerular podocytes upon injury. Kidney Int 87: 1176–1190, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xu W, Ge Y, Liu Z, Gong R: Glycogen synthase kinase 3β dictates podocyte motility and focal adhesion turnover by modulating paxillin activity: Implications for the protective effect of low-dose lithium in podocytopathy. Am J Pathol 184: 2742–2756, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sun YB, Qu X, Zhang X, Caruana G, Bertram JF, Li J: Glomerular endothelial cell injury and damage precedes that of podocytes in adriamycin-induced nephropathy. PLoS One 8: e55027, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Patrakka J, Tryggvason K: New insights into the role of podocytes in proteinuria. Nat Rev Nephrol 5: 463–468, 2009 [DOI] [PubMed] [Google Scholar]
- 36.Shankland SJ: The podocyte’s response to injury: Role in proteinuria and glomerulosclerosis. Kidney Int 69: 2131–2147, 2006 [DOI] [PubMed] [Google Scholar]
- 37.Wharram BL, Goyal M, Wiggins JE, Sanden SK, Hussain S, Filipiak WE, Saunders TL, Dysko RC, Kohno K, Holzman LB, Wiggins RC: Podocyte depletion causes glomerulosclerosis: Diphtheria toxin-induced podocyte depletion in rats expressing human diphtheria toxin receptor transgene. J Am Soc Nephrol 16: 2941–2952, 2005 [DOI] [PubMed] [Google Scholar]
- 38.Müller-Deile J, Schiffer M: The podocyte power-plant disaster and its contribution to glomerulopathy. Front Endocrinol (Lausanne) 5: 209, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eid AA, Gorin Y, Fagg BM, Maalouf R, Barnes JL, Block K, Abboud HE: Mechanisms of podocyte injury in diabetes: Role of cytochrome P450 and NADPH oxidases. Diabetes 58: 1201–1211, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kerjaschki D: Caught flat-footed: Podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108: 1583–1587, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hong DS, Kurzrock R, Supko JG, He X, Naing A, Wheler J, Lawrence D, Eder JP, Meyer CJ, Ferguson DA, Mier J, Konopleva M, Konoplev S, Andreeff M, Kufe D, Lazarus H, Shapiro GI, Dezube BJ: A phase I first-in-human trial of bardoxolone methyl in patients with advanced solid tumors and lymphomas. Clin Cancer Res 18: 3396–3406, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.de Zeeuw D, Akizawa T, Audhya P, Bakris GL, Chin M, Christ-Schmidt H, Goldsberry A, Houser M, Krauth M, Lambers Heerspink HJ, McMurray JJ, Meyer CJ, Parving HH, Remuzzi G, Toto RD, Vaziri ND, Wanner C, Wittes J, Wrolstad D, Chertow GM BEACON Trial Investigators : Bardoxolone methyl in type 2 diabetes and stage 4 chronic kidney disease. N Engl J Med 369: 2492–2503, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bryan HK, Olayanju A, Goldring CE, Park BK: The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem Pharmacol 85: 705–717, 2013 [DOI] [PubMed] [Google Scholar]
- 44.Choi BH, Kang KS, Kwak MK: Effect of redox modulating NRF2 activators on chronic kidney disease. Molecules 19: 12727–12759, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wakabayashi N, Itoh K, Wakabayashi J, Motohashi H, Noda S, Takahashi S, Imakado S, Kotsuji T, Otsuka F, Roop DR, Harada T, Engel JD, Yamamoto M: Keap1-null mutation leads to postnatal lethality due to constitutive Nrf2 activation. Nat Genet 35: 238–245, 2003 [DOI] [PubMed] [Google Scholar]
- 46.Miyazaki Y, Shimizu A, Pastan I, Taguchi K, Naganuma E, Suzuki T, Hosoya T, Yokoo T, Saito A, Miyata T, Yamamoto M, Matsusaka T: Keap1 inhibition attenuates glomerulosclerosis. Nephrol Dial Transplant 29: 783–791, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Reisman SA, Chertow GM, Hebbar S, Vaziri ND, Ward KW, Meyer CJ: Bardoxolone methyl decreases megalin and activates nrf2 in the kidney. J Am Soc Nephrol 23: 1663–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Chowdhry S, Zhang Y, McMahon M, Sutherland C, Cuadrado A, Hayes JD: Nrf2 is controlled by two distinct β-TrCP recognition motifs in its Neh6 domain, one of which can be modulated by GSK-3 activity. Oncogene 32: 3765–3781, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Jain AK, Jaiswal AK: GSK-3beta acts upstream of Fyn kinase in regulation of nuclear export and degradation of NF-E2 related factor 2. J Biol Chem 282: 16502–16510, 2007 [DOI] [PubMed] [Google Scholar]
- 50.Niture SK, Jain AK, Shelton PM, Jaiswal AK: Src subfamily kinases regulate nuclear export and degradation of transcription factor Nrf2 to switch off Nrf2-mediated antioxidant activation of cytoprotective gene expression. J Biol Chem 286: 28821–28832, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 51.Wang Z, Bao H, Ge Y, Zhuang S, Peng A, Gong R: Pharmacological targeting of GSK3β confers protection against podocytopathy and proteinuria by desensitizing mitochondrial permeability transition. Br J Pharmacol 172: 895–909, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Patel S, Doble BW, MacAulay K, Sinclair EM, Drucker DJ, Woodgett JR: Tissue-specific role of glycogen synthase kinase 3beta in glucose homeostasis and insulin action. Mol Cell Biol 28: 6314–6328, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ge Y, Si J, Tian L, Zhuang S, Dworkin LD, Gong R: Conditional ablation of glycogen synthase kinase 3β in postnatal mouse kidney. Lab Invest 91: 85–96, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Du Y, An S, Liu L, Li L, Zhou XJ, Mason RP, Mohan C: Serial non-invasive monitoring of renal disease following immune-mediated injury using near-infrared optical imaging. PLoS One 7: e43941, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Chugh S, Yuan H, Topham PS, Haydar SA, Mittal V, Taylor GA, Kalluri R, Salant DJ: Aminopeptidase A: a nephritogenic target antigen of nephrotoxic serum. Kidney Int 59: 601–613, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Takemoto M, Asker N, Gerhardt H, Lundkvist A, Johansson BR, Saito Y, Betsholtz C: A new method for large scale isolation of kidney glomeruli from mice. Am J Pathol 161: 799–805, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Dai Y, Gu L, Yuan W, Yu Q, Ni Z, Ross MJ, Kaufman L, Xiong H, Salant DJ, He JC, Chuang PY: Podocyte-specific deletion of signal transducer and activator of transcription 3 attenuates nephrotoxic serum-induced glomerulonephritis. Kidney Int 84: 950–961, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Tian X, Kim JJ, Monkley SM, Gotoh N, Nandez R, Soda K, Inoue K, Balkin DM, Hassan H, Son SH, Lee Y, Moeckel G, Calderwood DA, Holzman LB, Critchley DR, Zent R, Reiser J, Ishibe S: Podocyte-associated talin1 is critical for glomerular filtration barrier maintenance. J Clin Invest 124: 1098–1113, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Gong R, Rifai A, Ge Y, Chen S, Dworkin LD: Hepatocyte growth factor suppresses proinflammatory NFkappaB activation through GSK3beta inactivation in renal tubular epithelial cells. J Biol Chem 283: 7401–7410, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zhao Y, Zhao H, Zhang Y, Tsatralis T, Cao Q, Wang Y, Wang Y, Wang YM, Alexander SI, Harris DC, Zheng G: Isolation and epithelial co-culture of mouse renal peritubular endothelial cells. BMC Cell Biol 15: 40, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Terryn S, Jouret F, Vandenabeele F, Smolders I, Moreels M, Devuyst O, Steels P, Van Kerkhove E: A primary culture of mouse proximal tubular cells, established on collagen-coated membranes. Am J Physiol Renal Physiol 293: F476–F485, 2007 [DOI] [PubMed] [Google Scholar]
- 62.Gong R, Morris DJ, Brem AS: Variable expression of 11beta Hydroxysteroid dehydrogenase (11beta-HSD) isoforms in vascular endothelial cells. Steroids 73: 1187–1196, 2008 [DOI] [PubMed] [Google Scholar]
- 63.Zhan M, Usman IM, Sun L, Kanwar YS: Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol 26: 1304–1321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.