Abstract
Renal interstitial fibrosis and interstitial active inflammation are the main histologic features of renal allograft biopsy specimens. Fibrosis is currently assessed by semiquantitative subjective analysis, and color image analysis has been developed to improve the reliability and repeatability of this evaluation. However, these techniques fail to distinguish fibrosis from constitutive collagen or active inflammation. We developed an automatic, reproducible Fourier–transform infrared (FTIR) imaging–based technique for simultaneous quantification of fibrosis and inflammation in renal allograft biopsy specimens. We generated and validated a classification model using 49 renal biopsy specimens and subsequently tested the robustness of this classification algorithm on 166 renal grafts. Finally, we explored the clinical relevance of fibrosis quantification using FTIR imaging by comparing results with renal function at 3 months after transplantation (M3) and the variation of renal function between M3 and M12. We showed excellent robustness for fibrosis and inflammation classification, with >90% of renal biopsy specimens adequately classified by FTIR imaging. Finally, fibrosis quantification by FTIR imaging correlated with renal function at M3, and the variation in fibrosis between M3 and M12 correlated well with the variation in renal function over the same period. This study shows that FTIR-based analysis of renal graft biopsy specimens is a reproducible and reliable label–free technique for quantifying fibrosis and active inflammation. This technique seems to be more relevant than digital image analysis and promising for both research studies and routine clinical practice.
Keywords: histopathology, interstitial fibrosis, acute rejection, chronic allograft nephropathy, renal transplantation, renal pathology
Chronic allograft injury (CAI) remains the primary cause of renal allograft failure, despite improvements in immunosuppressive strategies. These therapies are on the basis of calcineurin inhibition, which may also induce chronic allograft lesions. Clinical expression of CAI is defined by a slow decline in renal function, high BP, and proteinuria. Several histologic features characterize CAI, including interstitial fibrosis (IF) and tubular atrophy (TA), collectively known as interstitial fibrosis tubular atrophy (IFTA), as well as glomerulosclerosis and fibrointimal hyperplasia of the vessels.1,2 Consequently, renal graft biopsies are essential to establish accurate diagnosis and prognosis.
However, the assessment of some histologic features, such as IF (i.e., IFTA) and inflammation, shows considerable interobserver variation, making comparison between biopsies unreliable. Consequently, the proposed Banff classification may not be valuable for standardized routine diagnostic practice, especially for IF and inflammatory infiltrates, where agreement as assessed by the κ-coefficient was reportedly 0.3 and 0.34, respectively.3
Several techniques on the basis of digital image analysis (DIA) have been developed to improve the reproducibility and precision of IF quantification.4–8 However, these techniques on the basis of collagen staining by Masson trichrome or red Sirius or immunostaining present an important limitation, namely the confusion of constitutive and pathologic collagen. Moreover, no single technique is capable of quantifying IF and inflammation at the same time.
Fourier–transform infrared (FTIR) spectroscopy is a candidate technique to overcome these limitations. Infrared analysis probes the intrinsic chemical composition of the tissue and can detect subtle molecular alterations associated with a disease. Thus, infrared spectra can be considered as tissue–specific spectroscopic signatures, characteristic of the histologic status of the sample. Two-dimensional mapping of tissue samples makes it possible to build, without staining, biochemical spectral images with a spatial resolution of a few micrometers. Contrary to routine histopathologic techniques, spectral imaging can be performed directly on paraffin-embedded tissues, avoiding possible alterations induced by chemical dewaxing.9–13 Moreover, FTIR analysis can be totally automatized, thereby avoiding inherent operator variability. This technique has proven effective to discriminate between tumor and normal tissues14,15 and between benign and malignant lesions14,16 and also, identify drug resistance in cancer.17,18 Infrared spectroscopy has also been used to identify renal stones19,20 and characterize renal tumor tissues.21 Nonetheless, to the best of our knowledge, no study has been published to date showing the possible contribution of infrared imaging to the characterization of nontumor renal tissue, particularly in terms of IF and inflammation.
In this retrospective study, we had two objectives. First, we assessed the ability of FTIR microimaging to classify fibrosis and inflammation regions in renal biopsies using a prediction model on the basis of linear discriminant analysis (LDA). Second, the classification model was further used to quantify these regions of interest and investigate the existence of a relation between the measurements thus obtained and clinical parameters.
Results
FTIR Classification Robustness Assessment
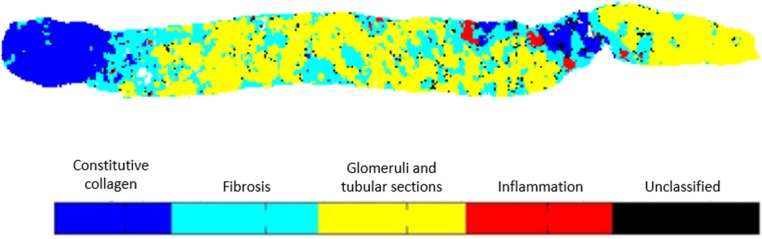
Because principal components analysis (PCA) was performed to reduce the data dimensionality, the first step in the construction of the prediction model was to determine the optimal number of principal components (PCs) to be used as inputs of the LDA model. Different models were constructed with the number of PCs varying from four to 20 (data not shown). During the validation phase, the optimal prediction accuracy was obtained with 15 PCs. Using this configuration, the LDA model aimed to classify each image pixel into one of four classes, namely constitutive collagen, fibrosis, glomeruli and tubular sections, or inflammation as seen in Figure 1. In addition to these spectral classes assigned to specific tissue structures, a fifth class was dedicated to unclassified pixels, of which there remained very few. A color code was used to highlight the tissue organization.
Figure 1.
Construction of a spectral image by pixel classification into five classes. Each 25×25-μm side pixel has an infrared spectrum that is classified by the LDA model into one of four defined classes (constitutive collagen, fibrosis, glomeruli and tubular sections, and inflammation). The pixels not belonging to any of these four classes are grouped in a fifth class under the heading unclassified.
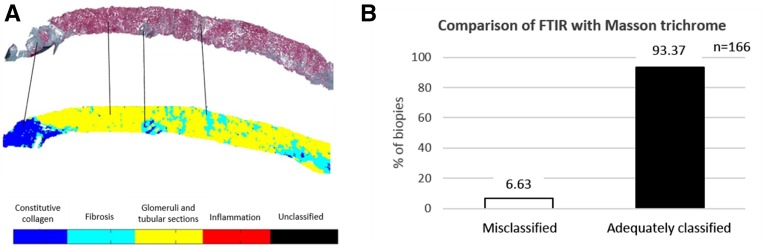
The quality of FTIR classification was determined by comparing the LDA images with Masson trichrome staining for the sample test set composed of 166 renal graft biopsies (Figure 2). For this step, three pathologists blindly examined for each biopsy the correspondence between the FTIR classification image and its serial Masson trichrome–stained sections. In total, 93.37% (155) of biopsies were correctly classified with regard to the reference standard method (Figure 2). Eleven biopsies (6.63%) were misclassified by FTIR analysis. The misclassification corresponded to patients with fixation problems precluding correct morphologic examination or a loss of material during section preparation. To further validate the qualitative assessment of fibrosis classification by FTIR, we also performed a comparison between FTIR images and second harmonic generation signal (details are provided in Supplemental Material, Supplemental Figure 2).
Figure 2.
Assessment of the robustness of classification by FTIR using the LDA model. (A) Fibrosis and constitutive collagen classes on the FTIR image of each biopsy from the test dataset were blindly compared with Masson trichrome–stained serial sections evaluated by three observers; (B) 93.7% of biopsies were adequately classified, whereas only 6.63% were misclassified.
These results showed that FTIR imaging combined with LDA data processing is an effective technique to discriminate fibrosis and constitutive collagen in contrast to other techniques using DIA.
Quality of Fibrosis Quantification by FTIR
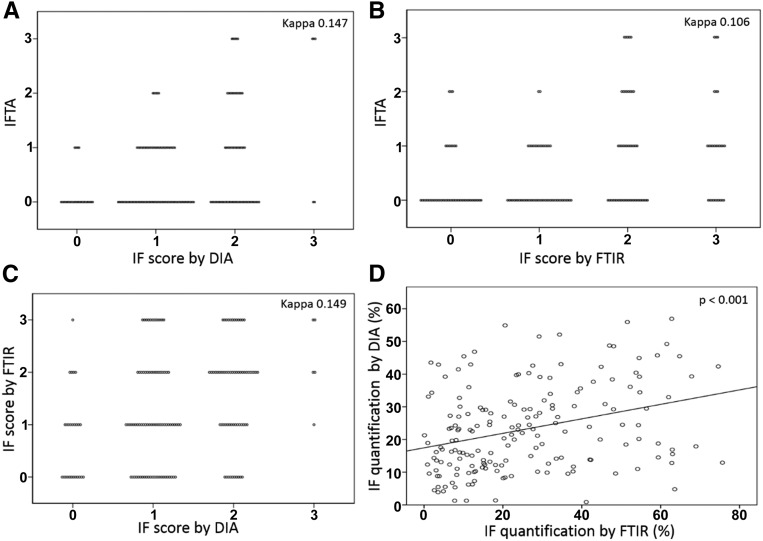
Three methods of IF quantification in renal graft biopsies were compared: (1) IF assessment by a pathologist (IFTA score) according to the Banff classification, (2) colorimetric quantification of IF on the basis of Masson trichrome staining computed by color deconvolution treatment, and (3) FTIR quantification of fibrosis on the basis of FTIR imaging combined with the LDA classification model developed earlier. To compare IFTA scoring (with zero, one, two, or three grades) with two other techniques of IF quantification, we established a score for DIA and infrared IF quantification. This score was on the basis of the Banff grading with regards to the percentage of the biopsy area occupied by fibrosis: 0<10%; 1= between 10% and 25%; 2= between 25% and 50%; and 3>50%. We found that the IFTA score did not show good agreement with the DIA score (κ=0.15) (Figure 3A) or the FTIR score (κ=0.11) (Figure 3B). Moreover, FTIR and DIA techniques of automated quantification of IF revealed mismatched scoring (κ=0.15) (Figure 3C), whereas the relative areas occupied by IF as measured by DIA and FTIR quantification were well correlated (R=0.323; P<0.001). However, although the results of these two techniques were correlated, the points on the graph appeared quite dispersed (Figure 3D). This dispersion seems to be related to the difference in section thickness and staining variability, sometimes with high or weak color saturation on Masson trichrome staining.
Figure 3.
Comparison of the IF score and quantification by three techniques. The IF score as estimated by visual inspection according to the Banff classification (IFTA) was compared with the IF score as quantified by (A) DIA or (B) FTIR using the LDA model. Score thresholds were homogenized for all techniques (0<10%; 1=10%–25%; 2=25%–50%; and 3>50%). (C) The IF score by DIA was compared with the IF score as quantified by FTIR, showing poor agreement between these two techniques, whereas (D) IF quantification (percentage) by DIA was correlated with IF quantification by FTIR using the LDA model (P<0.001).
Clinical Relevance of Fibrosis Quantification by FTIR
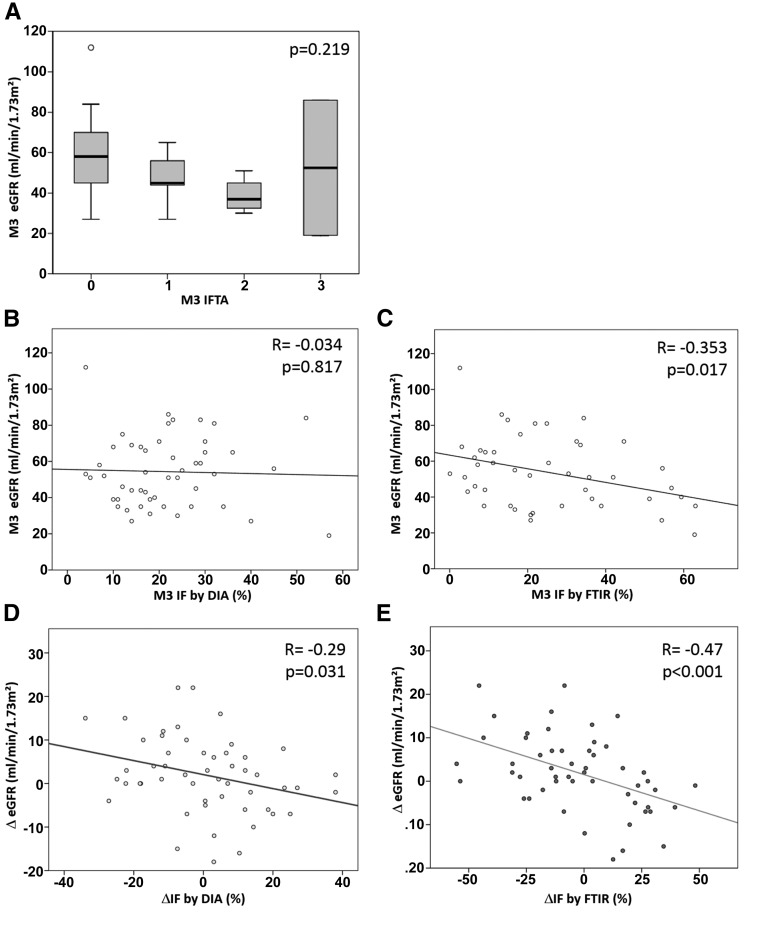
Considering FTIR as a good automated quantification technique for IF, we tested the clinical significance of FTIR IF quantification versus eGFR. For this purpose, we analyzed protocol renal graft biopsies performed at 3 months (M3) and M12 after transplantation from 53 graft recipients. The principal demographic and clinical characteristics of this cohort are summarized in Table 1. Each biopsy was analyzed by two pathologists and classified according the 2009 Banff classification.22 IF was quantified for each biopsy using the DIA and FTIR techniques. Histologic features and IF quantification data are shown in Table 2. IFTA scoring by the Banff classification was not associated with eGFR (P=0.22) (Figure 4A). In the same way, IF quantification by the DIA method was not correlated with eGFR (r=−0.034; P=0.82), whereas IF quantified by FTIR was correlated with eGFR (r=−0.353; P=0.02) (Figure 4, B and C).
Table 1.
Demographic and clinical characteristics of 106 renal graft biopsies from 53 graft recipients
| Characteristics | Means±SD or % (n) |
|---|---|
| Recipients | |
| Age, yr | 51.02±13.47 |
| Men | 50.9 (27) |
| BMI, kg/m2 | 25.73±4.21 |
| Donors | |
| Age, yr | 45.23±15.28 |
| Men | 45.3 (24) |
| Cause of death | |
| Trauma | 26.4 (14) |
| Vascular | 54.7 (29) |
| Cardiac arrest | 24.5 (13) |
| HBP | 18.9 (10) |
| Diabetes | 1.9 (1) |
| KDRI | 1.23±0.43 |
| Transplantation | |
| CIT, min | 1108±415 |
| Delayed graft recovery | 28.3 (15) |
| Initial IS regimen | |
| Cyclosporin | 75.5 (40) |
| Tacrolimus | 22.6 (12) |
| MMF | 94.3 (50) |
| Outcomes | |
| BPAR | 7.5 (4) |
| eGFR at M3, ml/min per 1.73 m2 | 54.38±19.73 |
| Proteinuria at M3, mg/d | 244.1±211.7 |
| eGFR at M12, ml/min per 1.73 m2 | 56.60±17.48 |
| Proteinuria at M12, mg/d | 233.4±247.13 |
BMI, body mass index; HBP, high BP; KDRI, kidney donor risk index; IS, immunosuppressor; MMF, mycophenolate mofetil; BPAR, biopsy–proven acute rejection.
Table 2.
Histologic features and IF quantification data
| Histologic Features | M3 | M12 |
|---|---|---|
| Interstitial inflammation | % (n) | % (n) |
| 0 | 84.9 (45) | 81.1 (43) |
| 1 | 11.3 (6) | 17 (9) |
| 2 | 1.9 (1) | 0 (0) |
| 3 | 1.9 (1) | 1.9 (1) |
| Inflammation by FTIR, % | 4.73±8.59 | 4.29±7.65 |
| Tubulitis | ||
| 0 | 81.1 (43) | 79.2 (42) |
| 1 | 11.3 (6) | 15.1 (8) |
| 2 | 5.7 (3) | 3.8 (2) |
| 3 | 1.9 (1) | 1.9 (1) |
| Chronic interstitial injuries | ||
| 0 | 90.5 (48) | 75.4 (40) |
| 1 | 5.7 (3) | 11.3 (6) |
| 2 | 3.8 (2) | 11.3 (6) |
| 3 | 0 (0) | 1.9 (1) |
| IFTA | ||
| 0 | 79.2 (42) | 59.8 (37) |
| 1 | 9.4 (5) | 20.8 (11) |
| 2 | 7.5 (4) | 7.5 (4) |
| 3 | 3.8 (2) | 1.9 (1) |
| Fibrosis by colorimetric, % | 21.55±11.5 | 21.89±11.63 |
| Fibrosis by FTIR, % | 25.26±18.58 | 22.73±17.97 |
| Fibrosis scoring by FTIR | ||
| 0 | 33.9 (18) | 28.3 (15) |
| 1 | 26.4 (14) | 35.8 (19) |
| 2 | 24.5 (13) | 22.6 (12) |
| 3 | 15.1 (8) | 13.2 (7) |
Figure 4.
Assessment of the effect of IF on renal function as quantified by visual rating, DIA, and FTIR quantification. (A) On biopsies performed M3 after transplantation (n=53), the IF score as estimated by visual inspection according to the Banff classification (IFTA) was not associated with the eGFR as estimated by the MDRD (P=0.22). (B) IF quantification by DIA was not correlated with eGFR (P=0.82), whereas (C) the IF quantification by FTIR was significantly correlated with eGFR (P=0.02). (D) The variation in IF quantification between M3 and M12 (Δfibrosis) was compared with the variation in eGFR over the same period (ΔeGFR) for both DIA and FTIR. ΔFibrosis as quantified by DIA was significantly correlated with ΔeGFR (P=0.03). (E) Similarly, Δfibrosis as quantified by FTIR was significantly correlated with ΔeGFR (P<0.001), with a better correlation coefficient.
To improve the clinical value of IF quantification by these two techniques, we investigated the relation between variations in IF and the values of eGFR between M3 and M12 (ΔeGFR) and between M3 and M24. The variations in IF as quantified by DIA on Masson trichrome–stained sections and FTIR analysis were correlated with ΔeGFR (Figure 4, D and E, Supplemental Figure 3).
Inflammation Quantification by FTIR
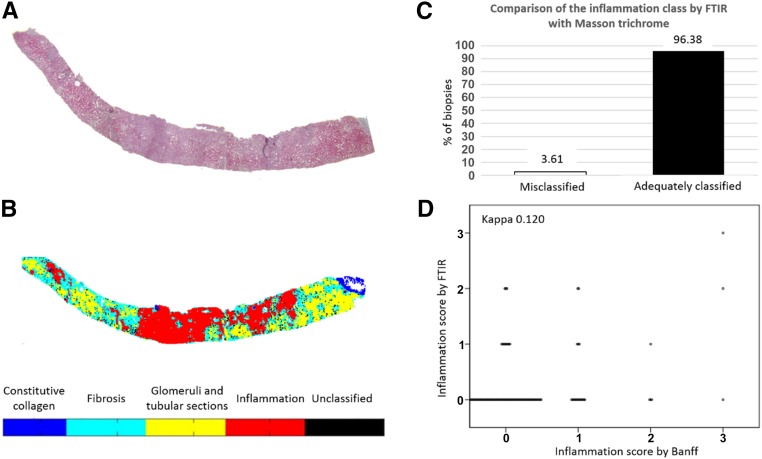
We integrated inflammation into the LDA classification model aiming to detect and quantify the relative area occupied by inflammatory cells on FTIR. Thus, a red cluster representing active inflammation was represented on FTIR images after processing by our LDA model. To verify the robustness of the inflammation classification by automated FTIR analysis, we used the same methodology as for IF. Briefly, three observers performed a pixel-to-pixel comparison focused on inflammatory areas for 166 renal graft biopsies evaluated by Masson trichrome and FTIR (Figure 5, A and B). We observed that 95.88% (161) of biopsies were adequately classified for inflammation by the FTIR classification and that only 2.73% (five) were misclassified (Figure 5C). Among five biopsies with inadequate classification for inflammation, three presented an aberrant FTIR signal with a single saturation signal of absorbance. Two others showed merging between inflammation and fibrosis. Thus, inflammation classification with our FTIR model seems useful to quantify active inflammation on renal biopsies. We then compared an inflammation score on the basis of the FTIR quantification (grade 0<10%; grade 1=10%–25%; grade 2=25%–50%; and grade 3>50%) with the visual assessment of inflammation scoring using the Banff classification. We found that the FTIR inflammation score was not in agreement with the Banff score (κ=0.12) (Figure 5D).
Figure 5.
Evaluation of the classification robustness of the LDA model for inflammation class and comparison with visual rating. Masson trichrome–stained serial sections of each biopsy from (A) the test set (n=166) were used to blindly compare with (B) the inflammation class on FTIR images; (C) >96% of biopsies were adequately classified. (D) The inflammation score as estimated by visual inspection according to the Banff classification showed poor agreement with the inflammation score as calculated from inflammation quantification by FTIR.
Discussion
In this study, we developed a new and original technique to identify and quantify IF and active inflammation on renal biopsies by FTIR spectroscopy. We built an LDA classification model of FTIR images on renal biopsies, making it possible in particular to detect active inflammation and IF areas, with discrimination of areas of constitutive collagen and normal parenchyma.
We assessed the robustness of our LDA classification model for IF compared with visual observation of Masson trichrome–stained sections. We report very good classification by the FTIR LDA model, with >90% of renal biopsies correctly classified according to the Masson trichrome evaluation. Indeed, focusing on collagen identification, we found satisfying results for FTIR, with >95% of biopsies correctly classified for IF. In contrast to other techniques, such as DIA, FTIR was able to distinguish pathologic collagen deposits (i.e., fibrosis) from constitutive collagen. Constitutive collagen is mainly found in the renal capsule, in the basement membranes (glomerular and tubular), and around the arteries. It may be a different type of collagen than fibrotic collagens. In the kidney, few studies have explored the distribution of different collagen types in the tubulointerstitial space and capsule. Type 6 collagen was found in the renal capsule and as a normal component of glomerular and extraglomerular matrix during the fetal period, but its expression was weaker in adult kidneys.23 In another report, type 3 collagen was found in the extraglomerular interstitium,24 but no data are available regarding the type of collagen in the renal capsule. In cirrhotic livers, collagen 6 was mainly found in the capsular fibrous tissue, whereas fibrosis was mainly composed of collagens 3–5.25 The different composition of collagen between fibrosis and constitutive extracellular matrix could explain the subtle difference in structure detected by infrared spectra. This ability is caused by an intrinsic discrimination of these two histologic structures as shown by centroid (spectra) comparison of these two classes (Supplemental Material). Thus, the FTIR classification makes it possible to specifically detect pathologic collagen, which represents a real improvement in histologic analysis.
In addition to its high power to detect fibrosis and inflammation, the FTIR technique is a fully automated, easy technique, usually requiring <1 hour to render a reliable result with high repeatability. No sample preparation is required, except to place 4- to 10-μm-thick sections on an infrared-compatible support. No human intervention is required for the FTIR acquisition and classification process. At the final step, the pathologist can select the area of interest to perform the quantification, usually by excluding the medulla, to maintain only the cortex area for analysis. A specific user–friendly software coupled with the infrared spectrometer device has been developed to integrate all steps from acquisition to final results. This technique could offer better spatial resolution by decreasing pixel size to ≤6.25×6.25 μm but at the cost of a considerable increase in acquisition time (6 hours per biopsy) with our experimentation. Nevertheless, we are currently developing FTIR acquisition with new devices, such as infrared focal plane arrays, making it possible to reduce acquisition time and consequently, increase the spatial resolution.26
It has been reported that visual assessment of IF with scoring (IFTA) according to the Banff classification suffers from poor interobserver reproducibility.3,27 Moreover, the use of a small number of grades to describe the severity of individual histologic injuries may lack sensitivity during the early stage of IFTA.28 To improve reproducibility and the performance of IF quantification, several techniques for computerized image analysis of IF in renal biopsies (stained by red Sirius or Masson trichrome) have been developed over the last decade.4,5,8,29–32 Recently, Servais and coworkers8,33,34 developed a semiautomated technique for quantification of IF by image analysis with a good reproducibility. Our FTIR classification model is able to quantify fibrosis by establishing the ratio of IF area to total area, with excellent reproducibility and the ability to discriminate pathologic deposition of collagen from constitutive collagen.
In our study, we compared the IFTA scoring of 166 renal graft biopsies according to the Banff classification with scoring on the basis of DIA and the automated FTIR fibrosis quantification technique. As expected, we observed poor agreement for both comparisons, underlining the high variability of Banff IFTA scoring compared with objective techniques. More surprisingly, we found poor agreement between IF scoring by DIA and FTIR. By contrast, the percentages of IF quantified by these two techniques were well correlated. Indeed, closer study of the dispersion of the data points showed good correlation at low levels of fibrosis and a poorer correlation at high levels of IF, with considerable spread for levels of fibrosis >30% of the total area. However, it should be underlined that the number of biopsies with high levels of fibrosis was lower than that of biopsies with little or no fibrosis. This feature could explain the good correlation between these two techniques, despite the relatively high dispersion of points.
Chronic allograft dysfunction defined by deterioration of renal function is a major cause of long–term graft failure after transplantation. Chronic histologic lesions could appear long before renal function decreases.35 Histologic features of chronic allograft nephropathy include TA, glomerulosclerosis, and arteriosclerosis and are dominated by IF.36,37 Consequently, early evaluation of IF before renal impairment is a central feature of renal graft monitoring. Indeed, protocol renal graft biopsies performed at M3 and M12 may make it possible to detect subclinical allograft rejection and evaluate the progression of chronic injury, especially fibrosis.6,38 Thus, accurate evaluation of fibrosis on renal graft biopsies is essential because of its repercussions on renal function and graft survival.1,39,40 Similarly, some authors have reported that quantification of fibrosis by DIA was predictive of renal outcome.7,31,41 To assess the accuracy of IF quantification by FTIR, we investigated the correlation between IF quantification and eGFR as estimated by the Modification of Diet in Renal Disease (MDRD) equation. Considering the high sensitivity of the FTIR technique, we initially focused on renal graft biopsies performed at M3 after transplantation and eGFR at the same time point. We observed an inverse correlation between the levels of fibrosis quantified by FTIR and renal function. In contrast, IF quantification by DIA failed to show any correlation with renal graft function at M3. Because of the reliability between IF and the decrease in renal function, we compared the course of renal function between M3 and M12 with the course of fibrosis over the same period by both DIA and FTIR analysis. Variations in IF as evaluated by DIA poorly correlated with variations in renal function, whereas variations in IF as quantified by FTIR correlated well with variations in renal function (P<0.001). These results show the great clinical relevance of the FTIR technique for IF quantification, which seems to be more relevant than the DIA technique that we used. We hypothesize that one of the reasons for this good result is related to the ability of FTIR to specifically detect pathologic collagen (fibrosis), whereas DIA quantifies both constitutive and pathologic collagen. In view of these results, the effect of sampling error might be lower than previously assumed in the estimation of IF.28,42 Consequently, FTIR quantification is an accurate technique for IF quantification for clinical application and management of patients with renal transplantation.
Furthermore, our LDA model was able to detect and quantify areas of active inflammation on renal biopsies, enabling an objective diagnosis of allograft rejection. This detection of active inflammation was robust, with >95% of renal graft biopsies adequately classified. Except for biopsies with aberrant FTIR signals, the smallest areas of inflammation were detected by our FTIR classification technique. Currently, evaluation of inflammation is performed by visual estimation in the same way as for fibrosis and with the same limitations. Moreover, the evaluation of inflammation is an important histologic feature that guides immunosuppressive therapy. Indeed, the immunosuppressive regimen is different depending on whether the level of inflammation is diagnosed as borderline modification (10%–25%) or high inflammation (>25%), defining cellular allograft rejection. However, it might be difficult for the pathologist to precisely determine the area occupied by inflammation. Moreover, unlike IF quantification, there is no automated technique to quantify inflammation. Schierwagen et al.43 developed a method to quantify polymorphonuclear leukocyte using a myeloperoxidase assay in rabbit tissue. This technique indirectly evaluates polymorphonuclear leukocyte activity in tissue but neglects lymphocyte infiltrates, which are predominant in allograft injuries. In a rat experimental model, Engstrand et al.44,45 reported a technique for lymphocyte quantification by cell propagation. Core biopsies were performed, and infiltrating cells were isolated using an in vitro culture system, allowing cells to propagate from biopsies to culture medium, where they were counted after immunochemical staining. Unfortunately, this elegant technique cannot be applied in routine. Like fibrosis quantification, DIA was used in another study to quantify inflammation areas but with poor discriminant capacity because of the inability to distinguish the noninflamed from mildly inflamed group.46 More recently, Klapczynski et al.47 developed a computer–assisted imaging algorithm, which notably performed neutrophil quantification after identification of these cells by a nuclear algorithm used to identify immunohistochemically stained neutrophils as positive nuclei and using immunohistochemical Nuclear Classifier. To the best of our knowledge, we are the first to develop a reproducible automated label–free technique to quantify active inflammation on renal graft biopsies.
In conclusion, we developed a new tool for histopathologic diagnosis and prognosis of renal injuries. This nondestructive label–free automated technique on the basis of FTIR analysis is easy to perform within 30–60 minutes, objectively quantifies fibrosis and inflammation, and was successfully applied to renal graft biopsy evaluation. In the future, this technique could be applied to other transplanted organs.
Concise Methods
Patients
Forty-eight indicated and protocol biopsies from renal graft recipients transplanted at the Reims University Hospital between 2008 and 2012 were used to construct and validate a classification model on the basis of infrared data.
We also included 166 additional renal graft biopsies to test the algorithm. These biopsies were obtained from renal graft recipients transplanted at the Reims University Hospital between 2008 and 2012 with a kidney from living or cadaveric donors. These 166 biopsies were obtained from 106 patients who had one or several renal graft biopsies, including protocol biopsies at M3 and M12 after transplantation or for an indicated cause. Immunosuppressive induction mainly comprised basiliximab (97% of patients) and antithymyglobulin (3%). The immunosuppressive regimen was composed of cyclosporin or tacrolimus for 86% and 12% of recipients, respectively, and an antimetabolite, mainly mycophenolate mofetil (98%).
Among 166 renal graft biopsies collected for this study, 106 were obtained from 53 patients at M3 and M12 after transplantation. Table 1 indicates the demographic and clinical characteristics of these patients. The remaining 60 renal graft biopsies were collected at either M3 or M12 or at another time for graft dysfunction.
Study Design
The percentage of IF was analyzed by three methods, namely (1) visual assessment of IF by three observers according to the 2009 Banff classification,48 (2) automated IF quantification with DIA on the basis of colorimetric deconvolution of a Masson trichrome–stained section, and (3) automated IF quantification by FTIR imaging combined with data processing.
First, the accuracy of the FTIR analysis was evaluated by comparison of the FTIR images with serial Masson trichrome–stained sections inspected by three observers. To improve the quality assessment of FTIR imaging, we also performed a comparison between FTIR images and second harmonic generation images collected on the same section by three observers (results are shown in Supplemental Material).
Second, the IF quantification by FTIR was compared with the IFTA Banff scoring and the IF quantification by DIA. Clinical significance of the infrared IF quantification by FTIR was studied by considering the renal function as a reference parameter. More precisely, we investigated correlations between (1) infrared IF quantification at M3 and eGFR at the same time point as estimated by the MDRD equation49 and (2) the variation in eGFR between M3 and M12 (ΔeGFR) and the variation in IF quantification by FTIR (Δfibrosis). We performed the same comparison with IF quantification by DIA to identify the most clinically relevant technique.
For the quantification of the active interstitial inflammation, the FTIR approach was compared with the Banff classification determined by three pathologists from Masson trichrome staining.
Sample Preparation
Renal graft biopsies were fixed in Dubosq–Brazil solution and embedded in paraffin. For each sample, a 2-μm-thick section and serial 10-μm-thick sections were cut. The 2-μm-thick sections were placed on poly–M-lysine–coated slides (SuperFrostII), deparaffinized, and stained by Masson trichrome, where collagen appears in green; 10-μm-thick sections were mounted on a calcium fluoride window (Crystran Ltd., Poole, United Kingdom) for infrared acquisition without additional treatment.
Histopathologic Assessment
For 214 renal biopsies, specimens revealed sufficient material for the evaluation of IF, TA, and interstitial inflammation.
For light microscopy, allograft biopsies were examined by pathologists according to the 2009 Banff criteria48 to evaluate several histologic features, including IF, interstitial inflammation, and the IFTA score reflecting both IF and TA scores. Results were expressed as grade of injury (grade 0, <10%; grade 1, 10%–25%; grade 2, 25%–50%; and grade 3, >50%).
Fibrosis Quantification by DIA
We developed a color image segmentation algorithm for automatic digital analysis of IF from Masson trichrome staining. For each biopsy, the tissue sections were imaged using a color camera (Spot Insight Color version 3.2.0; SPOT Imaging Solutions, a division of Diagnostic Instruments, Inc., Sterling Heights, MI) mounted on a upright light microscope (Aristoplan; Leitz Leica, Wetzlar, Germany). The images were acquired using a 20× objective (Leitz Leica). The acquisition software was Spot Advanced (version 3.2.2; Diagnostic Instruments, Inc.). For each biopsy, several images were acquired to image after concatenation the total area of the tissue, and a background image was acquired.
DIA was performed using the public domain National Institutes of Health (NIH) Image program ImageJ (developed at the US NIH and available at http://rsb.info.nih.gov/nih-image/). For each biopsy, the background was removed by outline detection; luminosity homogenization and contrast optimization were also performed using the background image. The medulla area was manually selected by the operator and removed from the image to retain only the cortex area. A color deconvolution algorithm was applied to separate the remaining area into three color channels, blue, red, and green, which were then binarized. The green channel reflected the presence of collagen as stained in green by Masson trichrome. Glomeruli and tubular and vascular sections corresponded to the red channel. The associated pixels representing the Bowman’s capsule, tubular basement membrane, and vascular wall areas were then removed from the green channel. In our DIA process, a step to remove pixels corresponding to the Bowman’s capsule, tubular basement, and vascular walls was included. This particular step is on the basis of a method of particle detection allowing identification of the perimeter of glomeruli, tubular sections, and vascular sections. Green pixels surrounding the close vicinity of the detected particles were considered as belonging to the Bowman’s capsule, tubular basement membranes, and vascular walls and consequently, erased. Thus, these constitutive histologic structures were not included in the fibrosis quantification according to the DIA process implemented.
Finally, the ratio of remaining area on the green channel to the total cortex area made it possible to obtain the percentage of fibrosis in the cortex biopsy. This process can be summarized in the five following steps: (1) acquisition and preprocessing of images; (2) image binarization, giving the total area; (3) color deconvolution in three channels (red, green, and blue); (4) binarization of each channel; and (5) subtraction of area associated with normal collagen from the green channel leaving the fibrosis area. The ratio of the image at step 5 to the image at step 2 gave the percentage of fibrosis (Supplemental Figure 1).
FTIR Data Collection
IR transmission spectral images were collected with a Spectrum Spotlight 2 Device coupled to a Spectrum One FTIR Spectrometer (both from PerkinElmer, Waltham, MA). The device is equipped with a nitrogen–cooled, 16-pixel line detector for imaging and a computer-controlled stage. A visible image of the sample was recorded, and the area of interest (total biopsy area) was selected. Spectral images were recorded with eight accumulations per spectrum, with a resolution of 4 cm−1. Each image pixel sampled a 25×25-μm area at the sample section, permitting the recording of detailed tissue structures. A background spectrum was collected (250 accumulations at 4-cm−1 resolution) on the calcium fluoride window. The microscope was isolated in vented Plexiglas housing to enable purging with dry air and eliminate atmospheric interferences. Each spectral image consisted of about 20,000 spectra, each containing 3282 values of absorbance spanning the spectral range of 720–4000 cm−1; >4×106 spectra were collected during this study.
Spectral Data Processing
Spectral images were subjected to preprocessing steps. First, spectral images were corrected from the contribution of atmospheric water vapor and CO2 absorption bands by a built-in function of the PerkinElmer Spotlight software. All additional data processing was carried out directly on spectral images using programs written in Matlab 8.2 software (Mathworks, Natick, MA). Spectra were analyzed in the fingerprint region (900–1800 cm−1), which has been proven to be the most informative for IR analysis of biologic samples.11,13 Spectra were processed by Savitzky–Golay smoothing using a third–order polynomial function and an 11–point window length. The correction of the contributions of paraffin and scattering effects in FTIR spectra and their normalization were performed by an automated processing method on the basis of extended multiplicative signal correction.11 This method has been successfully applied to infrared analysis of different human tissues.12,13
For additional supervised classification purposes, a training set composed of FTIR images acquired on 25 renal graft biopsies was created. For each image of the training set, spectra with similar characteristics and similar biomolecular properties were regrouped by K-means clustering. We tested K means with 4–12 clusters compared with Masson trichrome staining and fixed the optimal cluster number at five owing to the absence of new information beyond five clusters. To limit the sensitivity of K means to initialization, 30 replications were computed.
An experienced renal pathologist compared the infrared K–means images of the training set with a serial Masson trichrome–stained section to label each K-means cluster into one of four structures: (1) normal glomeruli and tubular sections, (2) constitutive collagen, (3) fibrosis, and (4) inflammation. All nonlabeled clusters were removed from additional analysis.
Spectra from each cluster were pooled to identify similarities between them. We performed PCA to reduce the data dimension. PCA is an unsupervised multivariate analysis technique that makes it possible to reduce the number of variables to a new set of latent variables that describes the main data variability.
To perform automated FTIR classification able to separately identify fibrosis and inflammation from constitutive collagen and normal glomeruli and tubular sections, LDA was applied on the PCA scores of the training dataset. LDA is a supervised classification method that seeks an optimal low–dimensional space, in which the projected data points are linearly separated into different classes.50 It aims to maximize the ratio of between-class variance to within-class variance.51–54
A five–class LDA model was chosen. Four classes assigned to specific tissue structures (i.e., [1] normal glomeruli and tubular sections, [2] constitutive collagen, [3] fibrosis, and [4] inflammation) were retained. The threshold of the pixel membership value was fixed at 0.5. An additional class corresponding to unclassified pixels (i.e., pixels with membership to one of four previous classes <0.5) was also considered to optimize the classification processing.
We underlined that the discrimination between classes was allowed by spectral differences of each histologic structure. These differences were visible on the reference spectra of each class named centroids. Indeed, constitutive collagen could be discriminated from fibrosis thanks to spectral differences on their centroids, reflecting biochemical and/or structural differences between these structures (details are in Supplemental Material).
Because the results of LDA are dependent on the number of PCs, several models, with 4–20 PCs, were constructed on the training dataset and then, applied on a validation dataset composed of 23 distinct renal graft biopsies previously labeled by a renal pathologist in the same way as the training dataset. The model with the best sensitivity for fibrosis and inflammation classes on the validation set was selected.
We explored the repeatability of this classification technique by running the LDA model five times on 20 biopsies. We observed very good repeatability, with an intraclass correlation coefficient of 1.00 (P<0.001).
Quantification of IF and Inflammation by FTIR
The LDA model was used to blindly quantify fibrosis in 166 renal graft biopsies. The quantification was on the basis of computing the ratio between the numbers of pixels attributed to fibrosis and the total number of pixels covering the tissue area (attributed to fibrosis, normal parenchyma, inflammation, and unclassified clusters). This quantification method excluded the constitutive collagen of the total surface. Thus, the kidney capsule is not considered as a functional area of the kidney.
Disclosures
None.
Supplementary Material
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015050601/-/DCSupplemental.
References
- 1.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Allen RD, Chapman JR: The natural history of chronic allograft nephropathy. N Engl J Med 349: 2326–2333, 2003 [DOI] [PubMed] [Google Scholar]
- 2.Nankivell BJ, Borrows RJ, Fung CL, O’Connell PJ, Chapman JR, Allen RD: Calcineurin inhibitor nephrotoxicity: Longitudinal assessment by protocol histology. Transplantation 78: 557–565, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Furness PN, Taub N Convergence of European Renal Transplant Pathology Assessment Procedures (CERTPAP) Project : International variation in the interpretation of renal transplant biopsies: Report of the CERTPAP Project. Kidney Int 60: 1998–2012, 2001 [DOI] [PubMed] [Google Scholar]
- 4.Pape L, Henne T, Offner G, Strehlau J, Ehrich JH, Mengel M, Grimm PC: Computer-assisted quantification of fibrosis in chronic allograft nephropaty by picosirius red-staining: A new tool for predicting long-term graft function. Transplantation 76: 955–958, 2003 [DOI] [PubMed] [Google Scholar]
- 5.Grimm PC, Nickerson P, Gough J, McKenna R, Stern E, Jeffery J, Rush DN: Computerized image analysis of Sirius Red-stained renal allograft biopsies as a surrogate marker to predict long-term allograft function. J Am Soc Nephrol 14: 1662–1668, 2003 [DOI] [PubMed] [Google Scholar]
- 6.Moreso F, Lopez M, Vallejos A, Giordani C, Riera L, Fulladosa X, Hueso M, Alsina J, Grinyó JM, Serón D: Serial protocol biopsies to quantify the progression of chronic transplant nephropathy in stable renal allografts. Am J Transplant 1: 82–88, 2001 [DOI] [PubMed] [Google Scholar]
- 7.Diaz Encarnacion MM, Griffin MD, Slezak JM, Bergstralh EJ, Stegall MD, Velosa JA, Grande JP: Correlation of quantitative digital image analysis with the glomerular filtration rate in chronic allograft nephropathy. Am J Transplant 4: 248–256, 2004 [DOI] [PubMed] [Google Scholar]
- 8.Servais A, Meas-Yedid V, Buchler M, Morelon E, Olivo-Marin JC, Lebranchu Y, Legendre C, Thervet E: Quantification of interstitial fibrosis by image analysis on routine renal biopsy in patients receiving cyclosporine. Transplantation 84: 1595–1601, 2007 [DOI] [PubMed] [Google Scholar]
- 9.Ly E, Cardot-Leccia N, Ortonne JP, Benchetrit M, Michiels JF, Manfait M, Piot O: Histopathological characterization of primary cutaneous melanoma using infrared microimaging: A proof-of-concept study. Br J Dermatol 162: 1316–1323, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Tfayli A, Piot O, Durlach A, Bernard P, Manfait M: Discriminating nevus and melanoma on paraffin-embedded skin biopsies using FTIR microspectroscopy. Biochim Biophys Acta 1724: 262–269, 2005 [DOI] [PubMed] [Google Scholar]
- 11.Ly E, Piot O, Wolthuis R, Durlach A, Bernard P, Manfait M: Combination of FTIR spectral imaging and chemometrics for tumour detection from paraffin-embedded biopsies. Analyst 133: 197–205, 2008 [DOI] [PubMed] [Google Scholar]
- 12.Wolthuis R, Travo A, Nicolet C, Neuville A, Gaub MP, Guenot D, Ly E, Manfait M, Jeannesson P, Piot O: IR spectral imaging for histopathological characterization of xenografted human colon carcinomas. Anal Chem 80: 8461–8469, 2008 [DOI] [PubMed] [Google Scholar]
- 13.Ly E, Piot O, Durlach A, Bernard P, Manfait M: Differential diagnosis of cutaneous carcinomas by infrared spectral micro-imaging combined with pattern recognition. Analyst 134: 1208–1214, 2009 [DOI] [PubMed] [Google Scholar]
- 14.Krishna CM, Sockalingum GD, Bhat RA, Venteo L, Kushtagi P, Pluot M, Manfait M: FTIR and Raman microspectroscopy of normal, benign, and malignant formalin-fixed ovarian tissues. Anal Bioanal Chem 387: 1649–1656, 2007 [DOI] [PubMed] [Google Scholar]
- 15.Salman A, Argov S, Ramesh J, Goldstein J, Sinelnikov I, Guterman H, Mordechai S: FT-IR microscopic characterization of normal and malignant human colonic tissues. Cell Mol Biol (Noisy-le-grand) 47: OL159–OL166, 2001 [PubMed] [Google Scholar]
- 16.Baker MJ, Trevisan J, Bassan P, Bhargava R, Butler HJ, Dorling KM, Fielden PR, Fogarty SW, Fullwood NJ, Heys KA, Hughes C, Lasch P, Martin-Hirsch PL, Obinaju B, Sockalingum GD, Sule-Suso J, Strong RJ, Walsh MJ, Wood BR, Gardner P, Martin FL: Using Fourier transform IR spectroscopy to analyze biological materials. Nat Protoc 9: 1771–1791, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Krishna CM, Kegelaer G, Adt I, Rubin S, Kartha VB, Manfait M, Sockalingum GD: Combined Fourier transform infrared and Raman spectroscopic approach for identification of multidrug resistance phenotype in cancer cell lines. Biopolymers 82: 462–470, 2006 [DOI] [PubMed] [Google Scholar]
- 18.Hughes C, Brown MD, Clarke NW, Flower KR, Gardner P: Investigating cellular responses to novel chemotherapeutics in renal cell carcinoma using SR-FTIR spectroscopy. Analyst 137: 4720–4726, 2012 [DOI] [PubMed] [Google Scholar]
- 19.Guerra-Lopez JR, Guida JA, Della Vedova CO: Infrared and Raman studies on renal stones: The use of second derivative infrared spectra. Urol Res 38: 383–390, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Primiano A, Persichilli S, Gambaro G, Ferraro PM, D'Addessi A, Cocci A, Schiattarella A, Zuppi C, Gervasoni J: FT-IR analysis of urinary stones: A helpful tool for clinician comparison with the chemical spot test. Dis Markers 2014: 176165, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sablinskas V, Urboniene V, Ceponkus J, Laurinavicius A, Dasevicius D, Jankevicius F, Hendrixson V, Koch E, Steiner G: Infrared spectroscopic imaging of renal tumor tissue. J Biomed Opt 16: 096006, 2011 [DOI] [PubMed] [Google Scholar]
- 22.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM 3rd, Bracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff '09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed] [Google Scholar]
- 23.Magro G, Grasso S, Colombatti A, Lopes M: Immunohistochemical distribution of type VI collagen in developing human kidney. Histochem J 28: 385–390, 1996 [DOI] [PubMed] [Google Scholar]
- 24.Razzaque MS, Koji T, Taguchi T, Harada T, Nakane PK: In situ localization of type III and type IV collagen-expressing cells in human diabetic nephropathy. J Pathol 174: 131–138, 1994 [DOI] [PubMed] [Google Scholar]
- 25.Chen W, Rock JB, Yearsley MM, Hanje AJ, Frankel WL: Collagen immunostains can distinguish capsular fibrous tissue from septal fibrosis and may help stage liver fibrosis. Appl Immunohistochem Mol Morphol 22: 735–740, 2014 [DOI] [PubMed] [Google Scholar]
- 26.Bassan P, Weida MJ, Rowlette J, Gardner P: Large scale infrared imaging of tissue micro arrays (TMAs) using a tunable Quantum Cascade Laser (QCL) based microscope. Analyst 139: 3856–3859, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Veronese FV, Manfro RC, Roman FR, Edelweiss MI, Rush DN, Dancea S, Goldberg J, Gonçalves LF: Reproducibility of the Banff classification in subclinical kidney transplant rejection. Clin Transplant 19: 518–521, 2005 [DOI] [PubMed] [Google Scholar]
- 28.Serón D, Moreso F, Fulladosa X, Hueso M, Carrera M, Grinyó JM: Reliability of chronic allograft nephropathy diagnosis in sequential protocol biopsies. Kidney Int 61: 727–733, 2002 [DOI] [PubMed] [Google Scholar]
- 29.Ruifrok AC, Johnston DA: Quantification of histochemical staining by color deconvolution. Anal Quant Cytol Histol 23: 291–299, 2001 [PubMed] [Google Scholar]
- 30.Ruifrok AC, Katz RL, Johnston DA: Comparison of quantification of histochemical staining by hue-saturation-intensity (HSI) transformation and color-deconvolution. Appl Immunohistochem Mol Morphol 11: 85–91, 2003 [DOI] [PubMed] [Google Scholar]
- 31.Servais A, Meas-Yedid V, Buchler M, Morelon E, Olivo-Marin JC, Thervet E: Quantification of interstitial fibrosis by image analysis on routine renal biopsy 1 year after transplantation in patients managed by C2 monitoring of cyclosporine microemulsion. Transplant Proc 39: 2560–2562, 2007 [DOI] [PubMed] [Google Scholar]
- 32.Farris AB, Adams CD, Brousaides N, Della Pelle PA, Collins AB, Moradi E, Smith RN, Grimm PC, Colvin RB: Morphometric and visual evaluation of fibrosis in renal biopsies. J Am Soc Nephrol 22: 176–186, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Meas-Yedid V, Servais A, Noel LH, Panterne C, Landais P, Herve N, Brousse N, Kreis H, Legendre C, Thervet E, Olivo-Marin JC, Morelon E: New computerized color image analysis for the quantification of interstitial fibrosis in renal transplantation. Transplantation 92: 890–899, 2011 [DOI] [PubMed] [Google Scholar]
- 34.Servais A, Meas-Yedid V, Toupance O, Lebranchu Y, Thierry A, Moulin B, Etienne I, Presne C, Hurault de LB, Le Pogamp P, Le Meur Y, Glotz D, Hayem C, Olivo Marin JC, Thervet E: Interstitial fibrosis quantification in renal transplant recipients randomized to continue cyclosporine or convert to sirolimus. Am J Transplant 9: 2552–2560, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Legendre C, Thervet E, Skhiri H, Mamzer-Bruneel MF, Cantarovich F, Noël LH, Kreis H: Histologic features of chronic allograft nephropathy revealed by protocol biopsies in kidney transplant recipients. Transplantation 65: 1506–1509, 1998 [DOI] [PubMed] [Google Scholar]
- 36.Racusen LC, Solez K, Colvin RB, Bonsib SM, Castro MC, Cavallo T, Croker BP, Demetris AJ, Drachenberg CB, Fogo AB, Furness P et al. : The Banff 97 working classification of renal allograft pathology. Kidney Int 55: 713–723, 1999 [DOI] [PubMed] [Google Scholar]
- 37.Dodd SM: Chronic allograft nephropathy: The inevitable outcome of renal transplantation? Curr Top Pathol 92: 37–60, 1999 [DOI] [PubMed] [Google Scholar]
- 38.Yilmaz S, Tomlanovich S, Mathew T, Taskinen E, Paavonen T, Navarro M, Ramos E, Hooftman L, Häyry P: Protocol core needle biopsy and histologic Chronic Allograft Damage Index (CADI) as surrogate end point for long-term graft survival in multicenter studies. J Am Soc Nephrol 14: 773–779, 2003 [DOI] [PubMed] [Google Scholar]
- 39.Chapman JR: Longitudinal analysis of chronic allograft nephropathy: Clinicopathologic correlations. Kidney Int Suppl 99: S108–S112, 2005 [DOI] [PubMed] [Google Scholar]
- 40.Nankivell BJ, Fenton-Lee CA, Kuypers DR, Cheung E, Allen RD, O’Connell PJ, Chapman JR: Effect of histological damage on long-term kidney transplant outcome. Transplantation 71: 515–523, 2001 [DOI] [PubMed] [Google Scholar]
- 41.Sund S, Grimm P, Reisaeter AV, Hovig T: Computerized image analysis vs semiquantitative scoring in evaluation of kidney allograft fibrosis and prognosis. Nephrol Dial Transplant 19: 2838–2845, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Sund S, Reisaeter AV, Fauchald P, Bentdal O, Hall KS, Hovig T: Living donor kidney transplants: A biopsy study 1 year after transplantation, compared with baseline changes and correlation to kidney function at 1 and 3 years. Nephrol Dial Transplant 14: 2445–2454, 1999 [DOI] [PubMed] [Google Scholar]
- 43.Schierwagen C, Bylund-Fellenius AC, Lundberg C: Improved method for quantification of tissue PMN accumulation measured by myeloperoxidase activity. J Pharmacol Methods 23: 179–186, 1990 [DOI] [PubMed] [Google Scholar]
- 44.Engstrand M, Johnsson C, Larsson E, Tufveson G, Korsgren O: Quantification of lymphocytes propagating from rat-kidney allografts--a tool to monitor anti-rejection treatment. Transpl Immunol 10: 31–36, 2002 [DOI] [PubMed] [Google Scholar]
- 45.Engstrand M, Larsson E, Naghibi M, Tufveson G, Korsgren O, Johnsson C: Lymphocyte propagation from biopsies of kidney allografts. Transplant Immunol 16: 215–219, 2006 [DOI] [PubMed] [Google Scholar]
- 46.Lauronen J, Häyry P, Paavonen T: An image analysis-based method for quantification of chronic allograft damage index parameters. APMIS 114: 440–448, 2006 [DOI] [PubMed] [Google Scholar]
- 47.Klapczynski M, Gagne GD, Morgan SJ, Larson KJ, Leroy BE, Blomme EA, Cox BF, Shek EW: Computer-assisted imaging algorithms facilitate histomorphometric quantification of kidney damage in rodent renal failure models. J Pathol Inform 3: 20, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Sis B, Mengel M, Haas M, Colvin RB, Halloran PF, Racusen LC, Solez K, Baldwin WM 3rdBracamonte ER, Broecker V, Cosio F, Demetris AJ, Drachenberg C, Einecke G, Gloor J, Glotz D, Kraus E, Legendre C, Liapis H, Mannon RB, Nankivell BJ, Nickeleit V, Papadimitriou JC, Randhawa P, Regele H, Renaudin K, Rodriguez ER, Seron D, Seshan S, Suthanthiran M, Wasowska BA, Zachary A, Zeevi A: Banff '09 meeting report: Antibody mediated graft deterioration and implementation of Banff working groups. Am J Transplant 10: 464–471, 2010 [DOI] [PubMed]
- 49.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D Modification of Diet in Renal Disease Study Group : A more accurate method to estimate glomerular filtration rate from serum creatinine: A new prediction equation. Ann Intern Med 130: 461–470, 1999 [DOI] [PubMed] [Google Scholar]
- 50.Zwielly A, Mordechai S, Sinielnikov I, Salman A, Bogomolny E, Argov S: Advanced statistical techniques applied to comprehensive FTIR spectra on human colonic tissues. Med Phys 37: 1047–1055, 2010 [DOI] [PubMed] [Google Scholar]
- 51.Pieters S, Vander Heyden Y, Roger JM, D'Hondt M, Hansen L, Palagos B, De Spiegeleer B, Remon JP, Vervaet C, De Beer T: Raman spectroscopy and multivariate analysis for the rapid discrimination between native-like and non-native states in freeze-dried protein formulations. Eur J Pharm Biopharm 85: 263–271, 2013 [DOI] [PubMed] [Google Scholar]
- 52.Xu L, Deng DH, Cai CB: Predicting the age and type of tuocha tea by fourier transform infrared spectroscopy and chemometric data analysis. J Agric Food Chem 59: 10461–10469, 2011 [DOI] [PubMed] [Google Scholar]
- 53.Khanmohammadi M, Bagheri Garmarudi A, Samani S, Ghasemi K, Ashuri A: Application of linear discriminant analysis and Attenuated Total Reflectance Fourier Transform Infrared microspectroscopy for diagnosis of colon cancer. Pathol Oncol Res 17: 435–441, 2011 [DOI] [PubMed] [Google Scholar]
- 54.Khanmohammadi M, Bagheri Garmarudi A, de la Guardia M: Feature selection strategies for quality screening of diesel samples by infrared spectrometry and linear discriminant analysis. Talanta 104: 128–134, 2013 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.