Abstract
Mitochondrial dysfunction in kidney cells has been implicated in the pathogenesis of CKD. Mitochondrial DNA (mtDNA) copy number is a surrogate measure of mitochondrial function, and higher mtDNA copy number in peripheral blood has been associated with lower risk of two important risk factors for CKD progression, diabetes and microalbuminuria. We evaluated whether mtDNA copy number in peripheral blood associates with incident CKD in a population-based cohort of middle-aged adults. We estimated mtDNA copy number using 25 high-quality mitochondrial single nucleotide polymorphisms from the Affymetrix 6.0 array. Among 9058 participants, those with higher mtDNA copy number had a lower rate of prevalent diabetes and lower C-reactive protein levels and white blood cell counts. Baseline eGFR did not differ significantly by mtDNA copy number. Over a median follow-up of 19.6 years, 1490 participants developed CKD. Higher mtDNA copy number associated with lower risk of incident CKD (highest versus lowest quartile: hazard ratio 0.65; 95% confidence interval, 0.56 to 0.75; P<0.001) after adjusting for age, sex, and race. After adjusting for additional risk factors of CKD, including prevalent diabetes, hypertension, C-reactive protein level, and white blood cell count, this association remained significant (highest versus lowest quartile: hazard ratio 0.75; 95% confidence interval, 0.64 to 0.87; P<0.001). In conclusion, higher mtDNA copy number associated with lower incidence of CKD independent of traditional risk factors and inflammation biomarker levels in this cohort. Further research on modifiable factors influencing mtDNA copy number may lead to improvement in the prevention and treatment of CKD.
Keywords: chronic kidney disease, mitochondria, epidemiology and outcomes, renal function decline
CKD affects 13% of adults in the United States and has few effective treatments.1,2 Identifying novel risk factors for incident CKD may reveal underlying pathophysiology and lead to improvement in prevention and treatment strategies. Mitochondria have a key role in cellular energy production. Recently, mitochondrial dysfunction in podocytes and kidney tubular cells has been implicated in the pathogenesis of CKD.3–5 Molecules that target mitochondrial function, including thiazolidinediones, have been proposed as therapeutic agents for protecting kidney function.6–9 However, studies on measures of mitochondrial dysfunction and CKD at the population level have been limited.
Mitochondrial dysfunction is characterized by mitochondrial fragmentation and the loss of mitochondrial DNA (mtDNA) integrity.3 mtDNA copy number has been used as a surrogate measure of mitochondrial function because mitochondrial integrity is difficult to measure.10 In population-based studies, higher mtDNA copy number in peripheral blood has been associated with lower risk of mortality.11 This association was also observed in patients undergoing hemodialysis.12 Regarding kidney function, higher mtDNA copy number in peripheral blood has been associated with lower risk of diabetes, a risk factor of CKD, and lower prevalence of albuminuria, a marker of kidney damage.13,14 Evaluating the association between mtDNA copy number in peripheral blood and kidney function can yield insight into the relationship between mitochondrial dysfunction in blood cells and CKD. We estimated mtDNA copy number using microarray probe intensities of mitochondrial single nucleotide polymorphisms (SNPs). We evaluated the cross-sectional association between mtDNA copy number and prevalent CKD defined as eGFR<60 ml/min per 1.73m2 and the prospective association between mtDNA copy number and incident CKD in white and black Americans in the Atherosclerosis Risk in Communities (ARIC) Study.
Results
Among the 9244 participants with and without prevalent CKD, defined as eGFR<60 ml/min per 1.73m2, those with CKD (n=183) were older, more likely to be black, had prevalent diabetes and hypertension, higher white blood cell (WBC) count, and higher levels of high-sensitivity C-reactive protein (hsCRP; all P<0.001, Supplemental Table 1). Those with prevalent CKD had lower mtDNA copy number adjusted for age, sex, self-reported race, and study center (Model 1, beta in SD of mtDNA copy number −0.54; 95% confidence interval [95% CI], −0.68 to −0.39; Supplemental Table 2). In the model further adjusted for clinical risk factors (prevalent coronary heart disease, diabetes, and hypertension; smoking status; body mass index [BMI]; serum albumin; hemoglobin A1c [HbA1c]; and log-transformed WBC count and hsCRP), this association remained significant (Model 4, beta in SD of mtDNA copy number −0.37; 95% CI, −0.53 to −0.22).
Among 9058 participants without stage G3 CKD (eGFR≥60 ml/min per 1.73m2) at baseline, the distributions of sex, race, baseline age, BMI, and eGFR were similar by quartiles of mtDNA copy number (Table 1). In contrast, participants with mtDNA copy number in the higher quartiles had lower prevalence of smoking, diabetes, and coronary heart disease, and lower baseline WBC count and hsCRP levels than those in quartile 1 (all P<0.001). mtDNA copy number had a moderate negative correlation with WBC count (Spearman r = −0.23).
Table 1.
Baseline characteristics of participants with eGFR≥60 ml/min per 1.73m2
| Participant Characteristics | Q1 | Q2 | Q3 | Q4 | P value |
|---|---|---|---|---|---|
| n | 2265 | 2264 | 2265 | 2264 | |
| Age, mean (SD) | 56.5 (5.8) | 56.5 (5.7) | 56.5 (5.6) | 56.8 (5.6) | 0.06 |
| Sex, female, % (n) | 56.7 (1284) | 57.4 (1299) | 57.3 (1298) | 55.7 (1260) | 0.62 |
| Race, black, % (n) | 19.6 (443) | 20.4 (461) | 22.3 (504) | 20.3 (459) | 0.14 |
| eGFR, ml/min per 1.73 m2, mean (SD) | 97.2 (14.3) | 97.5 (14.0) | 97.7 (13.6) | 97.4 (13.4) | 0.55 |
| Prevalent diabetes, % (n) | 17.6 (398) | 14.3 (324) | 12.5 (283) | 11.6 (263) | <0.001 |
| Prevalent hypertension, % (n) | 35.3 (799) | 33.3 (755) | 33.3 (755) | 32.9 (745) | 0.33 |
| Smoking status, % (n) | <0.001 | ||||
| Current smoker | 30.7 (696) | 25.4 (574) | 20.9 (473) | 18.2 (412) | |
| Former smoker | 34.3 (777) | 36.8 (834) | 39 (883) | 39.4 (892) | |
| Never smoked | 35 (792) | 37.8 (856) | 40.1 (909) | 42.4 (960) | |
| Prevalent coronary heart disease, % (n) | 6.5 (147) | 4.9 (112) | 4.7 (106) | 3.2 (72) | <0.001 |
| BMI, kg/m2, mean (SD) | 27.7 (5.4) | 28.1 (5.5) | 27.9 (5.3) | 27.9 (5.3) | 0.39 |
| WBC count, 103/μl, median (1st, 3rd quartile) | 6.4 (5.3, 7.7) | 6.0 (5, 7.2) | 5.6 (4.8, 6.7) | 5.3 (4.5, 6.4) | <0.001 |
| HbA1c, median (1st, 3rd quartile)a | 5.4 (5.2, 5.9) | 5.4 (5.2, 5.8) | 5.4 (5.2, 5.8) | 5.4 (5.2, 5.8) | 0.93 |
| Serum albumin, mean (SD)a | 4.18 (0.29) | 4.18 (0.29) | 4.19 (0.28) | 4.18 (0.28) | 0.40 |
| hsCRP, median (1st, 3rd quartile)a | 2.4 (1.1, 5.3) | 2.4 (1.1, 4.8) | 2.1 (1.0, 4.7) | 2.0 (1.0, 4.1) | <0.001 |
n=9058.
Variables are available at visit two only (n=8135).
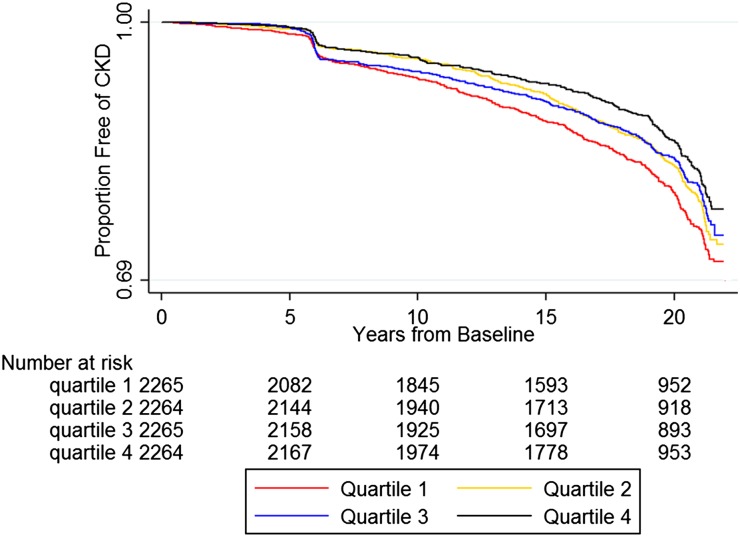
Over a median of 19.6 years of follow-up, 1490 participants developed CKD. Higher quartiles of mtDNA copy number were significantly associated with lower incidence of CKD (incident rate ratio of quartile [Q] 4 versus Q1 0.69; 95% CI, 0.60 to 0.79; p trend <0.001; Table 2). Figure 1 presents the Kaplan–Meier estimates of the proportion free of CKD by quartiles of mtDNA copy number. Those in Q4 clearly had fewer events than those in Q1 throughout the follow-up period, although the pattern for Q2 and Q3 was less clear.
Table 2.
Incident CKD by quartiles of mtDNA copy number
| Q1 | Q2 | Q3 | Q4 | P trend | |
|---|---|---|---|---|---|
| Number of events | 437 | 374 | 360 | 319 | |
| Person-year | 37,326 | 38,833 | 38,739 | 39,722 | |
| Incident CKD per 1000 person-year | 11.7 | 9.6 | 9.3 | 8.0 | |
| Incident rate ratio (95% CI) | 1.00 | 0.82 (0.72 to 0.94) | 0.79 (0.69 to 0.91) | 0.69 (0.60 to 0.79) | <0.001 |
n=9058.
Figure 1.
Kaplan–Meier estimates of the proportion free of CKD by quartiles of mtDNA copy number. We defined the baseline of this study as the visit where mtDNA copy number levels were measured. Of the 9058 participants included in the analysis, we measured mtDNA copy number levels at visit one (1987–89) for 449 participants, at visit two (1990–92) for 8582 participants, and at visit four (1996–98) for 27 participants. Of the 1490 incident CKD events, we ascertained 439 events on the basis of the visit-based definition of eGFR<60 ml/min per 1.73 m2 with at least 25% drop at a follow-up visit. The rest (1051 events) we ascertained on the basis of either CKD hospitalization codes or ESRD events. The drop in the Kaplan–Meier curves around year 6 was mostly from participants with visit two as the baseline who were assessed as having incident CKD at visit four, approximately 6 years after visit two, on the basis of a decline in eGFR.
We conducted a race-specific analysis to assess potential differences in the association between mtDNA copy number and incident CKD between populations. We detected no significant difference between whites and blacks (whites: hazard ratio [HR] of Q4 versus Q1 0.68; 95% CI, 0.58 to 0.81; p trend <0.001; blacks: HR Q4 versus Q1 0.58; 95% CI, 0.44 to 0.78; p trend <0.001; P for interaction between self-reported race and mtDNA copy number 0.18; Table 3). Given the lack of significant interaction between self-reported race and mtDNA copy number, we conducted subsequent analyses on incident CKD in the combined population and adjusted for self-reported race.
Table 3.
Association between mitochondrial DNA copy number and incident CKD
| Modela | n | Q1 | Q2 HR (95% CI) | Q3 HR (95% CI) | Q4 HR (95% CI) | P for trend |
|---|---|---|---|---|---|---|
| Model 1 | ||||||
| Whites | 7191 | 1.00 | 0.78 (0.66, 0.92) | 0.75 (0.63, 0.88) | 0.68 (0.58, 0.81) | <0.001 |
| Blacks | 1867 | 1.00 | 0.89 (0.69, 1.16) | 0.87 (0.67, 1.13) | 0.58 (0.44, 0.78) | <0.001 |
| Combined | 9058 | 1.00 | 0.81 (0.71, 0.93) | 0.78 (0.68, 0.9) | 0.65 (0.56, 0.75) | <0.001 |
| Model 2 | 9058 | 1.00 | 0.86 (0.75, 0.98) | 0.88 (0.76, 1.01) | 0.76 (0.66, 0.89) | <0.001 |
| Model 3 | 9058 | 1.00 | 0.89 (0.77, 1.02) | 0.95 (0.82, 1.09) | 0.80 (0.69, 0.92) | 0.001 |
| Model 4 | 8135 | 1.00 | 0.83 (0.72, 0.96) | 0.92 (0.79, 1.07) | 0.75 (0.64, 0.87) | <0.001 |
| Sensitivity analysis | ||||||
| Model 4 with time varying covariatesb | 8135 | 1.00 | 0.83 (0.71, 0.96) | 0.92 (0.79, 1.07) | 0.75 (0.64, 0.87) | <0.001 |
| Model 4 excluding WBC count ≥10 × 103/μl | 7874 | 1.00 | 0.83 (0.71, 0.97) | 0.93 (0.80, 1.09) | 0.75 (0.64, 0.88) | <0.001 |
| Model 4 adjusting for platelet count and % of monocytes, lymphocytes, and neutrophils | 4233 | 1.00 | 0.86 (0.68, 1.09) | 0.88 (0.69, 1.12) | 0.72 (0.56, 0.93) | 0.004 |
| Model 4 only including participants without microalbuminuriac at visit four (approximately 6 years after baseline) | 6063 | 1.00 | 0.84 (0.70, 1.01) | 0.87 (0.72, 1.05) | 0.77 (0.64, 0.94) | 0.002 |
Model 1 adjusted for age, sex, and study center. The combined model included self-reported race as a covariate. P for interaction between self-reported race and mtDNA copy number: 0.18. Model 2 added log-transformed WBC count. Model 3 added baseline eGFR, prevalent coronary heart disease, diabetes, hypertension, smoking status, and BMI. Model 4 added serum albumin, HbA1c, and log-transformed hsCRP that were available at visit two only.
Time-varying covariates: incident diabetes and hypertension.
Microalbuminuria was defined as urinary albumin-to-creatinine ratio <30 mg/g.
Because we had estimated mtDNA copy number from peripheral blood, Model 2 adjusted for WBC count. Higher mtDNA copy number remained significantly associated with lower incidence of CKD (HR of Q4 versus Q1 0.76; 95% CI, 0.66 to 0.89, P for trend <0.001). This association remained similar in subsequent analyses with additional adjustment. Model 3 adjusted for baseline eGFR and other risk factors of incident CKD. Model 4 additionally adjusted for serum albumin, HbA1c, and hsCRP (HR with Q1 as the reference, Q2 0.83; 95% CI, 0.72 to 0.96; Q3 0.92; 95% CI, 0.79 to 1.07; Q4 0.75; 95% CI, 0.64 to 0.87; P for trend <0.001). We detected no statistically significant interaction between mtDNA copy number and prevalent diabetes (P for interaction 0.39). In the sensitivity analyses including incident diabetes and hypertension as time-varying covariates and excluding participants with WBC count ≥10,000 cells/μl, we observed similar associations between mtDNA copy number and incident CKD. In the subsample adjusting for platelet count and percentages of monocytes, lymphocytes, and neutrophils (n=4233) and the subsample without microalbuminuria at visit four (approximately 6 years after the assessment of mtDNA copy number, n=6063), those in the highest quartile of mtDNA copy number remained having significantly lower risk for incident CKD than those in Q1.
Discussion
Main Findings
We have shown prevalent CKD (defined as eGFR<60 ml/min per 1.73m2) was associated with lower mtDNA copy number in peripheral blood and identified higher mtDNA copy number as associated with lower risk of incident CKD independent of known risk factors and biomarkers of systemic inflammation (WBC count and hsCRP) in a community-based cohort of white and black Americans. This finding suggests measures of mitochondrial dysfunction in blood cells may be informative of the systematic factors related to the pathophysiology of incident CKD.
In the Context of the Literature
mtDNA copy number is a surrogate measure of mitochondrial function,10 and, when estimated from peripheral blood, largely reflects mtDNA copy number in WBCs and platelets.15 This study extends previous research on the association between mtDNA copy number in peripheral blood and CKD. Lower mtDNA copy number had a significant cross-sectional association with higher prevalence of microalbuminuria in a population without CKD.13 Patients with diabetic nephropathy had lower mtDNA content in peripheral blood mononuclear cells than patients with diabetes without nephropathy.16 These findings were consistent with the results from our cross-sectional analysis on the association between prevalent CKD and lower mtDNA copy number. To our best knowledge, this is the first study that has demonstrated a prospective association between mtDNA copy number and incident CKD in a large cohort of white and black Americans.
Several lines of evidence can potentially explain the association between higher mtDNA copy number in peripheral blood and lower risk of incident CKD. First, mitochondrial dysfunction in kidney cells has been implicated in the pathogenesis of CKD3–5 and proposed as a therapeutic target for atherosclerotic renovascular disease.8,17 Mitochondrial dysfunction can be a consequence of oxidative stress.18 In functional studies, exposure to hydrogen peroxide, a reactive oxygen species, resulted in damage to the DNA replication enzyme (polymerase γ) in the mitochondria and a reduction in mtDNA copy number.19,20 Exposure to TNF-α, an inflammatory cytokine, also generated reactive oxygen species and reduced mtDNA copy number in myocytes.21 Cross-sectional studies of patients with CKD have shown that lower mitochondrial function, indicated by metabolites from urine and gene expression from peripheral blood, correlated with more severe CKD.22,23 In this study, the association between mtDNA copy number and incident CKD was slightly attenuated after controlling for WBC count, a marker of systematic inflammation, but remained independent of WBC count and hsCRP. These results suggest mtDNA copy number may represent the effect of cumulative exposure to oxidative stress that is not captured in the one-time measures of WBC count and hsCRP.
Second, mitochondria have been increasingly recognized as an important player in the regulation of apoptosis and autophagy.24–27 Mitochondrial dysfunction can weaken a cell’s capacity to respond to a range of metabolic processes, including additional oxidative stress and fatty acid overload, and can contribute to progressive disease.3,28 A transgenic mouse model has shown that mitochondrial dysfunction in podocytes induced by TGF-β signaling preceded the manifestation of segmental glomerulosclerosis.29 In addition, diabetic mouse models and in vitro studies have demonstrated that hyperglycemia induced mitochondrial fragmentation in podocytes, endothelial cells, and renal tubular cells.7,30 Finally, WBCs are involved in the kidney’s response to injury as evidenced by leukocyte infiltration in kidney tissues of patients with CKD and in experimental models.31–33 Lower mtDNA copy number might alter the role of WBCs and platelets in injury response. The inclusion of mtDNA copy number derived from WBCs and platelets in experiments investigating the role of mitochondrial dysfunction in CKD can increase our understanding on the potential role of these cells in the pathogenesis of CKD and may lead to novel insight for the prevention or treatment of CKD.
Strengths and Limitations
The strengths of this study include a large sample size with both white and black Americans and a long follow-up period of 19.6 years. Our incident CKD definition is highly specific.34 The estimate of mtDNA copy number was validated using quantitative polymerase chain reaction. Some limitations warrant mentioning. Although we controlled for many risk factors of CKD, albuminuria was not available at baseline. Although the association between mtDNA copy number and incident CKD remained significant in the subsample without microalbuminuria at a subsequent visit approximately 6 years later, we could not directly evaluate whether the association between mtDNA copy number and incident CKD was independent of albuminuria at baseline. mtDNA copy number was estimated from genomic DNA derived from buffy coat, which contains largely WBCs and platelets without differentiating between cell types. Although the association between mtDNA copy number and incident CKD was independent of platelet count and percentages of monocytes, lymphocytes, and neutrophils, we could not determine the contribution of different types of WBCs and platelets to the overall mtDNA copy number. Future studies are warranted to investigate mtDNA copy number by cell type to elucidate the potential contribution of mitochondrial dysfunction in specific cell types to the pathogenesis of CKD. This study used a one-time measure of mtDNA copy number in peripheral blood. Longitudinal measures of mtDNA copy number may provide insight into the relationship between mitochondrial dynamic in peripheral blood and kidney function.
Conclusions
Higher mtDNA copy number in peripheral blood is significantly associated with lower risk of incident CKD. Future studies are warranted to evaluate whether mtDNA copy number in peripheral blood may reveal systemic factors related to the pathogenesis of CKD and uncover targets for prevention and treatment.
Concise Methods
Study Population
The ARIC study is a prospective study of 15,792 individuals aged 45–65 at visit one (1987–89) from four communities in the United States.35 Our analyzed sample included participants with Affymetrix Genome-wide Human SNP 6.0 data for the estimation of mtDNA copy number and data in incident CKD and covariates. In total, estimates of mtDNA copy number were available in 11,480 participants. Details for quality control and methods of estimation are reported in the Supplemental Material. We further excluded individuals without serum creatinine for the estimation of eGFR at baseline (the visit where mtDNA copy number was estimated, n=1801), missing prevalent coronary heart disease (n=197) and other covariates (prevalent diabetes and hypertension status, smoking, BMI, and WBC count; n=149). Because altered mtDNA copy number (both higher and lower) in peripheral blood has been associated with renal cell carcinoma,36–38 we excluded participants with renal or renal pelvis cancer diagnosis on the basis of International Classification of Diseases (ICD) codes (ICD-9: 189.xx, ICD-10: C64, C65, C66, C68; n=89) obtained from the ARIC cohort surveillance study.35 The resulting analysis sample included 9244 participants for the cross-sectional study of mtDNA copy number and prevalent CKD, defined as eGFR<60 ml/min per 1.73m2. Of these participants, 183 had prevalent CKD, and three did not have incident CKD data, resulting in 9058 participants for the prospective study of mtDNA copy number and incident CKD.
Definitions of Prevalent and Incident CKD
We defined prevalent CKD as eGFR<60 ml/min per 1.73m2. We defined incident CKD using a validated definition as a composite outcome of (1) baseline eGFR≥60 ml/min per 1.73m2 with a drop to a level <60 ml/min per 1.73m2 at a follow-up visit with a concomitant 25% decline from baseline, (2) a hospitalization or death with an ICD diagnostic code indicating CKD obtained from the ARIC cohort surveillance study, or (3) incident ESRD identified through linkage with the US Renal Data System up to December 31, 2011. The hospitalization-based CKD definition has a sensitivity of 35.5% and a specificity of 95.7%.34 The ICD-9-CM and ICD-10-CM codes used for classifying CKD-related events are reported in Supplemental Table 3. We defined the baseline for incident CKD as the visit where blood samples were collected for the estimation of mtDNA copy number: 449 participants at visit one (1987–89), 8582 participants at visit two (1990–92), and 27 participants at visit four (1996–98).
Measures of mtDNA Copy Number
The methods for estimating mtDNA copy number are reported in detail in the Supplemental Material. Briefly, we isolated genomic DNA from buffy coat samples and hybridized the DNA to Affymetrix 6.0 microarrays according to the manufacturer’s protocol. Because sequence similarity between mitochondrial and nuclear DNA can adversely affect the accuracy of mtDNA copy number estimates,39 we curated 25 high-quality mitochondrial SNPs having probes with a perfect match to the annotated mitochondrial location. We used the median of the normalized intensity (log R ratio) for homozygous calls of these 25 mitochondrial SNPs corrected for GC content40 as the initial estimate of mtDNA copy number. To correct for batch effects, DNA quality, and starting DNA quantity, we generated principal components using the normalized probe intensities of 43,316 autosomal SNPs. We adjusted the initial estimate of the mtDNA copy number for principal components, age, sex, and study center using linear regression. We used the standardized residuals from this linear model as the estimate of relative mtDNA copy number in the present analyses.
Measures of Covariates
Race was on the basis of self-report. We removed genetic outliers on the basis of principal component analysis, as reported in the Supplemental Material. We measured all other covariates at the visit where whole-blood samples were collected for the estimation of mtDNA copy number. We defined diabetes as the use of diabetes medication, a fasting glucose level ≥126 mg/dl, a random glucose ≥200 mg/dl, or self-reported physician diagnosis of diabetes. We defined hypertension as a systolic blood pressure ≥140 mmHg or a diastolic blood pressure ≥90 mmHg measured at the baseline visit, or the use of antihypertensive medications. We defined prevalent coronary heart disease as a history of definite or probable myocardial infarction or cardiac procedure. Smoking status was on the basis of self-report. We measured HbA1c using high performance liquid chromatography.41 We measured serum albumin levels using a Roche Modular P800 Chemistry Analyzer. We measured hsCRP in serum using a latex-particle enhanced immunoturbidimetric assay kit (Roche Diagnostics, Indianapolis, IN) and we read this on the Roche Modular P800 Chemistry analyzer (Roche Diagnostics).
Statistical Analyses
We compared baseline characteristics of participants using t tests for nonskewed continuous variables, Wilcoxon tests for skewed continuous variables, and chi-squared tests for categorical variables. We plotted Kaplan–Meier estimates by quartiles of mtDNA copy number. We estimated incident rate ratios of higher quartiles of mtDNA copy number versus Q1 using Poisson regression.
For multivariate analysis, we used linear regression to evaluate the cross-sectional association between prevalent CKD and mtDNA copy number as a continuous variable. In Model 1, we adjusted for age, sex, self-reported race, and study center. In Model 2, we added log-transformed WBC count because mtDNA copy number from peripheral blood is largely from WBCs.15 Model 3 included additional factors related to CKD (prevalent diabetes, hypertension, and coronary heart disease; BMI; and smoking status). Model 4 included factors available only at visit two of the ARIC study (serum albumin, HbA1c, and hsCRP).
We evaluated the prospective association between mtDNA copy number and incident CKD using Cox regression, and categorized mtDNA copy number by quartiles. We used the same covariates in the cross-sectional analysis and in the prospective analysis. In addition, we added baseline eGFR to Models 3 and 4. Because lower mtDNA copy number measured from peripheral blood has been associated with diabetes, one of the strongest risk factors of incident CKD,42,43 we tested for the interaction between mtDNA copy number and prevalent diabetes on the basis of Model 4. We also conducted sensitivity analyses on the basis of Model 4. First, to evaluate potential mediating effect from incident diabetes and hypertension, we included incident diabetes and hypertension as time-varying covariates. Second, to evaluate potential confounding due to infection, we excluded participants with WBC count ≥10,000 cell/μl at baseline (n=261). Third, the blood samples used for the estimation of mtDNA copy number included largely WBCs and platelets. In a subsample (n=4233) of those included in Model 4, platelet count and percentages of monocytes, lymphocytes, and neutrophils were available. We evaluated the association between mtDNA copy number and incident CKD in this subsample, including platelet count and percentages of WBC subtypes as covariates to evaluate confounding due to blood cell types. Finally, because measures of albuminuria were not available at baseline, to provide insight into whether the association between mtDNA copy number and incident CKD might be independent of albuminuria, we re-evaluated the association between mtDNA copy number and incident CKD in the subsample without microalbuminuria, defined as urinary albumin-to-creatinine ratio ≥30mg/g, at visit four (approximately 6 years after baseline, n=6063). We obtained the P value for trend for each model by using mtDNA copy number as a continuous predictor. We performed comparison of baseline characteristics using R. We performed all other analyses using Stata 13.1.
Disclosures
None.
Supplementary Material
Acknowledgments
The authors thank the staff and participants of the Atherosclerosis Risk in Communities study for their important contributions.
A.T. was supported by National Institutes of Health/National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Renal Disease Epidemiology Training Grant T32DK007732. The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C).
Reagent for C-reactive protein was donated by Roche Diagnostics. Reagent for serum albumin was donated by Asahi Kasei Corporation. Assays of C-reactive protein and serum albumin were supported by NIDDK R01 DK089174.
An abstract reporting the results of this study was submitted to Kidney Week 2015.
Some of the data reported here have been supplied by the US Renal Data System. The interpretation and reporting of these data are the responsibility of the authors and in no way should be seen as an official policy or interpretation of the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060661/-/DCSupplemental.
References
- 1.Kidney Disease. Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 2.USRDS : USRDS 2013 Annual Data Report: Atlas of Chronic Kidney Disease and End-Stage Renal Disease in the United States, Bethesda, MD, National Institutes of Health, National Institute of Diabetes and Digestive and Kidney Diseases, 2013 [Google Scholar]
- 3.Zhan M, Brooks C, Liu F, Sun L, Dong Z: Mitochondrial dynamics: regulatory mechanisms and emerging role in renal pathophysiology. Kidney Int 83: 568–581, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Small DM, Coombes JS, Bennett N, Johnson DW, Gobe GC: Oxidative stress, anti-oxidant therapies and chronic kidney disease. Nephrology (Carlton) 17: 311–321, 2012 [DOI] [PubMed] [Google Scholar]
- 5.Che R, Yuan Y, Huang S, Zhang A: Mitochondrial dysfunction in the pathophysiology of renal diseases. Am J Physiol Renal Physiol 306: F367–F378, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Sarafidis PA, Stafylas PC, Georgianos PI, Saratzis AN, Lasaridis AN: Effect of thiazolidinediones on albuminuria and proteinuria in diabetes: a meta-analysis. Am J Kidney Dis 55: 835–847, 2010 [DOI] [PubMed] [Google Scholar]
- 7.Sun L, Xie P, Wada J, Kashihara N, Liu FY, Zhao Y, Kumar D, Chugh SS, Danesh FR, Kanwar YS: Rap1b GTPase ameliorates glucose-induced mitochondrial dysfunction. J Am Soc Nephrol 19: 2293–2301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Eirin A, Ebrahimi B, Zhang X, Zhu XY, Woollard JR, He Q, Textor SC, Lerman A, Lerman LO: Mitochondrial protection restores renal function in swine atherosclerotic renovascular disease. Cardiovasc Res 103: 461–472, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zhan M, Usman IM, Sun L, Kanwar YS: Disruption of renal tubular mitochondrial quality control by Myo-inositol oxygenase in diabetic kidney disease. J Am Soc Nephrol 26: 1304–1321, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Malik AN, Czajka A: Is mitochondrial DNA content a potential biomarker of mitochondrial dysfunction? Mitochondrion 13: 481–492, 2013 [DOI] [PubMed] [Google Scholar]
- 11.Ashar FN, Moes A, Moore AZ, Grove ML, Chaves PH, Coresh J, Newman AB, Matteini AM, Bandeen-Roche K, Boerwinkle E, Walston JD, Arking DE: Association of mitochondrial DNA levels with frailty and all-cause mortality. J Mol Med (Berl) 93: 177–186, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Rao M, Li L, Demello C, Guo D, Jaber BL, Pereira BJ, Balakrishnan VS HEMO Study Group : Mitochondrial DNA injury and mortality in hemodialysis patients. J Am Soc Nephrol 20: 189–196, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Lee JE, Park H, Ju YS, Kwak M, Kim JI, Oh HY, Seo JS: Higher mitochondrial DNA copy number is associated with lower prevalence of microalbuminuria. Exp Mol Med 41: 253–258, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee HK, Song JH, Shin CS, Park DJ, Park KS, Lee KU, Koh CS: Decreased mitochondrial DNA content in peripheral blood precedes the development of non-insulin-dependent diabetes mellitus. Diabetes Res Clin Pract 42: 161–167, 1998 [DOI] [PubMed] [Google Scholar]
- 15.Zhang ZW, Cheng J, Xu F, Chen YE, Du JB, Yuan M, Zhu F, Xu XC, Yuan S: Red blood cell extrudes nucleus and mitochondria against oxidative stress. IUBMB Life 63: 560–565, 2011 [DOI] [PubMed] [Google Scholar]
- 16.Czajka A, Ajaz S, Gnudi L, Parsade CK, Jones P, Reid F, Malik AN: Altered Mitochondrial Function, Mitochondrial DNA and Reduced Metabolic Flexibility in Patients With Diabetic Nephropathy. EBioMedicine 2: 499–512, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Textor SC, Lerman LO: Paradigm Shifts in Atherosclerotic Renovascular Disease: Where Are We Now? J Am Soc Nephrol 26: 2074–2080, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Navarro-González JF, Mora-Fernández C, Muros de Fuentes M, García-Pérez J: Inflammatory molecules and pathways in the pathogenesis of diabetic nephropathy. Nat Rev Nephrol 7: 327–340, 2011 [DOI] [PubMed] [Google Scholar]
- 19.Lee CF, Liu CY, Hsieh RH, Wei YH: Oxidative stress-induced depolymerization of microtubules and alteration of mitochondrial mass in human cells. Ann N Y Acad Sci 1042: 246–254, 2005 [DOI] [PubMed] [Google Scholar]
- 20.Graziewicz MA, Day BJ, Copeland WC: The mitochondrial DNA polymerase as a target of oxidative damage. Nucleic Acids Res 30: 2817–2824, 2002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Suematsu N, Tsutsui H, Wen J, Kang D, Ikeuchi M, Ide T, Hayashidani S, Shiomi T, Kubota T, Hamasaki N, Takeshita A: Oxidative stress mediates tumor necrosis factor-alpha-induced mitochondrial DNA damage and dysfunction in cardiac myocytes. Circulation 107: 1418–1423, 2003 [DOI] [PubMed] [Google Scholar]
- 22.Granata S, Zaza G, Simone S, Villani G, Latorre D, Pontrelli P, Carella M, Schena FP, Grandaliano G, Pertosa G: Mitochondrial dysregulation and oxidative stress in patients with chronic kidney disease. BMC Genomics 10: 388, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zaza G, Granata S, Rascio F, Pontrelli P, Dell’Oglio MP, Cox SN, Pertosa G, Grandaliano G, Lupo A: A specific immune transcriptomic profile discriminates chronic kidney disease patients in predialysis from hemodialyzed patients. BMC Med Genomics 6: 17, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Rambold AS, Lippincott-Schwartz J: Mechanisms of mitochondria and autophagy crosstalk. Cell Cycle 10: 4032–4038, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Varanita T, Soriano ME, Romanello V, Zaglia T, Quintana-Cabrera R, Semenzato M, Menabò R, Costa V, Civiletto G, Pesce P, Viscomi C, Zeviani M, Di Lisa F, Mongillo M, Sandri M, Scorrano L: The OPA1-dependent mitochondrial cristae remodeling pathway controls atrophic, apoptotic, and ischemic tissue damage. Cell Metab 21: 834–844, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Garcia-Souza LF, Oliveira MF: Mitochondria: biological roles in platelet physiology and pathology. Int J Biochem Cell Biol 50: 156–160, 2014 [DOI] [PubMed] [Google Scholar]
- 27.Gomes LC, Di Benedetto G, Scorrano L: During autophagy mitochondria elongate, are spared from degradation and sustain cell viability. Nat Cell Biol 13: 589–598, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Weinberg JM: Mitochondrial biogenesis in kidney disease. J Am Soc Nephrol 22: 431–436, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Daehn I, Casalena G, Zhang T, Shi S, Fenninger F, Barasch N, Yu L, D’Agati V, Schlondorff D, Kriz W, Haraldsson B, Bottinger EP: Endothelial mitochondrial oxidative stress determines podocyte depletion in segmental glomerulosclerosis. J Clin Invest 124: 1608–1621, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Wang W, Wang Y, Long J, Wang J, Haudek SB, Overbeek P, Chang BH, Schumacker PT, Danesh FR: Mitochondrial fission triggered by hyperglycemia is mediated by ROCK1 activation in podocytes and endothelial cells. Cell Metab 15: 186–200, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Anders HJ, Ninichuk V, Schlöndorff D: Progression of kidney disease: blocking leukocyte recruitment with chemokine receptor CCR1 antagonists. Kidney Int 69: 29–32, 2006 [DOI] [PubMed] [Google Scholar]
- 32.Ascon DB, Lopez-Briones S, Liu M, Ascon M, Savransky V, Colvin RB, Soloski MJ, Rabb H: Phenotypic and functional characterization of kidney-infiltrating lymphocytes in renal ischemia reperfusion injury. J Immunol 177: 3380–3387, 2006 [DOI] [PubMed] [Google Scholar]
- 33.Eardley KS, Kubal C, Zehnder D, Quinkler M, Lepenies J, Savage CO, Howie AJ, Kaur K, Cooper MS, Adu D, Cockwell P: The role of capillary density, macrophage infiltration and interstitial scarring in the pathogenesis of human chronic kidney disease. Kidney Int 74: 495–504, 2008 [DOI] [PubMed] [Google Scholar]
- 34.Grams ME, Rebholz CM, McMahon B, Whelton S, Ballew SH, Selvin E, Wruck L, Coresh J: Identification of incident CKD stage 3 in research studies. Am J Kidney Dis 64: 214–221, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.ARIC : The Atherosclerosis Risk in Communities (ARIC) Study: design and objectives. The ARIC investigators. Am J Epidemiol 129: 687–702, 1989 [PubMed] [Google Scholar]
- 36.Hofmann JN, Hosgood HD 3rd, Liu CS, Chow WH, Shuch B, Cheng WL, Lin TT, Moore LE, Lan Q, Rothman N, Purdue MP: A nested case-control study of leukocyte mitochondrial DNA copy number and renal cell carcinoma in the Prostate, Lung, Colorectal and Ovarian Cancer Screening Trial. Carcinogenesis 35: 1028–1031, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Purdue MP, Hofmann JN, Colt JS, Hoxha M, Ruterbusch JJ, Davis FG, Rothman N, Wacholder S, Schwartz KL, Baccarelli A, Chow WH: A case-control study of peripheral blood mitochondrial DNA copy number and risk of renal cell carcinoma. PLoS One 7: e43149, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Xing J, Chen M, Wood CG, Lin J, Spitz MR, Ma J, Amos CI, Shields PG, Benowitz NL, Gu J, de Andrade M, Swan GE, Wu X: Mitochondrial DNA content: its genetic heritability and association with renal cell carcinoma. J Natl Cancer Inst 100: 1104–1112, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Malik AN, Shahni R, Rodriguez-de-Ledesma A, Laftah A, Cunningham P: Mitochondrial DNA as a non-invasive biomarker: accurate quantification using real time quantitative PCR without co-amplification of pseudogenes and dilution bias. Biochem Biophys Res Commun 412: 1–7, 2011 [DOI] [PubMed] [Google Scholar]
- 40.Diskin SJ, Li M, Hou C, Yang S, Glessner J, Hakonarson H, Bucan M, Maris JM, Wang K: Adjustment of genomic waves in signal intensities from whole-genome SNP genotyping platforms. Nucleic Acids Res 36: e126, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Selvin E, Coresh J, Zhu H, Folsom A, Steffes MW: Measurement of HbA1c from stored whole blood samples in the Atherosclerosis Risk in Communities study. J Diabetes 2: 118–124, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Xu FX, Zhou X, Shen F, Pang R, Liu SM: Decreased peripheral blood mitochondrial DNA content is related to HbA1c, fasting plasma glucose level and age of onset in type 2 diabetes mellitus. Diabet Med 29: e47–e54, 2012 [DOI] [PubMed] [Google Scholar]
- 43.Bash LD, Coresh J, Köttgen A, Parekh RS, Fulop T, Wang Y, Astor BC: Defining incident chronic kidney disease in the research setting: The ARIC Study. Am J Epidemiol 170: 414–424, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.