Abstract
The Kidney Allocation System (KAS), a major change to deceased donor kidney allocation, was implemented in December 2014. Goals of KAS included directing the highest-quality organs to younger/healthier recipients and increasing access to deceased donor kidney transplantation (DDKT) for highly sensitized patients and racial/ethnic minorities. Using national registry data, we compared kidney distribution, DDKT rates for waitlist registrants, and recipient characteristics between January 1, 2013, and December 3, 2014 (pre-KAS) with those between December 4, 2014, and August 31, 2015 (post-KAS). Regional imports increased from 8.8% pre-KAS to 12.5% post-KAS; national imports increased from 12.7% pre-KAS to 19.1% post-KAS (P<0.001). The proportion of recipients >30 years older than their donor decreased from 19.4% to 15.0% (P<0.001). The proportion of recipients with calculated panel-reactive antibody =100 increased from 1.0% to 10.3% (P<0.001). Overall DDKT rate did not change as modeled using exponential regression adjusting for candidate characteristics (P=0.07). However, DDKT rate (incidence rate ratio, 95% confidence interval) increased for black (1.19; 1.13 to 1.25) and Hispanic (1.13; 1.05 to 1.20) candidates and for candidates aged 18–40 (1.47; 1.38 to 1.57), but declined for candidates aged >50 (0.93; 0.87 to 0.98 for aged 51–60 and 0.90; 0.85 to 0.96 for aged >70). Delayed graft function in transplant recipients increased from 24.8% pre-KAS to 29.9% post-KAS (P<0.001). Thus, in the first 9 months under KAS, access to DDKT improved for minorities, younger candidates, and highly sensitized patients, but declined for older candidates. Delayed graft function increased substantially, possibly suggesting poorer long-term outcomes.
Keywords: transplantation, transplant outcomes, chronic kidney disease
On December 4, 2014, major changes to the algorithm for deceased donor kidney allocation priority were implemented, under the name Kidney Allocation System (KAS). Major changes included crediting time on dialysis prior to listing as accumulated wait time, preferentially allocating kidneys with kidney donor profile index (KDPI)<20% to the healthiest 20% of candidates as determined by Estimated Post Transplant Survival score, and increasing priority for candidates with calculated panel reactive antibody (CPRA)≥98%.1,2 Goals of the KAS included improving access to transplantation for highly sensitized candidates and for racial minorities (who face increased risk of delays prior to listing),3 and improving allograft utility (i.e., improving allograft longevity and reducing death with a functioning graft) by allocating the best organs to candidates with the best predicted post-transplant survival.1,2 The KAS represents the most significant change to the kidney allocation algorithm in over 20 years.2
Prior to implementation of KAS, a simulation study suggested that KAS would increase access to deceased donor kidney transplantation (DDKT) for highly sensitized candidates and younger candidates, with minimal changes in access by race or ethnicity.2 However, changes to allocation policy can have complicated and unintended effects.4,5 Bray and colleagues predicted that KAS might be unsuccessful in increasing DDKT access for highly sensitized candidates (particularly among female and minority candidates), and might increase cold ischemia time (CIT).5,6
In order to understand early changes to deceased donor kidney allocation and utilization in the 9 months following KAS implementation, we performed a retrospective study of DDKT waitlist registrants and recipients using national registry data. The goals of our study were to compare DDKT utilization, transplant rate, and early post-transplant outcomes from the pre-KAS era (prior to December 4, 2014) to the post-KAS era (December 4, 2015 and onwards), as well as secular trends within the post-KAS era.
Results
Distribution of Deceased Donor Kidneys Pre- and Post-KAS
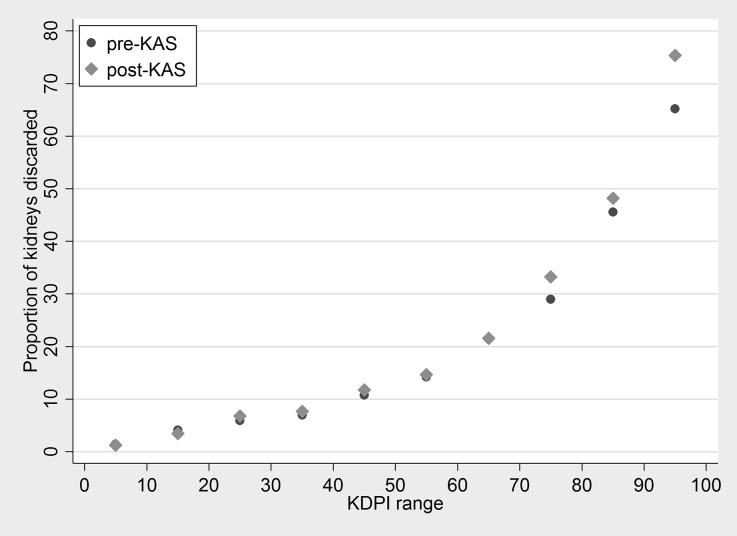
In the pre-KAS era, there were 28,514 deceased donor kidneys offered for transplant, of which 5190 (18.2%) were discarded. In the post-KAS era, there were 11,900 deceased donor kidneys offered for transplant, of which 2344 (19.7%) were discarded (P<0.001). In an unadjusted analysis, odds of discard increased 10% in the post-KAS era (odds ratio [OR] =1.10; 95% confidence interval [95% CI], 1.03 to 1.18; P<0.01). The increase in discards was observed only among kidneys with KDPI of ≥70 (Figure 1). In a model adjusting for donor KDPI and year of recovery, there was no evidence of change in discard among kidneys with a KDPI<70 (OR=1.02; 95% CI, 0.92 to 1.14; P=0.7), but odds of discard increased by 29% among kidneys with a KDPI of 70 or higher (OR=1.29; 95% CI, 1.15 to 1.43; P≤0.001).
Figure 1.
Proportion of discards among deceased donor kidneys offered for transplantation, pre- and post-KAS, stratified by KDPI range. Each point represents ten KDPI units. After implementation of KAS, the discard rate increased among kidneys with KDPI≥70.
There were 20,692 kidney-only deceased donor transplants in the pre-KAS era, and 8481 in the post-KAS era. The proportion of regional imports increased from 8.8% to 12.5%; the proportion of national imports increased from 12.7% to 19.1% (χ2 P<0.001). Median (interquartile range [IQR]) CIT was 15.8 (10.7–21.8) hours in the pre-KAS era and 16.8 (11.4–23.2) hours in the post-KAS era (rank-sum P<0.001). CIT increased by an average of 6% in the post-KAS era (proportional increase =1.06; 95% CI, 1.04 to 1.08; P<0.001). After adjusting for export status, there was no evidence of change in CIT associated with KAS (proportional increase =1.00; 95% CI, 0.99 to 1.02; P=0.99), indicating that the increase in CIT was fully mediated by the increase in regional and national exports.
Characteristics of DDKT Recipients Pre- and Post-KAS
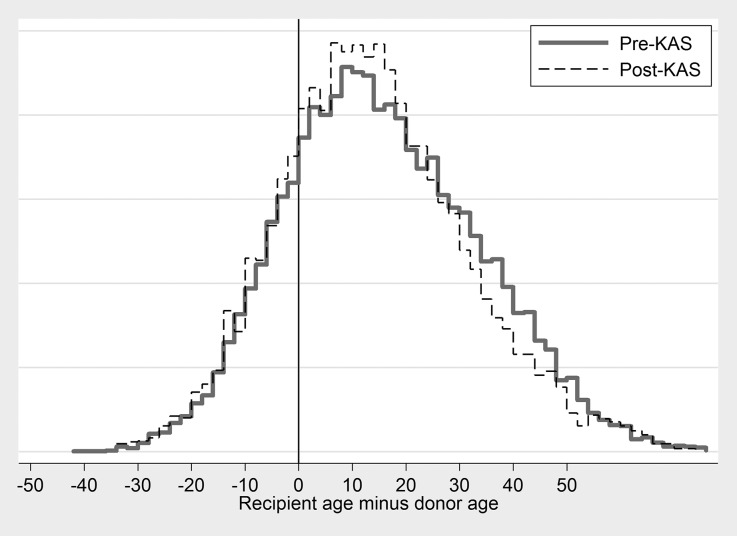
Age at transplant decreased from median (IQR)=55 (43–63) pre-KAS to 52 (40–62) post-KAS (P<0.001; Table 1). The proportion of black recipients increased from 31.7% pre-KAS to 37.2% post-KAS (P<0.001); the proportion who were Hispanic increased from 16.9% pre-KAS to 18.4% post-KAS (P<0.001). For comparison, 33.4% of active waitlist registrants on January 1, 2015 were black and 20.4% were Hispanic. The proportion of zero-mismatch recipients declined from 8.5% pre-KAS to 4.5% post-KAS (P<0.001). The correlation between donor age and recipient age increased from 0.35 pre-KAS to 0.38 post-KAS; the proportion of recipients who were more than 30 years older than their donor decreased from 19.4% pre-KAS to 15.0% post-KAS (P<0.001; Figure 2).
Table 1.
Characteristics of deceased donor kidney transplant recipients, pre-KAS (January 1, 2013–December 3, 2014) and post-KAS (December 4, 2014–August 31, 2015)
| Pre-KAS n=20,692 | Post-KAS n=8481 | P Value | |
|---|---|---|---|
| Median (IQR) age | 55 (44–63) | 52 (40–62) | <0.001 |
| Pediatric (%) | 4.3 | 4.0 | 0.3 |
| Female (%) | 39.1 | 40.6 | 0.02 |
| Race/ethnicity (%) | <0.001 | ||
| Black | 31.7 | 37.2 | |
| Hispanic | 16.9 | 18.4 | |
| All others | 51.3 | 44.4 | |
| Final CPRA (%) | <0.01 | ||
| 0 | 58.7 | 53.9 | |
| 1–20 | 9.9 | 9.6 | |
| 21–80 | 16.6 | 14.2 | |
| 81–97 | 11.5 | 7.2 | |
| 98 | 0.9 | 1.2 | |
| 99 | 1.3 | 3.6 | |
| 100 | 1.0 | 10.3 | |
| Blood type (%) | <0.001 | ||
| O | 45.7 | 46.0 | |
| A | 36.5 | 34.7 | |
| B | 12.8 | 13.2 | |
| AB | 5.0 | 6.2 | |
| Share type (%) | <0.001 | ||
| Local | 78.6 | 68.4 | |
| Regional | 8.8 | 12.5 | |
| National | 12.7 | 19.1 | |
| Zero-mismatch (%) | 8.5 | 4.5 | <0.001 |
| Median (IQR) CIT* | 15.8 (10.7–21.8) | 16.4 (11.8–23.2) | <0.001 |
After the implementation of KAS, DDKT recipients were more likely to be younger, to be black or Hispanic, to have a CPRA of 100, and to receive a kidney transplantation through a regional or national share (all P<0.001).
Figure 2.
Distribution of recipient age minus donor age, pre- and post-KAS. The correlation between donor age and recipient age increased from 0.35 to 0.38. The proportion of recipients who were more than 30 years older than their donor decreased from 19.4% pre-KAS to 15.0% post-KAS (P<0.001).
CPRA and Access to Transplantation
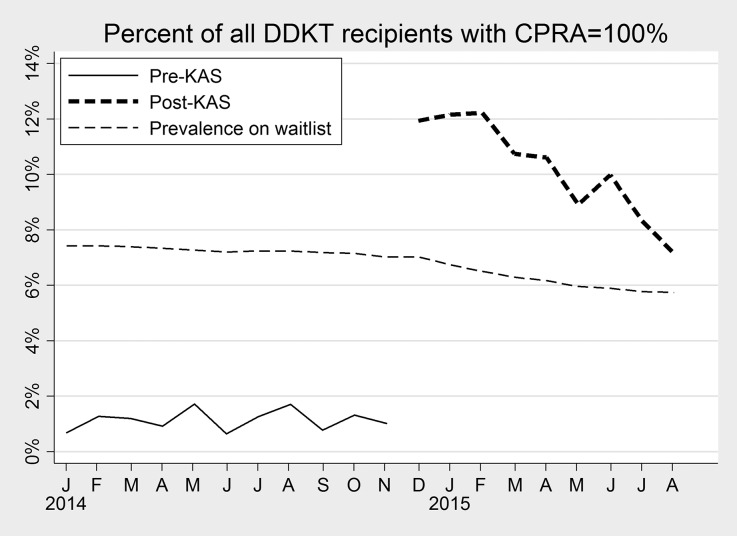
The proportion of recipients with a CPRA of 0 decreased from 58.7% pre-KAS to 53.9% post-KAS, and the proportion with a CPRA of 81–97 decreased from 11.5% to 7.2%; conversely, the proportion of recipients with a CPRA of 99 increased from 1.3% to 3.6%, and the proportion with a CPRA of 100 increased from 1.0% to 10.3% (P<0.001; Table 1). Calculated month-to-month, the proportion of transplant recipients with CPRA=100 remained <2% from January to November 2014 (Figure 3). However, this proportion increased to 13.2% in December 2014, declining gradually to 7.1% in August 2015 (Figure 3). During the post-KAS era, the proportion of recipients with CPRA=100 declined by 7.2% per month, suggesting a trend toward a steady state; the decline was statistically significant (OR=0.93; 95% CI, 0.90 to 0.95; P<0.001). The proportion of waitlist registrants with CPRA=100 declined slightly pre-KAS from 7.4% in January 2014 to 7.0% in December 2014, declining further to 5.7% in August 2015 (Figure 3). The amplified decline in waitlist prevalence of CPRA=100 post-KAS was statistically significant (P<0.001).
Figure 3.
Proportion of all DDKT recipients with CPRA=100% by month. Before implementation of KAS in December 2014, less than 2% of DDKT recipients had CPRA=100%. For comparison, the prevalence of CPRA=100% among waitlist registrants on December 1, 2014 was 7.0%. In the first month of KAS implementation, 11.9% of recipients had CPRA=100; this proportion declined to 7.1% in August 2015.
KDPI Distribution
Overall, there was no change in the distribution of KDPI of transplanted kidneys (median (IQR) KDPI=42 (22–63) pre-KAS versus 42 (22–63) post-KAS, rank-sum P=0.4). KDPI declined from median (IQR) 33 (17–51) pre-KAS to 21 (10–45) post-KAS for recipients aged 18–40 (P<0.001), but increased from 39 (21–56) to 41 (22–59) for recipients aged 41–50; from 44 (25–63) to 49 (32–67) for recipients aged 51–60; and from 52 (29–73) to 55 (36–73) for recipients aged 61–70 (all P<0.05; Table 2). KDPI increased from median (IQR) 35 (18–53) to 44 (30–59) for recipients with CPRA=80–97 (P<0.001; Table 2) but there was no change for recipients with CPRA≥98 (pre-KAS =35 [18–54], post-KAS=34 [16–54], P=0.6).
Table 2.
Distribution of kidney donor profile index (KDPI) for various categories of deceased donor transplant recipients, pre- and post-KAS
| Subgroup | Pre-KAS | Post-KAS | P Value |
|---|---|---|---|
| All registrants | 42 (22–63) | 42 (22–63) | 0.4 |
| Nonblack, non-Hispanic | 42 (22–64) | 42 (22–63) | 0.9 |
| Black | 43 (24–63) | 44 (23–63) | 0.8 |
| Hispanic | 40 (21–61) | 40 (19–60) | 0.2 |
| Male | 42 (22–64) | 43 (22–63) | 0.6 |
| Female | 42 (22–63) | 42 (21–62) | 0.5 |
| Age, yrs | |||
| <18 | 12 (6–22) | 11 (7–22) | 0.7 |
| 18–40 | 33 (17–51) | 21 (10–45) | <0.001 |
| 41–50 | 39 (21–56) | 41 (22–59) | 0.05 |
| 51–60 | 44 (25–63) | 49 (32–67) | <0.001 |
| 61–70 | 52 (29–73) | 55 (36–73) | <0.001 |
| >70 | 60.5 (37–79) | 63 (40.5–78) | 0.3 |
| CPRA | |||
| ≤80 | 44 (23–65) | 44 (22–64) | 0.6 |
| 81–97 | 35 (18–53) | 44 (30–59) | <0.001 |
| 98–100 | 35 (18–54) | 34 (16–54) | 0.6 |
KDPI is a marker of kidney quality, with lower KDPI indicating higher quality. Data are presented as median (IQR). In the post-KAS era, KDPI decreased for recipients aged 18–40; it increased for recipients aged ≥41 and for recipients with CPRA=80–97.
DDKT Rate Pre-KAS
In both the pre-KAS and post-KAS eras, transplant rate varied widely with candidate characteristics (Table 3). In the pre-KAS era, candidates of ABO type A (incidence rate ratio (IRR) =1.56; 95% CI, 1.51 to 1.60) or AB (IRR=2.57; 95% CI, 2.42 to 2.74) and pediatric candidates (IRR=6.97; 95% CI, 6.52 to 7.44) had a higher rate of DDKT (all P<0.001). Candidates of ABO type B (IRR=0.89; 95% CI, 0.85 to 0.93), and black (IRR=0.90; 95% CI, 0.87 to 0.93), and Hispanic candidates (IRR=0.76; 95% CI, 0.73 to 0.79) had a lower rate DDKT (all P<0.001). Increased CPRA was associated with slightly lower DDKT rate for CPRA 0–80; CPRA exceeding the threshold of 80 was associated with a 5.1-fold increase in DDKT rate, but the DDKT rate declined sharply with higher CPRA in the range 80–100 (IRR per 10 units=0.28; 95% CI, 0.26 to 0.29; P<0.001). Overall, the transplant rate declined over time by 2% per year in the pre-KAS era (IRR=0.96; 95% CI, 0.94 to 0.99; P<0.01).
Table 3.
Characteristics associated with transplant rate, stratified into pre-KAS versus post-KAS eras
| Pre-KAS | Post-KAS | |||
|---|---|---|---|---|
| Risk factor | Transplant IRR (95% CI) | P Value | Transplant IRR (95% CI) | P Value |
| Calendar time (per yr) | 0.96a (0.94 to 0.99) | <0.01 | 1.13a (1.01 to 1.27) | 0.03 |
| Female sex | 0.98 (0.96 to 1.01) | 0.3 | 0.99 (0.95 to 1.04) | 0.8 |
| ABO type | ||||
| A (versus O) | 1.56a (1.51 to 1.60) | <0.001 | 1.44a (1.37 to 1.51) | <0.001 |
| B | 0.89a (0.85 to 0.93) | <0.001 | 0.90a (0.84 to 0.97) | <0.01 |
| AB | 2.57a (2.42 to 2.74) | <0.001 | 2.72a (2.48 to 2.99) | <0.001 |
| Black | 0.90a (0.87 to 0.93) | <0.001 | 1.15a (1.10 to 1.21) | <0.001 |
| Hispanic | 0.76a (0.73 to 0.79) | <0.001 | 0.99 (0.94 to 1.06) | 0.9 |
| Pediatric | 6.97a (6.52 to 7.44) | <0.001 | 5.56a (4.98 to 6.20) | <0.001 |
| CPRA (per 10 units): 0–80 | 0.99a (0.98 to 1) | <0.01 | 1.01 (1 to 1.02) | 0.2 |
| Rate increase if CPRA>80 | 5.14a (4.72 to 5.60) | <0.001 | 0.95 (0.79 to 1.16) | 0.6 |
| CPRA (per 10 units): 80–100 | 0.28a (0.26 to 0.29) | <0.001 | 1.24a (1.12 to 1.37) | <0.001 |
| Wait time (log yrs) | 1.13a (1.12 to 1.14) | <0.001 | 0.89a (0.88 to 0.90) | <0.001 |
Transplant rate was modeled using exponential regression. IRR>1 indicates a higher rate of transplant (better access to DDKT); IRR<1 indicates a lower rate (worse access to DDKT). Candidates of ABO type A or AB and pediatric candidates had greater rate of DDKT. Black candidates had a lower rate of DDKT than other patients in the pre-KAS era, but a higher rate than other patients in the post-KAS era. Hispanic candidates had a lower rate of kidney transplantation in the pre-KAS era, but no difference from other patients in the post-KAS era.
P<0.05.
DDKT Rate Post-KAS
In the post-KAS era, candidates of ABO type A (IRR=1.44; 95% CI, 1.37 to 1.51) or AB (IRR=2.72; 95% CI, 2.48 to 2.99) and pediatric candidates (IRR=5.55; 95% CI, 4.98 to 6.20) continued to enjoy a higher rate of DDKT (all P<0.001). Candidates of ABO type B (IRR=0.90; 95% CI, 0.84 to 0.97) had a lower rate of DDKT than other patients, while blacks (IRR=1.15; 95% CI, 1.09 to 1.21) had a higher rate of DDKT than other patients (P<0.01). In contrast to the pre-KAS era, in the post-KAS era there was no association between CPRA and transplant rate for candidates with CPRA<80 (P=0.3), but the DDKT rate increased with higher CPRA in the range 80–100 (IRR per 10 units=1.24; 95% CI, 1.12 to 1.37; P<0.001).
Changes in DDKT Rate from Pre-KAS to Post-KAS
Adjusting for candidate characteristics as well as wait time, there was no overall change in DDKT rate associated with KAS (IRR=1.04; 95% CI, 0.10 to 1.08; P=0.7; Table 4). However, there were statistically significant changes in transplant rate for some candidate subgroups. Transplant rate declined by 8% for nonblack candidates (IRR=0.92; 95% CI, 0.87 to 0.96; P<0.001), but increased by 19% for black candidates (IRR=1.19; 95% CI, 1.13 to 1.25; P<0.001). DDKT rate increased by 13% for Hispanic candidates (IRR=1.13; 95% CI, 1.05 to 1.20), and by 26% for candidates of blood type AB (IRR=1.26; 95% CI, 1.13 to 1.41; P<0.001). The DDKT rate increased by 47% for candidates aged 18–40 (IRR=1.47; 95% CI, 1.38 to 1.57; P<0.001) and by 17% for candidates aged 41–50 (IRR=1.17; 95% CI, 1.09 to 1.24; P<0.001). However, the DDKT rate declined by 7% for candidates aged 51–60 (IRR=0.93; 95% CI, 0.87 to 0.98; P<0.001), by 10% for candidates aged 61–70 (IRR=0.90; 95% CI, 0.85 to 0.96; P<0.001), and by 24% for candidates aged >70 (IRR=0.76; 95% CI, 0.68 to 0.85; P<0.001).
Table 4.
Changes in rate of DDKT following implementation of KAS, for various subgroups of candidates
| Subgroup | Transplant IRR (95% CI) | P Value |
|---|---|---|
| All registrants | 1.04 (1 to 1.08) | 0.07 |
| Nonblack, non-Hispanic | 0.92a (0.87 to 0.96) | <0.001 |
| Black | 1.19a (1.13 to 1.25) | <0.001 |
| Hispanic | 1.13a (1.05 to 1.20) | <0.001 |
| Male | 1.01 (0.96 to 1.05) | 0.8 |
| Female | 1.09a (1.03 to 1.14) | <0.01 |
| ABO type | ||
| O | 1.04 (0.99 to 1.09) | 0.1 |
| A | 1.00 (0.95 to 1.05) | 0.9 |
| B | 1.06 (0.98 to 1.14) | 0.2 |
| AB | 1.26a (1.13 to 1.41) | <0.001 |
| Age, yrs | ||
| <18 | 1.03 (0.90 to 1.17) | 0.7 |
| 18–40 | 1.47a (1.38 to 1.57) | <0.001 |
| 41–50 | 1.17a (1.09 to 1.24) | <0.001 |
| 51–60 | 0.93a (0.87 to 0.98) | 0.01 |
| 61–70 | 0.90a (0.85 to 0.96) | <0.001 |
| >70 | 0.76a (0.68 to 0.85) | <0.001 |
IRRs were modeled using exponential regression, adjusted for candidate characteristics as well as log wait time. IRR>1 indicates increased rate of transplant in the post-KAS era; IRR<1 indicates a decreased rate.
P<0.05.
Delayed Graft Function Pre- and Post-KAS
Incidence of delayed graft function (DGF) increased from 24.8% in the pre-KAS era to 29.9% in the post-KAS era. In an unadjusted multilevel model accounting for center-level variation in DGF, odds of DGF were 35% higher under KAS (OR=1.35; 95% CI, 1.26 to 1.46; P<0.001). Adjustment for recipient characteristics did not attenuate this association (OR=1.35; 95% CI, 1.25 to 1.46; P<0.001). Further adjustment for CIT, share type (regional/national versus local), HLA mismatch, and donation after cardiac death attenuated the association slightly to 30% (OR=1.30; 95% CI, 1.20 to 1.41).
Discussion
In this national study of deceased donor kidney distribution and early outcomes in the first 9 months following implementation of KAS, the rate of deceased donor transplantation increased for blacks, Hispanics, and adults aged 18–50, and decreased for pediatric patients and adults aged >50. The proportion of transplant recipients with CPRA=100 increased dramatically from 2% pre-KAS to 12% immediately following implementation of KAS, but declined to 7% by August 2015. The proportion of regional and national exports increased. Odds of discard increased by 29% among kidneys with KDPI of 70 or higher. Incidence of DGF increased in the post-KAS era, explained partly by increases in CIT.
In light of these findings, it is worth speculating about whether KAS has been successful so far in accomplishing its intended purpose. In some aspects, it has been: the rate of transplantation increased for black and Hispanic patients, perhaps as a result of the new method of crediting wait time prior to listing for purposes of allocation priority, and highly sensitized candidates have achieved greater access to transplantation in the post-KAS era. On the other hand, some changes are perhaps less salutary. Candidates with a CPRA of 100, formerly disadvantaged, are now actually overrepresented among transplant recipients, perhaps more than was originally intended with the priority added for this vulnerable population. That said, the proportion of transplant recipients with CPRA=100 has decreased over the past few months, suggesting that a bolus effect may be occurring. Also troubling are the increases in early post-KAS discard and DGF.
Our results should be understood in the context of unavoidable limitations of our study. As with all observational studies, our results are vulnerable to confounding, although we have adjusted for known and potential confounders collected by the Organ Procurement and Transplantation Network (OPTN). Our comparison of the pre-KAS era to the post-KAS era may also be affected by secular trends. However, we have described some of these secular trends in our study; moreover, as the greatest change in the past 20 years following a long period of relative stasis in kidney allocation policy, KAS is likely significant enough to have overcome any short-term secular trends due to gradual changes in kidney supply and demand.
Most importantly, with only 9 months of data in the post-KAS era, we cannot be sure that the trends we have observed will continue. Indeed, our data suggest that the increase of recipients with CPRA=100 was at least partly a transient bolus effect, although even after this bolus there remains a much higher transplant rate for this previously vulnerable population. Some other trends identified in our study may also attenuate or disappear in the long term. Nevertheless, even if this is the case, we believe that it is important to document short-term trends. Any allocation policy change will result in short-term effects to organ distribution, some possibly unintended, and understanding these effects might inform future policy changes. While long-term studies of waitlist and post-transplant outcomes will be necessary to fully evaluate KAS, our study identifies important changes to kidney distribution in the early post-KAS era that affect how providers manage their waitlists, how patients make decisions about organ offers, and even possibly how policymakers should re-evaluate the new priority for highly sensitized patients.
Changes to allocation policy involve a great deal of time, energy, and expense.7 In the best case, allocation policy changes can lead to clear improvements in utilization. For example, following the Share-35 change to liver allocation, there were decreases in liver discard rate, geographic disparities, and waitlist mortality, with no evidence of change to post-transplant outcomes.8 However, in absence of a decrease in discard, changes to allocation policy merely shift resources from one group of patients to another. In the face of inadequate organ supply, allocation will always involve winners and losers, and what constitutes a fair allocation strategy may not be clear-cut.9 For example, pediatric candidates have substantially higher rates of DDKT than adults. Whether this is fair reflects not an objective cost/benefit calculus, but the values of the transplant community and the broader public.
Disparities in access to organ transplantation (with regards to demographics, ABO/HLA compatibility, sensitization, and other characteristics) persist,10–12 and organ allocation policy is an appropriate tool to address these disparities. However, allocation policy changes alone cannot solve the problem of organ shortages. Moreover, policy changes can easily lead to unintended consequences. The mixed record of KAS in its first 9 months of implementation underscores the need to increase the deceased donor organ pool, as well as the need to reduce the reliance on DDKT by improving access to and understanding of live donor kidney transplantation. The increase in DGF in the post-KAS era suggests that careful monitoring of graft survival is needed. Further policy changes may be required to maximize utility of the limited supply of deceased donor kidneys.
Concise Methods
Study Population
This study used data from the Scientific Registry of Transplant Recipients (SRTR). The SRTR data system includes data on all donor, waitlisted candidates, and transplant recipients in the United States, submitted by the members of the OPTN, and has been described elsewhere. The Health Resources and Services Administration, US Department of Health and Human Services provides oversight to the activities of the OPTN and SRTR contractors.
The study population consisted of kidney-only waitlist registrants and deceased donor kidney-only transplant recipients between January 1, 2013 and August 31, 2015, as well as deceased donor kidneys recovered over the same period. The time period from January 1, 2013 to December 3, 2014 (pre-KAS) was compared with the time period from December 4, 2014 to August 31, 2015 (post-KAS).
Distribution of Deceased Donor Kidneys Pre- and Post-KAS
We compared the discard rate of deceased donor kidneys pre-KAS versus post-KAS by using a chi-squared test and by logistic regression, adjusting for KDPI (to account for any differences in kidney quality) and date of organ recovery (to account for possible secular trends apart from KAS), with an interaction term to separately test change in discard for kidneys with KDPI<70 and KDPI≥70. We compared the proportion of regional and national imports pre-KAS versus post-KAS using a chi-squared test. We modeled the change in CIT (modeled with the functional form of ln[CIT+1]) pre-KAS versus post-KAS, both unadjusted and adjusting for regional and national import. In order to account for delays in reporting of CIT, CIT analyses included only transplants which occurred on or before April 30, 2015.
Characteristics of DDKT Transplants Pre- and Post-KAS
We compared characteristics of DDKT recipients (age, gender, race/ethnicity characterized as black/Hispanic/all others, final CPRA, ABO blood type, share type [locally allocated within the organ procurement organization, shared within a multistate region, or nationally shared], and CIT) pre-KAS versus post-KAS using rank-sum tests for continuous variables and χ2 tests for binary and categorical variables.
CPRA and Access to Transplantation
Because exploratory data analysis indicated a post-KAS increase in the proportion of recipients with CPRA=100, we calculated the proportion of such recipients (as well as the proportion of prevalent waitlist registrants with CPRA=100) separately for each month during the study period to see whether this proportion varied over time post-KAS. We modeled secular trends in the proportion of DDKT registrants/recipients with CPRA=100 during the post-KAS era using logistic regression.
KDPI Distribution
We compared DDKT kidney quality, as measured by the KDPI, pre- versus post-KAS for various recipient subgroups using rank-sum tests.
DDKT Rate Pre- and Post-KAS
To understand changes in access to DDKT in the post-KAS era, we modeled KAS-associated change in rate of DDKT among waitlist registrants using exponential (constant-hazard) regression as previously described,8 censoring for mortality and other waitlist removals, adjusting for calendar year, log accumulated waitlist time, gender, ABO blood type, race/ethnicity, current age (classified as pediatric [<18 years] versus adult), and CPRA with a spline at 80. In addition to calculating an overall change in transplant rate, we calculated subgroup-specific changes in transplant rate using interaction terms, with separate models for separate subgroups (e.g., the model of race interaction was different from the model of gender interaction).
DGF Pre- and Post-KAS
We compared incidence of DGF pre- versus post-KAS using multilevel logistic regression, with a random intercept at the center level to account for potential baseline differences in DGF by center.13 We created two models, one unadjusted and one adjusting for CPRA, ABO blood type, race/ethnicity, gender, and age at transplant. In order to account for delays in reporting of DGF, DGF analyses included only transplants which occurred on or before April 30, 2015.
Statistical Analyses
All analyses were performed using Stata 14.0/MP for Linux (StataCorp., College Station, TX).
Disclosures
None.
Acknowledgments
This work was supported by grant number K24DK101828 from the National Institute of Diabetes and Digestive and Kidney Diseases.
The analyses described here are the responsibility of the authors alone and do not necessarily reflect the views or policies of the Department of Health and Human Services, nor does mention of trade names, commercial products or organizations imply endorsement by the US Government. The data reported here have been supplied by the Minneapolis Medical Research Foundation as the contractor for the Scientific Registry of Transplant Recipients (SRTR). The interpretation and reporting of these data are the responsibility of the author(s) and in no way should be seen as an official policy of or interpretation by the SRTR or the US Government.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Matas AJ, Smith JM, Skeans MA, Thompson B, Gustafson SK, Stewart DE, Cherikh WS, Wainright JL, Boyle G, Snyder JJ, Israni AK, Kasiske BL: OPTN/SRTR 2013 Annual Data Report: kidney. Am J Transplant 15[Suppl 2]: 1–34, 2015 [DOI] [PubMed] [Google Scholar]
- 2.Israni AK, Salkowski N, Gustafson S, Snyder JJ, Friedewald JJ, Formica RN, Wang X, Shteyn E, Cherikh W, Stewart D, Samana CJ, Chung A, Hart A, Kasiske BL: New national allocation policy for deceased donor kidneys in the United States and possible effect on patient outcomes. J Am Soc Nephrol 25: 1842–1848, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Schold JD, Gregg JA, Harman JS, Hall AG, Patton PR, Meier-Kriesche HU: Barriers to evaluation and wait listing for kidney transplantation. Clin J Am Soc Nephrol 6: 1760–1767, 2011 [DOI] [PubMed] [Google Scholar]
- 4.Massie AB, Zeger SL, Montgomery RA, Segev DL: The effects of DonorNet 2007 on kidney distribution equity and efficiency. Am J Transplant 9: 1550–1557, 2009 [DOI] [PubMed] [Google Scholar]
- 5.Bray RA, Gebel HM: The new kidney allocation system (KAS) and the highly sensitized patient: expect the unexpected. Am J Transplant 14: 2917, 2014 [DOI] [PubMed] [Google Scholar]
- 6.Bray RA, Brannon P, Breitenbach C, Bryan C, Chen DF, Lai J, McRacken T, Kirk A, Kaplan B, Pearson T, Gebel HM: The new OPTN kidney allocation policy: potential for inequitable access among highly sensitized patients. Am J Transplant 15: 284–285, 2015 [DOI] [PubMed] [Google Scholar]
- 7.Bromberg JS, Halloran PF: Nine things you might not say or hear in transplantation. Am J Transplant 9: 11–13, 2009 [DOI] [PubMed] [Google Scholar]
- 8.Massie AB, Chow EK, Wickliffe CE, Luo X, Gentry SE, Mulligan DC, Segev DL: Early changes in liver distribution following implementation of Share 35. Am J Transplant 15: 659–667, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Matas AJ: Allocation or rationing--word choice is crucial. Am J Transplant 9: 9–10, 2009 [DOI] [PubMed] [Google Scholar]
- 10.Mathur AK, Ashby VB, Sands RL, Wolfe RA: Geographic variation in end-stage renal disease incidence and access to deceased donor kidney transplantation. Am J Transplant 10: 1069–1080, 2010 [DOI] [PubMed] [Google Scholar]
- 11.Ashby VB, Kalbfleisch JD, Wolfe RA, Lin MJ, Port FK, Leichtman AB: Geographic variability in access to primary kidney transplantation in the United States, 1996-2005. Am J Transplant 7: 1412–1423, 2007 [DOI] [PubMed] [Google Scholar]
- 12.Gordon EJ, Ladner DP, Caicedo JC, Franklin J: Disparities in kidney transplant outcomes: a review. Semin Nephrol 30: 81–89, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Orandi BJ, James NT, Hall EC, Van Arendonk KJ, Garonzik-Wang JM, Gupta N, Montgomery RA, Desai NM, Segev DL: Center-level variation in the development of delayed graft function after deceased donor kidney transplantation. Transplantation 99: 997–1002, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]