Abstract
Podocytes are specialized epithelial cells of the kidney blood filtration barrier that contribute to permselectivity via a series of interdigitating actin–rich foot processes. Positioned between adjacent projections is a unique cell junction known as the slit diaphragm, which is physically connected to the actin cytoskeleton via the transmembrane protein nephrin. Evidence indicates that tyrosine phosphorylation of the intracellular tail of nephrin initiates signaling events, including recruitment of cytoplasmic adaptor proteins Nck1 and Nck2 that regulate actin cytoskeletal dynamics. Nephrin tyrosine phosphorylation is altered in human and experimental renal diseases characterized by pathologic foot process remodeling, prompting the hypothesis that phosphonephrin signaling directly influences podocyte morphology. To explore this possibility, we generated and analyzed knockin mice with mutations that disrupt nephrin tyrosine phosphorylation and Nck1/2 binding (nephrinY3F/Y3F mice). Homozygous nephrinY3F/Y3F mice developed progressive proteinuria accompanied by structural changes in the filtration barrier, including podocyte foot process effacement, irregular thickening of the glomerular basement membrane, and dilated capillary loops, with a similar but later onset phenotype in heterozygous animals. Furthermore, compared with wild-type mice, nephrinY3F/Y3F mice displayed delayed recovery in podocyte injury models. Profiling of nephrin tyrosine phosphorylation dynamics in wild-type mice subjected to podocyte injury indicated site-specific differences in phosphorylation at baseline, injury, and recovery, which correlated with loss of nephrin-Nck1/2 association during foot process effacement. Our results define an essential requirement for nephrin tyrosine phosphorylation in stabilizing podocyte morphology and suggest a model in which dynamic changes in phosphotyrosine-based signaling confer plasticity to the podocyte actin cytoskeleton.
Keywords: nephrin, cell signaling, cytoskeleton, glomerular disease, cell biology and structure, podocyte
Blood filtration in the kidney is critically dependent on the size–selective barrier wall of the glomerulus, which is composed of an inner layer of fenestrated endothelium, a glomerular basement membrane (GBM), and an outer layer of podocytes. Differentiated podocytes adopt a unique and complex three–dimensional architecture that is fundamental to their function.1,2 Their cell bodies extend numerous microtubule–based major processes, which branch into an interdigitating network of smaller actin–rich foot processes bridged by slit diaphragms that serve as the final filtration element to limit loss of urinary protein. Podocyte injury has emerged as a significant contributor to many forms of renal disease and is characterized by remodeling of the actin cytoskeleton, loss of slit diaphragms, and foot process effacement, leading to proteinuria.3
The slit diaphragm is a specialized cell-cell junction that is anchored to the actin cytoskeleton via a series of transmembrane proteins, including the central scaffolding protein nephrin.4 Mutations in nephrin in humans and rodents lead to foot process effacement and proteinuria.5 The Finminor mutation, which results in near-complete truncation of the short cytoplasmic tail of nephrin, causes a similar disease phenotype as complete loss of nephrin,6 suggesting that this segment is essential for nephrin function. Within this segment, there exists a number of highly conserved tyrosine (Y) residues (Table 1) that, on phosphorylation by Fyn kinase,7–9 serve as docking sites for intracellular signaling proteins (Figure 1A).10 Through recruitment of actin adaptors, such as p85/PI3K,11,12 the Cas/Crk complex,13 and Nck1/2,8,9,14 nephrin phosphorylation is postulated to facilitate direct and dynamic connection to the podocyte cytoskeleton. Moreover, Nck enhances nephrin phosphorylation via activation of Fyn,15 and loss of Nck within podocytes leads to reduced nephrin tyrosine phosphorylation and widespread foot process effacement,15,16 inferring a reciprocal relationship between Nck and nephrin in the maintenance of podocyte structure.
Table 1.
Conserved tyrosine residues in human, mouse, and rat nephrin
| Human | Mouse | Rat |
|---|---|---|
| 1114-YEESa | 1128-YEESa | 1127-YEESa |
| 1138-YYRS | 1153-YYSM | 1152-YYSM |
| 1158-YSRG | 1172-YRQA | 1171-YHQG |
| 1176 – YDEVb | 1191-YDEVb | |
| 1183-YPPS | 1198-YGPP | 1194-YGPP |
| 1193-YDEVa,b | 1208-YDEVa,b | 1204-YDEVa,b |
| 1216-YDLR | 1212-YDLR | |
| 1210-YQDP | 1225-YEDP | |
| 1217-YDQVa,b | 1232-YDQVa,b | 1228-YDQVa,b |
The human Finminor mutation (R1109X) results in deletion of all indicated tyrosine residues.
Conserved between all three species.
YDxV motifs.
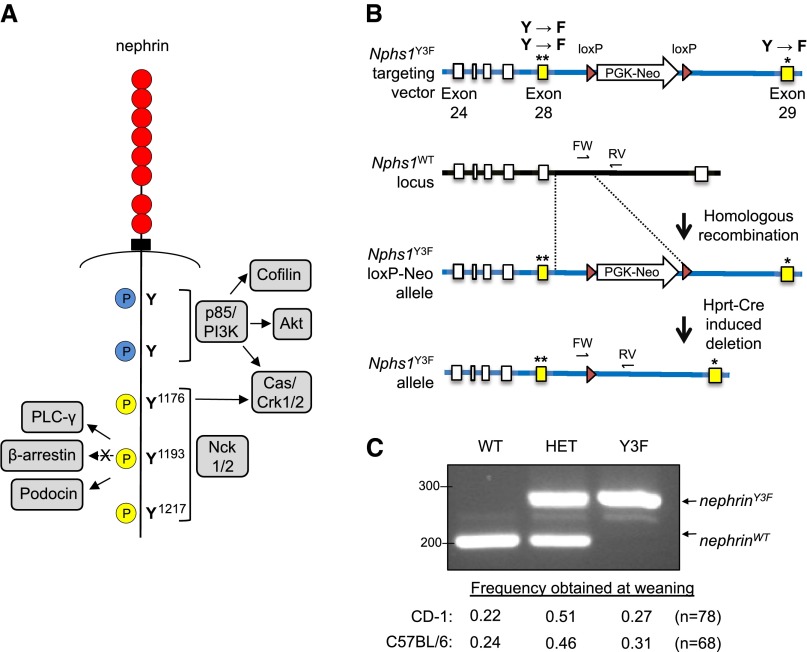
Figure 1.
Generation of nephrin-Y3F mice. (A) Diagram of human nephrin protein indicating the positions of phosphorylated (P) tyrosine (Y) residues within the cytoplasmic tail of nephrin with several binding partners as indicated. The three mouse nephrin tyrosines corresponding to human Y1176, Y1193, and Y1217 (yellow) were mutated to phenylalanine (F) to create nephrin-Y3F. (B) Schematic of the Y3F targeting vector used to introduce the modified exons (28 and 29; yellow) into the WT murine Nphs1 locus by homologous recombination. A loxP-flanked PGK-Neo selection cassette was later removed via Hprt-Cre. (C) PCR analysis of the Nphs1WT and Nphs1Y3F alleles using FW and RV primers (as in B) on genomic DNA samples from representative nephrinWT/WT, nephrinWT/Y3F (HET), and nephrinY3F/Y3F animals. All genotypes on both CD-1 and C57Bl/6 genetic backgrounds are obtained with normal Mendelian frequency. *, **Indicate presence of Y to F mutations.
Nephrin is tyrosine phosphorylated during glomerulogenesis and throughout life,8,16,17 and decreases in nephrin phosphorylation on Y1217 are seen coincident with foot process effacement in human kidney diseases, including minimal change disease (MCD)18 and membranous nephropathy.19 Similarly, reduced phosphorylation of the downstream survival factor Akt on serine (S) 473 can be observed in MCD,20,21 and inactivation of the actin binding protein cofilin is detected in patients with MCD, membranous nephropathy, and FSGS.22 These observations raise the intriguing possibility that uncoupling of phosphonephrin signaling from actin leads to pathologic disruptions in foot process morphology. However, additional investigation is required to determine whether perturbations in nephrin tyrosine phosphorylation directly contribute to the development of disease as a result of altered downstream signaling or rather, if decreases in nephrin tyrosine phosphorylation occur during the progression of disease simply as a consequence of changes in other signaling pathways.
To resolve the significant question of whether nephrin tyrosine phosphorylation directly controls podocyte function, we generated mice deficient in phosphonephrin signaling by altering the three tyrosine phosphorylation sites that mediate Nck binding (denoted nephrinY3F). NephrinY3F/Y3 mice develop widespread foot process effacement, basement membrane alterations, and defects in filtration barrier integrity, and they show delayed recovery within experimental models of podocyte injury. Our findings provide compelling evidence for an ongoing requirement for nephrin phosphotyrosine–based signaling in stabilization and restoration of podocyte cytoarchitecture and suggest that there may be value in the pursuit of therapeutic strategies that can selectively enhance nephrin tyrosine phosphorylation.
Results
Generation of NephrinY3F/Y3F Knockin Mice
To directly investigate the role of nephrin tyrosine phosphorylation in podocyte biology, we generated knockin mice in which the Y residues within the three Nck-binding YDxV motifs of nephrin were converted to phenylalanine (F), an amino acid that mimics the structure of tyrosine but cannot undergo phosphorylation (Figure 1A). These motifs correspond to tyrosine residues 1191, 1208, and 1232 in mouse and are herein referred to using the human amino acid numbering system with analogous positions at 1176, 1193, and 1217 (Table 1). A targeting vector containing appropriate nucleotide point mutations was constructed (Figure 1B), and the three mutations (herein denoted Y3F) were incorporated into the endogenous Nphs1 locus by homologous recombination. Successful targeting led to the generation of nephrinwild type (WT)/Y3F mice on a mixed C57BL/6:129 background, which we subsequently bred onto the outbred CD-1 background for more than six generations and independently bred onto the C57BL/6N background for more than five generations. When we intercrossed heterozygous nephrinWT/Y3F mice, we observed the birth of nephrinWT/WT (WT), nephrinWT/Y3F, and nephrinY3F/Y3F (Y3F) animals (Figure 1C) in the expected Mendelian ratios on both backgrounds. The presence of the three mutations was verified by DNA sequencing (data not shown).
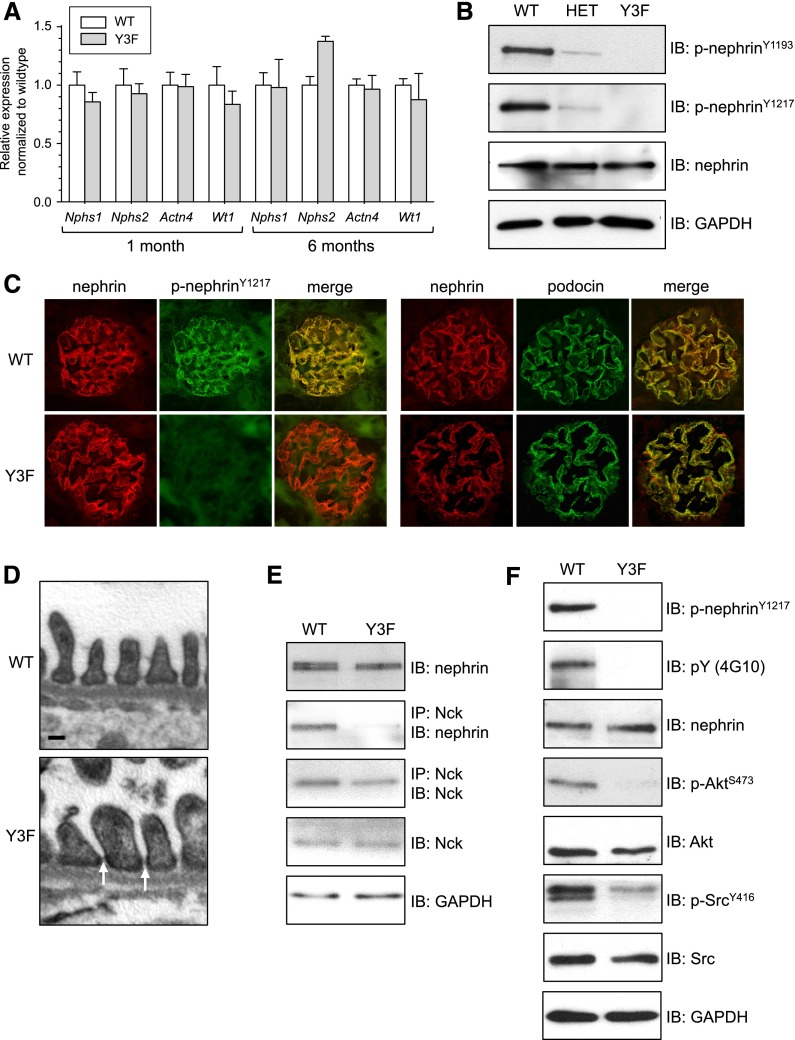
The expression and biochemical function of the mutant nephrin allele were examined in both CD-1 and C57BL/6 mice, with comparable results seen in both genetic backgrounds (Figure 2) (data not shown). We first isolated mRNA from the kidney cortex of 1- and 6-month-old nephrinWT/WT and nephrinY3F/Y3F animals and used quantitative PCR to compare the amount of mRNA expression of the two Nphs1 alleles as well as the podocyte markers Nphs2, Actn4, and Wt1 (Figure 2A). We observed similar amounts of Nphs1 mRNA in both young and aged nephrinWT/WT and nephrinY3F/Y3F animals, suggesting that insertion of the knockin mutation did not affect nephrin promoter activity or mRNA stability. There were no significant differences in expression of the other podocyte markers. We next isolated glomeruli from 1-month-old nephrinWT/WT, nephrinWT/Y3F, and nephrinY3F/Y3F animals and used Western blot analysis to monitor nephrin expression and tyrosine phosphorylation (Figure 2B). Comparable levels of total nephrin relative to GAPDH were seen in all genotypes. Moreover, using our phosphospecific nephrin antibodies developed to recognize Y1176, Y1193, or Y1217,16 we detected strong phosphorylation of nephrin in WT animals, which was severely decreased in nephrinWT/Y3F animals and completely absent from Y3F animals (Figure 2B).
Figure 2.
Validation of expression and function of Nphs1Y3F mutant allele. (A) Real–time PCR analysis of mRNA levels in glomeruli isolated from 1- and 6-month-old nephrinWT/WT and nephrinY3F/Y3F animals for Nphs1 and podocyte markers Nphs2, Actn4, and Wt1. For each gene, expression in WT animals was adjusted to 1.0. (B) Nephrin protein expression and phosphorylation was evaluated using immunoblotting (IB) for the indicated antibodies on glomeruli isolated from nephrinWT/WT, nephrinWT/Y3F (HET), and nephrinY3F/Y3F mice. (C) Dual-immunofluorescence staining for total nephrin (red) and phosphonephrin (Y1217; green) or total nephrin (red) and podocin (green) on kidney sections of 1-month-old WT and Y3F animals. (D) Transmission EM of 1-month-old WT and Y3F animals showing slit diaphragms (arrows). Scale bar, 100 nm. (E) IB for nephrin and Nck in anti-Nck immunoprecipitates (IPs) from WT and Y3F glomerular lysates showing disruption of nephrin-Nck interaction in Y3F mice. (F) IB of glomerular lysates (10 μg) from WT and Y3F mice showing altered nephrin phosphosignaling to Akt and Src. Similar results were obtained in CD-1 and C57BL/6 mice. All results shown are from CD-1 mice, except in E, which is from C57BL/6 mice.
Next, we investigated nephrin localization within the glomerulus of these animals via immunofluorescence staining, which revealed that both nephrin-WT and nephrin-Y3F proteins were expressed in a pattern characteristic of podocyte proteins (Figure 2C). Nephrin was phosphorylated on Y1217 in nephrinWT/WT animals, whereas we could not detect any signal above background in nephrinY3F/Y3F animals (Figure 2C). Given that nephrin phosphorylation can affect nephrin-podocin interactions,17,23 we performed dual-immunofluorescence staining for nephrin and podocin (Figure 2C) and found that both proteins were similarly expressed in a continuous pattern in the glomeruli of nephrinWT/WT and nephrinY3F/Y3F animals. In accordance with this staining pattern, transmission electron microscopy (EM) revealed the presence of slit diaphragm structures in nephrinY3F/Y3F animals (Figure 2D).
Finally, we examined the effect of the mutant nephrin-Y3F protein on nephrin signaling by Western blot analysis of glomerular lysates isolated from nephrinWT/WT and nephrinY3F/Y3F animals. Phosphorylation of these tyrosines is required for nephrin-Nck binding in vitro8,9,14; thus, we validated that this interaction was disrupted in vivo using immunoprecipitation (Figure 2E). Consistent with the role of Nck recruitment in promoting nephrin tyrosine phosphorylation,15 we observed a significant reduction in total nephrin tyrosine phosphorylation in nephrinY3F/Y3F animals using a pan-phosphotyrosine antibody (Figure 2F). We also found decreased activation of nephrin downstream signaling targets, with reduced phospho-Akt S473 and phospho-Src Y416 levels in nephrinY3F/Y3F animals compared with nephrinWT/WT animals (Figure 2F). Together, these analyses show that expression levels and localization of the mutant nephrin-Y3F protein are comparable with WT nephrin and that absence of the three YDxV tyrosine phosphorylation sites compromises a significant degree of nephrin phosphotyrosine signaling in vivo.
CD-1 NephrinY3F/Y3F Mice Develop Severe Proteinuria, GBM Alterations, and Foot Process Effacement
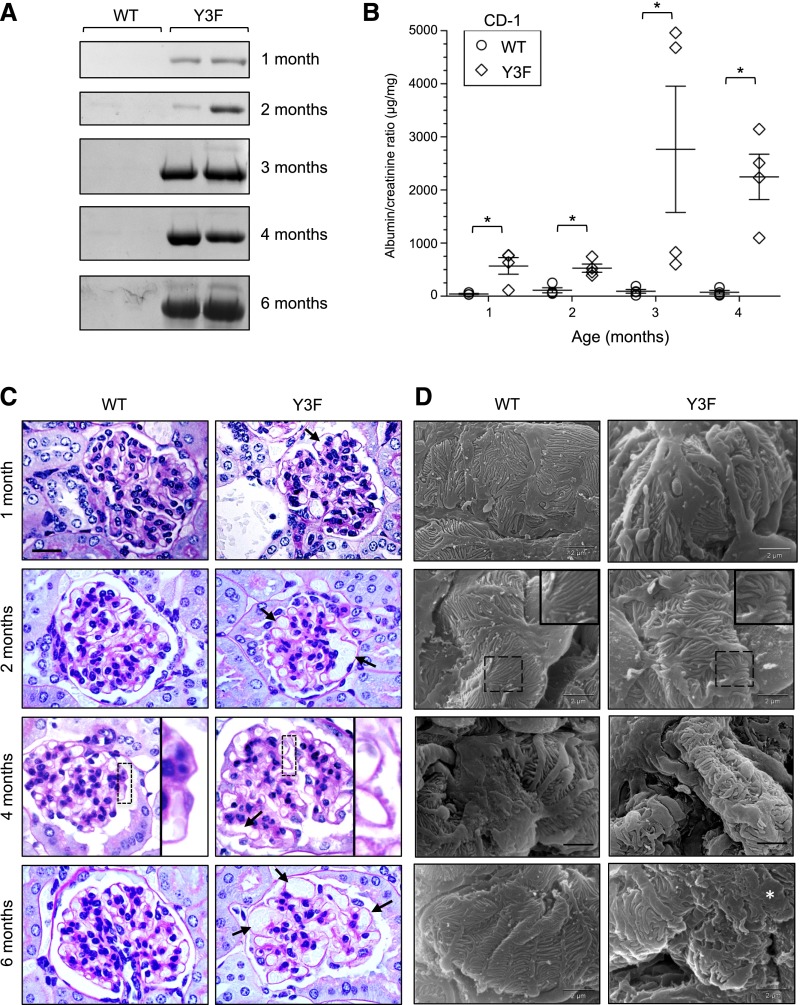
Monitoring of nephrinY3F/Y3F mice revealed that, by 1 month of age on the CD-1 background, most male animals showed significant albuminuria, which progressed markedly with age, as seen by both Coomassie blue staining after SDS-PAGE (Figure 3A) and measurement of the albumin-to-creatinine ratio (ACR) (Figure 3B). Interestingly, a subset of heterozygous nephrinWT/Y3F mice also showed signs of proteinuria by 4 months of age (Supplemental Figure 1A), suggesting that even partial reduction of nephrin tyrosine phosphorylation can induce glomerular damage over time.
Figure 3.
CD-1 nephrinY3F/Y3F mice develop proteinuria, GBM alterations, and foot process effacement. (A) Coomassie-stained gels of consecutive urine samples from nephrinWT/WT and nephrinY3F/Y3F animals. (B) Quantification using ACR (n=4 per genotype). *P<0.05 by ANOVA. (C) Light microscopy analysis of periodic acid–Schiff-stained sections of WT and Y3F animals. Y3F animals show capillary loop dilation (arrows in 1, 2 and 6 months) and GBM spikes (inset in 4 months). (D) Scanning EM analysis of podocyte morphology in WT and Y3F animals. Y3F animals begin to show branched and disorganized foot processes (inset in 2 months), which progress to severe foot process disorganization and effacement (asterisk at 6 months). Scale bars, 20 μm in C; 2 μm in D.
Histologic examination of glomeruli from nephrinWT/WT and nephrinY3F/Y3F mice by light microscopy was performed at four time points: at the earliest sign of proteinuria (1 month), at the onset of moderate proteinuria (2 months), after the progression to severe proteinuria (4 months), and finally, after a period of chronic proteinuria (6 months). At 1 month of age, we observed that glomeruli within nephrinY3F/Y3F mice contained enlarged capillary loops, which became much more extensive by 2 months of age (Figure 3C). At 4 months, there also appeared to be an irregular thickening of the GBM, and we observed pronounced GBM bulges or spikes in nephrinY3F/Y3F animals. By 6 months, glomeruli from nephrinY3F/Y3F were hypocellular, with numerous dilated capillary loops. Importantly, the mean arterial pressure was not different between nephrinWT/WT and proteinuric nephrinY3F/Y3F mice at 4 months of age (Table 2), indicating that the vascular phenotype is not simply a result of increased BP. Furthermore, serum creatinine and BUN were not different between nephrinWT/WT and nephrinY3F/Y3F mice ≤6 months of age, suggesting that the Y3F mutation did not affect on the GFR.
Table 2.
BPs of proteinuric male nephrinY3F/Y3F mice on different genetic backgrounds
| C57BL/6 10 mo | CD-1 4 mo | |||
|---|---|---|---|---|
| WT, n=3 | Y3F, n=3 | WT/HET, n=4 | Y3F, n=4 | |
| Weight, g | 48.9±0.8 | 46.6±2.4 | 49.2±3.2 | 47.1±4.7 |
| Systolic, mmHg | 109±8 | 101±5 | 106±4 | 105±9 |
| Diastolic, mmHg | 74±7 | 70±2 | 80±3 | 78±7 |
| Mean arterial pressure, mmHg | 86±8 | 80±3 | 88±3 | 87±8 |
| Heart rate, beats per min | 498±24 | 510±20 | 571±43 | 543±56 |
Ultrastructural examination using scanning EM revealed differences in podocyte foot process architecture between nephrinWT/WT and nephrinY3F/Y3F mice. At 1 and 2 months of age, we found that foot processes within nephrinY3F/Y3F mice adopted haphazard orientations, and they appeared short, thick, and wavy (Figure 3D). At 4 months of age, foot processes in nephrinY3F/Y3F mice showed signs of retraction, and by 6 months, nephrinY3F/Y3F mice displayed widespread podocyte foot process effacement.
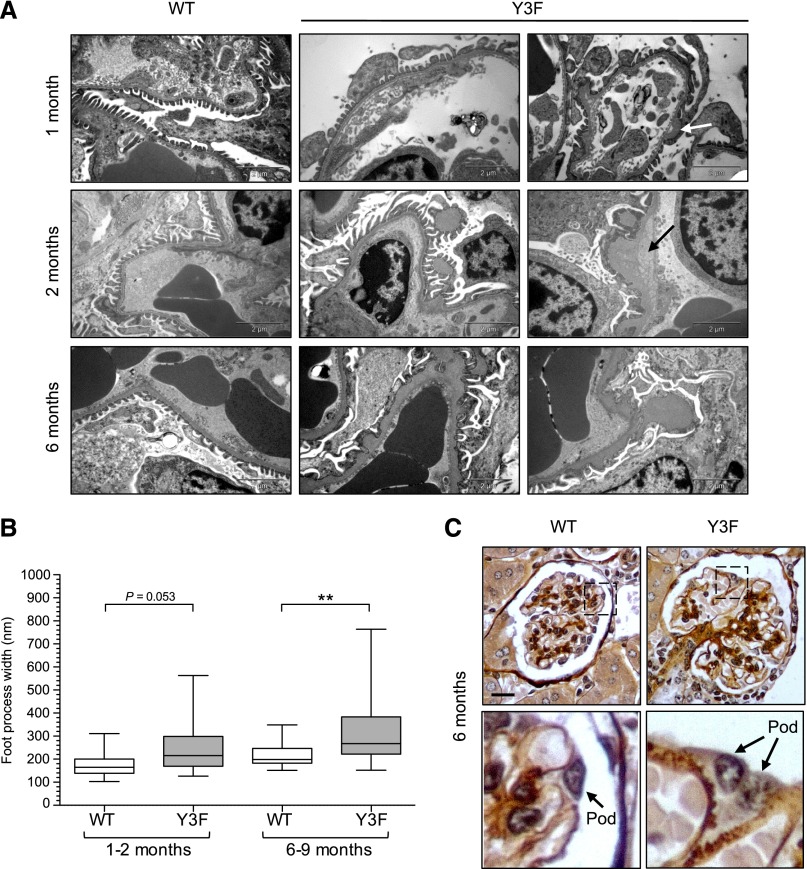
Additional analysis using transmission EM and subsequent quantification of foot process widths revealed varying degrees of damage within glomeruli of individual nephrinY3F/Y3F animals ranging from podocytes that appeared almost normal to those where the majority of foot processes had undergone significant spreading, particularly as the animals aged (Figure 4, A and B). The GBM bulges were associated with regions of clearing, and they appeared in some cases to be detached from the main aspect of the GBM (Figure 4A). By 6 months, there was a diffuse thickening of most of the GBM, which showed an irregular scalloped contour. The nature of the GBM thickening in nephrinY3F/Y3F mice was further confirmed by silver staining of glomeruli at 6 months of age (Figure 4C). Notably, these GBM changes seemed to parallel the onset and progression of foot process alterations in nephrinY3F/Y3F mice as seen in Figure 3. Altogether, the morphologic alterations seen in the glomeruli of nephrinY3F/Y3F mice support the notion that nephrin tyrosine phosphorylation is required to maintain filtration barrier function.
Figure 4.
Progressive GBM abnormalities in CD-1 nephrinY3F/Y3F mice. (A) Transmission EM depicting changes in basement membrane morphology in nephrinY3F/Y3F animals compared with nephrinWT/WT animals. At 1 and 2 months of age, there are focal regions of thickened GBM and evidence of splitting (arrows) in Y3F animals, and at 6 months, numerous subepithelial humps associated with extensive regions of effacement can be seen. (B) Box and whisker (5%–95%) plots of individual foot process widths measured via transmission EM (n=2–3 mice analyzed per group with a minimum of 50 measurements per mouse). **P<0.01 by ANOVA. (C) Light microscopy analysis of silver-stained sections from 6-month-old WT and Y3F mice. Diffuse light brown staining highlights the expanded GBM and membrane humps in Y3F mice (lower panel). Scale bars, 2 μm in A; 20 μm in C.
C57BL/6 NephrinY3F/Y3F Mice Develop Mild Albuminuria and a Delayed Glomerular Phenotype
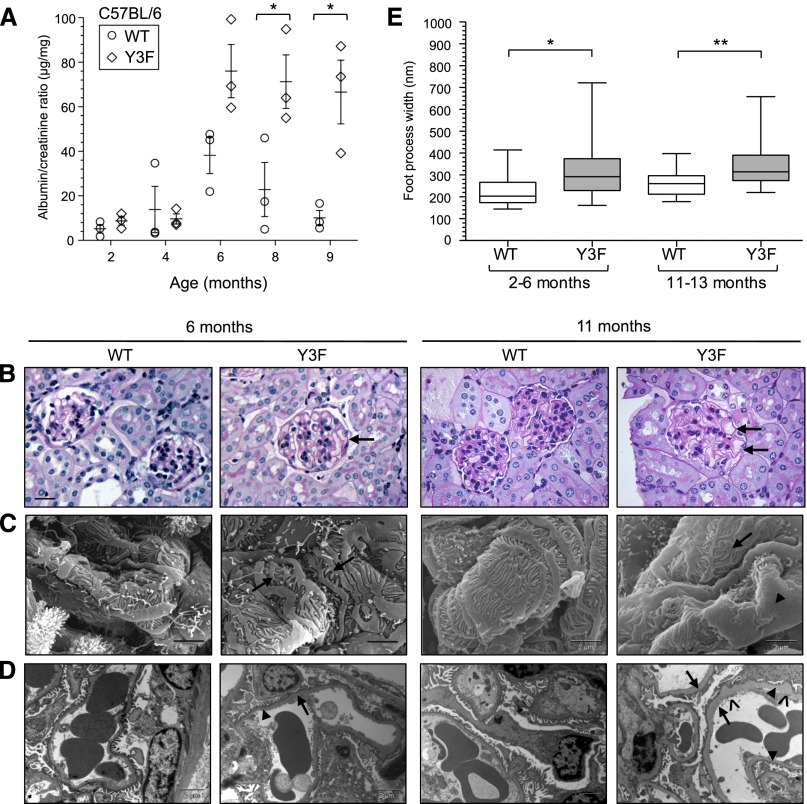
In parallel with our analysis of the nephrin-Y3F mutation on the CD-1 background, we also investigated the effects of reduced nephrin tyrosine phosphorylation on the inbred C57BL/6N background. C57BL/6 nephrinY3F/Y3F mice developed a milder and later onset phenotype than CD-1 nephrinY3F/Y3F mice, and the phenotype was more pronounced in male versus female mice, both of which are consistent with several reports describing strain- and sex-specific differences in the severity of renal disease in genetically modified mice.24–30 C57BL/6 nephrinY3F/Y3F mice showed significant albuminuria at >6 months of age (Figure 5A).
Figure 5.
C57BL/6 nephrinY3F/Y3F mice develop mild proteinuria and delayed glomerular phenotype. (A) Quantification of urine samples from male nephrinWT/WT and nephrinY3F/Y3F animals using ACR (n=3 per genotype). *P<0.05 by ANOVA. (B) Light microscopy analysis of periodic acid–Schiff-stained WT and Y3F animals at 6 and 11 months of age highlighting the presence of dilated capillary loops (arrows). (C). Scanning EMs depicting abnormal appearance of podocytes in Y3F animals, wherein foot processes appear short, broad, and curved (arrows) with significant regions of effacement at 11 months (arrowhead). (D) Transmission EM analysis of GBM morphology in WT and Y3F animals showing progressive segmental changes with regions of foot process preservation (arrowheads) adjacent to effacement (arrows) in addition to increased matrix deposition resulting in spike-like protrusions on the epithelial face of the GBM (^). (E) Box and whisker (5%–95%) plots of individual foot process widths measured via transmission EM of mice within the indicated age groups (n=2–4 mice analyzed per group with a minimum of 50 measurements per mouse). Scale bars, 20 μm in B; 2 μm in C and D. *P<0.05 by ANOVA; **P<0.01 by ANOVA.
Detailed analysis of the kidneys of C57BL/6 nephrinWT/WT and nephrinY3F/Y3F animals was performed at 6 and 11 months of age. Similar to the glomerular phenotype observed on the CD-1 background, C57BL/6 nephrinY3F/Y3F mice showed histologic signs of gross capillary dilation, which worsened as the mice aged, when proteinaceous casts could also be observed in the tubules of some nephrinY3F/Y3F animals (Figure 5B) (data not shown). Average BP remained unchanged between C57BL/6 nephrinWT/WT and proteinuric nephrinY3F/Y3F animals at 10 months of age (Table 2). Examination of podocyte ultrastructure using scanning EM revealed an array of foot process alterations in nephrinY3F/Y3F mice at 6 months of age, which by 11 months of age, appeared as broad, short, and disorganized projections with more extensive regions of effacement (Figure 5C). Transmission EM highlighted segmental regions of foot process effacement in nephrinY3F/Y3F mice at 6 months of age that were widespread by 11 months of age (Figure 5, D and E), and these regions tended to be associated with areas of increased GBM thickness (Figure 5D). Interestingly, GBM abnormalities were also observed in aged heterozygous C57BL/6 nephrinWT/Y3F mice (Supplemental Figure 1, B and C), consistent with the heterozygous phenotype seen on the CD-1 background.
C57BL/6 NephrinY3F/Y3F Mice Show Impaired Recovery from Podocyte Injury
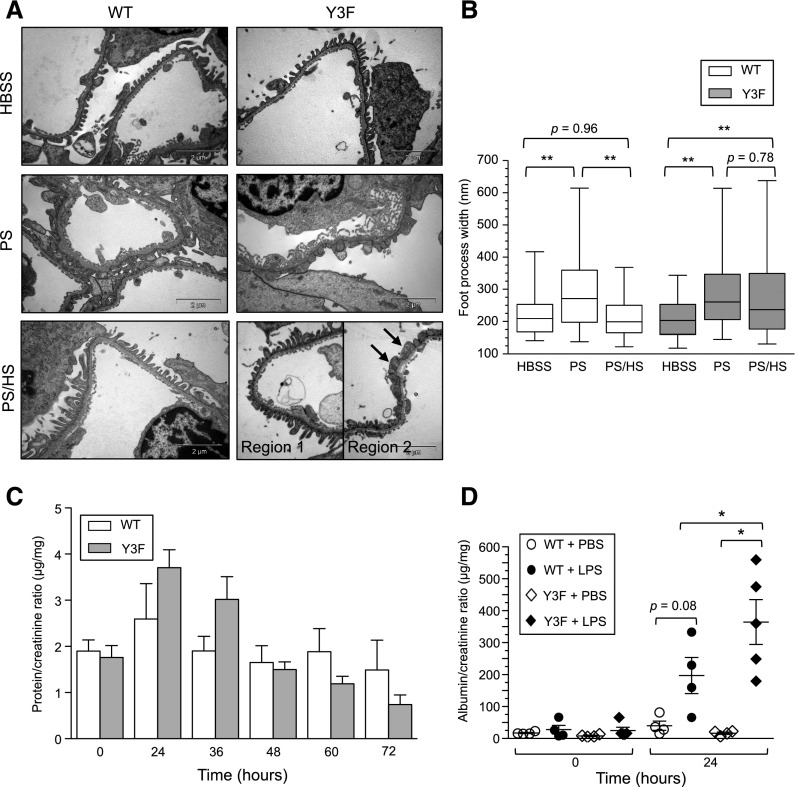
The above findings clearly show the importance of nephrin tyrosine phosphorylation in preservation of glomerular function, and we reasoned that this might reflect a requirement for nephrin phosphorylation in controlling dynamic actin regulation and podocyte remodeling that occur throughout life in response to hemodynamic strain and other insults. To directly test this, we used two models of reversible foot process effacement within young C57BL/6 nephrinY3F/Y3F mice. We first used the protamine sulfate model, where perfusion of protamine sulfate causes rapid foot process effacement, which can be reversed through subsequent perfusion with heparin sulfate.8,31 Kidneys within nephrinWT/WT and nephrinY3F/Y3F mice were perfused with HBSS control buffer, protamine sulfate, or protamine sulfate followed by heparin sulfate, fixed, and analyzed by EM. Compared with buffer-treated animals, mice from both genotypes perfused with protamine sulfate showed extensive foot process spreading (Figure 6, A and B). Interestingly, however, although podocyte foot process architecture was restored to baseline after heparin sulfate treatment in nephrinWT/WT animals, this recovery was impaired in the majority of nephrinY3F/Y3F glomeruli analyzed.
Figure 6.
C57BL/6 nephrinY3F/Y3F mice display delayed recovery in podocyte injury models. (A) Transmission EM of foot processes in nephrinWT/WT and nephrinY3F/Y3F mice perfused with HBSS, protamine sulfate (PS), or protamine sulfate followed by heparin sulfate (PS/HS). PS induces foot process spreading in both genotypes. A proportion of foot processes is restored with HS in Y3F mice (region 1), although the majority remains spread (region 2, arrows). Images are representative of two mice of each genotype per condition. Scale bar, 2 μm. (B) Box and whisker (5%–95%) plots of individual foot process widths measured via transmission EM (n=2 kidneys analyzed per treatment with a minimum of 60 measurements per mouse). Comparisons were made between treatments as indicated. (C) Quantification of total urinary protein normalized to urine creatinine shows that Y3F mice have an enhanced response to LPS–induced podocyte injury at 24 and 36 hours compared with control (Y3F: n=8–13 per time point; WT: n=4–6 per time point except at 60 hours, where n=3). (D) Quantification using ACR at 0 and 24 hours (n=4–5 per genotype). *P<0.05 by ANOVA; **P<0.01 by ANOVA.
We next examined the LPS model of acute injury, whereby LPS induces transient mild albuminuria within 24 hours after injection, which correlates with foot process effacement.31 NephrinWT/WT and nephrinY3F/Y3F mice were injected with PBS or LPS, and spot urine samples were collected at regular intervals ≤72 hours after injection. Urine protein was increased at 24 hours post-LPS injection in nephrinY3F/Y3F mice compared with control mice, and although proteinuria started to decline >24 hours in both genotypes, nephrinY3F/Y3F mice still showed elevated levels at 36 hours post-injection (Figure 6C). Quantitation of the urinary ACRs in mice before (0 hours) and 24 hours after injection showed that LPS treatment induced a significant increase in albuminuria in nephrinY3F/Y3F mice compared with nephrinWT/WT mice and that injection of PBS alone did not alter urinary protein levels in either genotype (Figure 6D). Collectively these findings are consistent with a defect in foot process restoration in nephrinY3F/Y3F mice.
Nephrin Tyrosine Phosphorylation and Binding to Nck Are Altered during Podocyte Effacement
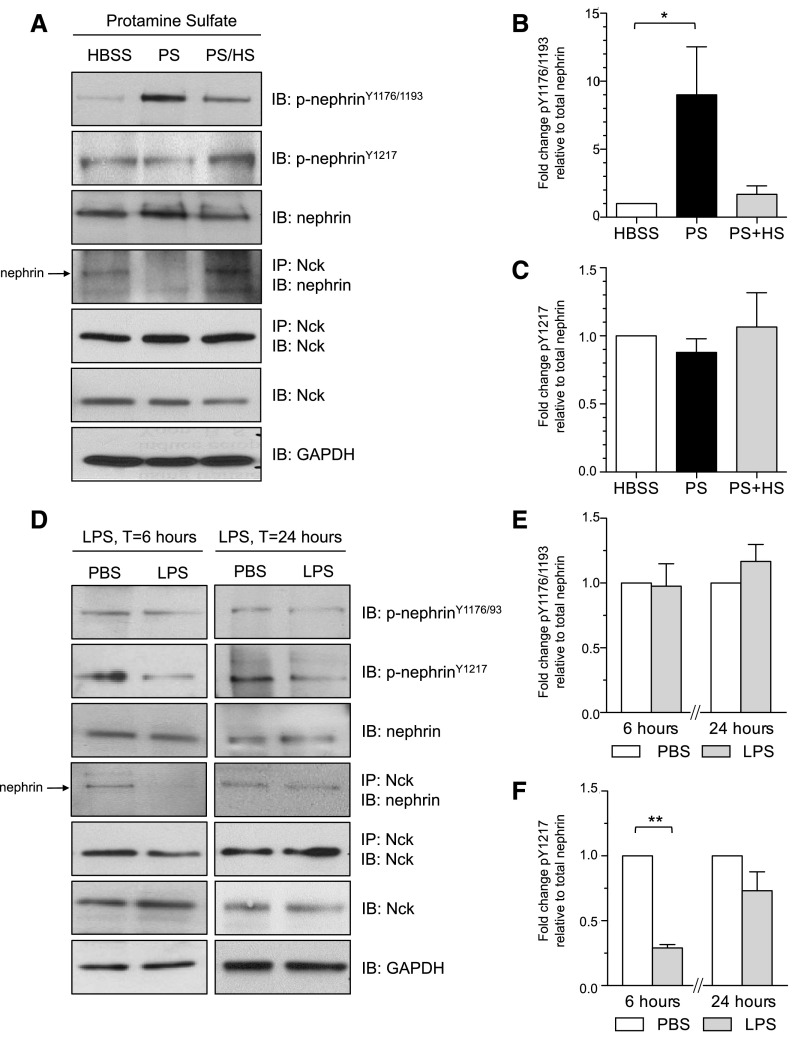
The recovery defect in nephrinY3F/Y3F mice prompted us to explore the dynamics of nephrin signaling that occur coincident with foot process actin remodeling in these injury models. Glomeruli were isolated from WT mice exposed to protamine sulfate or LPS challenge and immunoblotted with phosphospecific nephrin antibodies recognizing Y1176/Y1193 and Y1217.16 Within the protamine sulfate model, we observed a significant increase in nephrin phosphorylation on Y1176/Y1193 after protamine sulfate perfusion, which decreased after heparin sulfate recovery (Figure 7, A and B). Relative to protamine sulfate–treated mice, Y1176/Y1193 was weakly phosphorylated in glomeruli perfused with buffer alone. By contrast, Y1217 was highly phosphorylated with buffer alone and after heparin sulfate treatment, and a modest decrease in phosphorylation on this tyrosine was observed after protamine sulfate perfusion (Figure 7, A and C). Strikingly, this transient decrease in phosphorylation of Y1217 after protamine sulfate was paralleled by reduced interaction between nephrin and Nck, which was likewise restored after heparin sulfate treatment (Figure 7A). Similar findings were obtained within the LPS model. At 6 hours post-LPS injection, which precedes marked proteinuria, we detected a significant decrease in nephrin phosphorylation on Y1217 and reduced association of nephrin with Nck, both of which were restored by 24 hours when proteinuria peaks and subsequently resolves (Figure 7, D and F). No changes in Y1176/Y1193 were detected at either time point analyzed (Figure 7, D and E). These results further support a role for nephrin phosphotyrosine–based signaling pathways in preservation and restoration of foot process architecture and filtration barrier function in the face of podocyte injury.
Figure 7.
Immunoblot (IB) analysis of nephrin tyrosine phosphorylation and Nck binding in glomeruli isolated from WT mice exposed to podocyte injury. (A) Nephrin tyrosine phosphorylation and nephrin/Nck coimmunoprecipitation (IP) after injection with HBSS, protamine sulfate (PS), or protamine sulfate followed by heparin sulfate (PS/HS). Interaction of nephrin with Nck is decreased on PS perfusion. (B and C) Densitometric quantitation of results in A shows a significant increase in nephrin tyrosine phosphorylation on Y1176/1193 but not Y1217 in PS-treated samples. (D) Nephrin tyrosine phosphorylation and nephrin/Nck IP 6 and 24 hours after injection with PBS or LPS. Interaction of nephrin with Nck is decreased at 6 hours after LPS injection. (E and F) Densitometric quantitation of results in D shows a significant decrease in nephrin tyrosine phosphorylation on Y1217 but not Y1176/1193 at 6 hours after LPS injection. The fold change in nephrin phosphorylation was normalized to control samples (i.e., HBSS or PBS). Mice were analyzed individually, and results are representative of three to five mice per treatment. *P<0.05; **P<0.01.
Discussion
The potential for the cytoplasmic tail of nephrin to be regulated by tyrosine phosphorylation has been recognized since the initial characterization of nephrin.6,32 Given the clinical relevance of alterations in nephrin expression and function, numerous efforts have been made to determine the role of this phosphorylation. Our nephrinY3F knockin mouse model now provides unequivocal evidence that phosphonephrin signaling does not seem to be involved in the initial assembly of foot processes but is, instead, critical for stabilization of podocyte cytoarchitecture and restoration after injury. Our results further highlight the central role of nephrin-Nck interaction in regulation of foot process morphology.
The progressive phenotype of nephrinY3F/Y3F mice highlights the linkage between the slit diaphragm, the podocyte-GBM adhesion complex, and the cytoskeletal backbone of foot processes. The actin cytoskeleton is proposed to serve as a mechanotransduction apparatus to facilitate tight coregulation of podocyte contractility and GBM adhesion in response to fluctuating hemodynamic forces during filtration.33,34 Crucial intermediates that link the slit diaphragm and GBM together with the cytoskeletal backbone are the PINCH-ILK-parvin complex and α-actinin-4.35,36 Of note, Nck can complex with PINCH-ILK,37 and it plays a direct role in cell adhesion and spreading.38,39 In the nephrin-Y3F mutant, which cannot bind Nck, integration of the slit diaphragm with the mechanotransduction machinery seems to be compromised but incompletely severed. As such, tethering of the slit diaphragm to the actin cytoskeleton via mechanisms other than phosphonephrin-Nck1/2 interactions could explain why congenital nephrosis does not manifest in these mice (as it does in Nck-deficient animals9). Nevertheless, although this tenuous integration may be sufficient for early podocyte morphogenesis, the weakened linkage between the slit diaphragm, actin cytoskeleton, and GBM adhesion complex in the absence of phosphonephrin signaling renders podocytes more vulnerable and less adaptable to increased hemodynamic stress in mature animals, thereby leading to foot process effacement and albuminuria. The enhanced response to LPS-induced damage in nephrinY3F/Y3F mice further supports this notion. With time, the loss of podocyte contractile tone and adhesiveness causes the GBM to characteristically protrude at its external aspect closest to podocytes, and similarly, the capillary loops become more prone to gross dilation.33 The bulging of the GBM and ballooning of the glomerular tuft are among the most striking pathologies in nephrinY3F/Y3F mice, and analogous structures have been reported in mice lacking other podocyte proteins essential for binding to actin or the GBM.29,40–44
An additional characteristic of nephrinY3F/Y3F animals is the disorganized patterning of foot processes. Process formation in podocytes shares biologic features of that within neurons, wherein common cytoskeletal elements and signaling proteins are used to generate elaborate cell–based projections.45 Intriguingly, mice deficient in Nck expression in neurons display axon guidance defects.46 Although conditional deletion of Nck in podocytes results in profound congenital effacement9 (which may reflect the involvement of Nck in alternate pathways involving key structural proteins, such as β1-integrin47), it is important to note that foot process formation still occurs, even in the complete absence of nephrin.48,49 We, therefore, suggest that Nck has a comparable role in pathfinding downstream of phosphonephrin during the maturation of podocyte foot processes and that this altered patterning further compromises the stability of the slit diaphragm in nephrinY3F/Y3F mice.
The spectrum of glomerular pathologies associated with the nephrin-Y3F mutation is portrayed on both the CD-1 and C57BL/6 backgrounds, despite the differences in onset and severity. C57BL/6 mice have lower BPs than age–matched CD-1 mice,50 which may reflect reduced glomerular strain, although this is unlikely to be the sole determinant, and identification of modifier alleles within these backgrounds remains an area of intense interest. It is noteworthy that the nephrin-Y3F protein seems to have a dominant negative effect in the heterozygous state, and this may be relevant in patients with nephrotic syndrome who carry only a single nephrin mutation and those who present outside the neonatal period.51 Beyond the kidney, nephrin is expressed and functional in several tissues, including the brain, testis, and pancreas.48,52 However, nephrinY3F/Y3F mice are fertile, and they do not display any overt changes in locomotor activity or coordination (data not shown), implying a unique requirement for nephrin phosphorylation in the mature podocyte.
The delayed phenotype of nephrinY3F/Y3F mice on the C57BL/6 background allowed us to use these animals to explore the dynamics and overall necessity of nephrin tyrosine phosphorylation in experimental models of effacement, proteinuria, and recovery, which mimic the podocyte injury associated with acquired glomerulopathies. NephrinY3F/Y3F mice undergo foot process spreading on protamine sulfate perfusion and enhanced LPS–induced proteinuria, clearly showing that tyrosine phosphorylation of nephrin on these sites is not required to actively promote effacement in these models.53 In concordance with our findings, a number of recent works has established a nephrin-independent pathway resulting in Rac activation as an important pathomechanism to drive effacement.13,54,55 Instead, we find that foot process recovery is impaired in nephrinY3F/Y3F animals in both injury models, similar to that observed in mice lacking the actin regulators synaptopodin and cofilin.56,57 Taken together, our data support a model wherein nephrin tyrosine phosphorylation is required to counteract normal podocyte stress and loss of this phosphorylation, as seen in nephrinY3F/Y3F mice and some human diseases, leads to irreversible injury and chronic disease.
The injury phases in the disease models studied herein are coincident with dephosphorylation of nephrin Y1217 and uncoupling from Nck as well as decreased pS473Akt in the LPS model.20 Reductions in nephrin phosphorylation, Nck/p85 binding, and Akt activation have also been detected in the puromycin aminonucleoside model of reversible podocyte injury12,14,16,18 in parallel with increased expression of the phosphatase PTP1B, which can dephosphorylate nephrin Y1217.58 By contrast, phosphorylation of nephrin on Y1176/Y1193 is increased with protamine sulfate as noted previously.7,8 However, this change does not occur in the LPS model, and it does not seem to be essential for effacement; thus, the significance of this site remains to be elucidated. We posit that differential phosphorylation on particular tyrosines may facilitate preferential recruitment of specific signaling proteins to nephrin, such as Nck, as well as p85/PI3K, β-arrestin, and Cas/Crk, which in turn, control distinct processes that regulate foot process remodeling. In the context of Nck, YDxV phosphopeptides show similar binding affinities for the isolated SH2 domains59; however, Y1217 is found within a YDQV motif in contrast to the YDEV motif of both Y1176 and Y1193, and Nck seems to have the highest affinity for Y1217 in vivo.9 In summary, because induced loss of Nck in podocytes results in rapid demise of the filtration barrier16 and the inability to induce phosphorylation on Y1217 and subsequent Nck binding in nephrinY3F/Y3F mice impairs the rapid actin remodeling required for reformation of foot processes, we refine the existing model to include Nck as a critical signaling axis between nephrin Y1217 and the actin cytoskeleton to maintain podocyte plasticity. Establishment of this causal relationship supports the development of novel therapeutic approaches for human proteinuric kidney diseases that selectively preserve and/or enhance nephrin phosphorylation and thereby, directly target the podocyte actin cytoskeleton.
Concise Methods
Generation of NephrinY3F Mice
To generate nephrinWT/Y3F mice (official designation B6;129Nphs1<Tm1.1>/Njns), three A→T nucleotide substitutions were engineered into a construct containing the region from exon 24 to exon 29 of Nphs1, resulting in Y→F missense mutations at p.Y1191F, p.Y1208F, and p.Y1232F. Homologous recombination of the targeting vector into C57BL/6Jx129S6SvEv ES cells produced two independent clones. We initially examined nephrinY3F/Y3F mice derived from both clones and saw similar phenotypes (data not shown), and therefore, we selected one of the clones for additional study. All of the animals used in this study were derived from that clone. After verification of germline transmission, mice were crossed to B6.129S1 Hprt-Cre mice to remove the floxed PGK-neomycin selection cassette. The resulting offspring were crossed once to C57BL/6J and subsequently maintained on the C57BL/6N background (475; Charles River Canada).
Mice were genotyped by PCR using Nphs1Y3F-FW (5′-GCATATGTGAACGCATGAGG-3′) and Nphs1Y3F-RV (5′-GAAGGTGGTTGGTTGCAGTT-3′) primers. PCR cycling conditions used were 95°C for 5 minutes and then 35 cycles of 94°C (30 seconds), 59°C (30 seconds), and 72°C (45 seconds) followed by a final extension at 72°C for 5 minutes. DNA products were examined on 2% agarose gels. B6;129 nephrinY3F/Y3F mice were bred onto the CD-1 (472; Charles River Canada) background for six generations to generate CD-1.B6;129Nphs1<Tm1.1>/Njns mice before intercrossing heterozygous animals. Data include results from animals between generations 6 and 8.
There were similar histologic findings in male and female nephrinY3F/Y3F mice, and data from both sexes are represented in this study. Because the onset of proteinuria was generally earlier in males, male mice were used for all urine analyses, with the exception of the LPS study. The age and sex of mice used in specific injury models are reported in the description of each model.
Animals were housed on a 12-hour/12-hour artificial light-dark cycle. They had free access to standard chow (2014; Harlan Teklad) and drinking water.
Evaluation of Proteinuria
Mice were placed into a metabolic cage until they urinated freely. Urine samples (2 or 5 μl) were diluted in 1× SDS sample buffer, separated by 10% SDS-PAGE, and stained with Coomassie brilliant blue R. The urinary ACR was determined using the Albuwell M ELISA (Exocell) and Creatinine Companion (Exocell) Kits according to the manufacturer’s instructions. For time course recovery studies, the total urinary protein-to-creatinine ratio (in micrograms per milligram) was determined using standard protocols on the basis of a modified Bradford assay60 and the Jaffe reaction.
Histologic and Ultrastructural Analyses
For histology, kidneys were halved and fixed in 10% buffered formalin overnight before being embedded in paraffin; 4-μm sections were cut and stained with periodic acid–Schiff or periodic acid–ammoniacal silver61 to visualize the basement membrane. Slides were viewed on a Leica DM100 (Leica Microsystems, Buffalo Grove, IL). Images were prepared for presentation using Adobe Photoshop CS5 (Adobe Systems, Inc., San Jose, CA).
For EM, sagittal slices (scanning EM) and small pieces (transmission EM) of kidney tissue were fixed in 0.1 M sodium cacodylate buffer containing 4% paraformaldehyde and 2% gluteraldehyde (Electron Microscopy Sciences), postfixed in 1% OsO4, and dehydrated through graded ethanols. Transmission EM samples were embedded in Quetol–Spurr resin. Ultrathin sections were cut and stained with uranyl acetate and lead citrate and viewed using an FEI CM100 TEM. For scanning EM, samples were critical point dried and sputter coated with gold. Samples were viewed using an FEI XL30 SEM or Hitachi S-540 SEM (Hitachi, Yokohama, Japan).
LPS Injection
Nonproteinuric female C57BL/6 nephrinWT/WT and nephrinY3F/Y3F mice between the ages of 3 and 6 months were used for this experiment. Mice were injected intraperitoneally with 200 μg LPS (1 mg/ml in PBS; L2630; Sigma-Aldrich, St. Louis, MO) or an equal volume of PBS. Spot urine samples were collected before injection, at 24 hours after injection, and for recovery analysis, every 12 hours until 72 hours after injection. For biochemical analyses, mice were euthanized with CO2 at 6 and 24 hours after injection; kidneys were removed and then, immediately processed for glomerular isolation.
Protamine Sulfate Injury Model
Nonproteinuric male and female C57BL/6 nephrinWT/WT and nephrinY3F/Y3F mice ages 8–12 weeks old were used in this model. All perfusion solutions were maintained at 37°C in a water bath. Mice were anesthetized with a 2%:98% mix of isoflurane:oxygen and kept warm with a lamp.
The abdomen was opened, and a 27-gauge tube was inserted into the abdominal aorta just below the renal arteries. The inferior vena cava was then nicked below the renal veins, and 10 ml warmed HBSS was infused at a rate of 5 ml/min by hand. The kidneys were then perfused at a rate of 4.5 ml/min using a Pump 11 Elite Infusion Syringe Pump (Harvard Apparatus, Holliston, MA) via the abdominal aorta serially with HBSS for 2 minutes, protamine sulfate (2 mg/ml; Sigma-Aldrich) for 15 minutes, HBSS for 2 minutes, and heparin sulfate (800 μg/ml) for 15 minutes. Kidneys were immediately processed for glomerular isolation or placed in appropriate fixative to be further processed for EM. Foot process widths were measured using ImageJ.
Antibodies
The commercial antibodies used were as follows: mouse anti-GAPDH (G041; Applied Biologic Materials Inc.), guinea pig anti-nephrin (20R-NP002; Fitzgerald Inc.), rabbit anti–pS473 Akt (4060; Cell Signaling Technology, Danvers, MA), rabbit anti-Akt (4691; Cell Signaling Technology), rabbit anti–pY416 (active) Src (2101; Cell Signaling Technology), rabbit anti–pY1217 human nephrin (also recognizes mouse pY1232; 2423–1; Epitomics), rabbit anti–pY1193 human nephrin (also recognizes mouse pY1208),16 mouse anti–β-actin (A5441; Sigma-Aldrich), rabbit anti-podocin (P0372; Sigma-Aldrich), and rabbit anti-Src (Ab-529; Abcam, Inc., Cambridge, MA). Rabbit antinephrin was described previously.17 Rabbit anti-Nck62 was a gift from Louise Larose. Secondary antibodies for immunofluorescence from Invitrogen (Carlsbad, CA) were goat anti–rabbit Alexa Fluor 488 (A11008) and goat anti–guinea pig Alexa Fluor 594 (A11076). Secondary antibodies for immunoblotting from Bio-Rad (Hercules, CA) were goat anti–mouse HRP (170–6516) and goat anti–rabbit HRP (170–6515).
Glomerular Isolation and Lysis
Glomeruli were isolated from mice of the indicated genotypes (one kidney cortex from each of two mice per group) by differential sieving, pelleted, and resuspended in 600 μl RIPA lysis buffer of 50 mM Tris (pH 7.5), 150 mM NaCl, 10% glycerol, 1% NP-40, 0.25% Na-deoxycholate, 0.1% SDS, and 1 mM EDTA supplemented with fresh inhibitors. Alternatively, glomeruli were isolated from minced kidney cortex incubated in 1 mg/ml type 4 collagenase (Worthington) in a 37°C water bath with slight agitation for 30 minutes. Digested tissue was passed through a 100-μm nylon sieve (Thermo Fisher Scientific, Vernon Hills, IL) and washed with ice-cold PBS. Blood was removed by brief incubation in ack lysis buffer of 150 mM NH4Cl, 10 mM KHCO3, and 0.1 mM EDTA, and glomeruli were pelleted by spinning at 350×g for 1 minute. Glomeruli were then resuspended in cold PLC lysis buffer of 10% glycerol, 50 mM Hepes, 150 mM NaCl, 1.5 mM MgCl2, 1 mM EGTA, 10 mM NaPPi, 100 mM NaF, and 1% Triton X-100 supplemented with fresh inhibitors, sonicated for 10 seconds on ice, incubated on ice for 5 minutes, and then, centrifuged at 14,000×g for 10 minutes at 4°C. Protein concentrations were determined using a BCA Protein Assay (Pierce, Rockford, IL). Supernatants were mixed with appropriate amounts of 5× SDS sample buffer and incubated at 100°C for 5 minutes.
Immunoprecipitation
For immunoprecipitation of Nck, protein A beads (Invitrogen) were first conjugated to Nck antibodies to avoid interference from IgG heavy chain during immunoblotting as described elsewhere63 with the following alterations: 0.5 μl antibody was incubated per 100 μl beads in 500 μl PBS for 1 hour at room temperature. Excess unbound antibody was removed by washing three times in PBS followed by spinning at 3000×g for 3 minutes; 100 μl 1.3 mg/ml DSS (Thermo Fisher Scientific) in DMSO was added to crosslink the antibody and beads in 500 μl PBS and left for an additional 1 hour at room temperature. Conjugated beads were successively washed four times in TBS (0.02 M Tris base and 0.15 M NaCl) and an additional four times in 100 mM glycine to remove unconjugated antibody. Conjugated beads were also washed four times in TBS before use; 100 μl conjugated anti-Nck beads were incubated with 2 mg glomerular lysate for 5 hours at 4°C.
Immunoblotting
Protein samples were resolved on 8% or 12% SDS-PAGE gels with 100 μg protein loaded as total lysate. Proteins were transferred to PVDF membrane (EMD Millipore, Billerica, MA) and blocked in 5% skim milk or 5% BSA in 1× TBST. Membranes were incubated with primary antibodies overnight at 4°C: rabbit: anti-Nck (1:1000), anti-nephrin (1:1000), anti-pY1217 (1:2500), anti-pY1193 (1:2500), anti-pY1176/1193 (1:2500), anti-pS473 Akt (1:2000), anti-Akt (1:1000), anti-pY416 Src (1:1000), and anti-Src (1:1000); mouse: anti-GAPDH (1:1000) and anti–β-actin (1:2000). After washing, membranes were incubated with HRP–conjugated secondary antibody diluted 1:10,000 in TBST for 1 hour at room temperature. Signals were detected using ECL (Pierce) or Luminata Crescendo (EMD Millipore). Blots were imaged using a ChemiDoc XRS+ (Bio-Rad) or exposed to film (Pierce). Densitometry was performed using ImageLab version 2.0 analysis software (Bio-Rad).
Indirect Immunofluorescence of Tissue Sections
Kidneys were flash frozen in Cryomatrix (Fisher Scientific, Waltham, MA); 6-μm sections were dried at room temperature for 10 minutes, fixed, and permeabilized in acetone at −20°C for 10 minutes. When using the phosphonephrin antibodies, phosSTOP Tablets (Roche, Basel, Switzerland) were added to all solutions. All subsequent steps were carried out at room temperature. Slides were blocked for 1 hour in 10% goat serum and then, incubated with primary antibodies for 1 hour (guinea pig anti-nephrin [1:100], rabbit anti–pY1217-nephrin [1:50], or anti-podocin [1:100]). After three washes in PBS, slides were incubated with secondary antibodies for 1 hour (goat anti–rabbit Alexa 488 and goat anti–guinea pig Alexa 594; both 1:400). Slides were washed and mounted using Prolong Gold Antifade Mounting Medium (Invitrogen). Epifluoresence images were obtained using Volocity software version 5.3.2 (Improvision) on a DMIRE2 Microscope (Leica Microsystems) using a 63× oil immersion objective. Stacks were captured at 0.2-μm z intervals, and then, deconvolved using an iterative restoration function (95% confidence interval with 15 iterations) in Volocity.
Quantitative Real–Time PCR
For mRNA isolation, kidney cortex was dissected from nephrinWT/WT (n=3) and nephrinY3F/Y3F (n=3) mice, immediately flash frozen, and stored at −80°C. mRNA was isolated using TriZol (Invitrogen), and mRNA concentrations were measured on a nanodrop (Thermo Fisher Scientific) after DNase I (Invitrogen) treatment. RNA integrity was verified on a Bioanalzyer (Agilent Technologies, Santa Clara, CA); all samples used for analysis had an RIN value of at least seven. cDNA synthesis was performed on 1 μg RNA with the Superscript II Kit (Invitrogen) using random primers. Quantitative RT-PCR reactions were carried out using SsoFast EvaGreen Supermix (Bio-Rad) on a CFX96 Real–Time PCR Detection System (Bio-Rad). Primer sequences used in this study are listed in Table 3. Standard curves were generated for all primer pairs used, and melt curves were examined to verify that there was a single amplicon. No reverse transcription and no template controls were included in all runs. Expression differences were calculated using the ΔCt method corrected for primer efficiency. Differences were normalized to the reference genes Gapdh and Hprt. All calculations were performed in CFX Manager 3.0 (Bio-Rad).
Table 3.
Primer sequences used for real–time PCR analysis
| Gene | Forward Primer (5′-3′) | Reverse Primer (5′-3′) | Size, bp |
|---|---|---|---|
| Actn4 | ATGGTCTCCGACATCAACAA | TCATTCAGCAGCCATTCTTC | 77 |
| Nphs1 | GGAAGAGGTGTCATATCGCC | CTCAAAGGGCAGAGAACCAG | 250 |
| Nphs2 | TCCATGAGGTGGTAACCAAA | CTTTGGACACATGGGCTAGA | 111 |
| Wt1 | ACGTCCTTTCATGTGTGCAT | TTTCTCACCAGTGTGCTTCC | 94 |
| Gapdh | AGGTCGGTGTGAACGGATTTG | GGGGTCGTTGATGGCAACA | 95 |
| Hprt | CATGGACTGATTATGGACAGGACTG | ATCCAGCAGGTCAGCAAAGAACT | 126 |
BP Measurements
Mice were anesthetized with a 2%:98% mix of isoflurane:oxygen. Body temperature was maintained at 37°C using a battery–operated rectal thermometer combined with a 100-W heating lamp. A small incision was made, and a 1.2-F catheter (FTS-1211B-0018; Scisense Inc.) was inserted into the right carotid artery, through the aortic valve, and into the left ventricle. Pressures were digitized at a sampling rate of 2000 Hz and recorded using iWorx analytic software (Labscribe2, Dover, NH).
Statistical Analyses
Values are presented as means±SEMs. Differences between two groups were analyzed by t test, and differences between more than two groups were analyzed by ANOVA using SAS version 9.4 (SAS Institute Inc., Cary, NC). Statistical analyses of quantitative RT-PCR data were performed in CFX Manager software (Bio-Rad). Graphs were prepared using Graphpad Prism version 5.0 (GraphPad Software, La Jolla, CA). P<0.05 was considered statistically significant.
Study Approval
Animal studies were approved by the University of Guelph Animal Care Committee and carried out in accordance with Canadian Council on Animal Care protocols.
Disclosures
None.
Supplementary Material
Acknowledgments
This manuscript is dedicated to the memory of Dr. Tony Pawson, in whose laboratory this signaling journey was initiated. We thank Dr. Joel Henderson for helpful discussions, Dr. Louise Larose for Nck antibodies, and Doug Holmyard, Sandy Smith, and Lily Morikawa for technical assistance. We also thank Jenn Randall, Martha Smith, and the University of Guelph Central Animal Facility staff for assistance with animal husbandry and experiments. The University of Connecticut Health Center Gene Targeting and Transgenic Facility was instrumental in the creation of nephrinWT/Y3F mice.
This work was supported by grants from the Kidney Foundation of Canada (to N.J.), Canadian Institutes of Health Research (to N.J.), and the Sick Kids Foundation (to N.J.). L.A.N. and C.E.M. were supported by Natural Sciences and Engineering Research Council of Canada Alexander Graham Bell Canada Graduate Doctoral Scholarships. C.E.M., M.J.P., and A.K.C. were supported by Ontario Graduate Scholarships. N.J. holds a Tier II Canada Research Chair in Eukaryotic Cellular Signaling and was the recipient of a New Investigator Award from the Kidney Research Scientist Core Education and National Training Program.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
This article contains supplemental material online at http://jasn.asnjournals.org/lookup/suppl/doi:10.1681/ASN.2015091048/-/DCSupplemental.
References
- 1.Greka A, Mundel P: Cell biology and pathology of podocytes. Annu Rev Physiol 74: 299–323, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Scott RP, Quaggin SE: Review series: The cell biology of renal filtration. J Cell Biol 209: 199–210, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kerjaschki D: Caught flat-footed: Podocyte damage and the molecular bases of focal glomerulosclerosis. J Clin Invest 108: 1583–1587, 2001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Grahammer F, Schell C, Huber TB: The podocyte slit diaphragm--from a thin grey line to a complex signalling hub. Nat Rev Nephrol 9: 587–598, 2013 [DOI] [PubMed] [Google Scholar]
- 5.Welsh GI, Saleem MA: Nephrin-signature molecule of the glomerular podocyte? J Pathol 220: 328–337, 2010 [DOI] [PubMed] [Google Scholar]
- 6.Kestilä M, Lenkkeri U, Männikkö M, Lamerdin J, McCready P, Putaala H, Ruotsalainen V, Morita T, Nissinen M, Herva R, Kashtan CE, Peltonen L, Holmberg C, Olsen A, Tryggvason K: Positionally cloned gene for a novel glomerular protein--nephrin--is mutated in congenital nephrotic syndrome. Mol Cell 1: 575–582, 1998 [DOI] [PubMed] [Google Scholar]
- 7.Harita Y, Kurihara H, Kosako H, Tezuka T, Sekine T, Igarashi T, Ohsawa I, Ohta S, Hattori S: Phosphorylation of nephrin triggers Ca2+ signaling by recruitment and activation of phospholipase C-gamma1. J Biol Chem 284: 8951–8962, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Verma R, Kovari I, Soofi A, Nihalani D, Patrie K, Holzman LB: Nephrin ectodomain engagement results in Src kinase activation, nephrin phosphorylation, Nck recruitment, and actin polymerization. J Clin Invest 116: 1346–1359, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Jones N, Blasutig IM, Eremina V, Ruston JM, Bladt F, Li H, Huang H, Larose L, Li SS, Takano T, Quaggin SE, Pawson T: Nck adaptor proteins link nephrin to the actin cytoskeleton of kidney podocytes. Nature 440: 818–823, 2006 [DOI] [PubMed] [Google Scholar]
- 10.New LA, Martin CE, Jones N: Advances in slit diaphragm signaling. Curr Opin Nephrol Hypertens 23: 420–430, 2014 [DOI] [PubMed] [Google Scholar]
- 11.Huber TB, Hartleben B, Kim J, Schmidts M, Schermer B, Keil A, Egger L, Lecha RL, Borner C, Pavenstädt H, Shaw AS, Walz G, Benzing T: Nephrin and CD2AP associate with phosphoinositide 3-OH kinase and stimulate AKT-dependent signaling. Mol Cell Biol 23: 4917–4928, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhu J, Sun N, Aoudjit L, Li H, Kawachi H, Lemay S, Takano T: Nephrin mediates actin reorganization via phosphoinositide 3-kinase in podocytes. Kidney Int 73: 556–566, 2008 [DOI] [PubMed] [Google Scholar]
- 13.George B, Verma R, Soofi AA, Garg P, Zhang J, Park TJ, Giardino L, Ryzhova L, Johnstone DB, Wong H, Nihalani D, Salant DJ, Hanks SK, Curran T, Rastaldi MP, Holzman LB: Crk1/2-dependent signaling is necessary for podocyte foot process spreading in mouse models of glomerular disease. J Clin Invest 122: 674–692, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li H, Zhu J, Aoudjit L, Latreille M, Kawachi H, Larose L, Takano T: Rat nephrin modulates cell morphology via the adaptor protein Nck. Biochem Biophys Res Commun 349: 310–316, 2006 [DOI] [PubMed] [Google Scholar]
- 15.New LA, Keyvani Chahi A, Jones N: Direct regulation of nephrin tyrosine phosphorylation by Nck adaptor proteins. J Biol Chem 288: 1500–1510, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jones N, New LA, Fortino MA, Eremina V, Ruston J, Blasutig IM, Aoudjit L, Zou Y, Liu X, Yu GL, Takano T, Quaggin SE, Pawson T: Nck proteins maintain the adult glomerular filtration barrier. J Am Soc Nephrol 20: 1533–1543, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Li H, Lemay S, Aoudjit L, Kawachi H, Takano T: SRC-family kinase Fyn phosphorylates the cytoplasmic domain of nephrin and modulates its interaction with podocin. J Am Soc Nephrol 15: 3006–3015, 2004 [DOI] [PubMed] [Google Scholar]
- 18.Uchida K, Suzuki K, Iwamoto M, Kawachi H, Ohno M, Horita S, Nitta K: Decreased tyrosine phosphorylation of nephrin in rat and human nephrosis. Kidney Int 73: 926–932, 2008 [DOI] [PubMed] [Google Scholar]
- 19.Ohashi T, Uchida K, Asamiya Y, Tsuruta Y, Ohno M, Horita S, Nitta K: Phosphorylation status of nephrin in human membranous nephropathy. Clin Exp Nephrol 14: 51–55, 2010 [DOI] [PubMed] [Google Scholar]
- 20.Zhang SY, Kamal M, Dahan K, Pawlak A, Ory V, Desvaux D, Audard V, Candelier M, BenMohamed F, Matignon M, Christov C, Decrouy X, Bernard V, Mangiapan G, Lang P, Guellaën G, Ronco P, Sahali D: c-mip impairs podocyte proximal signaling and induces heavy proteinuria. Sci Signal 3: ra39, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Canaud G, Bienaimé F, Viau A, Treins C, Baron W, Nguyen C, Burtin M, Berissi S, Giannakakis K, Muda AO, Zschiedrich S, Huber TB, Friedlander G, Legendre C, Pontoglio M, Pende M, Terzi F: AKT2 is essential to maintain podocyte viability and function during chronic kidney disease. Nat Med 19: 1288–1296, 2013 [DOI] [PubMed] [Google Scholar]
- 22.Ashworth S, Teng B, Kaufeld J, Miller E, Tossidou I, Englert C, Bollig F, Staggs L, Roberts IS, Park JK, Haller H, Schiffer M: Cofilin-1 inactivation leads to proteinuria--studies in zebrafish, mice and humans. PLoS One 5: e12626, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Quack I, Rump LC, Gerke P, Walther I, Vinke T, Vonend O, Grunwald T, Sellin L: beta-Arrestin2 mediates nephrin endocytosis and impairs slit diaphragm integrity. Proc Natl Acad Sci U S A 103: 14110–14115, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Johnstone DB, Ikizler O, Zhang J, Holzman LB: Background strain and the differential susceptibility of podocyte-specific deletion of Myh9 on murine models of experimental glomerulosclerosis and HIV nephropathy. PLoS One 8: e67839, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kang JS, Wang XP, Miner JH, Morello R, Sado Y, Abrahamson DR, Borza DB: Loss of alpha3/alpha4(IV) collagen from the glomerular basement membrane induces a strain-dependent isoform switch to alpha5alpha6(IV) collagen associated with longer renal survival in Col4a3-/- Alport mice. J Am Soc Nephrol 17: 1962–1969, 2006 [DOI] [PubMed] [Google Scholar]
- 26.Gao F, Maiti S, Sun G, Ordonez NG, Udtha M, Deng JM, Behringer RR, Huff V: The Wt1+/R394W mouse displays glomerulosclerosis and early-onset renal failure characteristic of human Denys-Drash syndrome. Mol Cell Biol 24: 9899–9910, 2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Nishino T, Sasaki N, Nagasaki K, Ahmad Z, Agui T: Genetic background strongly influences the severity of glomerulosclerosis in mice. J Vet Med Sci 72: 1313–1318, 2010 [DOI] [PubMed] [Google Scholar]
- 28.Sachs N, Claessen N, Aten J, Kreft M, Teske GJ, Koeman A, Zuurbier CJ, Janssen H, Sonnenberg A: Blood pressure influences end-stage renal disease of Cd151 knockout mice. J Clin Invest 122: 348–358, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Baleato RM, Guthrie PL, Gubler MC, Ashman LK, Roselli S: Deletion of CD151 results in a strain-dependent glomerular disease due to severe alterations of the glomerular basement membrane. Am J Pathol 173: 927–937, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Long DA, Kolatsi-Joannou M, Price KL, Dessapt-Baradez C, Huang JL, Papakrivopoulou E, Hubank M, Korstanje R, Gnudi L, Woolf AS: Albuminuria is associated with too few glomeruli and too much testosterone. Kidney Int 83: 1118–1129, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Pippin JW, Brinkkoetter PT, Cormack-Aboud FC, Durvasula RV, Hauser PV, Kowalewska J, Krofft RD, Logar CM, Marshall CB, Ohse T, Shankland SJ: Inducible rodent models of acquired podocyte diseases. Am J Physiol Renal Physiol 296: F213–F229, 2009 [DOI] [PubMed] [Google Scholar]
- 32.Lahdenperä J, Kilpeläinen P, Liu XL, Pikkarainen T, Reponen P, Ruotsalainen V, Tryggvason K: Clustering-induced tyrosine phosphorylation of nephrin by Src family kinases. Kidney Int 64: 404–413, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Kriz W, Lemley KV: A potential role for mechanical forces in the detachment of podocytes and the progression of CKD. J Am Soc Nephrol 26: 258–269, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Endlich N, Endlich K: The challenge and response of podocytes to glomerular hypertension. Semin Nephrol 32: 327–341, 2012 [DOI] [PubMed] [Google Scholar]
- 35.Dai C, Stolz DB, Bastacky SI, St-Arnaud R, Wu C, Dedhar S, Liu Y: Essential role of integrin-linked kinase in podocyte biology: Bridging the integrin and slit diaphragm signaling. J Am Soc Nephrol 17: 2164–2175, 2006 [DOI] [PubMed] [Google Scholar]
- 36.Zha D, Chen C, Liang W, Chen X, Ma T, Yang H, Goor H, Ding G: Nephrin phosphorylation regulates podocyte adhesion through the PINCH-1-ILK-α-parvin complex. BMB Rep 46: 230–235, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tu Y, Li F, Goicoechea S, Wu C: The LIM-only protein PINCH directly interacts with integrin-linked kinase and is recruited to integrin-rich sites in spreading cells. Mol Cell Biol 19: 2425–2434, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chaki SP, Barhoumi R, Berginski ME, Sreenivasappa H, Trache A, Gomez SM, Rivera GM: Nck enables directional cell migration through the coordination of polarized membrane protrusion with adhesion dynamics. J Cell Sci 126: 1637–1649, 2013 [DOI] [PubMed] [Google Scholar]
- 39.Clouthier DL, Harris CN, Harris RA, Martin CE, Puri MC, Jones N: Requisite role for Nck adaptors in cardiovascular development, endothelial-to-mesenchymal transition, and directed cell migration. Mol Cell Biol 35: 1573–1587, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Chase SE, Encina CV, Stolzenburg LR, Tatum AH, Holzman LB, Krendel M: Podocyte-specific knockout of myosin 1e disrupts glomerular filtration. Am J Physiol Renal Physiol 303: F1099–F1106, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Gross O, Beirowski B, Harvey SJ, McFadden C, Chen D, Tam S, Thorner PS, Smyth N, Addicks K, Bloch W, Ninomiya Y, Sado Y, Weber M, Vogel WF: DDR1-deficient mice show localized subepithelial GBM thickening with focal loss of slit diaphragms and proteinuria. Kidney Int 66: 102–111, 2004 [DOI] [PubMed] [Google Scholar]
- 42.Girgert R, Martin M, Kruegel J, Miosge N, Temme J, Eckes B, Müller GA, Gross O: Integrin α2-deficient mice provide insights into specific functions of collagen receptors in the kidney. Fibrogenesis Tissue Repair 3: 19, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Sachs N, Kreft M, van den Bergh Weerman MA, Beynon AJ, Peters TA, Weening JJ, Sonnenberg A: Kidney failure in mice lacking the tetraspanin CD151. J Cell Biol 175: 33–39, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cho AR, Uchio-Yamada K, Torigai T, Miyamoto T, Miyoshi I, Matsuda J, Kurosawa T, Kon Y, Asano A, Sasaki N, Agui T: Deficiency of the tensin2 gene in the ICGN mouse: An animal model for congenital nephrotic syndrome. Mamm Genome 17: 407–416, 2006 [DOI] [PubMed] [Google Scholar]
- 45.Kobayashi N, Gao SY, Chen J, Saito K, Miyawaki K, Li CY, Pan L, Saito S, Terashita T, Matsuda S: Process formation of the renal glomerular podocyte: Is there common molecular machinery for processes of podocytes and neurons? Anat Sci Int 79: 1–10, 2004 [DOI] [PubMed] [Google Scholar]
- 46.Fawcett JP, Georgiou J, Ruston J, Bladt F, Sherman A, Warner N, Saab BJ, Scott R, Roder JC, Pawson T: Nck adaptor proteins control the organization of neuronal circuits important for walking. Proc Natl Acad Sci U S A 104: 20973–20978, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Pozzi A, Jarad G, Moeckel GW, Coffa S, Zhang X, Gewin L, Eremina V, Hudson BG, Borza DB, Harris RC, Holzman LB, Phillips CL, Fassler R, Quaggin SE, Miner JH, Zent R: Beta1 integrin expression by podocytes is required to maintain glomerular structural integrity. Dev Biol 316: 288–301, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Putaala H, Soininen R, Kilpeläinen P, Wartiovaara J, Tryggvason K: The murine nephrin gene is specifically expressed in kidney, brain and pancreas: Inactivation of the gene leads to massive proteinuria and neonatal death. Hum Mol Genet 10: 1–8, 2001 [DOI] [PubMed] [Google Scholar]
- 49.Rantanen M, Palmén T, Pätäri A, Ahola H, Lehtonen S, Aström E, Floss T, Vauti F, Wurst W, Ruiz P, Kerjaschki D, Holthöfer H: Nephrin TRAP mice lack slit diaphragms and show fibrotic glomeruli and cystic tubular lesions. J Am Soc Nephrol 13: 1586–1594, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Leelahavanichkul A, Yan Q, Hu X, Eisner C, Huang Y, Chen R, Mizel D, Zhou H, Wright EC, Kopp JB, Schnermann J, Yuen PS, Star RA: Angiotensin II overcomes strain-dependent resistance of rapid CKD progression in a new remnant kidney mouse model. Kidney Int 78: 1136–1153, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Lemley KV: Yet more ways to skin a cat: Nephrin mutations outside the neonatal period. J Am Soc Nephrol 19: 1837–1838, 2008 [DOI] [PubMed] [Google Scholar]
- 52.Juhila J, Lassila M, Roozendaal R, Lehtonen E, Messing M, Langer B, Kerjaschki D, Verbeek JS, Holthofer H: Inducible nephrin transgene expression in podocytes rescues nephrin-deficient mice from perinatal death. Am J Pathol 176: 51–63, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Patrakka J, Tryggvason K: Nephrin--a unique structural and signaling protein of the kidney filter. Trends Mol Med 13: 396–403, 2007 [DOI] [PubMed] [Google Scholar]
- 54.Blattner SM, Hodgin JB, Nishio M, Wylie SA, Saha J, Soofi AA, Vining C, Randolph A, Herbach N, Wanke R, Atkins KB, Gyung Kang H, Henger A, Brakebusch C, Holzman LB, Kretzler M: Divergent functions of the Rho GTPases Rac1 and Cdc42 in podocyte injury. Kidney Int 84: 920–930, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Schaldecker T, Kim S, Tarabanis C, Tian D, Hakroush S, Castonguay P, Ahn W, Wallentin H, Heid H, Hopkins CR, Lindsley CW, Riccio A, Buvall L, Weins A, Greka A: Inhibition of the TRPC5 ion channel protects the kidney filter. J Clin Invest 123: 5298–5309, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Asanuma K, Kim K, Oh J, Giardino L, Chabanis S, Faul C, Reiser J, Mundel P: Synaptopodin regulates the actin-bundling activity of alpha-actinin in an isoform-specific manner. J Clin Invest 115: 1188–1198, 2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Garg P, Verma R, Cook L, Soofi A, Venkatareddy M, George B, Mizuno K, Gurniak C, Witke W, Holzman LB: Actin-depolymerizing factor cofilin-1 is necessary in maintaining mature podocyte architecture. J Biol Chem 285: 22676–22688, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Aoudjit L, Jiang R, Lee TH, New LA, Jones N, Takano T: Podocyte protein, nephrin, is a substrate of protein tyrosine phosphatase 1B. J Signal Transduct 2011: 376543, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Blasutig IM, New LA, Thanabalasuriar A, Dayarathna TK, Goudreault M, Quaggin SE, Li SS, Gruenheid S, Jones N, Pawson T: Phosphorylated YDXV motifs and Nck SH2/SH3 adaptors act cooperatively to induce actin reorganization. Mol Cell Biol 28: 2035–2046, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Zor T, Selinger Z: Linearization of the Bradford protein assay increases its sensitivity: Theoretical and experimental studies. Anal Biochem 236: 302–308, 1996 [DOI] [PubMed] [Google Scholar]
- 61.Feugas P, Morikawa L: Periodic acid-ammoniacal silver (PAAS) method for the demonstration of glomerular basement membranes using the microwave oven. J Histotechnol 2: 105–107, 1989 [Google Scholar]
- 62.Lussier G, Larose L: A casein kinase I activity is constitutively associated with Nck. J Biol Chem 272: 2688–2694, 1997 [DOI] [PubMed] [Google Scholar]
- 63.Qoronfleh MW, Ren L, Emery D, Perr M, Kaboord B: Use of immunomatrix methods to improve protein-protein interaction detection. J Biomed Biotechnol 2003: 291–298, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.