Abstract
A single determination of eGFR associates with subsequent mortality risk. Prior decline in eGFR indicates loss of kidney function, but the relationship to mortality risk is uncertain. We conducted an individual–level meta-analysis of the risk of mortality associated with antecedent eGFR slope, adjusting for established risk factors, including last eGFR, among 1.2 million subjects from 12 CKD and 22 other cohorts within the CKD Prognosis Consortium. Over a 3-year antecedent period, 12% of participants in the CKD cohorts and 11% in the other cohorts had an eGFR slope <−5 ml/min per 1.73 m2 per year, whereas 7% and 4% had a slope >5 ml/min per 1.73 m2 per year, respectively. Compared with a slope of 0 ml/min per 1.73 m2 per year, a slope of −6 ml/min per 1.73 m2 per year associated with adjusted hazard ratios for all-cause mortality of 1.25 (95% confidence interval [95% CI], 1.09 to 1.44) among CKD cohorts and 1.15 (95% CI, 1.01 to 1.31) among other cohorts during a follow-up of 3.2 years. A slope of +6 ml/min per 1.73 m2 per year also associated with higher all–cause mortality risk, with adjusted hazard ratios of 1.58 (95% CI, 1.29 to 1.95) among CKD cohorts and 1.43 (95% CI, 1.11 to 1.84) among other cohorts. Results were similar for cardiovascular and noncardiovascular causes of death and stronger for longer antecedent periods (3 versus <3 years). We conclude that prior decline or rise in eGFR associates with an increased risk of mortality, independent of current eGFR.
Keywords: chronic kidney disease, glomerular filtration rate, mortality, epidemiology, outcomes
CKD affects 10%–16% of the global population.1,2 Numerous studies have reported the significant association of low eGFR at a single time point with mortality,3–9 a more frequent occurrence than ESRD, even among patients with late stages of CKD.10 Recently, there has been great interest in whether a decline in eGFR adds information to mortality risk assessment beyond eGFR at a single time point. Clinicians are often faced with a situation in which current eGFR is known along with its past trajectory. Thus, a clinically relevant question is whether past trajectory of eGFR can provide additional information beyond current eGFR.11,12
A surprising finding in previous studies was that an increase in eGFR was associated with an increased risk of mortality. Whether these observations are generalizable is uncertain, because they were on the basis of data from single centers13,14 and/or cohorts with mean baseline eGFR values of ≥50 ml/min per 1.73 m2.11–16 Improvement in eGFR in a CKD population might show different associations with mortality than that in a general population cohort. In addition, the U-shaped association might be driven by confounding factors, such as weight loss or heart failure. Thus, a comprehensive investigation about eGFR increase and mortality risk is warranted.
The objective of the study was to use meta-analysis to address two clinically relevant questions: given patients presenting with a particular eGFR, does the prior eGFR trajectory provide additional prognostic information with respect to mortality risk beyond the present eGFR per se, and if so, what is the shape of this relationship?
Results
Associations with eGFR Slope
Over a 3-year antecedent period, median (interquartile range [IQR]) numbers of creatinine measurements were 7 (IQR, 7–7) in the CKD and 5 (IQR, 4–5) in the other (general population/high cardiovascular risk) cohorts; 12% of participants in the CKD and 11% of participants in the other cohorts had an eGFR slope <−5 ml/min per 1.73 m2 per year, whereas 7% and 4% experienced an eGFR slope >+5 ml/min per 1.73 m2 per year during the antecedent period, respectively. There were no consistent differences in the age or sex distribution between subjects with antecedent slopes of <−5, ≥−5 to ≤+5, and >+5 ml/min per 1.73 m2 year; however, black subjects tended to be in the <−5 ml/min per 1.73 m2 category (Supplemental Table 1, Table 1). Subjects with annual slopes <−5 ml/min per 1.73 m2 per year had a higher prevalence of elevated albuminuria, were more often diabetic, and were more likely to have a history of cardiovascular disease (CVD) compared with subjects in the stable or increasing eGFR slope categories (Supplemental Table 2).
Table 1.
Cohort characteristics and outcomes: characteristics of the CKD (n=12) and other (general population and high cardiovascular risk; n=22) cohorts that could provide data for a 3-year antecedent period
| Variable | Total Sample | CKD Cohorts | Other Cohorts |
|---|---|---|---|
| N | 1,277,217 | 249,977 | 1,027,240 |
| Median no. SCre (IQR) | 5 (4–5) | 7 (7–7) | 5 (4–5) |
| Slope <−5 ml/yr | |||
| N, % | 11 | 12 | 11 |
| Age (SD), yr | 58 (17) | 73 (11) | 54 (17) |
| Women, % | 49 | 9 | 60 |
| Black, % | 4 | 15 | 1 |
| Slope ≥−5 to ≤5 ml/yr | |||
| N, % | 84 | 80 | 85 |
| Age (SD), yr | 59 (17) | 76 (10) | 55 (16) |
| Women, % | 48 | 9 | 56 |
| Black, % | 2 | 9 | 0 |
| Slope >5 ml/yr | |||
| N, % | 5 | 7 | 4 |
| Age (SD), yr | 57 (19) | 73 (10) | 50 (17) |
| Women, % | 48 | 11 | 63 |
| Black, % | 3 | 10 | 1 |
| Mean (SD) follow-up,a yr | 3.2 (4.0) | 3 (1) | 3 (4) |
| ACM events | 102,477 | 57,269 | 45,208 |
| CVM eventsb | 8231 | 340 | 7891 |
Slope <−5 ml/yr is the declining eGFR group with an annualized eGFR slope of <−5 ml/min per 1.73 m2 per year. N, % is the proportion of the cohort belonging to a given slope category. Slope ≥−5 to ≤5 ml/yr is the stable eGFR group with an annualized GFR between ≥−5 and ≤5 ml/min per 1.73 m2 per year. Slope >5 ml/yr is the increasing eGFR group with an annualized eGFR slope of >5 ml/min per 1.73 m2 per year. SCre, serum creatinine measurements available during the antecedent period; CVM, cardiovascular mortality.
Follow-up time refers to the at-risk period subsequent to the 3-year antecedent interval.
Not all cohorts could provide data with respect to CVM (Supplemental Table 2).
After adjustment, lower current eGFR, younger age, black race, higher total cholesterol, the presence of diabetes, and the presence of albuminuria (severely increased only in CKD cohorts; moderately increased and severely increased in the other cohorts) were associated with antecedent slope <−5 ml/min per 1.73 m2 per year (Supplemental Table 3). Factors associated with an eGFR slope >+5 ml/min per 1.73 m2 per year included higher current (last) eGFR, women, history of CVD, and the presence of albuminuria (severely increased only in CKD cohorts; moderately increased and severely increased in the other cohorts).
All-Cause Mortality
Among cohorts with 3-year antecedent data, 102,477 of 1,277,217 subjects died (8%) over a mean follow-up time of 3.2 years (Supplemental Table 4, Table 1). Among 12 CKD cohorts, 57,269 of 249,977 subjects died (23%), whereas among 22 other cohorts, 45,208 of 1,027,240 subjects died (4%). After antecedent intervals of 1 and 2 years, 223,979 of 1,765,589 (13%) and 158,617 of 1,597,849 (10%) subjects died, respectively (Supplemental Table 5).
Risk of All-Cause Mortality Associated with a Decline in eGFR
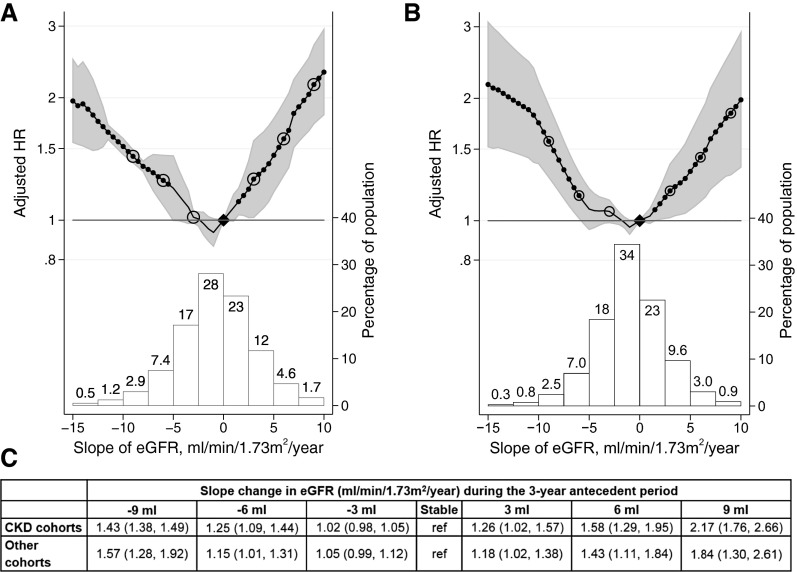
Compared with subjects with no change in eGFR over the antecedent 3-year period, a slope of −6 ml/min per 1.73 m2 per year was associated with hazard ratios (HRs) for all-cause mortality (ACM) of 1.25 (95% confidence interval [95% CI], 1.09 to 1.44) and 1.15 (95% CI, 1.01 to 1.31) among members of CKD and other cohorts, respectively (Figure 1, Supplemental Table 6). The risk of ACM associated with an annual eGFR decline was attenuated with shorter antecedent periods (corresponding to smaller absolute eGFR declines) (Supplemental Figure 1).
Figure 1.
HRs of ACM and change in eGFR. Analyses are shown for (A) CKD cohorts and (B) other (general population and high cardiovascular risk) cohorts. C depicts the adjusted HRs for the open circles in A and B. The upper panels of A and B depict meta-analyzed HRs for ACM associated with various annualized rates of eGFR. The reference group for calculation of HRs was patients with stable eGFR values (i.e., slope =0 ml/min per 1.73 m2 per year). Black circles indicate statistical significance compared with the reference (diamonds). The HR for eGFR slope was adjusted for age, sex, race (black versus nonblack), systolic BP, total cholesterol, diabetes, history of CVD, and current (last) eGFR. The lower panels of A and B illustrate histograms of the distribution of eGFR slopes among members of the CKD and other cohorts.
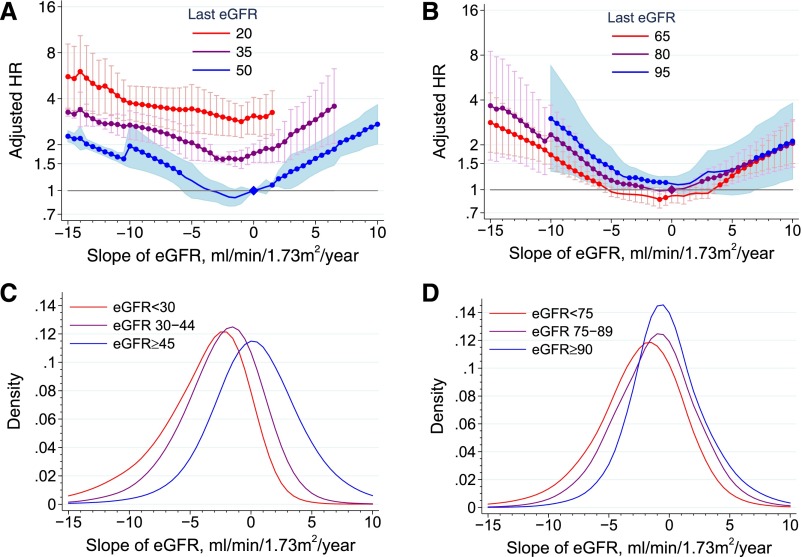
For both CKD and other cohorts, there was no statistically significant interaction of current eGFR and antecedent eGFR slope with ACM (P for interaction =0.17 and 0.19, respectively) (Figure 2). Higher current albuminuria was associated with higher ACM risk. Among albuminuria strata, the association between antecedent eGFR slope and ACM mortality overlapped only in the extremes of the eGFR slope distribution in the CKD cohorts and was roughly parallel by level of albuminuria in the other cohorts, suggesting a similar absence of interaction between current albuminuria and antecedent eGFR decline (P for interaction =0.67 [moderately increased albuminuria] and 0.45 [severely increased albuminuria] for CKD cohorts and P for interaction =0.44 [moderately increased albuminuria] and 0.14 [severely increased albuminuria] for other cohorts) (Supplemental Figure 2).
Figure 2.
Interaction of eGFR slope and current value of eGFR. Analyses are shown for the CKD cohorts (A and C) and the other (B and D; general population and high cardiovascular risk) cohorts. In A and B, meta–analyzed adjusted HRs for ACM associated with various annualized rates of eGFR within strata of current eGFR are depicted. For CKD and other cohorts, the current eGFR strata were set at 20, 35, and 50 ml/min per 1.73 m2 and 65, 80, and 95 ml/min per 1.73 m2, respectively. The reference group for calculation of HRs was patients with stable eGFR values (i.e., a slope =0 ml/min per 1.73 m2 per year). The HR for eGFR slope was adjusted for age, sex, race (black versus nonblack), systolic BP, total cholesterol, diabetes, history of CVD, and current (last) eGFR. C and D illustrate kernel density plots of the distribution of eGFR slopes with current eGFR strata among members of the cohorts.
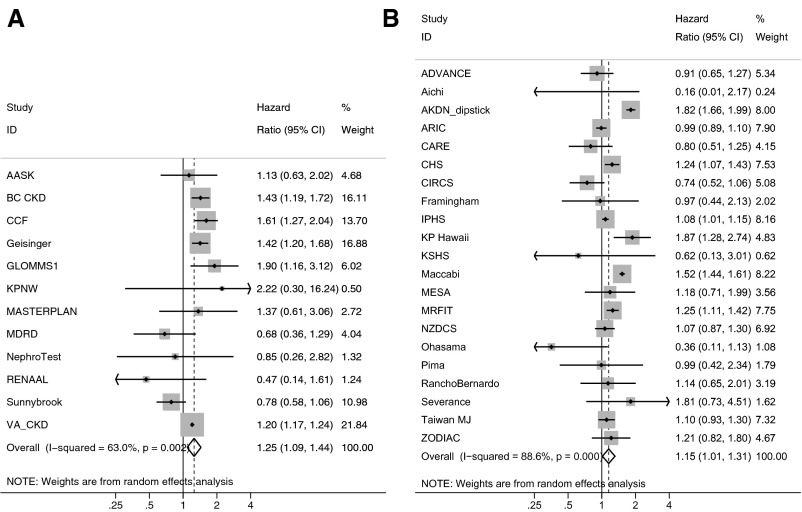
The risk associated with an eGFR slope of −6 ml/min/ per 1.73 m2 per year over the 3-year antecedent period showed heterogeneity (Figure 3). Among CKD cohorts, metaregression suggested that differences in follow-up time (with higher HRs associated with shorter follow-up) and median age (with higher HRs associated with older age) may have accounted for some heterogeneity (Supplemental Figure 3), whereas for the other cohorts, heterogeneity was not explained by metaregression (Supplemental Figure 4).
Figure 3.
Forest plot of HRs associated with a 6 ml/min per 1.73 m2 per year decline in eGFR (an eGFR slope of −6 ml/min per 1.73 m2 per year) over a 3-year antecedent period. Analyses are shown for (A) CKD cohorts and (B) other (general population and high cardiovascular risk) cohorts. Adjusted HRs within each cohort for ACM associated with an annualized decline of the eGFR of 6 ml/min per 1.73 m2 per year are depicted. The reference group for calculation of HRs was patients with stable eGFR values (i.e., a slope =0 ml/min per 1.73 m2 per year). The HR for eGFR slope was adjusted for age, sex, race (black versus nonblack), systolic BP, total cholesterol, diabetes, history of CVD, and current (last) eGFR. AASK, African American Study of Kidney Disease and Hypertension; ADVANCE, The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation Trial; Aichi, Aichi Workers’ Cohort; AKDN_dipstick, Alberta Kidney Disease Network; ARIC, Atherosclerosis Risk in Communities Study; BC CKD, British Columbia CKD Study; CARE, The Cholesterol and Recurrent Events Trial; CCF, Cleveland Clinic CKD Registry Study; CHS, Cardiovascular Health Study; CIRCS, Circulatory Risk in Communities Study; Framingham, Framingham Heart Study; Geisinger, Geisinger CKD Study; GLOMMS 1, Grampian Laboratory Outcomes, Morbidity and Mortality Studies 1; IPHS, Ibaraki Prefectural Health Study; KP Hawaii, Kaiser Permanente Hawaii Cohort; KPNW, Kaiser Permanente Northwest; KSHS, Kangbuk Samsung Health Study; MASTERPLAN, Multifactorial Approach and Superior Treatment Efficacy in Renal Patients with the Aid of a Nurse Practitioner; MDRD, Modification of Diet in Renal Disease Study; MESA, Multi-Ethnic Study of Atherosclerosis; MRFIT, Multiple Risk Factor Intervention Trial; NephroTest, NephroTest Study; NZDCS, New Zealand Diabetes Cohort Study; Ohasama, Ohasama Study; Pima, Pima Indian Study; RanchoBernardo, Rancho Bernardo Study; RENAAL, Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan; Severance, Severance Cohort Study; Sunnybrook, Sunnybrook Cohort; Taiwan MJ, Taiwan MJ Cohort Study; VA CKD, Veterans Administration CKD Study; ZODIAC, Zwolle Outpatient Diabetes Project Integrating Available Care.
For the CKD cohorts, absolute risk of ACM was higher with greater antecedent decline in eGFR, but current eGFR was relatively more important in determining the absolute mortality risk. Absolute risk of ACM in the other cohorts was low (Supplemental Table 7).
Risk of ACM Associated with an Increase in eGFR
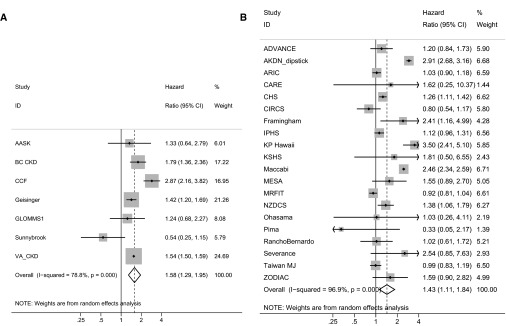
ACM risk associations of antecedent eGFR increase were at least as strong as those for eGFR decline and mortality (Figure 1). Compared with subjects with no change in eGFR over the antecedent 3-year period, a slope of +6 ml/min per 1.73 m2 per year was associated with HRs for ACM of 1.58 (95% CI, 1.29 to 1.95) for the CKD cohorts and 1.43 (95% CI, 1.11 to 1.84) among members of the other cohorts (Figure 1, Supplemental Table 6). The risk associated with an eGFR slope of +6 ml/min per 1.73 m2 per year over the 3-year antecedent period showed heterogeneity across both CKD and other cohorts (Figure 4). The absolute risk of ACM was higher among members of the CKD versus the other cohorts, with current eGFR being a more important risk factor than antecedent slope (Supplemental Table 7).
Figure 4.
Forest plot of HRs associated with a 6 ml/min per 1.73 m2 per year increase in eGFR (an eGFR slope of +6 ml/min per 1.73 m2 per year) over a 3-year antecedent period. Analyses are shown for (A) CKD cohorts and (B) other (general population and high cardiovascular risk) cohorts. Adjusted HRs within each cohort for ACM associated with an annualized increase of the eGFR of 6 ml/min per 1.73 m2 per year are depicted. The reference group for calculation of HRs was patients with stable eGFR values (i.e., slope =0 ml/min per 1.73 m2 per year). The HR for eGFR slope was adjusted for age, sex, race (black versus nonblack), systolic BP, total cholesterol, diabetes, history of CVD, and current (last) eGFR. AASK, African American Study of Kidney Disease and Hypertension; ADVANCE, The Action in Diabetes and Vascular Disease: Preterax and Diamicron Modified Release Controlled Evaluation Trial; AKDN_dipstick, Alberta Kidney Disease Network; ARIC, Atherosclerosis Risk in Communities Study; BC CKD, British Columbia CKD Study; CARE, The Cholesterol and Recurrent Events Trial; CCF, Cleveland Clinic CKD Registry Study; CHS, Cardiovascular Health Study; CIRCS, Circulatory Risk in Communities Study; Framingham, Framingham Heart Study; Geisinger, Geisinger CKD Study; GLOMMS 1, Grampian Laboratory Outcomes, Morbidity and Mortality Studies 1; IPHS, Ibaraki Prefectural Health Study; KP Hawaii, Kaiser Permanente Hawaii Cohort; KSHS, Kangbuk Samsung Health Study; MESA, Multi-Ethnic Study of Atherosclerosis; MRFIT, Multiple Risk Factor Intervention Trial; NZDCS, New Zealand Diabetes Cohort Study; Ohasama, Ohasama Study; Pima, Pima Indian Study; RanchoBernardo, Rancho Bernardo Study; Severance, Severance Cohort Study; Sunnybrook, Sunnybrook Cohort; Taiwan MJ, Taiwan MJ Cohort Study; VA CKD, Veterans Administration CKD Study; ZODIAC, Zwolle Outpatient Diabetes Project Integrating Available Care.
The association of eGFR increase and mortality remained significant in all sensitivity analyses. Participants with positive eGFR slopes in the other cohorts had a trend toward higher risk of both cardiovascular and noncardiovascular mortality, although risk associations were attenuated (Table 2). Similarly, the increased risk of ACM associated with a positive eGFR slope in the antecedent period persisted when we included a measure, the root mean squared error (RMSE), of each individual’s variation around the eGFR slope line as a covariate in the Cox model (Supplemental Figure 5) or when the model was stratified by RMSE (Supplemental Figure 6). Although weight loss of >2.0 kg was associated with increased odds of eGFR rise, excluding subjects who lost >2.0 kg during the antecedent 3 years did not alter the U-shaped relationship between antecedent eGFR slope and ACM (Supplemental Figure 7). Excluding patients with diabetes and either adjusting for or stratifying by use of renin-angiotensin system–inhibiting medications in the antecedent period made no meaningful difference in the risk associations (Supplemental Figures 8–10).
Table 2.
Adjusted HRs for cardiovascular mortality and noncardiovascular mortality subsequent to an eGFR slope during a 3-year antecedent period for the other (general/high risk) cohorts (among 14 cohorts with available data)
| Outcome | Slope Change in eGFR (ml/min per 1.73 m2 per yr) during the 3-yr Antecedent Period | ||||||
|---|---|---|---|---|---|---|---|
| −9 | −6 | −3 | Stable | 3 | 6 | 9 | |
| CV mortality | |||||||
| Other cohorts | 1.33 (1.17 to 1.52) | 1.10 (0.98 to 1.22) | 1.08 (0.97 to 1.21) | Reference | 1.12 (1.02 to 1.22) | 1.27 (1.10 to 1.46) | 1.46 (1.16 to 1.84) |
| Non-CV mortality | |||||||
| Other cohorts | 1.29 (1.17 to 1.43) | 1.09 (1.03 to 1.15) | 1.03 (0.95 to 1.13) | Reference | 1.02 (0.95 to 1.09) | 1.08 (0.95 to 1.23) | 1.31 (1.00 to 1.73) |
Data are presented as adjusted HR (95% CI). CV, cardiovascular. The HR for eGFR slope was adjusted for age, sex, race (black versus nonblack), systolic BP, total cholesterol, diabetes, history of CVD, and current (last) eGFR.
Analyses using percentage change of eGFR rather than slope are shown in Supplemental Figure 11. Because a given absolute change in eGFR represents a higher percentage change for persons with lower current eGFR values and because the CKD cohorts had, in general, lower current eGFR, the distribution of percentage decline is shifted to the left for the CKD relative to the other cohorts, such that a greater number of persons in the CKD cohorts experienced a ≥30% reduction in eGFR over 3 years. Nonetheless, risk associations were similar to slightly stronger when prior eGFR trajectory was assessed as a percentage change rather than slope (Supplemental Figure 11). Compared with an adjusted Cox model without eGFR slope, the addition of the latter resulted in a marginal improvement in the discrimination with respect to ACM: pooled estimates for the resulting change of c statistics were 0.003 (95% CI, −0.000 to 0.007) and 0.002 (95% CI, 0.001 to 0.004) for the CKD and other cohorts, respectively (Supplemental Table 8).
Discussion
In this analysis of >1.2 million subjects and >100,000 deaths, we found that antecedent eGFR slope over a 3-year period, whether positive or negative, exhibited a statistically significant association with ACM, cardiovascular mortality, and noncardiovascular mortality. These associations were observed even after adjustment for current eGFR (last eGFR in the antecedent period), suggesting that there is modest incremental information in the prior eGFR trajectory beyond eGFR measured at a single time point. In general, large changes in eGFR were unusual (11% for <−5 ml/min per 1.73 m2 per year and 5% for >5 ml/min per 1.73 m2 per year), but associated with the highest risk of mortality, whereas lesser changes were more common, but associated with smaller risks. Antecedent improvement in eGFR was associated with a mortality risk similar in magnitude to antecedent decline. This association persisted in numerous sensitivity analyses, suggesting that rapid change in creatinine-based eGFR—whether for the worse or the better—may be a poor prognostic sign. The relationship between antecedent eGFR slope and ACM was apparent across the entire spectrum of current eGFR, but at least within CKD cohorts, current eGFR had a much greater effect on absolute mortality risk than did prior trajectory.
Previous studies have shown that low eGFR measured at a single time point is an important risk factor for ACM.3,5,17–19 We sought to evaluate whether prior change in eGFR contributes independently to ACM prognosis in the clinical setting, where last eGFR value is known. Previous studies have investigated this association from a clinical trial perspective, adjusting for the first eGFR. The latter is relevant for the situation where two subjects begin a clinical trial at the same eGFR value, but one maintains a stable eGFR, whereas the other subject’s eGFR either falls or rises.20 In contrast, adjustment for last eGFR during the antecedent period, as per this analysis, replicates the clinical scenario, whereby ACM risk is compared between two patients who present with the same eGFR value, but one has had a stable eGFR, and the other has either fallen or risen to that value. Similar to previous work, in which adjustments were made for either the first or last eGFR in the antecedent period, we found a U-shaped relationship between eGFR slope and subsequent ACM risk.11,13–16 Direct, quantitative comparison between the results of these investigations and our own investigations are hampered by different indices of renal function change, different antecedent periods, and the use of rates, in some studies, rather than HRs to quantify mortality risk. However, Turin et al.12 found adjusted HRs for ACM of 1.14 and 1.68 for 4-ml/min per 1.73 m2 per year declining and increasing slopes, respectively, compared with subjects with a stable eGFR value in a Canadian population–based study. These values are qualitatively similar to those for the other (general population and high risk) cohorts in this analysis. The small quantitative difference may be caused by differences in the set of adjustment factors used in the two studies. Note that data from the latter cohort were included in this analysis.
Several mechanisms may underlie the association of antecedent change in eGFR and mortality. In principle, change in creatinine-based eGFR may reflect either change in true GFR—caused by progression or remission of CKD or onset or recovery from acute kidney disease—or change in nonfiltration determinants of serum creatinine, such as muscle wasting or malnutrition. A steeper antecedent eGFR decline has been traditionally held to signify past decline in true GFR. Thus, in this study, the true GFR for individuals with a steeper eGFR decline in the antecedent period may have continued to decline in the follow-up period below the last or current true GFR, and lower true GFR per se is expected to be associated with mortality. Alternatively, an antecedent decline in true GFR may simply reflect a more severe comorbidity profile.12,14 For example, although we adjusted for diabetes and our findings were qualitatively similar after excluding subjects with diabetes, we did not adjust for severity of diabetes, a key determinant of both true GFR decline and mortality risk.21,22 Similarly, episodes of acute coronary syndrome or congestive heart failure may increase the risk of death and also, cause true GFR decline.23,24 However, we observed similar associations of antecedent eGFR decline with non-CVD mortality as we did with CVD mortality (albeit in the limited cohorts with these data). Previous investigations have suggested that variability in the eGFR itself may be associated with higher ACM risk.25 However, in this study, with individual residual eGFR variation expressed as the RMSE, we found little attenuation of the effect of decreasing (or increasing) eGFR slope on ACM.
The association between increasing eGFR and mortality is less intuitive. Rather than indicating improving true GFR, a rising eGFR may be an indicator of declining muscle mass or malnutrition, with the latter being responsible for the increase in ACM risk. However, exclusion of subjects who lost weight attenuated the risk of ACM on both ends of the eGFR slope spectrum but did not eliminate the U shape. Furthermore, a previous study reported an association between higher ACM risk and positive eGFR slope using cystatin C as a filtration marker, although cystatin C levels are less affected by muscle mass than creatinine, suggesting that a rising eGFR may reflect a rising true GFR.16 A rising prior true GFR may be caused by recovery from acute kidney disease associated with an acute illness, and it was the latter that was responsible for the observed increase in ACM risk rather than the rising true GFR per se. Finally, a rising true GFR could be seen with hyperfiltration in remnant nephrons, which could be associated with subsequent kidney disease progression, but it is not generally hypothesized to be associated with mortality. Because single-nephron GFR cannot be measured in humans, this mechanism remains speculative.
The strengths of this analysis include its large sample size with geographically diverse general population, high CVD risk, and CKD cohorts with current eGFR values that spanned a wide spectrum. We used an index of eGFR change that is commonly used in the clinical setting, the annualized eGFR slope, and in sensitivity analyses, the percentage change in eGFR. We estimated ACM risks with a uniform meta–analytic approach using individual-level data across collaborating cohorts. Our study also has limitations. The general/high-risk cohorts enrolled generally younger persons and were less representative with respect to elderly individuals than the CKD cohorts. As in all observational studies, residual confounding is possible, and we captured only certain comorbidities. Laboratory assays were not uniform, but where possible, serum creatinine measures were calibrated to isotope dilution mass spectrometry standards. Variation in cohort study design as well as study population might introduce heterogeneity, but the relative consistency across cohorts, despite these variations, points toward the robustness of our findings. Finally, P values close to the nominal level of significance may be prone to type 1 error given the number of statistical tests involved in our analyses.
In conclusion, compared with patients with a stable eGFR, those with either an antecedent rise or fall in values were at increased risk of subsequent mortality. Prior change of eGFR over 3 years contributed additional information regarding mortality risk beyond the current eGFR itself. However, these incremental risks were clinically meaningful only for large eGFR changes, which were uncommon. Future research could focus on new filtration markers or direct GFR measurement to help to elucidate the nature of the relationship between rising eGFR and mortality risk.
Concise Methods
Cohort Selection Criteria
The Chronic Kidney Disease Progression Consortium includes cohorts in which the presence of CKD was required for cohort entry and those in which entry was determined by factors other than CKD (general population and high–CVD risk cohorts; i.e., other cohorts).3–5,8,18 This study involved 35 cohorts (13 CKD and 22 other) and included subjects ≥18 years of age who had repeated serum creatinine measurements during antecedent intervals from 1 to 3 years in duration. For the main analysis, we included 34 cohorts (12 CKD and 22 other) that could provide data for a 3-year antecedent period. This study was approved by the Institutional Review Board at the Johns Hopkins Bloomberg School of Public Health.
Antecedent Change in eGFR
eGFR was calculated using the Chronic Kidney Disease Epidemiology Collaboration 2009 creatinine equation.26 In cohorts without standardization of creatinine measurement to isotope dilution mass spectrometry, reported creatinine levels were multiplied by 0.95.27 For each participant, annualized eGFR slope (milliliters per minute per 1.73 m2 per year) was derived from ordinary least squares10 regression using all eGFR measurements available during the antecedent period. This study focuses on the population with available data in the 3-year antecedent interval (results for 1- and 2-year periods are presented in Supplemental Material). Rapidly declining, stable, and rapidly increasing eGFR slope were defined as antecedent slopes of <−5, −5 to +5, and >+5 ml/min per 1.73 m2 per year, respectively.28
Assessment of Baseline Covariates
Within the antecedent period, we considered the last eGFR as the current eGFR. The last eGFR measurement was taken at 3±0.5 years (i.e., between 2.5 and 3.5 years after the first available eGFR). All covariates were assessed within 1 year before the last eGFR measurement during the antecedent period. Diabetes was defined as fasting glucose ≥7.0 mmol/L (126 mg/dl), nonfasting glucose ≥11.1 mmol/L (200 mg/dl), hemoglobin A1c ≥6.5%, use of antiglycemic drugs, or self-reported diabetes. Prior myocardial infarction, coronary revascularization, heart failure, or stroke was considered as a history of CVD. Albuminuria was categorized as none, moderately increased, or severely increased.29
Assessment of Outcomes
The primary study outcome was ACM occurring subsequent to the antecedent time period, with time at risk starting at the last measurement of eGFR (current). In Supplemental Material, we analyzed cardiovascular and noncardiovascular mortality when data were available (i.e., for 14 of the other cohorts).
Statistical Analyses
We performed two–stage meta-analyses, whereby each cohort was first analyzed separately and then pooled using random effect models (Supplemental Appendix 1). We imputed missing values of covariates (except eGFR) using cohort–specific mean values. Covariates that were completely missing for a particular cohort were excluded from the regression model for that cohort. We assessed heterogeneity with the I2 statistic8 and random effects meta–regression analyses. Because the distributions of antecedent eGFR slope may be different among other and CKD cohorts, we a priori designed the meta-analyses to be stratified by cohort type.
Within each cohort, we estimated the adjusted HRs of ACM according to GFR slope with piecewise linear splines (knots at −10, −5, −3, −1, +1, and +3 ml/min per 1.73 m2 per year). Cox models were adjusted for age, sex, race (black versus nonblack), systolic BP, total cholesterol, diabetes, history of CVD, and current eGFR. Adjustment for albuminuria was done only in secondary analyses, because albuminuria was not measured in conjunction with the last available eGFR in several cohorts. Forest plots of HR estimates at eGFR slopes of −6 and +6 ml/min per 1.73 m2 per year were constructed (chosen as representative values within the rapid declining and rising eGFR slope categories, respectively). Differential effects of current eGFR and albuminuria on the relationship between change in eGFR and ACM were evaluated with interaction terms. We computed the base-case cumulative hazard of ACM at 1, 3, 5, and 10 years after baseline (Supplemental Appendix 2). Absolute risk was calculated by multiplying the meta–analyzed adjusted HRs for eGFR slopes of −6, −4, −2, 0, +2, +4, and +6 ml/min per 1.73 m2 per year by the pooled base-case cumulative hazard. The improvement in discrimination with respect to ACM was assessed with the difference in c statistics for an adjusted model with and without eGFR slope as a covariate.
Because of an observed risk increase with antecedent increase in eGFR, we conducted several sensitivity analyses. First, we evaluated the associations of antecedent eGFR slope with cardiovascular (death caused by myocardial infarction, heart failure, stroke, or sudden cardiac death) and noncardiovascular (all other etiologies) mortality. Second, we assessed the effect of individual residual eGFR variability. We used the RMSE as an indicator of the variation of an individual’s eGFR values around his or her ordinary least squares regression line. The RMSE was included as a covariate and then, a stratifying variable (categorized as <5, 5–10, and >10). Third, to explore whether increasing eGFR reflected weight loss, we excluded subjects with antecedent weight loss >2 kg over the 3-year period. Fourth, to evaluate whether the U–shaped risk relationship might represent diabetes–associated glomerular hyperfiltration, we repeated analyses excluding persons with diabetes mellitus. Fifth, analyses were repeated according to whether individuals had ever been exposed to renin-angiotensin-system blocking medications in the antecedent interval as a covariate in the Cox model and then, a stratifying variable. Analyses were performed using Stata/SE 13 software (StataCorp., College Station, TX; www.stata.com). P values <0.05 were considered statistically significant.
Disclosures
None.
Supplementary Material
Acknowledgments
The Chronic Kidney Disease Prognosis Consortium (CKD-PC) Data Coordinating Center is funded, in part, by a program grant from the US National Kidney Foundation (funding sources include AbbVie and Amgen, Inc.) and National Institute of Diabetes and Digestive and Kidney Diseases Grant R01DK100446-01. A variety of sources have supported enrollment and data collection, including laboratory measurements, as well as follow-up in the collaborating cohorts of the CKD-PC. These funding sources include government agencies, such as the National Institutes of Health, and medical research councils as well as foundations and industry sponsors, and they are listed in Supplemental Appendix 3. Individual cohort and collaborator support is listed in Supplemental Appendix 3.
The funders had no role in the design, analysis, or interpretation of this study and did not contribute to the writing of this report or the decision to submit the article for publication.
Group members are listed by study. African American Study of Kidney Disease and Hypertension: Jackson T. Wright Jr., Case Western Reserve University; Lawrence J. Appel, Johns Hopkins University; Tom Greene, University of Utah; and Brad C. Astor, University of Wisconsin. The Action in Diabetes and Vascular Disease Study: Preterax and Diamicron Modified Release Controlled Evaluation: Stephen MacMahon, George Institute; John Chalmers, George Institute; Hisatomi Arima, George Institute; and M.W., George Institute, University of Oxford, University of Sydney, and Johns Hopkins Bloomberg School of Public Health. Aichi Workers’ Cohort: H.Y., Fujita Health University; Kentaro Yamashita, Nagoya Medical Center; Hideaki Toyoshima, Anjo Kosei Hospital; and Koji Tamakoshi, Nagoya University. Alberta Kidney Disease Network: M.T., University of Calgary; Brenda Hemmelgarn, University of Calgary; Matt James, University of Calgary; and Tanvir Chowdhury Turin, University of Calgary. Atherosclerosis Risk in Communities Study: J.C., Johns Hopkins University; K.M., Johns Hopkins University; M.E.G., Johns Hopkins University; and Yingying Sang, Johns Hopkins University. Australian Diabetes, Obesity, and Lifestyle Study (AusDiab): Robert C. Atkins, Monash University; Kevan R. Polkinghorne, Monash University; and Steven Chadban, University of Sydney. Beaver Dam CKD: Anoop Shankar; Ronald Klein, University of Wisconsin; Barbara E.K. Klein, University of Wisconsin; and Kristine E. Lee, University of Wisconsin. British Columbia CKD Study: Adeera Levin, British Columbia Provincial Renal Agency and University of British Columbia and Ognjenka Djurdjev, British Columbia Provincial Renal Agency and Provincial Health Services. The Cholesterol and Recurrent Events Trial: M.T., University of Calgary; Frank M. Sacks, Harvard School of Public Health; and Gary C. Curhan, Channing Division of Network Medicine/Renal Division, Brigham and Women's Hospital, Harvard Medical School, Harvard School of Public Health. Cardiovascular and Renal Outcome in CKD 2–4 Patients - The Fourth Homburg evaluation (CARE for HOMe): Adam M. Zawada, Saarland University Medical Center; Kyrill S. Rogacev, Saarland University Medical Center; Sarah Seiler, Saarland University Medical Center; and Gunnar H. Heine, Saarland University Medical Center. Cleveland Clinic CKD Registry Study: Sankar D. Navaneethan, Cleveland Clinic; J.N., Glickman Urological and Kidney Institute; and Jesse D. Schold, Cleveland Clinic. Cardiovascular Health Study: Michael Shlipak, University of California, San Francisco and San Francisco Veterans Affairs Medical Center; Mark J. Sarnak, Tufts Medical Center; and Ronit Katz, University of Washington. Circulatory Risk in Communities Study: Hiroyasu Iso, Osaka University; A.K., Osaka Center for Cancer and Cardiovascular Disease Prevention; Hironori Imano, Osaka University; and Kazumasa Yamagishi, University of Tsukuba. Chronic Renal Impairment in Birmingham: David C. Wheeler, University College London; Jonathan Emberson, University of Oxford; Jonathan N. Townend, Queen Elizabeth Hospital Birmingham; and Martin J. Landray, University of Oxford. Epidemiologische Studie zu Chancen der Verhuetung, Frueherkennung und optimierten Therapie chronischer Erkrankungen in der aelteren Bevölkerung (ESTHER): D.R., German Cancer Research Center and Ulm University; Hermann Brenner, German Cancer Research Center; Heiko Müller, German Cancer Research Center; and Ben Schöttker, German Cancer Research Center. Framingham Heart Study: C.S.F., Center for Population Studies and Brigham and Women’s Hospital and Harvard Medical School; Shih-Jen Hwang, National Heart, Lung, and Blood Institute; James B. Meigs, Massachusetts General Hospital; and Ashish Uphadhay, Boston University Medical Center. Geisinger CKD Study: Jamie Green, Geisinger Medical Center; H. Lester Kirchner, Geisinger Medical Center; Robert Perkins, Geisinger Medical Center; and Alex R. Chang, Geisinger Medical Center. Grampian Laboratory Outcomes, Morbidity and Mortality Study 1: C.B., University of Aberdeen; Angharad Marks, University of Aberdeen; Nick Fluck, National Health Service (NHS) Grampian; and Gordon J. Prescott, University of Aberdeen. Gubbio: Massimo Cirillo, University of Salerno. The Nord-Trøndelag Health Study: Stein Hallan, Norwegian University of Science and Technology and St. Olav University; Knut Aasarød, Norwegian University of Science and Technology and St. Olav University Hospital; Cecilia M. Øien, Norwegian University of Science and Technology; and Maria Radtke, Norwegian University of Science and Technology and St. Olavs University Hospital. Ibaraki Prefectural Health Study: Fujiko Irie, Ibaraki Prefectural Office; Hiroyasu Iso, Osaka University; Toshimi Sairenchi, Dokkyo Medical University School of Medicine; and Kazumasa Yamagishi, University of Tsukuba. Kaiser Permanente Northwest: David H. Smith, Kaiser Permanente Northwest; Micah L. Thorp, Kaiser Permanente Northwest; and Eric S. Johnson, Kaiser Permanente Northwest. Kaiser Permanente Hawaii Cohort: Brian J. Lee, Kaiser Permanente Hawaii Region. Kangbuk Samsung Health Study: Eliseo Guallar, Johns Hopkins University; S.R., Sunkgyunkwan University School of Medicine; Yoosoo Chang, Kangbuck Samsung Hospital, Sungkyunkwan University, School of Medicine; Juhee Cho, Sungkyunkwan University; and Hocheol Shin, Kangbuck Samsung Hospital, Sungkyunkwan University, School of Medicine. Maccabi: Gabriel Chodick, Maccabi Healthcare Services; Varda Shalev, Maccabi Healthcare Services and Tel Aviv University; Yair C. Birnbaum, Maccabi Healthcare Services; and Bracha Shainberg, Maccabi Healthcare Services. Multifactorial Approach and Superior Treatment Efficacy in Renal Patients with the Aid of a Nurse Practitioner Study: Jack F.M. Wetzels, Radboud University Medical Centre; Peter J. Blankestijn, University Medical Center Utrecht; and Arjan D. van Zuilen, University Medical Center Utrecht. Modification of Diet in Renal Disease Study: Mark J. Sarnak, Tufts Medical Center; Andrew S. Levey, Tufts Medical Center; L.A.I, Tufts Medical Center; and Vandana Menon, Tufts Medical Center. Multi-Ethnic Study of Atherosclerosis: Michael Shlipak, University of California, San Francisco and San Francisco Veterans Affairs Medical Center; Mark J. Sarnak, Tufts Medical Center; Ronit Katz, University of Washington; and C.A.P., University of California and San Francisco Veterans Affairs Medical Center. Multiple Risk Factor Intervention Trial: A.I., Minneapolis Veterans Affairs Health Care System; and James D. Neaton, University of Minnesota. NephroTestStudy: Marc Froissart, Institut National de la Santé et de la Recherche Médicale (Inserm) U1018; Benedicte Stengel, Inserm U1018, University of Paris Sud, and Université Versailles Saint Quentin (USVQ); Marie Metzger, Inserm U1018 and University of Paris Sud-11; Jean-Philippe Haymann, Sorbonne Universités, Université Pierre et Marie Curie University of Paris 06, Assistance Publique-Hôpitaux de Paris; Pascal Houillier, Assistance Publique-Hôpitaux de Paris, Paris Descartes University; and Martin Flamant, Assistance Publique-Hôpitaux de Paris. New Zealand Diabetes Cohort Study: C. Raina Elley, University of Auckland; Timothy Kenealy, University of Auckland; Simon A. Moyes, University of Auckland; John F. Collins, Auckland District Health Board; and Paul L. Drury, Auckland District Health Board. Ohasama Study: Takayoshi Ohkubo, Teikyo University; Hirohito Metoki, Tohoku University; Masaaki Nakayama, Fukushima Medical University; Masahiro Kikuya, Tohoku University; and Yutaka Imai, Tohoku University. Okinawa 83/93: Kunitoshi Iseki, University Hospital of the Ryukyus. Pima Indian Study: Robert G. Nelson, National Institute of Diabetes and Digestive and Kidney Diseases; and William C. Knowler, National Institute of Diabetes and Digestive and Kidney Diseases. Prevention of Renal and Vascular Endstage Disease Study: R.T.G., University Medical Center Groningen; Stephan J.L. Bakker, University Medical Center Groningen; Hans L. Hillege, University Medical Center Groningen; and Pim van der Harst, University Medical Center Groningen. Rancho Bernardo Study: Simerjot K. Jassal, University of California, San Diego and Veterans Affairs San Diego Healthcare; Jaclyn Bergstrom, University of California, San Diego; Joachim H. Ix, University of California, San Diego and Veterans Affairs San Diego Healthcare; and Elizabeth Barrett-Connor, University of California, San Diego. Reduction of Endpoints in Non-Insulin Dependent Diabetes Mellitus with the Angiotensin II Antagonist Losartan: Hiddo J. Lambers Heerspink, University of Groningen; Barry E. Brenner, Brigham and Women's Hospital and Harvard School of Medicine; and Dick de Zeeuw, University of Groningen. Severance Cohort Study: S.H.J., Yonsei University; Heejin Kimm, Yonsei University; and Yejin Mok, Yonsei University. Sunnybrook Cohort: Navdeep Tangri, University of Manitoba; Maneesh Sud, University of Toronto; and D.N., University of Toronto. Taiwan MJ Cohort Study: Chi-Pang Wen, China Medical University Hospital; Sung-Feng Wen, University of Wisconsin; Chwen-Keng Tsao, MJ Health Management Institution; and Min-Kuang Tsai, National Health Research Institutes. ULSAM: Johan Ärnlöv, Uppsala University; Lars Lannfelt, Uppsala University; and Anders Larsson, Uppsala University. Veterans Administration CKD Study: Csaba P. Kovesdy, Memphis Veterans Affairs Medical Center and University of Tennessee Health Science Center; and Kamyar Kalantar-Zadeh, University of California Irvine Medical Center. Zwolle Outpatient Diabetes Project Integrating Available Care: Henk J. Bilo, Isala Clinics; Nanno Kleefstra, Isala Clinics; Klaas H. Groenier, Isala Clinics; Hanneke Joosten, Isala Clinics; and I.D., Isala Clinics. CKD-PC Steering Committee: J.C., Johns Hopkins Bloomberg School of Public Health; R.T.G., University Medical Center Groningen; P.E.d.J., University Medical Center Groningen; Kunitoshi Iseki, University Hospital of the Ryukyus; Andrew S. Levey, Tufts Medical Center; K.M., Johns Hopkins Bloomberg School of Public Health; Mark J. Sarnak, Tufts Medical Center; Benedicte Stengel, l'Institut National de la Santé et de la Recherche Médicale U1018, University of Paris Sud, and l'Université Versailles Saint Quentin (USVQ); D.G.W., University of Alabama at Birmingham; and M.W., George Institute, University of Oxford, University of Sydney, and Johns Hopkins Bloomberg School of Public Health. CKD-PC Data Coordinating Center: Shoshana H. Ballew (Coordinator), Johns Hopkins Bloomberg School of Public Health; J.C. (Principal Investigator), Johns Hopkins Bloomberg School of Public Health; M.E.G. (Director of Nephrology Initiatives), Johns Hopkins Bloomberg School of Public Health; K.M. (Director), Johns Hopkins Bloomberg School of Public Health; Yingying Sang (Lead Programmer), Johns Hopkins Bloomberg School of Public Health; and M.W. (Senior Statistician), George Institute, University of Oxford, University of Sydney, and Johns Hopkins Bloomberg School of Public Health.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015060688/-/DCSupplemental.
References
- 1.Kidney Disease: Improving Global Outcomes (KDIGO) CKD Work Group : KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kidney Int Suppl 3: 1–150, 2013 [Google Scholar]
- 2.Levey AS, Atkins R, Coresh J, Cohen EP, Collins AJ, Eckardt KU, Nahas ME, Jaber BL, Jadoul M, Levin A, Powe NR, Rossert J, Wheeler DC, Lameire N, Eknoyan G: Chronic kidney disease as a global public health problem: Approaches and initiatives - a position statement from Kidney Disease Improving Global Outcomes. Kidney Int 72: 247–259, 2007 [DOI] [PubMed] [Google Scholar]
- 3.van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey A, de Jong P, Gansevoort RT, van der Velde M, Matsushita K, Coresh J, Astor BC, Woodward M, Levey AS, de Jong PE, Gansevoort RT, Levey A, El-Nahas M, Eckardt KU, Kasiske BL, Ninomiya T, Chalmers J, Macmahon S, Tonelli M, Hemmelgarn B, Sacks F, Curhan G, Collins AJ, Li S, Chen SC, Hawaii Cohort KP, Lee BJ, Ishani A, Neaton J, Svendsen K, Mann JF, Yusuf S, Teo KK, Gao P, Nelson RG, Knowler WC, Bilo HJ, Joosten H, Kleefstra N, Groenier KH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with all-cause and cardiovascular mortality. A collaborative meta-analysis of high-risk population cohorts. Kidney Int 79: 1341–1352, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Gansevoort RT, Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J Chronic Kidney Disease Prognosis Consortium : Lower estimated GFR and higher albuminuria are associated with adverse kidney outcomes. A collaborative meta-analysis of general and high-risk population cohorts. Kidney Int 80: 93–104, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, Jong PE, Coresh J, Astor BC, Matsushita K, Gansevoort RT, van der Velde M, Woodward M, Levey AS, de Jong PE, Coresh J, El-Nahas M, Eckardt KU, Kasiske BL, Wright J, Appel L, Greene T, Levin A, Djurdjev O, Wheeler DC, Landray MJ, Townend JN, Emberson J, Clark LE, Macleod A, Marks A, Ali T, Fluck N, Prescott G, Smith DH, Weinstein JR, Johnson ES, Thorp ML, Wetzels JF, Blankestijn PJ, van Zuilen AD, Menon V, Sarnak M, Beck G, Kronenberg F, Kollerits B, Froissart M, Stengel B, Metzger M, Remuzzi G, Ruggenenti P, Perna A, Heerspink HJ, Brenner B, de Zeeuw D, Rossing P, Parving HH, Auguste P, Veldhuis K, Wang Y, Camarata L, Thomas B, Manley T Chronic Kidney Disease Prognosis Consortium : Lower estimated glomerular filtration rate and higher albuminuria are associated with mortality and end-stage renal disease. A collaborative meta-analysis of kidney disease population cohorts. Kidney Int 79: 1331–1340, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cheng TY, Wen SF, Astor BC, Tao XG, Samet JM, Wen CP: Mortality risks for all causes and cardiovascular diseases and reduced GFR in a middle-aged working population in Taiwan. Am J Kidney Dis 52: 1051–1060, 2008 [DOI] [PubMed] [Google Scholar]
- 7.Foley RN, Murray AM, Li S, Herzog CA, McBean AM, Eggers PW, Collins AJ: Chronic kidney disease and the risk for cardiovascular disease, renal replacement, and death in the United States Medicare population, 1998 to 1999. J Am Soc Nephrol 16: 489–495, 2005 [DOI] [PubMed] [Google Scholar]
- 8.Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, Coresh J, Gansevoort RT Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: A collaborative meta-analysis. Lancet 375: 2073–2081, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sarnak MJ, Levey AS, Schoolwerth AC, Coresh J, Culleton B, Hamm LL, McCullough PA, Kasiske BL, Kelepouris E, Klag MJ, Parfrey P, Pfeffer M, Raij L, Spinosa DJ, Wilson PW American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention : Kidney disease as a risk factor for development of cardiovascular disease: A statement from the American Heart Association Councils on Kidney in Cardiovascular Disease, High Blood Pressure Research, Clinical Cardiology, and Epidemiology and Prevention. Circulation 108: 2154–2169, 2003 [DOI] [PubMed] [Google Scholar]
- 10.Keith DS, Nichols GA, Gullion CM, Brown JB, Smith DH: Longitudinal follow-up and outcomes among a population with chronic kidney disease in a large managed care organization. Arch Intern Med 164: 659–663, 2004 [DOI] [PubMed] [Google Scholar]
- 11.Turin TC, Coresh J, Tonelli M, Stevens PE, de Jong PE, Farmer CK, Matsushita K, Hemmelgarn BR: One-year change in kidney function is associated with an increased mortality risk. Am J Nephrol 36: 41–49, 2012 [DOI] [PubMed] [Google Scholar]
- 12.Turin TC, Coresh J, Tonelli M, Stevens PE, de Jong PE, Farmer CK, Matsushita K, Hemmelgarn BR: Change in the estimated glomerular filtration rate over time and risk of all-cause mortality. Kidney Int 83: 684–691, 2013 [DOI] [PubMed] [Google Scholar]
- 13.Al-Aly Z, Zeringue A, Fu J, Rauchman MI, McDonald JR, El-Achkar TM, Balasubramanian S, Nurutdinova D, Xian H, Stroupe K, Abbott KC, Eisen S: Rate of kidney function decline associates with mortality. J Am Soc Nephrol 21: 1961–1969, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Perkins RM, Bucaloiu ID, Kirchner HL, Ashouian N, Hartle JE, Yahya T: GFR decline and mortality risk among patients with chronic kidney disease. Clin J Am Soc Nephrol 6: 1879–1886, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Matsushita K, Selvin E, Bash LD, Franceschini N, Astor BC, Coresh J: Change in estimated GFR associates with coronary heart disease and mortality. J Am Soc Nephrol 20: 2617–2624, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rifkin DE, Shlipak MG, Katz R, Fried LF, Siscovick D, Chonchol M, Newman AB, Sarnak MJ: Rapid kidney function decline and mortality risk in older adults. Arch Intern Med 168: 2212–2218, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fox CS, Matsushita K, Woodward M, Bilo HJG, Chalmers J, Heerspink HJL, Lee BJ, Perkins RM, Rossing P, Sairenchi T, Tonelli M, Vassalotti JA, Yamagishi K, Coresh J, de Jong PE, Wen C-P, Nelson RG Chronic Kidney Disease Prognosis Consortium : Associations of kidney disease measures with mortality and end-stage renal disease in individuals with and without diabetes: A meta-analysis. Lancet 380: 1662–1673, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Matsushita K, Mahmoodi BK, Woodward M, Emberson JR, Jafar TH, Jee SH, Polkinghorne KR, Shankar A, Smith DH, Tonelli M, Warnock DG, Wen CP, Coresh J, Gansevoort RT, Hemmelgarn BR, Levey AS Chronic Kidney Disease Prognosis Consortium : Comparison of risk prediction using the CKD-EPI equation and the MDRD study equation for estimated glomerular filtration rate. JAMA 307: 1941–1951, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Shlipak MG, Matsushita K, Ärnlöv J, Inker LA, Katz R, Polkinghorne KR, Rothenbacher D, Sarnak MJ, Astor BC, Coresh J, Levey AS, Gansevoort RT CKD Prognosis Consortium : Cystatin C versus creatinine in determining risk based on kidney function. N Engl J Med 369: 932–943, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Coresh J, Turin TC, Matsushita K, Sang Y, Ballew SH, Appel LJ, Arima H, Chadban SJ, Cirillo M, Djurdjev O, Green JA, Heine GH, Inker LA, Irie F, Ishani A, Ix JH, Kovesdy CP, Marks A, Ohkubo T, Shalev V, Shankar A, Wen CP, de Jong PE, Iseki K, Stengel B, Gansevoort RT, Levey AS CKD Prognosis Consortium : Decline in estimated glomerular filtration rate and subsequent risk of end-stage renal disease and mortality. JAMA 311: 2518–2531, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.UK Prospective Diabetes Study (UKPDS) Group : Intensive blood-glucose control with sulphonylureas or insulin compared with conventional treatment and risk of complications in patients with type 2 diabetes (UKPDS 33). Lancet 352: 837–853, 1998 [PubMed] [Google Scholar]
- 22.de Boer IH, Sun W, Cleary PA, Lachin JM, Molitch ME, Steffes MW, Zinman B DCCT/EDIC Research Group : Intensive diabetes therapy and glomerular filtration rate in type 1 diabetes. N Engl J Med 365: 2366–2376, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Sud M, Tangri N, Pintilie M, Levey AS, Naimark DM: ESRD and death after heart failure in CKD. J Am Soc Nephrol 26: 715–722, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sud M, Tangri N, Pintilie M, Levey AS, Naimark D: Risk of end-stage renal disease and death after cardiovascular events in chronic kidney disease. Circulation 130: 458–465, 2014 [DOI] [PubMed] [Google Scholar]
- 25.Perkins RM, Tang X, Bengier AC, Kirchner HL, Bucaloiu ID: Variability in estimated glomerular filtration rate is an independent risk factor for death among patients with stage 3 chronic kidney disease. Kidney Int 82: 1332–1338, 2012 [DOI] [PubMed] [Google Scholar]
- 26.Levey AS, Stevens LA, Schmid CH, Zhang YL, Castro AF 3rd, Feldman HI, Kusek JW, Eggers P, Van Lente F, Greene T, Coresh J CKD-EPI (Chronic Kidney Disease Epidemiology Collaboration) : A new equation to estimate glomerular filtration rate. Ann Intern Med 150: 604–612, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Levey AS, Coresh J, Greene T, Marsh J, Stevens LA, Kusek JW, Van Lente F Chronic Kidney Disease Epidemiology Collaboration : Expressing the Modification of Diet in Renal Disease Study equation for estimating glomerular filtration rate with standardized serum creatinine values. Clin Chem 53: 766–772, 2007 [DOI] [PubMed] [Google Scholar]
- 28.Kidney Disease Improving Global Outcomes (KDIGO) Work Group : KDIGO Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Chapter 2: Definition, identification, and prediction of CKD progression. Kidney Int Suppl 3: 63–72, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Miller WG, Bruns DE, Hortin GL, Sandberg S, Aakre KM, McQueen MJ, Itoh Y, Lieske JC, Seccombe DW, Jones G, Bunk DM, Curhan GC, Narva AS National Kidney Disease Education Program-IFCC Working Group on Standardization of Albumin in Urine : Current issues in measurement and reporting of urinary albumin excretion. Clin Chem 55: 24–38, 2009 [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.