Abstract
Glomerular apoptosis may contribute to diabetic nephropathy (dNP), but the pathophysiologic relevance of this process remains obscure. Here, we administered two partially disjunct polycaspase inhibitors in 8-week-old diabetic (db/db) mice: M-920 (inhibiting caspase-1, -3, -4, -5, -6, -7, and -8) and CIX (inhibiting caspase-3, -6, -7, -8, and -10). Notably, despite reduction in glomerular cell death and caspase-3 activity by both inhibitors, only M-920 ameliorated dNP. Nephroprotection by M-920 was associated with reduced renal caspase-1 and inflammasome activity. Accordingly, analysis of gene expression data in the Nephromine database revealed persistently elevated glomerular expression of inflammasome markers (NLRP3, CASP1, PYCARD, IL-18, IL-1β), but not of apoptosis markers (CASP3, CASP7, PARP1), in patients with and murine models of dNP. In vitro, increased levels of markers of inflammasome activation (Nlrp3, caspase-1 cleavage) preceded those of markers of apoptosis activation (caspase-3 and -7, PARP1 cleavage) in glucose-stressed podocytes. Finally, caspase-3 deficiency did not protect mice from dNP, whereas both homozygous and hemizygous caspase-1 deficiency did. Hence, these results suggest caspase-3-dependent cell death has a negligible effect, whereas caspase-1-dependent inflammasome activation has a crucial function in the establishment of dNP. Furthermore, small molecules targeting caspase-1 or inflammasome activation may be a feasible therapeutic approach in dNP.
Keywords: diabetic nephropathy, apoptosis, immunology, renal protection
Markers of apoptotic cell death in glomerular cells have repeatedly been linked with diabetic nephropathy (dNP).1 However, glomerular apoptosis is thought to be rare2 and unequivocal evidence for a causative role of apoptotic cell death is sparse at best. Conceptually, apoptosis, a caspase-3-dependent immunologically and inflammatorily silent form of cell death, is less likely than cell-death forms associated with inflammation to contribute to the manifestation of dNP, because dNP is closely linked with sterile inflammation.3,4 Clarifying the functional relevance of apoptosis and other cell-death forms in dNP may be of translational relevance, because several pharmaceutical approaches to target proapoptotic pathways are available or being investigated.5 Pyroptosis, for example, is a cell-death form associated with inflammation that results from unfettered inflammasome activation, and therapies restricting inflammasome activity are clinically used.6,7 Inflammasome activation has recently been associated and mechanistically linked with dNP.3 Whether inhibition of caspases using small molecules may be a feasible therapeutic approach and whether such an approach should target caspase-3 and apoptosis remains unknown.
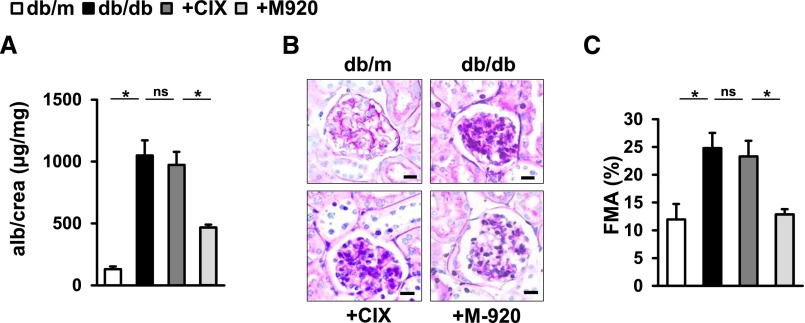
To ascertain the role of caspase activation and cell death in dNP and to evaluate the therapeutic potential of caspase inhibition in dNP, we employed two polycaspase inhibitors (M-920 and CIX) targeting a partially disjunct set of caspases. The CIX inhibitor targets caspases 3, 6, 7, 8, and 10,8,9 and the M-920 inhibitor targets caspases 1, 3, 4, 5, 6, 7, and 8.10 Treatment was initiated in 8-week-old db/db mice and mice were analyzed after 12 weeks of treatment. Only M-920 but not CIX ameliorated markers of dNP (albuminuria and extracellular matrix accumulation; Figure 1). Blood glucose levels and body weight did not differ among experimental mice (Supplemental Figure 1). These data indicate a critical role of caspases 1, 4, and 5—the caspases targeted by M-920, but not the CIX inhibitor—in dNP. To further dissect the role of caspase-1 versus caspases-4/5 we used corresponding inhibitors. Only caspase-1 inhibition, but not caspase-4/5 inhibition, efficiently ameliorated markers of dNP (albuminuria and extracellular matrix accumulation; Supplemental Figure 2), indicating that the inhibition of caspase-1 by the M-920 compound mediates protection from diabetic nephropathy.
Figure 1.
Differential effect of caspase inhibitors M-920 and CIX on diabetic nephropathy in db/db mice. (A) Albuminuria and extracellular matrix accumulation, as reflected by the fractional mesangial area (FMA), are decreased in M-920-treated db/db mice (+M-920; targeting caspases 1, 3, 4, 5, 6, 7, and 8) as compared with PBS-treated db/db mice (db/db). Treatment with CIX (+CIX; targeting caspases 3, 6, 7, 8, and 10) has no effect on these markers in db/db mice. Treatment was initiated at age 8 weeks and continued for 12 weeks. Mean value±SEM. At least six mice in each group. (B) FMA; periodic acid–Schiff–stained glomeruli (size bar: 20 µm). (C) FMA; mean value±SEM. *P<0.05. alb, albumin; crea, creatinine; db/m, nondiabetic control; ns, not significant.
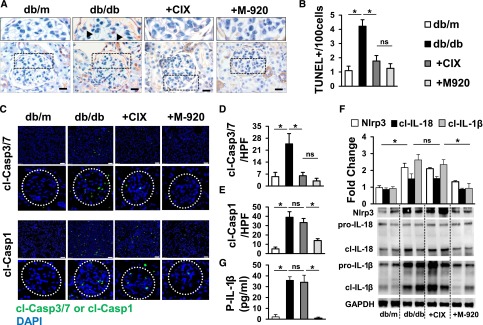
To exclude that the superior efficacy of the M-920 inhibitor reflects insufficient inhibition of glomerular cell death by CIX we conducted a TUNEL assay. Both caspase inhibitors efficiently reduced glomerular cell death as detected by the TUNEL assay (Figure 2, A and B). Of note, albuminuria and extracellular matrix expansion was evident in CIX-treated mice despite efficient prevention of cell death, implying that cell death inhibition per se is not sufficient to prevent dNP in mice. To ascertain the efficacy of caspase inhibition we determined in situ caspase-3/7 and caspase-1 activity. Congruent with the TUNEL results both interventions efficiently prevented caspase-3/7 activity (Figure 2, C and D). As expected on the basis of the profile of caspases targeted by the caspase inhibitors, only M-920, but not CIX, additionally reduced caspase-1 activity (Figure 2, C and E). In agreement with our recent results, we observed cleaved caspase-1 predominately within glomeruli, although we could also detect some cleaved caspase-1 in the tubulointerstitial compartment.3
Figure 2.
M-920 prevents inflammasome activation in addition to glomerular cell death. (A, B) Both caspase inhibitors (M-920 and CIX) decrease glomerular cell death in db/db mice as detected by the TUNEL assay. (A) Representative TUNEL-stained glomeruli (size bar: 20 µm Higher magnification (top) of glomeruli indicated by black dotted lines and TUNEL-positive cells indicated by black arrowheads. (C, D) Both M-920 and CIX decrease caspase-3/7 activity, as detected by the FLICA assay. However, only M-920, but not CIX, also reduces caspase-1 activity (C, E). (F) In db/db mice treated with M-920, but not in those treated with CIX, the cleaved forms of IL-1β and IL-18 and expression of Nlrp3 are reduced in renal cortex extracts. (G) Renal inflammasome inhibition in M-920-treated db/db mice is associated with lower plasma IL-1β levels. (B, D–G) Mean value±SEM of at least six mice in each group; (C) representative images of frozen sections incubated with FLICA probes – overviews (size bar: 20 µm) and higher (4×) magnification of glomeruli (glomeruli indicated by white dotted circles); (F) representative immunoblots; *P<0.05. cl-Casp1, cleaved caspase-1; cl-Casp3/7, cleaved caspase-3/7; cl-IL-1β, cleaved IL-1β; cl-IL-18, cleaved IL-18; DAPI, 4′,6-diamidino-2-phenylindole; db/m, nondiabetic control; GAPDH, glyceraldehyde-3-phosphate dehydrogenase; HPF, high-power field; ns, not significant; P-IL-1β, plasma-IL-1β.
Thus, the nephroprotective effect of M-920 may be related to the inhibition of caspase-1. Active caspase-1 interacts with PYCARD (N-terminal PYRIN-PAAD-DAPIN domain) or ASC (apoptosis-associated speck-like protein containing a CARD), forming the inflammasome complex, leading to proteolytic cleavage and activation of IL-1β and IL-18. Indeed, in db/db mice treated with M-920, but not in those treated with CIX, the cleaved forms of IL-1β and IL-18, as well as expression of Nlrp3, were reduced in renal cortex extracts (Figure 2F), which was associated with lower plasma IL-1β levels (Figure 2G). Taken together, these data provide experimental evidence that caspase-3/7-mediated cell death does not contribute to the manifestation of dNP in db/db mice. Rather, these data support a role of caspase-1 and inflammasome activation for establishment of dNP in db/db mice.
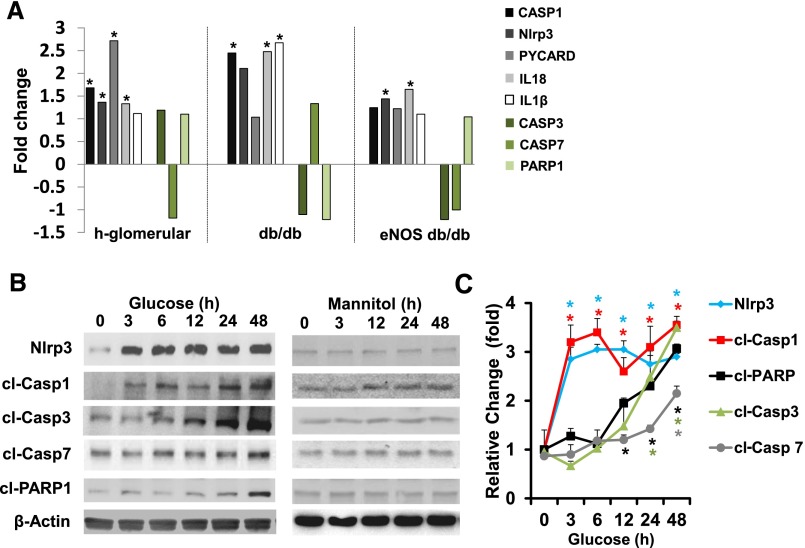
To gain insights into the role of inflammasome and apoptosis regulators in human dNP we screened the Nephromine database for glomerular expression of the inflammasome-related genes caspase-1 (CASP1), IL-18, IL-1β, PYCARD (ASC), and Nlrp3 (NLRP3) and compared these genes to the apoptosis-related genes caspase-3 (CASP3), caspase-7 (CASP7), and Parp-1 (PARP1) in healthy controls and individuals with dNP (Figure 3A). No apoptosis-related genes, only inflammasome-related regulators, were persistently induced in microdissected glomeruli of individuals with dNP (Figure 3A). Likewise, in two distinct mouse models of diabetic nephropathy, expression of inflammasome regulators, but not that of apoptosis regulators, was largely induced (Figure 3A). The expression pattern of inflammasome- and apoptosis-related genes supports a pivotal role of inflammasome activation for dNP. Previously, induction of caspase-3 or caspase-1 activation by high glucose concentrations has been reported in vitro.3,11,12 However, direct comparative analyses providing insight into the kinetics are lacking. Hence, we analyzed the temporal pattern of inflammasome and apoptosis activation upon glucose stimulation (25 mM) in murine podocytes in vitro. The inflammasome markers Nlrp3 and cleaved caspase-1 markedly increased within 3 hours, preceding the induction of cleaved PARP1 (12 hours), cleaved caspase-3 (24 hours), and cleaved caspase-7 (48 hours; Figure 3, B and C), although mannitol (25 mM) had no effect on inflammasome or apoptosis regulators at different time points (Figure 3B, Supplemental Figure 3). Thus, glucose-induced inflammasome activation precedes apoptosis activation in vitro, further supporting a leading role of inflammasome activation for glucose-induced cellular dysfunction.
Figure 3.
Inflammasome and apoptosis markers are differentially regulated in dNP and glucose-treated podocytes in vitro. We screened the Woroniecka cohort in the Nephromine database (human dNP) for glomerular expression of inflammasome- and apoptosis-related genes. (A) Only the inflammasome-related regulators, caspase-1 (CASP1), IL-18, ASC (PYCARD), and Nlrp3 (NLRP3), but not the apoptosis-related genes, caspase-3 (CASP3), caspase-7 (CASP7), and PARP-1 (PARP1) are significantly induced in microdissected glomeruli of patients with diabetes with dNP (h-glomerular) as compared with nondiabetic controls. Likewise, significant induction of inflammasome-related genes (CASP1, IL-18, and IL-1β for db/db C57BLKS mice, and CASP1 and IL-18 for eNOS−/− db/db mice), but not of apoptosis-related genes (CASP3, CASP7, and PARP1), is observed within the Nephromine Hodgin dataset as compared with nondiabetic db/m mice or eNOS+/+ m/m mice. The fold changes of the log2 median centered intensity from the Nephromine database (Life Technologies, Ann Arbor, MI) are used for graphical representation. High glucose (25 mM; [B, C]), but not mannitol (25 mM; [B]), induces the inflammasome markers Nlrp3 and cleaved caspase-1 within 3 hours in murine podocytes in vitro, preceding the induction of cleaved PARP1 (12 hours), cleaved caspase-3 (24 hours), and cleaved caspase-7 (48 hours; [C]). (B) Representative immunoblots of at least three repeat experiments; (C, E) mean value±SEM; *P<0.05. cl-Casp1, cleaved caspase-1; cl-Casp3, cleaved caspase-3; cl-Casp7, cleaved caspase-7; cl-PARP1, cleaved PARP1.
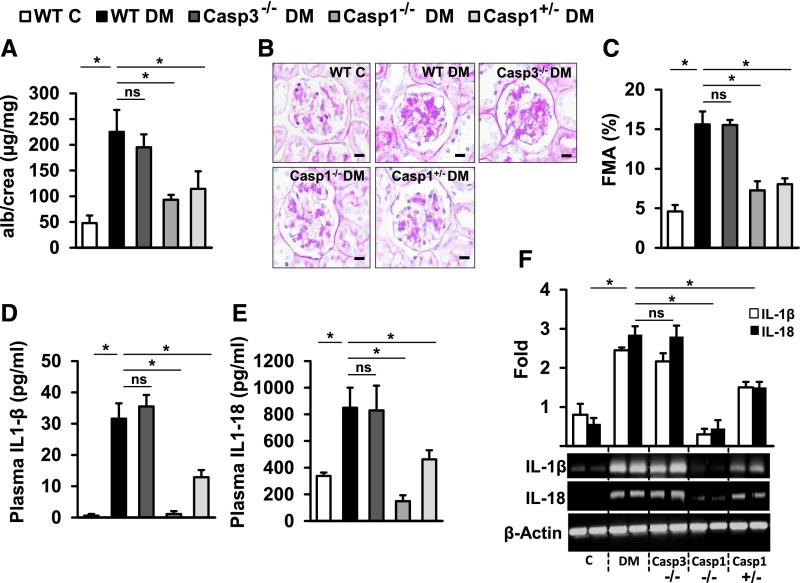
Although demonstrating a potential therapeutic value, the pharmacologic approach used to inhibit multiple caspases has limitations, such as the inhibition of caspases other than caspase-1 or caspase-3 or potentially off-target effects.13 In order to pinpoint causal relevance of caspase-1 or caspase-3 for dNP we directly compared mice with genetic caspase-1 or caspase-3 deficiency. We induced persistent hyperglycemia in uninephrectomized mice, and mice were followed up for 10 weeks. Blood glucose levels and body weight were similar in experimental groups (Supplemental Figure 4). Congruent with the above inhibitor studies, caspase-3 deficiency failed to protect mice against dNP (Figure 4, A–C), whereas—matching previous results3—caspase-1 deficiency was protective (Figure 4, A–C). Notably, even hemizygous caspase-1 mice (Casp1+/−) were protected from dNP (Figure 4, A–C), consistent with a gene dosage effect.14 As expected, IL-1β and IL-18 plasma levels and renal expression of IL-1β and IL-18 were unaffected in caspase-3−/− mice, but reduced in caspase-1−/− and caspase-1+/− mice (Figure 4, D–F).
Figure 4.
Caspase-3 deficiency fails to protect against diabetic nephropathy. (A) Compared with nondiabetic wild-type control mice (WT C), STZ induced diabetic mice (WT DM) show markedly increased albuminuria, and (B, C) increased fractional mesangial area (FMA). (A–C) Caspase-3 deficiency (Casp3−/−) fails to reduce albuminuria and the FMA in diabetic mice, whereas homozygous (Casp1−/−) or hemizygous (Casp1+/−) caspase-1 deficiency is protective. (D) IL-1β and (E) IL-18 plasma levels and (F) renal IL-1β/IL-18 expression are unaffected in caspase-3−/− mice, but are reduced in caspase-1+/−, and reduced to a larger extent in caspase-1−/− mice. (A, C–F) Mean value±SEM; at least six mice in each group; (B) representative periodic acid–Schiff–stained glomeruli (size bar: 20 µm); (F) representative agarose-gels are shown; *P<0.05. alb, albumin; C, control; crea, creatinine; ns, not significant.
These results demonstrate that dNP evolves despite prevention of glomerular cell death. Simultaneously, they emphasize the mechanistic relevance of the inflammasome in dNP, which is entirely consistent with the well-supported concept of sterile inflammation in dNP. Furthermore, these results are congruent with studies demonstrating inflammasome activation in human patients with diabetes and mouse models of dNP.15–17 Of note, a mechanistic relevance of inflammasome activation in animal models of dNP has recently been established.3 Furthermore, a recent observational study suggests that inhibition of inflammasome signaling improves renal function (on the basis of a decline in serum creatinine) in patients with established dNP.18 Likewise, the data obtained from the Nephromine dataset19 demonstrate that inflammasome, but not apoptosis, regulators are induced in glomeruli of patients with diabetes, which further supports a crucial role of inflammasome activation for dNP, not only in rodents, but also in humans.
At first sight these results are at odds with previous reports linking apoptosis with dNP.1,11 Of note, these studies frequently used a TUNEL assay to detect apoptosis.1,11,20,21 However, the TUNEL assay is not specific for apoptosis and additionally detects other cell-death forms.22,23 Importantly, both interventions, CIX and M-920, reduced glomerular cell death and in situ caspase-3/7 activity in this study, but the CIX inhibitor nevertheless failed to protect from dNP. Hence, although our results confirm the association of cell death, as detected by TUNEL, with dNP, they question the mechanistic relevance of glomerular cell death.
Although caspase-3 activation is not required for establishment of dNP in mice, we cannot exclude that caspase-3 activation may have an effect during later disease stages. Our in vitro analyses showing activation of caspase-3 and other apoptosis regulators (caspase-7, PARP1) in glucose-stressed podocytes are in agreement with earlier reports.1,11,24 Notably, the detailed kinetic analyses revealed that the induction of apoptosis regulators is preceded by Nlrp3 induction and caspase-1 activation in vitro, supporting a prominent role of inflammasome activation for glucose-induced cell damage. Of note, pyroptosis (cell death resulting from unfettered inflammasome activation) and apoptosis are not exclusive, and pathways leading to or triggered by these cell-death forms interact.6,25,26 Furthermore, we do not exclude a role of mitochondrial dysfunction. Indeed, mitochondrial dysfunction promotes inflammasome activation via increased ROS, thus contributing to glomerular dysfunction.3,27
On the basis of these data, we propose that inflammasome-mediated cellular dysfunction, potentially leading to pyroptosis, is primarily promoting diabetic glomerulopathy, although apoptosis may succeed at later stages. Indeed, a “switch” from pyroptosis to apoptosis may reflect an endogenous defense mechanism aiming to restrict further tissue inflammation. Following this hypothesis, which awaits experimental evaluation, specific inhibition of apoptosis may actually be disadvantageous.
The caspase-1-deficient mice used in this study also lack caspase-11, the murine ortholog for human caspase-4 and -5.28 Caspase-11 directly interacts with cytosolic lipopolysaccharides, causing noncanonical inflammasome activation and pyroptosis (a TUNEL-positive cell-death form).29 However, because dNP evolves despite prevention of TUNEL-positive cell death (this study) we suspect that the contribution of caspase-11 to the establishment of diabetic nephropathy is of minor relevance. This, however, needs to be evaluated in the future upon availability of suitable mouse models.
The protection of hemizygous caspase-1-deficient mice supports the notion that inhibition of caspase-1 or caspase-1-dependent mechanisms, in particular inflammasome activation, is a promising therapeutic target in dNP.3,18 Of note, small-molecule inflammasome inhibitors, similar to those used within this study, are being developed,30,31 which may increase the practicality of inflammasome inhibition in patients with diabetes. Furthermore, single-nucleotide polymorphisms within inflammasome-related genes have been identified and linked with inflammatory diseases.32 The analyses of inflammasome-associated single-nucleotide polymorphisms may aid in identifying patients at risk for dNP.
Concise Methods
For detail please see the Supplemental Material.
Mice
We obtained db/db (Lepr db/db), nondiabetic control db/m, Casp1−/−, and Casp3−/− mice from The Jackson Laboratory, Bar Harbor, ME. In this study we used littermates that had been backcrossed for at least 10 generations on a C57BL/6 background. We conducted all animal experiments following standards and procedures approved by the local Animal Care and Use Committee (Landesverwaltungsamt Halle, Germany).
dNP Model
In this study we analyzed two different diabetic models. We used db/db mice and uninephrectomized mice with persistent hyperglycemia (assured by continuous blood glucose levels >300 mg/dl) after low-dose streptozotocin injection (intraperitoneally, 40 mg/kg body wt, freshly dissolved in 0.05 mol/l sterile sodium citrate, pH 4.5, for 5 consecutive days). Mice were treated, euthanized, and analyzed as described previously.3,11 For additional information please see the Supplemental Material.
In Vivo Intervention Studies
A subset of mice was treated either with the polycaspase inhibitor M-920 (20 mg/kg body wt daily, intraperitoneally [ip])10 or with the polycaspase inhibitor CIX (peptide sequence: Ac-DEVD-CMK; 20 mg/kg body wt daily, ip), known not to affect IL-1β processing (Merck Millipore).8,9 For in vivo caspase-1 inhibition we used Z-WEHD-FMK (R&D Systems, 1 mg/kg body wt, ip, daily for 6 weeks) and for caspase-4/5 inhibition we used Ac-LEVD-CHO (Enzo Life Sciences, 1 mg/kg body wt, ip, daily for 6 weeks).33
Caspase-1 and Caspase-3/7 Activity Assay
We determined caspase-1 and caspase-3/7 activity on frozen kidney sections using the FLICA casp-1 and casp-3/7 assay (ImmunoChemistry Technologies LCC). Briefly, we prepared 5-µm-thick frozen kidney tissue sections and allowed them to air-dry. Then we fixed slides with acetone for 1 minute, rehydrated them by washing (twice for 5 minutes) in PBS-Tween, and blocked the slides for 20 minutes with blocking solution containing 20% aqua-block in media with 0.2% Tween. We decanted the blocking solution and then applied 50 µl of 3× FLICA working solution (freshly prepared by diluting 150× stock solution 1:50 in PBS) per section and incubated the slides at room temperature for 2 hours, protected from light. Tissues were washed with PBS-Tween (twice for 5 minutes). We mounted tissues with Vactashield mounting medium containing DAPI and fluorescent images were captured with an Olympus Bx43 Microscope (Olympus, Tokyo, Japan). Images analyses were done by using Image J software.
Statistical Analyses
The data are summarized as mean±SEM. We performed statistical analyses with the t test, ANOVA, or Mann–Whitney U test, as appropriate, and post hoc comparison with the method of Tukey. We used the Kolmogorov–Smirnov test or D’Agostino–Pearson normality test to determine whether the data are consistent with a Gaussian distribution. We used StatistiXL (www.statistixl.com) and Prism 5 (www.graphpad.com) software for statistical analyses. We accepted statistical significance at values of P<0.05.
Disclosures
None.
Supplementary Material
Acknowledgments
We thank Kathrin Deneser, Julia Judin, Juliane Friedrich, René Rudat, and Rumiya Makarova for excellent technical support.
This work was supported by grants from the Deutsche Forschungsgemeinschaft (IS 67/2-4 to B.I., TH 1789/1-1 to T.M., SFB1118 to P.P.N. and SH 849/1-2 to K.S.), the European Foundation for the Study of Diabetes (to B.I.), Hopp Stiftung and German Diabetes Center (to P.P.N.), the Stiftung für Pathobiochemie und Molekulare Diagnostik (to B.I.), and a DAAD scholarship to M.M.A.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060676/-/DCSupplemental.
References
- 1.Susztak K, Raff AC, Schiffer M, Böttinger EP: Glucose-induced reactive oxygen species cause apoptosis of podocytes and podocyte depletion at the onset of diabetic nephropathy. Diabetes 55: 225–233, 2006 [PubMed] [Google Scholar]
- 2.Kumar D, Robertson S, Burns KD: Evidence of apoptosis in human diabetic kidney. Mol Cell Biochem 259: 67–70, 2004 [DOI] [PubMed] [Google Scholar]
- 3.Shahzad K, Bock F, Dong W, Wang H, Kopf S, Kohli S, Al-Dabet MM, Ranjan S, Wolter J, Wacker C, Biemann R, Stoyanov S, Reymann K, Söderkvist P, Groß O, Schwenger V, Pahernik S, Nawroth PP, Gröne HJ, Madhusudhan T, Isermann B: Nlrp3-inflammasome activation in non-myeloid-derived cells aggravates diabetic nephropathy. Kidney Int 87: 74–84, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mudaliar H, Pollock C, Panchapakesan U: Role of Toll-like receptors in diabetic nephropathy. Clin Sci (Lond) 126: 685–694, 2014 [DOI] [PubMed] [Google Scholar]
- 5.Fischer U, Schulze-Osthoff K: Apoptosis-based therapies and drug targets. Cell Death Differ 12[Suppl 1]: 942–961, 2005 [DOI] [PubMed] [Google Scholar]
- 6.Miao EA, Rajan JV, Aderem A: Caspase-1-induced pyroptotic cell death. Immunol Rev 243: 206–214, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ozaki E, Campbell M, Doyle SL: Targeting the NLRP3 inflammasome in chronic inflammatory diseases: current perspectives. J Inflamm Res 8: 15–27, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mocanu MM, Baxter GF, Yellon DM: Caspase inhibition and limitation of myocardial infarct size: protection against lethal reperfusion injury. Br J Pharmacol 130: 197–200, 2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Maelfait J, Vercammen E, Janssens S, Schotte P, Haegman M, Magez S, Beyaert R: Stimulation of Toll-like receptor 3 and 4 induces interleukin-1beta maturation by caspase-8. J Exp Med 205: 1967–1973, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hotchkiss RS, Chang KC, Swanson PE, Tinsley KW, Hui JJ, Klender P, Xanthoudakis S, Roy S, Black C, Grimm E, Aspiotis R, Han Y, Nicholson DW, Karl IE: Caspase inhibitors improve survival in sepsis: a critical role of the lymphocyte. Nat Immunol 1: 496–501, 2000 [DOI] [PubMed] [Google Scholar]
- 11.Isermann B, Vinnikov IA, Madhusudhan T, Herzog S, Kashif M, Blautzik J, Corat MA, Zeier M, Blessing E, Oh J, Gerlitz B, Berg DT, Grinnell BW, Chavakis T, Esmon CT, Weiler H, Bierhaus A, Nawroth PP: Activated protein C protects against diabetic nephropathy by inhibiting endothelial and podocyte apoptosis. Nat Med 13: 1349–1358, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Lee, SH, Moon, SJ, Paeng, J, Kang, HY, Nam, BY, Kim, S, Kim, CH, Lee, MJ, Oh, HJ, Park, JT, Han, SH, Yoo, TH, Kang, SW: Podocyte hypertrophy precedes apoptosis under experimental diabetic conditions. Apoptosis 20: 1056–1071, 2015 [DOI] [PubMed]
- 13.Schotte P, Declercq W, Van Huffel S, Vandenabeele P, Beyaert R: Non-specific effects of methyl ketone peptide inhibitors of caspases. FEBS Lett 442: 117–121, 1999 [DOI] [PubMed] [Google Scholar]
- 14.Sarkar A, Hall MW, Exline M, Hart J, Knatz N, Gatson NT, Wewers MD: Caspase-1 regulates Escherichia coli sepsis and splenic B cell apoptosis independently of interleukin-1beta and interleukin-18. Am J Respir Crit Care Med 174: 1003–1010, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lee HM, Kim JJ, Kim HJ, Shong M, Ku BJ, Jo EK: Upregulated NLRP3 inflammasome activation in patients with type 2 diabetes. Diabetes 62: 194–204, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yang SM, Ka SM, Wu HL, Yeh YC, Kuo CH, Hua KF, Shi GY, Hung YJ, Hsiao FC, Yang SS, Shieh YS, Lin SH, Wei CW, Lee JS, Yang CY, Chen A: Thrombomodulin domain 1 ameliorates diabetic nephropathy in mice via anti-NF-κB/NLRP3 inflammasome-mediated inflammation, enhancement of NRF2 antioxidant activity and inhibition of apoptosis. Diabetologia 57: 424–434, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Sakai N, Wada T: Revisiting inflammation in diabetic nephropathy: the role of the Nlrp3 inflammasome in glomerular resident cells. Kidney Int 87: 12–14, 2015 [DOI] [PubMed] [Google Scholar]
- 18.Balasubramaniam G, Almond M, Dasgupta B: Improved renal function in diabetic patients with acute gout treated with anakinra. Kidney Int 88: 195–196, 2015 [DOI] [PubMed] [Google Scholar]
- 19.Martini S, Eichinger F, Nair V, Kretzler M: Defining human diabetic nephropathy on the molecular level: integration of transcriptomic profiles with biological knowledge. Rev Endocr Metab Disord 9: 267–274, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wang H, Madhusudhan T, He T, Hummel B, Schmidt S, Vinnikov IA, Shahzad K, Kashif M, Muller-Krebs S, Schwenger V, Bierhaus A, Rudofsky G, Nawroth PP, Isermann B: Low but sustained coagulation activation ameliorates glucose-induced podocyte apoptosis: protective effect of factor V Leiden in diabetic nephropathy. Blood 117: 5231–5242, 2011 [DOI] [PubMed] [Google Scholar]
- 21.Liu G, Sun Y, Li Z, Song T, Wang H, Zhang Y, Ge Z: Apoptosis induced by endoplasmic reticulum stress involved in diabetic kidney disease. Biochem Biophys Res Commun 370: 651–656, 2008 [DOI] [PubMed] [Google Scholar]
- 22.Fink SL, Cookson BT: Apoptosis, pyroptosis, and necrosis: mechanistic description of dead and dying eukaryotic cells. Infect Immun 73: 1907–1916, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Linkermann A, Chen G, Dong G, Kunzendorf U, Krautwald S, Dong Z: Regulated cell death in AKI. J Am Soc Nephrol 25: 2689–2701, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Lee SC, Han SH, Li JJ, Lee SH, Jung DS, Kwak SJ, Kim SH, Kim DK, Yoo TH, Kim JH, Chang SH, Han DS, Kang SW: Induction of heme oxygenase-1 protects against podocyte apoptosis under diabetic conditions. Kidney Int 76: 838–848, 2009 [DOI] [PubMed] [Google Scholar]
- 25.Lamkanfi M, Kanneganti TD: Caspase-7: a protease involved in apoptosis and inflammation. Int J Biochem Cell Biol 42: 21–24, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Shimada K, Crother TR, Karlin J, Dagvadorj J, Chiba N, Chen S, Ramanujan VK, Wolf AJ, Vergnes L, Ojcius DM, Rentsendorj A, Vargas M, Guerrero C, Wang Y, Fitzgerald KA, Underhill DM, Town T, Arditi M: Oxidized mitochondrial DNA activates the NLRP3 inflammasome during apoptosis. Immunity 36: 401–414, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhou R, Yazdi AS, Menu P, Tschopp J: A role for mitochondria in NLRP3 inflammasome activation. Nature 469: 221–225, 2011 [DOI] [PubMed] [Google Scholar]
- 28.Kayagaki N, Warming S, Lamkanfi M, Vande Walle L, Louie S, Dong J, Newton K, Qu Y, Liu J, Heldens S, Zhang J, Lee WP, Roose-Girma M, Dixit VM: Non-canonical inflammasome activation targets caspase-11. Nature 479: 117–121, 2011 [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Zhao Y, Shao F: Non-canonical activation of inflammatory caspases by cytosolic LPS in innate immunity. Curr Opin Immunol 32: 78–83, 2015 [DOI] [PubMed] [Google Scholar]
- 30.Coll RC, Robertson AA, Chae JJ, Higgins SC, Muñoz-Planillo R, Inserra MC, Vetter I, Dungan LS, Monks BG, Stutz A, Croker DE, Butler MS, Haneklaus M, Sutton CE, Núñez G, Latz E, Kastner DL, Mills KH, Masters SL, Schroder K, Cooper MA, O’Neill LA: A small-molecule inhibitor of the NLRP3 inflammasome for the treatment of inflammatory diseases. Nat Med 21: 248–255, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Abderrazak A, Couchie D, Mahmood DF, Elhage R, Vindis C, Laffargue M, Matéo V, Büchele B, Ayala MR, El Gaafary M, Syrovets T, Slimane MN, Friguet B, Fulop T, Simmet T, El Hadri K, Rouis M: Anti-inflammatory and antiatherogenic effects of the NLRP3 inflammasome inhibitor arglabin in ApoE2.Ki mice fed a high-fat diet. Circulation 131: 1061–1070, 2015 [DOI] [PubMed] [Google Scholar]
- 32.Pontillo A, Girardelli M, Kamada AJ, Pancotto JA, Donadi EA, Crovella S, Sandrin-Garcia P: Polimorphisms in inflammasome genes are involved in the predisposition to systemic lupus erythematosus. Autoimmunity 45: 271–278, 2012 [DOI] [PubMed] [Google Scholar]
- 33.Xia M, Boini KM, Abais JM, Xu M, Zhang Y, Li PL: Endothelial NLRP3 inflammasome activation and enhanced neointima formation in mice by adipokine visfatin. Am J Pathol 184: 1617–1628, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.