Abstract
HNF-1β is a tissue–specific transcription factor that is expressed in the kidney and other epithelial organs. Humans with mutations in HNF-1β develop kidney cysts, and HNF-1β regulates the transcription of several cystic disease genes. However, the complete spectrum of HNF-1β–regulated genes and pathways is not known. Here, using chromatin immunoprecipitation/next generation sequencing and gene expression profiling, we identified 1545 protein-coding genes that are directly regulated by HNF-1β in murine kidney epithelial cells. Pathway analysis predicted that HNF-1β regulates cholesterol metabolism. Expression of dominant negative mutant HNF-1β or kidney-specific inactivation of HNF-1β decreased the expression of genes that are essential for cholesterol synthesis, including sterol regulatory element binding factor 2 (Srebf2) and 3-hydroxy-3-methylglutaryl-CoA reductase (Hmgcr). HNF-1β mutant cells also expressed lower levels of cholesterol biosynthetic intermediates and had a lower rate of cholesterol synthesis than control cells. Additionally, depletion of cholesterol in the culture medium mitigated the inhibitory effects of mutant HNF-1β on the proteins encoded by Srebf2 and Hmgcr, and HNF-1β directly controlled the renal epithelial expression of proprotein convertase subtilisin–like kexin type 9, a key regulator of cholesterol uptake. These findings reveal a novel role of HNF-1β in a transcriptional network that regulates intrarenal cholesterol metabolism.
Keywords: polycystic kidney disease, lipids, transcription factors
Hepatocyte nuclear factor-1β (HNF-1β) is a tissue–specific transcription factor that is expressed in epithelial cells in the liver, kidney, genital tract, pancreas, lung, and intestine.1 In the mammalian kidney, HNF-1β is expressed in tubular epithelial cells in all segments of the nephrons and renal collecting ducts. HNF-1β contains an N–terminal dimerization domain, a Pit-1/Oct-1/Unc-86 homeodomain that mediates binding to the consensus sequence (5′-RGTTAATNATTAACM-3′), and a C–terminal transactivation domain.2 HNF-1β has been shown to function as either a transcriptional activator or a transcriptional repressor depending on the target gene and cellular context.2–4 One mechanism for transcriptional activation involves the recruitment of coactivators that include P/CAF, CBP, p300, and zyxin.2,5
HNF-1β is essential for the proper embryonic development of the kidney.6–8 In the developing mouse kidney, HNF-1β is expressed in nephron precursors and the branching ureteric bud that gives rise to the renal collecting system. Loss-of-function mutations in Hnf-1β cause renal agenesis, in part, because of reduced expression of Wnt9b, a ureteric bud–derived factor that is required for the induction of new nephrons.7 Expression of dominant negative mutant HNF-1β disrupts renal tubulogenesis because of deregulated expression of the target gene Socs3.4 HNF-1β also plays a role in nephron patterning through regulation of Notch signaling.9
Humans with heterozygous mutations in HNF-1β develop congenital kidney anomalies, including renal agenesis, hypoplasia/dysplasia, multicystic renal dysplasia, and glomerulocystic kidney disease.10,11 A common feature is the formation of kidney cysts derived from the renal tubules. This cystic phenotype is recapitulated in the mouse by transgenic expression of dominant negative mutant HNF-1β or kidney-specific inactivation of Hnf-1β.12,13 HNF-1β regulates the expression of genes encoding ciliary proteins that have been implicated in cyst formation, including PKD2 and PKHD1.12,13 Moreover, we have recently reported that HNF-1β regulates the activity of the Pkhd1 promoter in the kidney in vivo.14
In the adult kidney, HNF-1β is expressed in renal tubular epithelial cells composing the nephron and collecting ducts, where it regulates the expression of tissue-specific genes, including Ksp-cadherin, collectrin, and solute transporters.1,15 Several physiologically relevant gene targets have been identified in the kidney, primarily through identification of the consensus recognition sequence in candidate gene promoters (e.g., NKCC2, FXYD2, OAT3/4, and URAT1). However, the complete spectrum of genes and networks that are directly regulated by HNF-1β is still not known.
Here, we used chromatin immunoprecipitation (ChIP) followed by next generation sequencing (ChIP sequencing [ChIP-seq]) combined with gene expression profiling to identify genes that are directly regulated by HNF-1β in renal epithelial cells. These studies unexpectedly revealed that HNF-1β directly regulates the expression of multiple genes that are required for cholesterol synthesis. We also found evidence for a role of HNF-1β in the regulation of cholesterol uptake by transcriptional activation of proprotein convertase subtilisin–like kexin type 9 (Pcsk9).
Results
Identification of HNF-1β Binding Sites in Kidney Cells
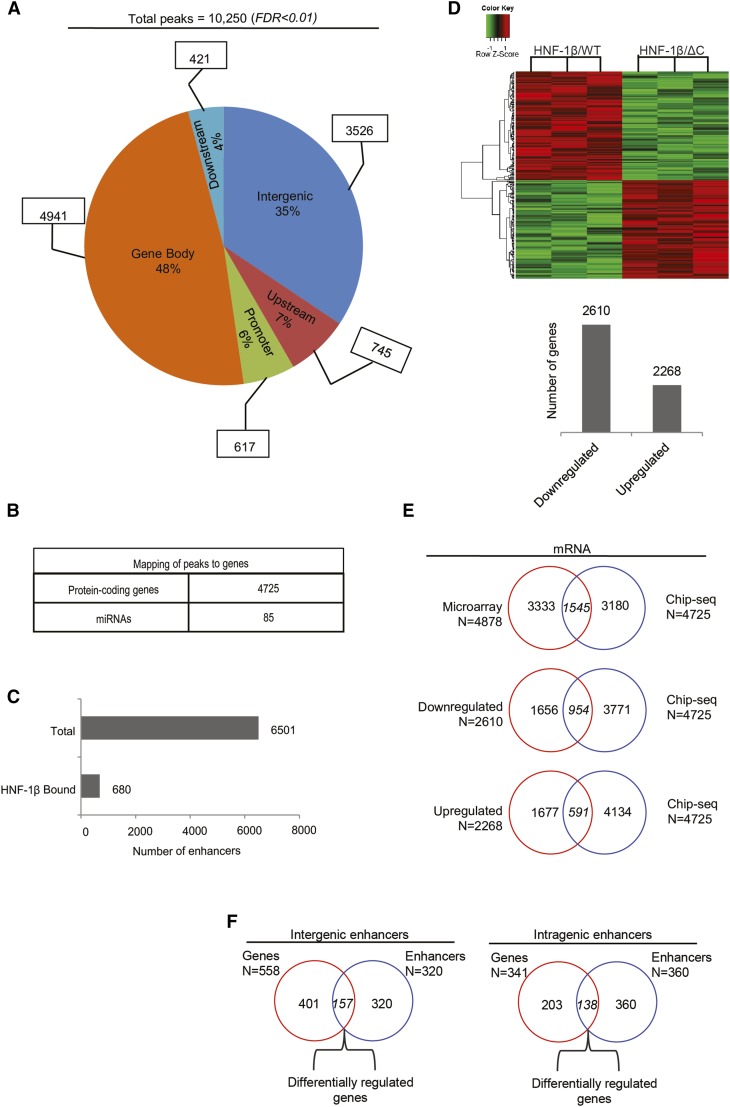
To identify HNF-1β binding sites at the whole-genome level, we performed ChIP-seq analysis on mIMCD3 renal epithelial cells. Chromatin was isolated from mIMCD3 cells, crosslinked, and immunoprecipitated with an anti–HNF-1β antibody. After reversing the crosslinks, the immunoprecipitated DNA was subjected to next generation sequencing. Binding sites were identified by enrichment of genomic sequences compared with input and immunoprecipitation with control IgG.16 Quality control of the ChIP-seq experiments is shown in Supplemental Table 1. We found a total of 10,250 peaks representing significantly enriched HNF-1β binding sites (FDR<0.01). We then determined the spatial distribution of the HNF-1β binding peaks relative to annotated genes in the mouse genome. The majority of HNF-1β binding sites were located close to or within genes (48% gene bodies, 6% gene promoters, 7% upstream regions, and 4% downstream regions) (Figure 1A). The remainder of the peaks (35%) mapped to intergenic domains.
Figure 1.
Genome-wide identification of genes that are directly regulated by HNF-1β in kidney cells. (A) Genome-wide identification of HNF-1β binding sites in chromatin from mIMCD3 renal epithelial cells. The pie chart shows the distribution of HNF-1β binding sites in the indicated genomic regions: promoter regions extending from the transcription start site (TSS) to −1 kb, upstream regions (−1 to −10 kb), gene bodies extending from the TSS to the transcription termination site (TTS), downstream regions (TTS to +5 kb), and intergenic regions (peaks outside of the classified regions). (B) Number of protein-coding genes and miRNAs that are mapped to HNF-1β binding sites. (C) Total number of active enhancers in the kidney identified in the mouse ENCODE Project (upper bar) and number of active enhancers corresponding to HNF-1β binding sites (lower bar). (D) Microarray analysis of mRNA expression in kidney cells expressing HNF-1βΔC. Heat map depicting genes that are differentially expressed in response to induction of the HNF-1βΔC mutant (FDR<0.05). Data shown are from three independent experiments. The numbers of upregulated and downregulated genes are indicated in the histogram. (E) Venn diagrams combining the ChIP-seq data and microarray data to identify direct mRNA targets that are differentially expressed in response to induction of the HNF-1βΔC mutant. (F) Mapping of enhancers to intergenic and intragenic domains. Venn diagrams show the number of genes located in close proximity to enhancers occupied by HNF-1β and differentially expressed in response to induction of the HNF-1βΔC mutant.
Next, we mapped the HNF-1β binding sites to known mRNA and miRNA transcripts. Binding sites were linked to a gene if they were located within 50 kb upstream of the transcription start site or within the body of the gene. Binding sites were linked to an miRNA if there was no intervening gene between the binding site and the miRNA. On the basis of these criteria, in total, 4725 mRNAs and 85 miRNAs were mapped to the HNF-1β binding peaks (Figure 1B). We performed quantitative ChIP to validate the ChIP-seq results for a representative sample of the target genes (Supplemental Figure 1A).
Active enhancers can be distinguished by epigenetic marks, such as histone H3 lysine 4 monomethylation and lysine 27 acetylation,17,18 as well as binding of RNA polymerase 2.19 We determined whether the HNF-1β binding sites that were identified by ChIP-seq overlapped with active enhancer marks in the kidney identified from the mouse ENCODE Project.20 Using this approach, in total, 6501 enhancers were identified in the mouse kidney, of which 680 were occupied by HNF-1β (Figure 1C). Supplemental Figure 1C depicts an example of an active enhancer located between Psat1 and Cep78 genes showing colocalization with HNF-1β binding.
To determine the consensus HNF-1β binding sequence in our ChIP-seq dataset, we extracted the sequence elements and performed motif analysis. When we examined binding sites in gene bodies and intergenic domains, only the half–site consensus motif for HNF-1β was over-represented. However, when we examined peaks extracted from promoter regions, the full HNF-1β consensus motif was significantly enriched (Supplemental Figure 1D).
Identification of Genes That Are Directly Regulated by HNF-1β
To identify genes that are regulated by HNF-1β, we performed microarray analysis on HNF-1β mutant cells. RNA was extracted from renal epithelial cells (53A cells) expressing a dominant negative HNF-1β mutant lacking the C–terminal transcriptional activation domain (HNF-1βΔC),2,4 and gene expression profiles were compared with uninduced cells. Expression of the HNF-1βΔC mutant altered the expression of 4878 mRNAs, of which 2610 were downregulated and 2268 were upregulated (Figure 1D). We validated the microarray results by performing real–time quantitative RT-PCR (qRT-PCR) analysis on a representative subset of mRNAs targets. We found a high correlation (Pearson coefficient =0.89) between the two datasets (Supplemental Figure 2A).
Next, we combined the gene expression profiles with the ChIP-seq data to identify direct HNF-1β targets. Genes that were differentially expressed in HNF-1β mutant cells and contained a nearby HNF-1β binding site were considered to be direct targets. Genes that were differentially expressed but did not contain a nearby HNF-1β binding site were considered to be indirect targets. Figure 1E shows that 1545 protein-coding genes were identified as direct targets of HNF-1β. Of these genes, 591 were upregulated and 954 were downregulated in HNF-1β mutant cells. This unbiased method identified multiple genes that have previously been identified as direct HNF-1β targets, including Pkhd1, Glis2, Pde4c, and Socs3, further establishing the validity of the approach (Supplemental Figure 2B).12
We also analyzed HNF-1β binding to active enhancers and correlated binding with changes in gene expression (Figure 1F). Active enhancers bound by HNF-1β were distributed between intragenic (n=360) and intergenic (n=320) domains. In addition, we found 341 unique genes that mapped to intragenic enhancers, of which 138 (40%) were differentially expressed in HNF-1β mutant cells. In total, 558 unique genes mapped 5′ or 3′ to the intergenic enhancers, of which 157 (28%) were differentially expressed. These data indicate that HNF-1β binds to active enhancers and regulates a subset of nearby genes.
HNF-1β Directly and Indirectly Regulates Genes Involved in Cholesterol Synthesis
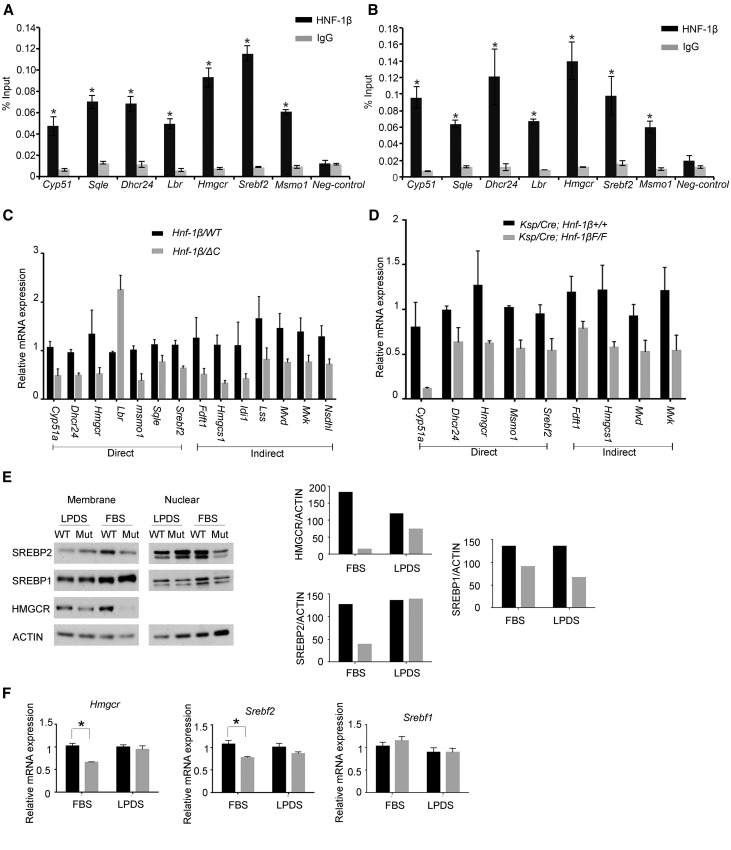
To gain insight into the biologic functions of the genes that are directly regulated by HNF-1β, we performed KEGG pathway analysis using WebGestalt software.21 Table 1 lists the highest scoring pathways. Genes that function in metabolic pathways were over-represented, and within this category, cholesterol biosynthesis was the top scoring pathway. Seven genes (Cyp51, Dhcr24, 3-hydroxy-3-methylglutaryl-CoA reductase [Hmgcr], Lbr, Msmo1, Sqle, and sterol regulatory element binding factor 2 [Srebf2]) representing 26% of the genes in the cholesterol biosynthesis pathway were identified as direct HNF-1β targets. Seven additional genes in the cholesterol biosynthesis pathway (Fdft1, Hmgcs1, Idi1, Lss, Mvd, Mvk, and Nsdhl) were downregulated in HNF-1β mutant cells but did not contain a nearby binding site and therefore, were considered to be indirect targets. Supplemental Figure 3 shows the locations of HNF-1β binding peaks within or near target genes. The enrichment of HNF-1β binding at the respective site for each target gene was confirmed by ChIP-quantitative PCR (qPCR) both in mIMCD3 cells and in vivo in the kidney (Figure 2, A and B). We also performed ChIP-qPCR analysis of the identified target genes in uninduced 53A cells and found similar enrichment of HNF-1β binding (Supplemental Figure 1B). The changes in the expression of direct and indirect target genes were confirmed by qRT-PCR in cells expressing the HNF-1βΔC mutant (Figure 2C). qRT-PCR expression analysis was also performed on kidneys from Ksp-Cre;Hnf-1βflox/flox mice, in which HNF-1β is specifically inactivated in renal tubules.13 Kidney-specific inactivation of HNF-1β inhibited the expression of key genes involved in cholesterol synthesis, including Hmgcr and Srebf2 (Figure 2D). To determine if the reduction in mRNA levels resulted in lower protein abundance, we performed Western blot assays. Expression of the HNF-1βΔC mutant significantly reduced the amount of active sterol regulatory element binding protein-2 (SREBP-2) in the nucleus and HMGCR in membranes (Figure 2E, FBS). These findings indicate that HNF-1β directly and indirectly regulates the renal expression of genes involved in cholesterol synthesis both in vitro and in vivo.
Table 1.
Deregulated pathways in HNF-1β mutant cells
| Pathways | Count | P Value | FDR |
|---|---|---|---|
| Metabolic pathways | 125 | <0.001 | 7.59E-37 |
| Pathways in cancer | 57 | <0.001 | 1.38E-27 |
| Wnt signaling | 31 | <0.001 | 9.79E-17 |
| Endocytosis | 35 | <0.001 | 1.20E-15 |
| Exon guidance | 27 | <0.001 | 4.98E-15 |
| Focal adhesion | 30 | <0.001 | 7.40E-13 |
| Basal cell carcinoma | 16 | <0.001 | 1.76E-11 |
| Adherens junction | 18 | <0.001 | 2.19E-11 |
| Melanogenesis | 20 | <0.001 | 4.22E-11 |
| Phosphatidylinositol signaling | 16 | <0.001 | 3.65E-09 |
| Glycerophospholipid metabolism | 16 | <0.001 | 4.98E-09 |
| Hedgehog signaling | 13 | <0.001 | 1.60E-08 |
| Leukocyte endothelial migration | 18 | <0.001 | 4.01E-08 |
| MAPK signaling | 27 | <0.001 | 4.90E-08 |
The analysis was performed on direct HNF-1β targets with P<0.05. Columns indicate KEGG pathway, gene count, P value, and FDR for the enrichment. FDR, false discovery rate; MAPK, mitogen-activated protein kinase, KEGG, Kyoto Encyclopedia of Genes and Genomes.
Figure 2.
HNF-1β regulates the expression of genes involved in cholesterol synthesis. ChIP showing occupancy of the indicated genes by endogenous HNF-1β in chromatin from (A) mIMCD3 cells and (B) 28-day-old mouse kidney. Enrichment of HNF-1β binding was calculated using the percentage input method and compared with control IgG. Error bars represent SEM (n=3). *P<0.05. (C) qRT-PCR analysis showing altered expression of genes involved in cholesterol synthesis in cells expressing the HNF-1βΔC mutant (gray bars) compared with wild-type cells (black bars). Direct targets indicate genes that are located near HNF-1β binding sites. Data shown are means±SEMs of three independent experiments. All pairwise comparisons were significantly different (P<0.05). (D) qRT-PCR analysis showing altered expression of genes involved in cholesterol synthesis in kidneys from 28-day-old HNF-1β mutant mice (Ksp/Cre;Hnf-1βF/F; gray bars) compared with control littermates (black bars). Data shown are means±SEMs of three independent experiments. All pairwise comparisons were significantly different (P<0.05). (E) Western blot analysis showing the expression of SREBP-2, SREBP-1, and HMGCR in wild-type cells (WT) and cells expressing the HNF-1βΔC mutant (Mut). Cells were cultured in FBS or LPDS for 48 hours before analysis. Right panel shows densitometric analysis of the HMGCR and nuclear fractions of SREBP1 and SREBP2 normalized to the levels of actin. (F) Expression of Hmgcr, Srebf1, and Srebf2 in wild-type cells (black bars) and cells expressing the HNF-1βΔC mutant (gray bars). Cells were cultured in either 10% FBS or 10% LPDS, induced for 48 hours, and then, subjected to qRT-PCR analysis. Error bars represent SEM. *P<0.05.
HNF-1β Regulates Cholesterol Synthesis
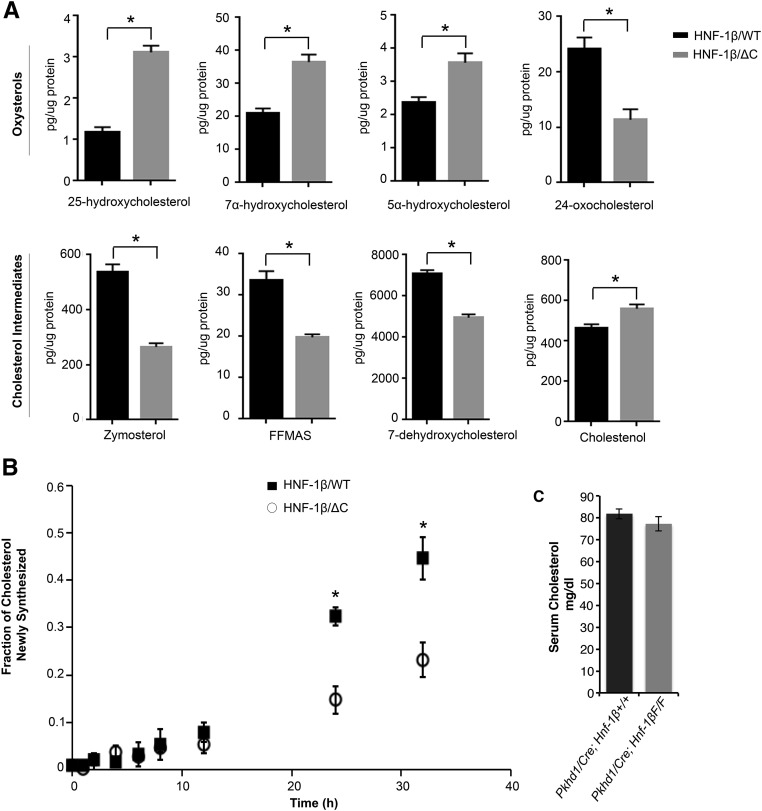
To determine if the observed changes in gene expression and protein levels affected sterol metabolism, we measured the level of sterols and oxysterols by HPLC-tandem mass spectrometry (MS).22 The total levels of cholesterol were not detectably different between HNF-1β mutant cells and controls. In contrast, the levels of major intermediates in sterol synthesis, including zymosterol, 7-dehydrocholesterol, and follicular fluid meiosis–activating sterol, were significantly decreased in HNF-1β mutant cells (P<0.05) (Figure 3A). Conversely, three oxysterols (7α-hydroxycholesterol, 5α-hydroxycholesterol, and 25-hydroxycholesterol) were increased (P<0.05) (Figure 3A). The decreased levels of cholesterol synthetic intermediates are consistent with the decreased expression of cholesterol biosynthetic genes in HNF-1β mutant cells (Supplemental Figure 4).
Figure 3.
Altered sterol levels and cholesterol synthesis rate in HNF-1β mutant cells. (A) Levels of cholesterol intermediates and oxysterols were measured by HPLC/MS in wild-type cells (black bars) and cells expressing the HNF-1βΔC mutant (gray bars). Cells were cultured in 10% FBS. Error bars indicate SEM. *P<0.05. (B) Cholesterol synthesis rate in wild-type cells (▪) and cells expressing the HNF-1βΔC mutant (○). Cells were labeled with D2O, and the incorporation of the isotope into cholesterol was measured at the indicated time points. Data shown are the means±SDs of three separate experiments. *P<0.05. (C) Serum cholesterol in 35-day-old HNF-1β mutant mice (Pkhd1/Cre;Hnf-1βF/F; gray bars) compared with control littermates (black bars). Data shown are means±SEMs of six independent experiments. FFMAS, follicular fluid meiosis-activating sterol.
Next, we directly measured the rate of cholesterol synthesis using deuterium (D2O) labeling.23 D2O (5%) was added to the culture media, and incorporation into cholesterol was measured at different time points by HPLC/MS. Mutant cells exhibited a 55% reduction in the cholesterol synthesis rate 36 hours after induction of HNF-1βΔC (Figure 3B). Taken together, these findings show that HNF-1β plays a significant role in regulating cholesterol synthesis in mouse kidney cells. Mutation of HNF-1β inhibits cholesterol synthesis, which alters the levels of cholesterol intermediates and metabolites.
To determine if the changes in renal cholesterol synthesis affected circulating cholesterol levels, we measured serum cholesterol in kidney–specific HNF-1β mutant mice. For these experiments, we used Pkhd1/Cre;Hnf-1βflox/flox mice with more slowly progressive cystic kidney disease, in which serum cholesterol could be measured before the onset of renal failure and its confounding effects on lipid metabolism. As shown in Figure 3C, serum cholesterol levels were not significantly different between HNF-1β mutant mice and control littermates, confirming that the kidney is not a major source of circulating cholesterol.
HNF-1β Regulates Pcsk9, a Regulator of Cholesterol Uptake
Because the presence of lipoproteins and cholesterol in the cell media can affect the expression of cholesterol biosynthetic genes,24 we performed qRT-PCR analysis on cells cultured in lipoprotein-depleted serum (LPDS). Incubation of HNF-1β mutant cells in LPDS prevented the downregulation of SREBP-2 and partially restored the expression of HMGCR at both the mRNA and protein levels (Figure 2, E and F). SREBP-1 protein was slightly downregulated under both conditions. These findings suggested that HNF-1β regulates cholesterol synthesis and that this regulation may be influenced by cholesterol uptake from the growth medium.
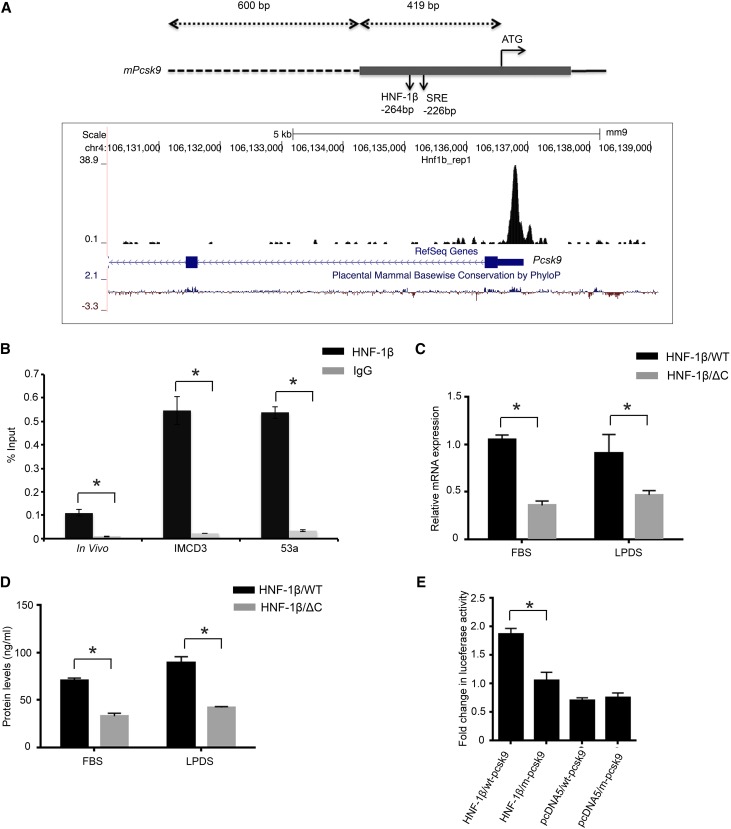
To further explore the mechanism, we re-examined the ChIP-seq dataset and identified a prominent HNF-1β binding site in the first exon of Pcsk9 in chromatin from renal epithelial cells (Figure 4A). This site was located 419 bp upstream from the start codon in close proximity to the sterol regulatory element. PCSK9 inhibits the expression of the LDL receptor on the cell surface and thereby, regulates cellular uptake of LDL cholesterol.25 We verified that Pcsk9 is a direct target gene of HNF-1β by quantitative ChIP-qPCR of chromatin from mIMCD3 cells, uninduced 53A cells, and kidney tissue (Figure 4B). To determine whether HNF-1β binding results in altered expression of Pcsk9, we measured Pcsk9 mRNA transcripts. qRT-PCR analysis showed that induction of mutant HNF-1β reduced the expression of Pcsk9 by approximately 50% (P<0.05). The decrease in Pcsk9 mRNA levels persisted in mutant cells cultured in LPDS (Figure 4C). PCSK9 is synthesized as an inactive proenzyme that undergoes proteolytic cleavage, and the catalytically active fragment is secreted from the cell.25,26 HNF-1β mutant cells exhibited a 50% reduction in the levels of active PCSK9 secreted in the media, which was similar under both FBS and LPDS conditions (Figure 4D). These results indicate that the regulation of Pcsk9 by HNF-1β is independent of the presence of lipoproteins in the culture media.
Figure 4.
HNF-1β regulates Pcsk9 expression and promoter activity. (A, upper panel) Schematic diagram of the mouse Pcsk9 promoter region showing the HNF-1β binding site and sterol regulatory element (SRE) relative to the ATG start codon. The gray bar indicates the first exon, the dashed line indicates the 5′ flanking sequence, and the line indicates the first intron. (A, lower panel) Genomic coordinates and size bar are shown at the top. HNF-1β binding peaks from ChIP-seq are shown in black. Blue boxes indicate Pcsk9 exons, and arrowheads indicate the direction of transcription. The bottom line indicates evolutionary sequence conservation. Data were visualized using the UCSC Genome Browser.54 (B) Quantitative ChIP-qPCR showing binding of HNF-1β to the indicated region of Pcsk9 in mIMCD3 cells, uninduced 53A cells, and adult kidney. Data shown are means±SEMs of three independent experiments. *P<0.05. (C) Expression of Pcsk9 mRNA in wild-type cells (black bars) and cells expressing the HNF-1βΔC mutant (gray bars) cultured in either FBS or LPDS. (D) ELISA of cleaved PCSK9 protein in wild-type cells (black bars) and cells expressing the HNF-1βΔC mutant (gray bars) cultured in FBS or LPDS. (E) Luciferase assays of Pcsk9 promoter activity. A DNA fragment extending 1019 bp upstream to the ATG start codon and containing 419 bp of the first exon and 600 bp of the 5′ flanking sequence was cloned into a luciferase reporter plasmid. mIMCD3 cells were transfected with equimolar amounts of wild-type (wt-pcsk9) or mutant (m-pcsk9) reporter plasmid and an HNF-1β or control (pcDNA5) expression plasmid. Luciferase activity was measured 48 hours after transfection. Luciferase activity was significantly reduced in m-pcsk9 transfected cells. Data shown are means±SEMs of three independent experiments. *P<0.05.
To test whether HNF-1β functions as a transcriptional activator of Pcsk9, we performed reporter gene assays. A genomic fragment containing the promoter and HNF-1β binding site was cloned into a luciferase reporter plasmid and transfected into mIMCD3 cells. Luciferase activity was increased 3.5-fold compared with the empty reporter plasmid (Figure 4E). Mutation of the HNF-1β binding sites reduced luciferase activity by 50%. These results indicate that HNF-1β directly stimulates Pcsk9 transcription in renal epithelial cells.
Discussion
In this study, we used an unbiased approach combining ChIP-seq and gene expression profiling to identify genes and networks that are directly regulated by the transcription factor HNF-1β in renal epithelial cells. ChIP-seq revealed a wide range of HNF-1β binding sites throughout the mouse genome. Most HNF-1β binding sites are located within or near protein-coding genes. In addition, the majority of sites within gene promoters contained the full HNF-1β consensus motif, whereas sites outside promoters predominantly contained the HNF-1β half-site. HNF-1β binding and regulatory activity may be strongest at gene promoters. We also found that approximately 10% of the total active enhancers in the kidney are occupied by HNF-1β. More occupied enhancers are located in intragenic regions than intergenic regions. Only a minority of genes (<50%) in close proximity to the mapped enhancers showed changes in expression in HNF-1β mutant cells. It is possible that some of the identified enhancers may regulate genes through long–range chromatin interactions and may not necessarily regulate the genes that are in closest proximity to HNF-1β–bound enhancers. Alternatively, the epigenetic marks of active chromatin in whole kidney may differ from the histone modifications in mIMCD3 cells.
By combining the mRNA microarray results with the ChIP-seq results, we identified 1545 protein-coding genes as direct targets of HNF-1β. Some of the identified HNF-1β targets have been previously shown to play a role in cyst formation and tubulogenesis (Pkhd1, Glis2, Cys1, and Socs3).4,27–29 To understand the biologic functions of the genes that are directly regulated by HNF-1β, we performed KEGG pathway analysis. Not surprisingly given the increased cell proliferation in kidney cysts, many HNF-1β target genes have known functions in cancer. Specifically, genes in the Wnt and hedgehog signaling pathways were identified. Consistent with this result, previous studies have shown that HNF-1β regulates the expression of Wnt9b, Glis2, and Glis3.7,30 An unexpected finding was that the largest number of protein-coding genes that are directly regulated by HNF-1β (n=125) functions in metabolic pathways. In particular, genes that are involved in cholesterol synthesis were over-represented in the dataset.
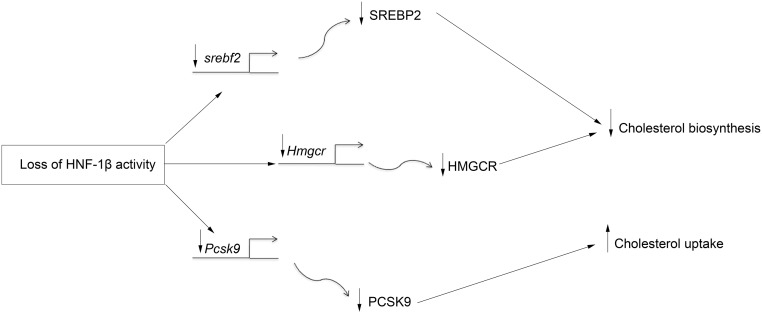
Cholesterol synthesis has been extensively studied in the liver, the source of circulating LDL. Less is known about the regulation of synthesis in peripheral tissues, such as the kidney. Here, we show that multiple genes in the cholesterol biosynthetic pathway, including Lbr, Cyp51, Dhcr24, Hmgcr, Msmo1, and Sqle, contain binding sites for HNF-1β and are downregulated in HNF-1β mutant cells, which suggests that HNF-1β directly regulates their expression. The HNF-1β gene targets include Hmgcr, which encodes HMG-CoA reductase, the enzyme that catalyzes the rate-limiting step in cholesterol synthesis. Seven additional genes involved in cholesterol synthesis (Fdft1, Hmgcs1, Idi1, Lss, Mvd, Mvk, and Nsdhl) were indirectly regulated by HNF-1β. The expression of these genes is regulated by SREBP-2. The gene encoding SREBP-2 (Srebf2) contains a prominent HNF-1β binding site in the first intron and a smaller peak in the promoter, and its expression is decreased in HNF-1β mutant cells and kidneys. Collectively, these findings show that HNF-1β regulates cholesterol synthesis directly through transactivation of genes, such as Hmgcr, as well as indirectly through transactivation of Srebf2 (Figure 5).
Figure 5.
Multilevel regulation of renal cholesterol metabolism by HNF-1β. Loss of HNF-1β inhibits the transcription of genes involved in cholesterol synthesis either directly (middle line) or indirectly through inhibition of Srebf2 (top line). In addition, loss of HNF-1β inhibits the transcription of Pcsk9, which may increase cholesterol uptake (bottom line). Not shown is that loss of HNF-1β also leads to increased levels of 25-hydroxycholesterol, which may further inhibit SREBP-2 activity.
Consistent with the alterations in gene transcription, the levels of HMGCR protein were reduced in HNF-1β mutant cells. This reduction resulted in a lower rate of cholesterol synthesis and reduced levels of major cholesterol biosynthetic intermediates. These findings show that HNF-1β plays a physiologically significant role in cholesterol synthesis in the kidney. In addition, the observed increase in oxysterols, such as 25-hydroxycholesterol, may contribute to the reduction in cholesterol synthesis in HNF-1β mutant cells. By antagonizing SREBP-2 transcriptional activity and promoting its retention in the ER, 25-hydroxycholesterol exerts a negative feedback on cholesterol synthesis.31
ChIP-seq also identified Pcsk9 as a direct transcriptional target of HNF-1β. PCSK9 is a serine protease that plays a crucial role in regulating cholesterol influx by degradation of the LDL receptor.25 Wild–type HNF-1β transactivates the Pcsk9 promoter, whereas mutation of HNF-1β inhibits the expression of Pcsk9 and reduces the amount of secreted active PCSK9. Secreted PCSK9 binds to the EGF repeats of the LDL receptor and promotes its degradation, which reduces cellular uptake of LDL cholesterol. Therefore, reduced production of active PCSK9 in HNF-1β mutant cells would be predicted to promote cholesterol uptake (Figure 5), which would mitigate the effects of inhibition of cholesterol synthesis and may explain why we did not detect changes in total cholesterol content. Consistent with a dependence on exogenous cholesterol, incubation of HNF-1β mutant cells in lipoprotein-deficient serum prevented the decrease in nuclear SREBP-2 and reduced the magnitude of the inhibition of HMGCR mRNA and protein.
HNF-1β is structurally related to HNF-1α, a transcription factor that has previously been shown to play a role in lipid metabolism in the liver.32 Inactivation of HNF-1α increases hepatic cholesterol synthesis accompanied by upregulation of Hmgcr and Fdft1, two key cholesterol synthetic genes. In contrast, we found that inhibition of HNF-1β in kidney cells leads to downregulation of Hmgcr and Fdft1 and reduces cholesterol synthesis. These findings indicate that HNF-1α and HNF-1β have opposing effects on cholesterol synthesis in the liver and kidney, respectively. The expression of Pcsk9 also seems to be differentially regulated by HNF-1α and HNF-1β. In the liver, expression of Pcsk9 is regulated by HNF-1α, whereas HNF-1β has no effect.33,34 In contrast, we found that HNF-1β is a strong activator of Pcsk9 in kidney cells. In the adult rat kidney, Pcsk9 is primarily expressed in the inner medulla,35 which overlaps with the expression of HNF-1β. In contrast, HNF-1α is restricted to proximal tubules and therefore, unlikely to be involved in expression of Pcsk9 in the inner medulla. Although the function of PCSK9 in the kidney is unclear, PCSK9 may regulate cholesterol uptake in renal epithelial cells similar to its function in hepatocytes. In addition, PCSK9 produced by transgenic overexpression in the kidney has also been shown to enter the bloodstream and inhibit the uptake of LDL cholesterol in the liver.36
Because the liver is the major source of LDL cholesterol, it is unlikely that HNF-1β–dependent renal cholesterol synthesis will influence circulating cholesterol levels. Indeed, we found that serum cholesterol levels were unaffected by deletion of HNF-1β in the kidney. Instead, HNF-1β seems to regulate intrarenal cholesterol metabolism. Previous studies have identified two other transcription factors, SREBP-2 and liver X receptor, that are expressed in the kidney and have known roles in cholesterol metabolism.37 In response to low cholesterol levels, proteolytic processing of SREBP-2 releases an N-terminal domain that binds sterol response elements and activates cholesterol synthetic genes. Liver X receptor is an orphan hormone receptor that mediates cholesterol efflux through transcriptional regulation of ABC transporters. Our studies show that intrarenal cholesterol metabolism is also regulated by the transcription factor HNF-1β and that this regulation is physiologically relevant, because mutations of HNF-1β alter sterol levels and the rate of cholesterol synthesis. Moreover, we found that the gene encoding SREBP-2 is itself activated by HNF-1β, which indicates that HNF-1β acts upstream in a transcriptional network that regulates cholesterol synthesis and uptake in kidney cells.
Cholesterol is an essential constituent of the plasma membrane, and its metabolic precursors play important roles in signaling pathways, such as hedgehog and Wnt signaling.38 These pathways are deregulated in HNF-1β mutant cells (Table 1), although whether the perturbations are directly related to the loss of HNF-1β activity or consequences of the abnormal sterol profile remains to be determined. Expression of HNF-1β is increased after ischemic kidney injury,39 a condition that is also associated with stimulation of renal cholesterol synthesis.40,41 One possibility is that the increase in renal cholesterol synthesis, which seems to have a cytoprotective function, is mediated by HNF-1β through activation of cholesterol biosynthetic genes.
In summary, we have identified a novel role of HNF-1β in the regulation of multiple steps in renal cholesterol metabolism (Figure 5). The apparently paradoxical effect of wild–type HNF-1β to both stimulate cholesterol synthesis and inhibit cholesterol uptake is reminiscent of the role of SREBP-2, which also activates transcription of cholesterol biosynthetic genes and Pcsk9. In the case of SREBP-2, the inhibition of cholesterol uptake is thought to militate against cholesterol overload in the liver, and it is possible that HNF-1β plays a similar role in the kidney. Additional studies will be needed to define the contribution of abnormal cholesterol metabolism to the pathogenesis of cystic kidney disease and other HNF-1β mutant phenotypes.
Concise Methods
Cell Lines and Animals
Wild–type mIMCD3 cells and its derived cell line (53A) that expresses HNF-1βΔC3 were grown to confluence in growth medium consisting of low-glucose DMEM (Invitrogen, Carlsbad, CA) supplemented with 10% FBS (US Biotechnologies); 53A cells were treated with mifepristone to induce expression of the HNF-1βΔC mutant as described previously,4 and vehicle-treated cells were used as a negative control. Kidney-specific inactivation of HNF-1β was achieved using Cre/LoxP recombination by crossing Ksp-Cre mice or Pkhd1-Cre mice with Hnf-1βflox/flox mice as previously described.13,14 All animal procedures were performed in accordance with the guidelines of the Institutional Animal Care and Use Committees of the University of Texas Southwestern Medical Center and the University of Minnesota Medical School.
Plasmids and Reporter Gene Assay
The HNF-1β binding region in the promoter of Pcsk9 was amplified from genomic DNA using the following primers: 5′-cggggtaccctgccgaacagtgccagactggg-3′ and 5′-cccaagcttcggggcgaggagaggtgcgc-3′. The 1019-bp amplified region was digested with KpnI and HindIII and cloned into pGL3-Basic to produce the wt-Pcsk9-Luc reporter plasmid. A mutant Pcsk9-Luc reporter plasmid containing a mutation of the HNF-1β binding site was generated by site-directed mutagenesis using the Quick-Change XL II Site-Directed Mutagenesis Kit (Stratagene) using the following primers: 5′-ccccatcggaagatcctctctgagttaccatgcaagggccccggtactaaaggatca-3′ and 5′-ctgatcctttagtaccggggcccttgcatggtaactcagagaggatcttccgatgggg-3′. The sequences of wild-type and mutant plasmids were verified by DNA sequencing. Reporter gene assays were performed by plating cells in six–well plastic dishes at a density of 1.5×105 cells per dish. After 24 hours, when cells had reached 50% confluence, cells were transfected with equimolar amounts of plasmid DNA using the Lipofectamine Plus Reagent (Invitrogen). Cells were lysed 48 hours after transfection and assayed for luciferase activity as previously described.3
Real-Time PCR
The 53A cells were cultured for 24 hours followed by induction of mutant HNF-1βΔC protein for 48 hours. Total RNA from cells or postnatal day 28 adult wild–type or HNF-1β mutant mouse kidneys was extracted using the RNeasy Mini Kit (Qiagen, Germantown, MD) according to the manufacturer’s protocol. cDNA was synthesized using the iScript cDNA Synthesis Kit (Bio-Rad, Hercules, CA), and real-time qPCR was performed with the iTAG Universal SYBER Green Supermix (Bio-Rad) using the CFX Connect Real-Time System (Bio-Rad). Gene expression levels were normalized to 18S rRNA. Primers used for qRT-PCR are listed in Supplemental Table 2.
Gene Expression Profiling
Microarray experiments on total RNA were performed at the Genomics and Microarray Core Facility at the University of Texas Southwestern Medical Center. Briefly, total RNA from three different samples of induced and uninduced 53A cells was extracted as described above. RNA was reversed transcribed, and the cDNA was fluorescently labeled and hybridized with Mouse Gene 1.0 ST Array (Affymetrix, Santa Clara, CA). Normalization of probe–level gene expression data across experiments was done using the RMA method available within the Bioconductor R package.42 An FDR value cutoff of 0.05 was applied to identify genes significantly expressed between conditions. Annotation and summary of genes were done using the Bioconductor R package.43 Microarray data were deposited in the Gene Expression Omnibus (accession no. GSE72033).
Quantitative ChIP-qPCR
ChIP assays were performed using the ChIP-IT High Sensitivity Kit (Active Motif) or the EZ ChIP Kit (EMD Millipore, Billerica, MA) according to the manufacturer’s protocol. Briefly, mIMCD3 cells, uninduced 53A cells, or mouse kidney tissue were crosslinked with 1% formaldehyde for 15 minutes at room temperature. Crosslinked tissues were homogenized into a single-cell suspension, and chromatin samples were extracted from the nuclei and sonicated. Immunoprecipitation was performed with 5 μg rabbit anti–HNF-1β (sc-22840; Santa Cruz Biotechnology, Santa Cruz, CA) and rabbit IgG (sc-2027; Santa Cruz Biotechnology) antibody as a negative control. Immunoprecipitated DNA or 1% of the input was diluted 1:20 in dH2O, and real-time PCR was performed in triplicate using gene-specific primers and SOSadvanced Cyber Green Supermix (Bio-Rad). ChIP-qPCR data were normalized using the percentage input method according to the following formula: 100×2((Ct(input) −6.644) − Ct(HNF-1β)). Mock immunoprecipitation (IgG) was used as a negative control; t test was used for statistical analysis. Primers used for ChIP-qPCR are listed in Supplemental Table 3.
ChIP Sequencing
Immunoprecipitation of chromatin bound to HNF-1β in mIMCD3 cells was carried out as described above. Two control (anti-IgG) and two experimental (anti–HNF-1β) samples from the immunoprecipitated DNA were sent for next generation sequencing. Sequencing was performed by the Genomics and Microarray Core Facility at the University of Texas Southwestern Medical Center using an Illumina platform. The quality of the raw sequencing data was assessed using FASTQC. Reads with >70% bases with quality lower than phred score of 20 were removed. Quality score–filtered reads were then aligned to the mouse reference genome NCBI37 (mmu9) using Burrows–Wheeler aligner.44 Identification of transcription factor binding sites was performed using the peak calling algorithm QuEST.45 A fold change cutoff of three was applied to identify significant peaks between experimental and background samples. The identified peaks were annotated using HOMER.46 The ChIP-seq data have been deposited in the Gene Expression Omnibus (accession no. GSE71250).
Motif Analyses
Sequence elements from the ChIP-Seq data were extracted, and prediction of the consensus binding motifs was performed using MEME-Chip.47 MEME software was used to predict de novo motifs that were statistically over-represented within a 200-bp region centered on the genomic coordinates where HNF-1β was bound. Analysis was carried out on peaks derived from three separate HNF-1β binding regions (promoter, intergenic, and intragenic) for which common and novel binding motifs were discovered.
HPLC-MS and Cholesterol Synthesis Rate
Cholesterol synthesis rate was analyzed in wild-type and HNF-1βΔC –expressing cells. Briefly, cells were seeded in 6-mm dishes, cultured in 10% FBS for 24 hours, and induced for 16 hours. D2O (5%) was then added to the media, and cells were harvested at different time points after D2O labeling (time =0, 1, 2, 4, 6, 8, 12, 24, and 32 hours). After harvesting, the cells were saponified in 1% KOH at 60°C for 2 hours. After saponification, the lipids were isolated using a Bligh–Dyer extraction48 and reconstituted in 300 μl methanol. To determine the synthesis rate, the isotopomer pattern of cholesterol was measured using HPLC/MS. A Shimadzu LC20A HPLC System (Shimadzu, Tokyo, Japan) was used with an Agilent C18 Poroshell Column (Agilent Technologies, Santa Clara, CA) with a linear gradient transitioning from 93% methanol:7% H2O to 100% methanol over 10 minutes.49 The cholesterol isotopic pattern was measured using an AB Sciex Qtrap 4000 (AB Sciex, Framingham, MA).49 The fraction of the cholesterol newly synthesized at each point was determined using isotopic spectral analysis by evaluating the M=0 to M=3 isotopomers.50,51 Cholesterol synthesis rate was estimated by fitting the relationship between fraction of newly synthesized and time to a first–order kinetic models.23 For sterols and oxysterols measurements, cells were seeded in six–well cell culture plates, induced for 48 hours, and subsequently processed for HPLC/MS analysis as previously described.22
Serum Cholesterol Measurements
Mice were anesthetized according to approved protocols, and blood from 35-day-old kidney–specific HNF-1β mutant mice (Pkhd1/Cre;Hnf-1βflox/flox) and control littermates was collected by cardiac puncture. Blood was separated by centrifugation, and 40 μl serum was sent to the University of Texas Southwestern Mouse Metabolic Phenotype Core for determination of cholesterol concentration. Total cholesterol was measured using VITROS CHOL Slides and the VITROS Chemistry Products Calibrator Kit 2 (Vitros 250; reference no. 166 9829; Vitros Chemistry Systems) as previously described.52
Cell Fractionation and Immunoblot Analyses
Pooled cell pellets from triplicate 10-cm plates were washed in PBS; resuspended in 0.5 ml buffer containing 10 mM HEPES-KOH (pH 7.4), 10 mM KCl, 1.5 mM MgCl2, 5 mM EDTA, 5 mM EGTA, 5 mM dithiothreitol, 0.1 mM leupeptin, and 250 mM sucrose; and supplemented with a protease inhibitor cocktail consisting of 1 mM dithiothreitol, 1 mM phenylmethylsulfonyl fluoride, 0.5 mM Pefabloc, 10 μg/ml leupeptin, 5 μg/ml pepstatin A, 25 μg/ml ALLN, and 10 μg/ml aprotinin. The cell suspension was homogenized by passing through a 22-gauge needle 30 times and centrifuged at 2200 rpm for 7 minutes at 4°C. The resulting postnuclear supernatants were further subjected to centrifugation at 100,000×g for 30 minutes at 4°C. The pellet fraction obtained from this spin (designated membranes) was resuspended in 100 μl buffer containing 10 mM Tris-HCl (pH 6.8), 100 mM NaCl, 1% (wt/vol) SDS, 1 mM EDTA, and 1 mM EGTA. The pellet obtained from the original 2200-rpm spin was resuspended in 0.3 ml buffer containing 20 mM HEPES-KOH (pH 7.6), 2.5% (vol/vol) glycerol, 0.42 M NaCl, 1.5 mM MgCl2, 1 mM EDTA, 1 mM EGTA, and the above mentioned protease inhibitor cocktail. The resuspended pellet was then rotated at 4°C for 1 hour and centrifuged at 100,000×g for 30 minutes at 4°C. The supernatant (designated nuclear extract) was precipitated overnight in 1.5 ml acetone at −20°C. The precipitated material was pelleted by centrifugation at 17,000×g for 15 minutes at 4°C and resuspended in 80 μl buffer containing 10 mM Tris-HCl (pH 6.8), 100 mM NaCl, 1% (wt/vol) SDS, 1 mM EDTA, and 1 mM EGTA. Protein concentrations of the membrane and nuclear extract fractions were measured using the BCA Protein Assay Kit (Pierce, Rockford, IL). Before SDS-PAGE, the membranes were mixed with an equal volume of buffer containing 62.5 mM Tris-HCl (pH 6.8), 15% (wt/vol) SDS, 8 M urea, 10% (vol/vol) glycerol, and 100 mM dithiothreitol as well as 66.7 μl 4× SDS loading buffer. The nuclear extracts were mixed with 26.7 μl 4× SDS loading buffer. All samples were incubated at 37°C for 30 minutes and subjected to 10% SDS-PAGE, after which the proteins were transferred to nitrocellulose membranes (GE Healthcare, Waukesha, WI). Immunoblot analysis was carried out with the following primary antibodies: IgG-211, a rabbit polyclonal antibody against SREBP-1 (amino acids 32–250); IgG-22D5, a rabbit mAb against SREBP-2 (amino acids 32–250); IgG-A9, a mouse mAb against the catalytic domain of hamster HMGCR (amino acids 450–887)53; and rabbit polyclonal antiactin antibody (Sigma-Aldrich, St. Louis, MO). Primary antibodies were detected with horseradish peroxidase–conjugated donkey anti–mouse or anti–rabbit (Jackson ImmunoResearch Laboratories, West Grove, PA) using SuperSignal West Pico Chemiluminescent Substrate (Thermo Fisher Scientific, Vernon Hills, IL) according to the manufacturer’s instructions.
ELISA
A LumiNunc Maxisorp White Assay Plate (NalgeNunc) was coated with rabbit anti–mouse PCSK9 polyclonal antibody (IgG-551C) diluted to 5 μg/ml in 100 μl 20 mM sodium phosphate (pH 7.5) and 100 mM sodium chloride (buffer A) and incubated overnight at 4°C. The plate was then washed three times with 350 μl PBS with Tween 20 (pH 7.4) and blocked with 150 ml 0.5% BSA in buffer A for 1 hour at room temperature with shaking. All subsequent plate washes were conducted three times in PBS with Tween 20 on a BioTek ELv405 Plate Washer (BioTek Instruments, Winooski, VT). All samples, standards, and antibodies were diluted in buffer A and 0.5% BSA. Media were diluted 1:10, and 100 μl were added in duplicate to the assay plate. Purified mouse PCSK9 was serially diluted in the range of 0.39–50 ng/ml and added in duplicate as standards. The plate was incubated 2.5 hours at 37°C with shaking. The plate was again washed, and 100 μl rabbit anti–mouse PCSK9 polyclonal antibody (IgG-552C; biotinylated with the EZ-Link Sulfo-NHS-Biotin Kit; Pierce) was added and incubated for 2 hours at room temperature with shaking. After an additional wash, 100 μl Avidin-HRP (1:40,000; Pierce) was added and incubated for 1 hour at room temperature. After a final wash, 100 μl SuperSignal ELISA Pico Substrate (Pierce) was added for 1 minute with shaking and luminescence quantified using a Thermo Fisher Luminoskan Ascent Luminometer (Thermo Fisher Scientific). Linear regression analysis of the standard curve was used to determine media concentrations.
Statistical Analyses
Statistical analyses were performed using t test for pairwise comparisons. P<0.05 was considered significant.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Patricia Cobo-Stark and Sachin Hajarnis for expert technical assistance, Zhendong Ma for assistance with chromatin immunoprecipitation sequencing, and Jeffery McDonald for assistance with liquid chromatography-tandem mass spectrometry. We also thank Jay Horton and David Russell for helpful discussions.
This work was supported by National Institutes of Health (NIH) Grant R37DK042921 (to P.I.) and University of Texas Southwestern O’Brien Kidney Research Core Center NIH Grant P30DK079328. K.A. and L.N. were supported by NIH Training Grant T32DK007257. M.P. was supported by Fondation pour la recherche médicale (FRM), European Community’s Seventh Framework Programmme FP7/2009 Agreement 241955 (SYSCILIA), and Agence Nationale pour le Recherche. R.D.-B. is an Early Career Scientist of the Howard Hughes Medical Institute and was supported by NIH Grant HL20948.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060607/-/DCSupplemental.
References
- 1.Igarashi P, Shao X, McNally BT, Hiesberger T: Roles of HNF-1beta in kidney development and congenital cystic diseases. Kidney Int 68: 1944–1947, 2005 [DOI] [PubMed] [Google Scholar]
- 2.Hiesberger T, Shao X, Gourley E, Reimann A, Pontoglio M, Igarashi P: Role of the hepatocyte nuclear factor-1beta (HNF-1beta) C-terminal domain in Pkhd1 (ARPKD) gene transcription and renal cystogenesis. J Biol Chem 280: 10578–10586, 2005 [DOI] [PubMed] [Google Scholar]
- 3.Gong Y, Ma Z, Patel V, Fischer E, Hiesberger T, Pontoglio M, Igarashi P: HNF-1beta regulates transcription of the PKD modifier gene Kif12. J Am Soc Nephrol 20: 41–47, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ma Z, Gong Y, Patel V, Karner CM, Fischer E, Hiesberger T, Carroll TJ, Pontoglio M, Igarashi P: Mutations of HNF-1beta inhibit epithelial morphogenesis through dysregulation of SOCS-3. Proc Natl Acad Sci U S A 104: 20386–20391, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi YH, McNally BT, Igarashi P: Zyxin regulates migration of renal epithelial cells through activation of hepatocyte nuclear factor-1β. Am J Physiol Renal Physiol 305: F100–F110, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Coffinier C, Gresh L, Fiette L, Tronche F, Schütz G, Babinet C, Pontoglio M, Yaniv M, Barra J: Bile system morphogenesis defects and liver dysfunction upon targeted deletion of HNF1beta. Development 129: 1829–1838, 2002 [DOI] [PubMed] [Google Scholar]
- 7.Lokmane L, Heliot C, Garcia-Villalba P, Fabre M, Cereghini S: vHNF1 functions in distinct regulatory circuits to control ureteric bud branching and early nephrogenesis. Development 137: 347–357, 2010 [DOI] [PubMed] [Google Scholar]
- 8.Haumaitre C, Barbacci E, Jenny M, Ott MO, Gradwohl G, Cereghini S: Lack of TCF2/vHNF1 in mice leads to pancreas agenesis. Proc Natl Acad Sci U S A 102: 1490–1495, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Massa F, Garbay S, Bouvier R, Sugitani Y, Noda T, Gubler MC, Heidet L, Pontoglio M, Fischer E: Hepatocyte nuclear factor 1β controls nephron tubular development. Development 140: 886–896, 2013 [DOI] [PubMed] [Google Scholar]
- 10.Bellanné-Chantelot C, Chauveau D, Gautier J-F, Dubois-Laforgue D, Clauin S, Beaufils S, Wilhelm J-M, Boitard C, Noël L-H, Velho G, Timsit J: Clinical spectrum associated with hepatocyte nuclear factor-1beta mutations. Ann Intern Med 140: 510–517, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Bingham C, Ellard S, Allen L, Bulman M, Shepherd M, Frayling T, Berry PJ, Clark PM, Lindner T, Bell GI, Ryffel GU, Nicholls AJ, Hattersley AT: Abnormal nephron development associated with a frameshift mutation in the transcription factor hepatocyte nuclear factor-1 beta. Kidney Int 57: 898–907, 2000 [DOI] [PubMed] [Google Scholar]
- 12.Hiesberger T, Bai Y, Shao X, McNally BT, Sinclair AM, Tian X, Somlo S, Igarashi P: Mutation of hepatocyte nuclear factor-1beta inhibits Pkhd1 gene expression and produces renal cysts in mice. J Clin Invest 113: 814–825, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Gresh L, Fischer E, Reimann A, Tanguy M, Garbay S, Shao X, Hiesberger T, Fiette L, Igarashi P, Yaniv M, Pontoglio M: A transcriptional network in polycystic kidney disease. EMBO J 23: 1657–1668, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Williams SS, Cobo-Stark P, Hajarnis S, Aboudehen K, Shao X, Richardson JA, Patel V, Igarashi P: Tissue-specific regulation of the mouse Pkhd1 (ARPKD) gene promoter. Am J Physiol Renal Physiol 307: F356–F368, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bai Y, Pontoglio M, Hiesberger T, Sinclair AM, Igarashi P: Regulation of kidney-specific Ksp-cadherin gene promoter by hepatocyte nuclear factor-1beta. Am J Physiol Renal Physiol 283: F839–F851, 2002 [DOI] [PubMed] [Google Scholar]
- 16.Landt SG, Marinov GK, Kundaje A, Kheradpour P, Pauli F, Batzoglou S, Bernstein BE, Bickel P, Brown JB, Cayting P, Chen Y, DeSalvo G, Epstein C, Fisher-Aylor KI, Euskirchen G, Gerstein M, Gertz J, Hartemink AJ, Hoffman MM, Iyer VR, Jung YL, Karmakar S, Kellis M, Kharchenko PV, Li Q, Liu T, Liu XS, Ma L, Milosavljevic A, Myers RM, Park PJ, Pazin MJ, Perry MD, Raha D, Reddy TE, Rozowsky J, Shoresh N, Sidow A, Slattery M, Stamatoyannopoulos JA, Tolstorukov MY, White KP, Xi S, Farnham PJ, Lieb JD, Wold BJ, Snyder M: ChIP-seq guidelines and practices of the ENCODE and modENCODE consortia. Genome Res 22: 1813–1831, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Spicuglia S, Vanhille L: Chromatin signatures of active enhancers. Nucleus 3: 126–131, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zentner GE, Tesar PJ, Scacheri PC: Epigenetic signatures distinguish multiple classes of enhancers with distinct cellular functions. Genome Res 21: 1273–1283, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kim TK, Hemberg M, Gray JM, Costa AM, Bear DM, Wu J, Harmin DA, Laptewicz M, Barbara-Haley K, Kuersten S, Markenscoff-Papadimitriou E, Kuhl D, Bito H, Worley PF, Kreiman G, Greenberg ME: Widespread transcription at neuronal activity-regulated enhancers. Nature 465: 182–187, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stamatoyannopoulos JA, Snyder M, Hardison R, Ren B, Gingeras T, Gilbert DM, Groudine M, Bender M, Kaul R, Canfield T, Giste E, Johnson A, Zhang M, Balasundaram G, Byron R, Roach V, Sabo PJ, Sandstrom R, Stehling AS, Thurman RE, Weissman SM, Cayting P, Hariharan M, Lian J, Cheng Y, Landt SG, Ma Z, Wold BJ, Dekker J, Crawford GE, Keller CA, Wu W, Morrissey C, Kumar SA, Mishra T, Jain D, Byrska-Bishop M, Blankenberg D, Lajoie BR, Jain G, Sanyal A, Chen KB, Denas O, Taylor J, Blobel GA, Weiss MJ, Pimkin M, Deng W, Marinov GK, Williams BA, Fisher-Aylor KI, Desalvo G, Kiralusha A, Trout D, Amrhein H, Mortazavi A, Edsall L, McCleary D, Kuan S, Shen Y, Yue F, Ye Z, Davis CA, Zaleski C, Jha S, Xue C, Dobin A, Lin W, Fastuca M, Wang H, Guigo R, Djebali S, Lagarde J, Ryba T, Sasaki T, Malladi VS, Cline MS, Kirkup VM, Learned K, Rosenbloom KR, Kent WJ, Feingold EA, Good PJ, Pazin M, Lowdon RF, Adams LB Mouse ENCODE Consortium : An encyclopedia of mouse DNA elements (Mouse ENCODE). Genome Biol 13: 418, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhang B, Kirov S, Snoddy J: WebGestalt: An integrated system for exploring gene sets in various biological contexts. Nucleic Acids Res 33: W741–W748, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McDonald JG, Smith DD, Stiles AR, Russell DW: A comprehensive method for extraction and quantitative analysis of sterols and secosteroids from human plasma. J Lipid Res 53: 1399–1409, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Mitsche MA, McDonald JG, Hobbs HH, Cohen JC: Flux analysis of cholesterol biosynthesis in vivo reveals multiple tissue and cell-type specific pathways. eLife 4: e07999, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Cerda SR, Wilkinson J 4th, Broitman SA: Regulation of cholesterol synthesis in four colonic adenocarcinoma cell lines. Lipids 30: 1083–1092, 1995 [DOI] [PubMed] [Google Scholar]
- 25.Horton JD, Cohen JC, Hobbs HH: PCSK9: A convertase that coordinates LDL catabolism. J Lipid Res 50[Suppl]: S172–S177, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Debose-Boyd RA, Horton JD: Opening up new fronts in the fight against cholesterol. eLife 2: e00663, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Attanasio M, Uhlenhaut NH, Sousa VH, O’Toole JF, Otto E, Anlag K, Klugmann C, Treier AC, Helou J, Sayer JA, Seelow D, Nürnberg G, Becker C, Chudley AE, Nürnberg P, Hildebrandt F, Treier M: Loss of GLIS2 causes nephronophthisis in humans and mice by increased apoptosis and fibrosis. Nat Genet 39: 1018–1024, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Hou X, Mrug M, Yoder BK, Lefkowitz EJ, Kremmidiotis G, D’Eustachio P, Beier DR, Guay-Woodford LM: Cystin, a novel cilia-associated protein, is disrupted in the cpk mouse model of polycystic kidney disease. J Clin Invest 109: 533–540, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Williams SS, Cobo-Stark P, James LR, Somlo S, Igarashi P: Kidney cysts, pancreatic cysts, and biliary disease in a mouse model of autosomal recessive polycystic kidney disease. Pediatr Nephrol 23: 733–741, 2008 [DOI] [PubMed] [Google Scholar]
- 30.De Vas MG, Kopp JL, Heliot C, Sander M, Cereghini S, Haumaitre C: Hnf1b controls pancreas morphogenesis and the generation of Ngn3+ endocrine progenitors. Development 142: 871–882, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Adams CM, Reitz J, De Brabander JK, Feramisco JD, Li L, Brown MS, Goldstein JL: Cholesterol and 25-hydroxycholesterol inhibit activation of SREBPs by different mechanisms, both involving SCAP and Insigs. J Biol Chem 279: 52772–52780, 2004 [DOI] [PubMed] [Google Scholar]
- 32.Shih DQ, Bussen M, Sehayek E, Ananthanarayanan M, Shneider BL, Suchy FJ, Shefer S, Bollileni JS, Gonzalez FJ, Breslow JL, Stoffel M: Hepatocyte nuclear factor-1alpha is an essential regulator of bile acid and plasma cholesterol metabolism. Nat Genet 27: 375–382, 2001 [DOI] [PubMed] [Google Scholar]
- 33.Li H, Dong B, Park SW, Lee HS, Chen W, Liu J: Hepatocyte nuclear factor 1alpha plays a critical role in PCSK9 gene transcription and regulation by the natural hypocholesterolemic compound berberine. J Biol Chem 284: 28885–28895, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shende VR, Wu M, Singh AB, Dong B, Kan CF, Liu J: Reduction of circulating PCSK9 and LDL-C levels by liver-specific knockdown of HNF1α in normolipidemic mice. J Lipid Res 56: 801–809, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Seidah NG, Benjannet S, Wickham L, Marcinkiewicz J, Jasmin SB, Stifani S, Basak A, Prat A, Chretien M: The secretory proprotein convertase neural apoptosis-regulated convertase 1 (NARC-1): Liver regeneration and neuronal differentiation. Proc Natl Acad Sci U S A 100: 928–933, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Luo Y, Warren L, Xia D, Jensen H, Sand T, Petras S, Qin W, Miller KS, Hawkins J: Function and distribution of circulating human PCSK9 expressed extrahepatically in transgenic mice. J Lipid Res 50: 1581–1588, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Proctor G, Jiang T, Iwahashi M, Wang Z, Li J, Levi M: Regulation of renal fatty acid and cholesterol metabolism, inflammation, and fibrosis in Akita and OVE26 mice with type 1 diabetes. Diabetes 55: 2502–2509, 2006 [DOI] [PubMed] [Google Scholar]
- 38.de Weille J, Fabre C, Bakalara N: Oxysterols in cancer cell proliferation and death. Biochem Pharmacol 86: 154–160, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Ogata K, Shimamura Y, Hamada K, Hisa M, Bun M, Okada N, Inoue K, Taniguchi Y, Ishihara M, Kagawa T, Horino T, Fujimoto S, Terada Y: Upregulation of HNF-1β during experimental acute kidney injury plays a crucial role in renal tubule regeneration. Am J Physiol Renal Physiol 303: F689–F699, 2012 [DOI] [PubMed] [Google Scholar]
- 40.Naito M, Bomsztyk K, Zager RA: Renal ischemia-induced cholesterol loading: Transcription factor recruitment and chromatin remodeling along the HMG CoA reductase gene. Am J Pathol 174: 54–62, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Johnson AC, Ware LB, Himmelfarb J, Zager RA: HMG-CoA reductase activation and urinary pellet cholesterol elevations in acute kidney injury. Clin J Am Soc Nephrol 6: 2108–2113, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gautier L, Cope L, Bolstad BM, Irizarry RA: affy--analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 20: 307–315, 2004 [DOI] [PubMed] [Google Scholar]
- 43.Du P, Kibbe WA, Lin SM: lumi: A pipeline for processing Illumina microarray. Bioinformatics 24: 1547–1548, 2008 [DOI] [PubMed] [Google Scholar]
- 44.Li H, Durbin R: Fast and accurate long-read alignment with Burrows-Wheeler transform. Bioinformatics 26: 589–595, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Valouev A, Johnson DS, Sundquist A, Medina C, Anton E, Batzoglou S, Myers RM, Sidow A: Genome-wide analysis of transcription factor binding sites based on ChIP-Seq data. Nat Methods 5: 829–834, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Heinz S, Benner C, Spann N, Bertolino E, Lin YC, Laslo P, Cheng JX, Murre C, Singh H, Glass CK: Simple combinations of lineage-determining transcription factors prime cis-regulatory elements required for macrophage and B cell identities. Mol Cell 38: 576–589, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Machanick P, Bailey TL: MEME-ChIP: Motif analysis of large DNA datasets. Bioinformatics 27: 1696–1697, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bligh EG, Dyer WJ: A rapid method of total lipid extraction and purification. Can J Biochem Physiol 37: 911–917, 1959 [DOI] [PubMed] [Google Scholar]
- 49.Quehenberger O, Armando AM, Brown AH, Milne SB, Myers DS, Merrill AH, Bandyopadhyay S, Jones KN, Kelly S, Shaner RL, Sullards CM, Wang E, Murphy RC, Barkley RM, Leiker TJ, Raetz CR, Guan Z, Laird GM, Six DA, Russell DW, McDonald JG, Subramaniam S, Fahy E, Dennis EA: Lipidomics reveals a remarkable diversity of lipids in human plasma. J Lipid Res 51: 3299–3305, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Hellerstein MK, Neese RA: Mass isotopomer distribution analysis at eight years: Theoretical, analytic, and experimental considerations. Am J Physiol 276: E1146–E1170, 1999 [DOI] [PubMed] [Google Scholar]
- 51.Kelleher JK, Kharroubi AT, Aldaghlas TA, Shambat IB, Kennedy KA, Holleran AL, Masterson TM: Isotopomer spectral analysis of cholesterol synthesis: Applications in human hepatoma cells. Am J Physiol 266: E384–E395, 1994 [DOI] [PubMed] [Google Scholar]
- 52.Allain CC, Poon LS, Chan CS, Richmond W, Fu PC: Enzymatic determination of total serum cholesterol. Clin Chem 20: 470–475, 1974 [PubMed] [Google Scholar]
- 53.Liscum L, Luskey KL, Chin DJ, Ho YK, Goldstein JL, Brown MS: Regulation of 3-hydroxy-3-methylglutaryl coenzyme A reductase and its mRNA in rat liver as studied with a monoclonal antibody and a cDNA probe. J Biol Chem 258: 8450–8455, 1983 [PubMed] [Google Scholar]
- 54.Kent WJ, Sugnet CW, Furey TS, Roskin KM, Pringle TH, Zahler AM, Haussler D: The human genome browser at UCSC. Genome Res 12: 996–1006, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.