Abstract
Climate change (global warming) is leading to an increase in heat extremes and coupled with increasing water shortage, provides a perfect storm for a new era of environmental crises and potentially, new diseases. We use a comparative physiologic approach to show that one of the primary mechanisms by which animals protect themselves against water shortage is to increase fat mass as a means for providing metabolic water. Strong evidence suggests that certain hormones (vasopressin), foods (fructose), and metabolic products (uric acid) function as survival signals to help reduce water loss and store fat (which also provides a source of metabolic water). These mechanisms are intricately linked with each other and stimulated by dehydration and hyperosmolarity. Although these mechanisms were protective in the setting of low sugar and low salt intake in our past, today, the combination of diets high in fructose and salty foods, increasing temperatures, and decreasing available water places these survival signals in overdrive and may be accelerating the obesity and diabetes epidemics. The recent discovery of multiple epidemics of CKD occurring in agricultural workers in hot and humid environments may represent harbingers of the detrimental consequences of the combination of climate change and overactivation of survival pathways.
Keywords: vasopressin, osmolality, obesity, metabolism, chronic kidney disease
The 21st century is bringing new challenges with population expansion, a decrease in natural resources, and climate change. Mean temperatures increased by 0.8°C since 1880, with two thirds of the change occurring since 1975, and they are projected to increase by 3°C to 4°C by the end of the 21st century.1,2 Temperature extremes have also increased by 75% because of climate change.3 Continued population growth and to a lesser extent, climate change have also resulted in decreasing water resources.4,5 Today, one half of the world population suffer moderate water shortage (i.e., 1.0–1.7 m3 water per person per year), and 10% have extreme water shortage (defined as <0.5 m3 per person per year), with the primary areas affected being Africa, southern and eastern Asia, and the Middle East.4
Increasing water shortage coupled with climate change increases the risk for dehydration-associated diseases. For example, there is increasing evidence that climate change may have a role in epidemics of CKD that are occurring among workers in hot environments.6 While this latter paper focuses on the sites of these epidemics and their relationship to local temperatures and changing climate, space constraints prevented it from being able to address a more central question on the biology of water conservation and how it relates to disease. Here we review how various species protect themselves from dehydration, and we identify nutrient, hormonal and metabolic pathways triggered by hyperosmolarity that link water conservation with survival. We also discuss how these pathways may predict diseases that will dominate the next millennium. Importantly, climate change, heat stress, and water shortage not only will affect kidney disease, but risk for metabolic diseases including obesity and diabetes.
How Animals Survive Water Shortage
The transition of vertebrates from sea based to land was associated with many adaptations, but some of the most important were mechanisms to conserve water, including ways to store water, minimize water loss, and generate water.7
Water Storage
Some terrestrial animals store water in their bladders. The water-holding frog (Cyclorana platycephala) of the Sandy Desert of Australia, for example, stores so much water that it may double its weight.8 These frogs were a favorite source of water for the Tiwi people during hot summers. Some frogs live 5 years without drinking water, which is because they utilize water stored in their bladders and also generate water during the metabolism of fat.7,8 The giant tortoise of the Galapagos Islands stores water in their urinary bladder. After rain, the tortoise voids their bladder urine (which contains urea and other waste products) and drinks copiously to refill their bladder with fresh water. When needed, the turtle reabsorbs the water through the bladder wall, while at the same time, excreting some of its wastes into it, and over time, the osmolarity of the bladder urine increases.9
Reduced Urinary Water Losses
Homer Smith6 proposed that the evolution from aquatic to terrestrial environments required efficient ways to excrete nitrogen to help minimize loss of water.10 Most aquatic animals excrete ammonia, the simplest nitrogen product, as their means for eliminating nitrogen waste products (ammoniotely). In contrast, ammonia is not an appropriate compound for nitrogen excretion by terrestrial animals, because its renal excretion requires 400 ml water per 1 gram ammonia and blood levels >0.05 mM are neurotoxic.10 Rather, urea excretion is common among land amphibians and mammals, because it is concentrated easily and with low toxicity. Most effective is excretion of uric acid (uricotely), which requires only 1/50 the amount of water as that for the excretion of ammonia. Excretion of uric acid is the principal mechanism for nitrogen excretion in birds, reptiles, and some amphibians.10 Here, the uric acid is precipitated in the cloaca, where the last water is absorbed, and then, the urate pellet is excreted.
Although ureotelic animals have obligate water loss to help excrete metabolic wastes, urinary loss is minimized by urinary concentration, a process largely driven by vasopressin (or vasotocin in lower vertebrates). Vasopressin reduces water excretion by allowing water reabsorption in the collecting ducts, but it also increases sodium and urea reabsorption. The reduction in urea excretion by vasopressin improves urinary concentration by increasing urea accumulation in the renal medulla, which aids water reabsorption.
Reducing Nonrenal Water Loss
Water loss also occurs through the skin and lungs, where it helps regulate body temperature when animals are exposed to heat. A lack of sweating can result in a marked rise in body temperature and circulatory collapse (heat shock). In contrast, excessive sweating without rehydration may result in hypernatremia and volume depletion.
Desert rodents minimize water loss by hiding during the day in burrows, where temperatures are lower and humidity is high. Lungfish coat themselves with slime to minimize water loss as they burrow and estivate in the mud. Estivating frogs (C. platycephala) form cocoons from sloughed epithelial layers of skin.11 Tree frogs decrease water loss by secreting an impermeable waxy material onto their skin.11 Lemurs estivate in tree hollows to avoid sun exposure and reduce their metabolism and water needs. The dromedary camel conserves water by minimizing sweating because of a reduction in sweat glands. The camel also does not pant and has adaptations in its nose that minimize respiratory losses of water.12,13 The consequence is significant diurnal variation in body temperature (as much as 6°C), with temperatures occasionally reaching 40°C on hot days.12 To prevent dehydration, camels ingest large volumes (up to 57 L) of water at one sitting. Despite these preventive measures, camels can become severely dehydrated.14
Metabolic Water
Water is also generated during fat and glycogen metabolism. Fat is anhydrous and contains only 10% water by weight,15 but when fat is oxidized, water and carbon dioxide are released. For every gram of fat metabolized, 1.12 ml water is generated.16 Liver or muscle glycogen also generates 0.6 ml water per gram of glycogen metabolized.17 Because glycogen is water soluble, it also releases potassium and water during metabolism, accounting for an additional 3 ml water per gram of glycogen metabolized.18,19 The marked diuresis after initiation of a low-carbohydrate diet is partially because of water released during glycogen metabolism.19 Although glycogen metabolism produces metabolic water, most organisms store more fat than glycogen. Thus, fat is the major source of metabolic water for most animals.
Metabolic water is used by many animals to survive periods of water shortage. Marine whales obtain much of their water from the burning of fat.20 Although capable of ingesting seawater and excreting a urine more concentrated than seawater, whales rarely use this method for obtaining water.20 Lungfish obtain water from fat metabolism while they estivate in the mud for 1–2 years. Desert rodents, such as the sand rat (Psammomys obesus), have high body fat, which they use to generate water during times of need. Larger desert animals, such as the camel and oryx, also use metabolic water, and in the oryx, this may account for 24% of its overall water needs.21
Some obligatory water loss by the lung occurs during fat metabolism because of the need to excrete carbon dioxide that may counter the gain of water provided during fat metabolism.22 However, animals like camels have developed techniques to reduce water loss from their airways and skin.12,13
Survival Mechanisms Associated with Dehydration
Because fat and glycogen act as storage for water, it is not surprising that survival mechanisms associated with starvation and water shortage have overlapping metabolic pathways. Here, we discuss some of these mechanisms.
Vasopressin: The Survival Hormone
Vasopressin is an old hormone, with its predecessor, vasotocin, appearing 700 million years ago.23 Although vasopressin reduces urinary water losses in response to a loss of intracellular or extracellular fluid, it has other actions that may aid water conservation.24 For example, vasopressin may also reduce nonrenal water loss25,26 by acting via V2 receptors in the lungs.27 Vasopressin also reduces fever because of antipyretic effects that reduce water loss.24 In frogs, vasotocin reduces water loss through the skin and stimulates water reabsorption from the bladder when frogs are exposed to dehydrating stimuli.11,23 In humans, however, the reduction of sweating in dehydrated individuals occurs via a vasopressin-independent mechanism.28,29
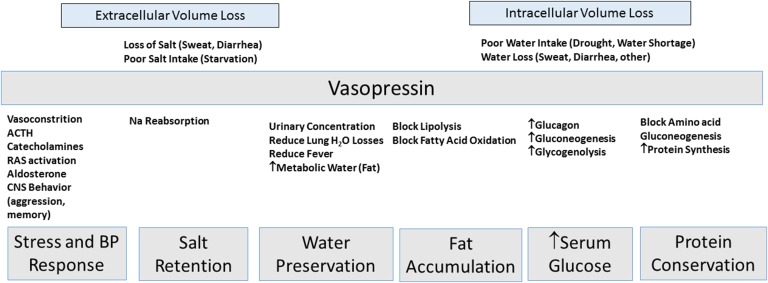
Vasopressin has other survival functions (Figure 1). Acute infusion of vasopressin increases serum glucose in humans,30 likely by stimulating glycogenolysis and gluconeogenesis.31–34 Vasopressin stimulates glucagon release from islet cells.34 Vasopressin stimulates sodium reabsorption in the cortical and outer medullary collecting ducts.24 Vasopressin also stimulates protein synthesis, cell proliferation, and cell hypertrophy in vitro.35
Figure 1.
Vasopressin, the ultimate survival hormone. Vasopressin may have originated as a survival hormone for situations where the organism suffered from either extracellular volume or intracellular volume loss. The effects of vasopressin include actions much greater than simply preventing the loss of water but also, include generating a stress response, increasing BP, stimulating protein synthesis, stimulating fat accumulation, and maintaining elevated serum glucose (insulin resistance) to provide energy to the brain. ACTH, adrenocorticotrophic hormone; CNS, central nervous system; RAS, renin angiotensin system.
Vasopressin also may stimulate fat accumulation. Vasopressin blocks fat oxidation31,32 and enhances fat accumulation by blocking lipolysis in fasting animals.32,35,36 In fasting animals, vasopressin reduces ketosis but increases glucose levels.32 Vasopressin enhances insulin resistance and fatty liver accumulation in the obese Zucker rat.37
Vasopressin secretion is associated with stress responses that improve chances for survival. For example, vasopressin acutely increases BP and induces vascular constriction via the V1a receptor.35 Vasopressin stimulates adrenocorticotrophic hormone release from the anterior pituitary via the V1b receptor38,39 and catecholamine release from the adrenal medulla, where both V1a and V1b receptors are expressed.40 Vasopressin activates the renin-angiotensin system35 and stimulates aldosterone release.35 These stress responses are associated with vasopressin–mediated behavioral changes that include aggression, anxiety, impulsivity, and memory.36,41,42
Fructose: The Survival Nutrient
The effect of vasopressin to stimulate fat accumulation (by blocking fat oxidation), increase blood glucose (via gluconeogenesis), increase BP, and stimulate stress responses is reminiscent of the effects of fructose.43 It is of interest that fructose, but not glucose, stimulates vasopressin release in humans.44,45 We recently showed that orally administered fructose augments circulating vasopressin levels (as determined by measuring copeptin, a validated biomarker for vasopressin46) and urinary concentration in dehydrated rats.47 Fructose also stimulates urinary sodium reabsorption48 and reduces urea excretion49 similar to vasopressin.
Dehydration also results in endogenous fructose production because of activation of the aldose reductase-sorbitol dehydrogenase (polyol) pathway.50 We found that acutely dehydrated mice show a blunted vasopressin response if endogenous fructose metabolism is abolished (by using fructokinase knockout mice) (C. Roncal-Jimenez et al., unpublished data). These studies emphasize a strong relationship between fructose and vasopressin.
We speculate that fructose may be a primary nutrient for survival, especially under conditions of reduced food or water availability. Indeed, the administration of fructose to fasting humans increases glucose levels (likely from the metabolism of fructose itself) and reduces ketosis, amino acid–induced gluconeogenesis, urinary nitrogen (ammonia and urea) excretion, and sodium excretion.49 These are the same effects observed when vasopressin is given to starving animals.32 Thus, fructose and vasopressin may act similarly to preserve water, salt, and fat while maintaining glucose levels as a source of energy for brain function. Viewed this way, the action of vasopressin to stimulate fat accumulation provides a mechanism for not only storing water but also, providing energy during times of food or water deprivation.
Uric Acid: The Metabolic Danger Signal
As discussed earlier, birds and reptiles excrete uric acid as their primary means for excreting nitrogen to minimize water loss.10 Despite uric acid being a potent extracellular antioxidant,51 the uric acid generated during fructose metabolism stimulates hepatic fat accumulation (by blocking fat oxidation) and gluconeogenesis, increases BP, and stimulates impulsivity in laboratory animals.52–56 In rodents, uric acid potentiates the effect of fructose to stimulate hepatic fat accumulation and gluconeogenesis.57,58 These data suggest that uric acid may also be a metabolic survival factor, which is consistent with observations that serum uric acid increases with both dehydration and starvation.59
Interestingly, the rise of uric acid that occurs with protein degradation and amino acid–induced gluconeogenesis is reversed with fructose in fasting humans.49 Likewise, although vasopressin reduces uric acid excretion in healthy subjects,60 in the syndrome of inappropriate antidiuretic hormone, serum uric acid is low, and urinary uric acid excretion is high.61,62 Thus, whether uric acid has a role in water handling remains unclear and deserves additional studies.
Dehydration in Humans
Dehydration in the Hot Environment
Humans have obligate daily water losses from the lungs (250 ml/d) and urine (350–500 ml/d). In hot conditions, water losses from sweat may increase to 3–4 L/h and 8 L over a 24-hour period.15 Subjects working in hot tropical environments acclimate by producing a higher sweat rate that is lower in sodium, thereby resulting in less increase in core temperature, and also, they have higher plasma volume, less oxygen utilization, and less lactate accumulation.63 However, this adaptation may result in greater water loss and increased risk for hyperosmolarity.63 To help counter water loss from sweat, subjects living in the tropics tend to have slightly higher core temperatures during the day, with a greater fall at night, showing a similar trend as that observed in camels.64
Dehydration develops easily in the hot environment. An increase in serum osmolarity of 10 mosM/kg occurs within 40 minutes of exercise in the heat65 or with water deprivation for 24 hours.66,67 The Tsimane Indians of the Amazon show evidence of dehydration in 40% of subjects, especially on days with high temperatures and strenuous physical activity, despite mean water intake of 6 L daily.68 Chronic recurrent dehydration is also common in sugar cane workers in Central America who work under hot and humid conditions.69–71 After dehydration occurs, mental and physical performance worsen,65,72,73 total sweat volume decreases,74 and relative water content of sweat decreases (reflected by higher sodium concentration).63 Energy intake also decreases, which results in a reduction in obligate osmoles required for excretion.67 Ultimately, confusion, seizures, and coma may develop.
Diseases Favored by Water Shortage and Climate Change
Heat Stroke and Acute Mortality
Heat waves increase the risk for heat stroke and heat-associated mortality.75–77 In 2015, >1400 deaths occurred from heat stroke in Andhra Pradesh, India.78 In a case-control study performed in Arizona, the risk for heat-associated death was 3.5-fold among agricultural workers and 2.3-fold in construction workers, and it was disproportionately higher in Native American and Hispanic American men.77
Kidney Stones
The risk of kidney stones is increased in subjects with low urine output because of the effect of urinary concentration to increase concentrations of poorly soluble constituents, like calcium oxalate and uric acid. There is a relationship between mean daily temperature and risk for kidney stones, especially when temperatures exceed 30°C.79
CKD
Heat stress doubles the risk for developing CKD among those working in hot environments.80 Recently, epidemics of CKD have been reported in India, Sri Lanka, Mexico, and Central America.81–86 The CKD observed in these areas is not because of the classic causes of CKD, such as diabetes or hypertension, but rather, seems to be a type of chronic tubulointerstitial disease.87,88 Although the roles of toxins and infections have not been completely ruled out, common risk factors for each of the epidemics are hot temperatures and recurrent dehydration that can be linked with climate change.89
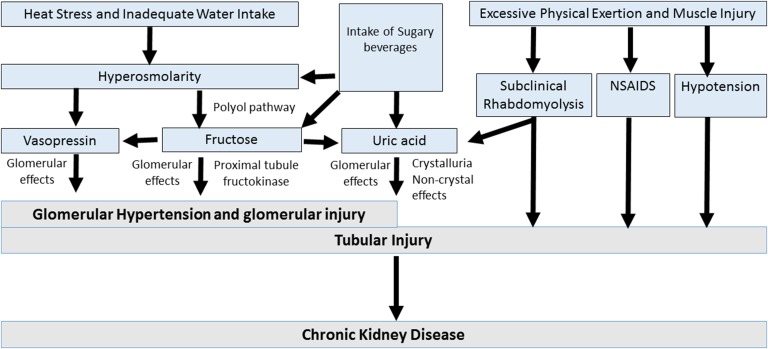
Although acute dehydration is known to cause a transient reduction in kidney function without permanent renal damage, chronic recurrent heat–induced dehydration causes CKD in mice.50 The mechanism for CKD may involve hyperosmolarity-induced alteration of fructose and vasopressin metabolism (Figure 2). The rise in serum osmolarity stimulates vasopressin and increases intrarenal fructose generation via activation of the aldose reductase pathway.50 The metabolism of fructose within the proximal tubule results in local oxidative stress, inflammation, and uric acid generation, which induce local injury.90 Experimental studies also document a role for vasopressin in CKD.91 An increase in serum and urinary uric acid also occurs with heat and exercise, which increases the risk for urinary urate crystal formation.89,92
Figure 2.
Potential mechanisms involved in heat stress–associated CKD. CKD occurring in response to recurrent dehydration may involve a variety of mechanisms. Central to the loss of water is the development of hyperosmolarity, which stimulates the release of vasopressin, and the generation of fructose in the kidney from activation of the polyol (aldose reductase-sorbitol dehydrogenase) pathway.50 Vasopressin acts to increase glomerular hydrostatic pressure and increases the risk for progression of kidney disease.91,123,124 Endogenous fructose production is also metabolized by fructokinase in the proximal tubule, resulting in tubular injury and the release of oxidants, uric acid, and chemokines.90 Fructose may also increase vasopressin levels,125 and likewise, rehydration with sugar beverages may provide additional fructose, with an amplification of the vasopressin and uric acid levels.47 Furthermore, other factors that may be involved include low–grade muscle injury associated with excessive physical exertion leading to subclinical rhabdomyolysis,126 an increased risk for nonsteroidal anti–inflammatory drug (NSAID) use, and rarely, hypotension from volume depletion. Volume depletion may also be associated with activation of the renin-angiotensin system and development of hypokalemia, which may also play a role in kidney disease.
The possibility that dehydration may be a risk factor for CKD should also be considered. Low urine output93,94 and high urine osmolarity95 predict risk for the progression of CKD. Low intake of plain water increases the risk for CKD, whereas intake of other beverages does not show the same effect.94 Likewise, high vasopressin levels (indicated by high plasma copeptin levels) are associated with increased risk for microalbuminuria.96,97 Currently, there is a randomized trial to determine if supplementation with water to increase urinary output to >3 L/d slows the progression of CKD.98
Obesity, Metabolic Syndrome, and Hypertension
As mentioned, fructose and vasopressin show similar effects to increase fat stores and conserve water (Figure 3). This suggests that transient elevations in serum osmolarity because of either a relative water deficit or a high-sodium diet might be associated with increased risk for obesity and metabolic syndrome. Evidence supporting hyperosmolarity as a risk factor for obesity and metabolic syndrome is increasing. First, obese subjects have elevated plasma sodium and plasma osmolarity.99 Second, plasma hypertonicity predicts the development of diabetes in subjects >70 years old.100 Third, subjects with metabolic syndrome and insulin resistance have elevated plasma copeptin levels.101–104 Fourth, elevated levels of plasma copeptin predict development of diabetes96,105 and obesity.96
Figure 3.
Modern diseases engaged by water shortage and global climate change. Although the vasopressin system was developed as a survival mechanism when the host lost either intracellular or extracellular volume, in modern society, it may, instead, be associated with the development of diseases. Climate change and water shortage triggered with diets high in fructose (sugar), salt, and umami foods may lead to overactivation of this pathway. The metabolic effects of high osmolarity may include the syndrome of obesity, metabolic syndrome, and diabetes. In contrast, recurrent dehydration and highly concentrated and acidic urine may increase the risk for crystallization of uric acid and chronic kidney damage.
Although inadequate hydration and hot temperatures facilitate hyperosmolarity, it could also be enhanced by a high intake of salt with a less than adequate intake of water. In this regard, low water intake predicts development of insulin resistance,106 whereas increasing water intake is associated with weight loss, at least in overweight subjects.107 High salt intake is also associated with obesity, metabolic syndrome, and diabetes108–112 and predicts these conditions independent of energy intake or intake of sugary beverages.112–114 Thus, the development of obesity is not simply because of greater intake of soft drinks consequent to salt-induced thirst, which has been suggested.115 Furthermore, subjects given a high-salt diet show reduced insulin sensitivity within 5 days.116 Conversely, hyperinsulinemia promotes distal tubular sodium retention.117
Hyperosmolarity likely increases the risk for obesity and metabolic syndrome by stimulating vasopressin (Figure 3). Indeed, water loading reduced fat content of the liver of obese Zucker rats coupled with a reduction in vasopressin levels.37 However, hyperosmolarity is likely acting via another pathway as well. We recently found that mice fed a high-salt diet for 5 months develop leptin resistance, obesity, and metabolic syndrome (M.A. Lanaspa et al., unpublished data). The mechanism was shown to be caused by hyperosmolarity-mediated upregulation of aldose reductase in the liver, which resulted in endogenous fructose generation via the polyol pathway. Importantly, mice unable to metabolize fructose because of genetic deletion of fructokinase were protected from developing metabolic syndrome and fatty liver, despite ingesting equal amounts of salt.
Hypertonicity also regulates BP and the immune system.118–120 Specifically, a high-salt diet activates a transcription factor, NF of activated T cells 5, that stimulates macrophages to sequester salt in the skin, thereby modulating BP. Salt-induced hypertonicity also activates T helper 17 lymphocytes involved in host defense.121
Summary
In summary, climate change and low water intake are increasing our risk for dehydration–associated kidney diseases, including kidney stones, heat stroke, and CKD. Hyperosmolarity, especially in a sedentary environment, may also increase the risk for obesity and diabetes. We speculate that hyperosmolarity triggers factors originally designed to aid survival by increasing fat stores and conserving water, such as vasopressin, endogenously produced fructose, and uric acid. Overactivation of these pathways may act in synergy with Western diets high in fructose-containing sugars, salt, and purine-rich foods to accelerate the obesity and diabetes epidemics (Figure 2).43,50,91,122 Similarly, recurrent dehydration and heat stress may also be playing a role in causing CKD via similar pathways.89
More studies are needed to investigate the effect of climate change and water shortage on kidney disease and diabetes and especially, the role of vasopressin, fructose, and uric acid. Intervention studies to improve worksite conditions and hydration among agricultural workers in tropical communities and other at–risk groups are recommended. Recognizing the importance of the kidney in climate change–associated disease will prepare nephrologists to face an increase in heat stress–associated kidney diseases predicted to occur in the next decades.
Disclosures
R.J.J. has several patents and patent applications related to lowering uric acid or blocking fructose metabolism in the treatment of metabolic diseases. R.J.J. and M.A.L. are members of a startup company, Colorado Research Partners LLC (Aurora, CO), that is trying to develop inhibitors of fructose metabolism. R.J.J. also has some shares with XORT Therapeutics (Calgary, AB, Canada), which is a startup company developing novel xanthine oxidase inhibitors. R.J.J. is on the Scientific Board of Amway as well.
Acknowledgments
This work was supported by Department of Defense grant PR130106 and National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) of the National Institutes of Health (NIH) grant R01DK108408-01A1. T.J. is funded by the NIH training grant NIDDK 5T32DK007446-34.
This paper is considered a contribution by the University of Colorado Climate Change and Health consortium.
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.NASA: Global Temperatures. Earth Observatory. Available at: http://earthobservatory.nasa.gov/Features/WorldOfChange/decadaltemp.php. Accessed April 20, 2016
- 2.Hansen J, Ruedy R, Sato M, Lo K: Global surface temperature change. Rev Geophys 48: RG4004, 2010 [Google Scholar]
- 3.Fischer EM, Knutti R: Anthropogenic contribution to global occurrence of heavy precipitation and high temperature extremes. Nat Clim Chang 5: 560–565, 2015 [Google Scholar]
- 4.Kummu M, Ward PJ, de Moel H, Varis O: Is physical water scarcity a new phenomenon? Global assessment of water shortage over the last two millennia. Environ Res Lett 5: 1–10, 2010
- 5.Vörösmarty CJ, Green P, Salisbury J, Lammers RB: Global water resources: Vulnerability from climate change and population growth. Science 289: 284–288, 2000 [DOI] [PubMed] [Google Scholar]
- 6.Glaser J, Lemery J, Rajagopalan B, Diaz HF, Garcia-Trabanino R, Taduri G, Madero M, Amarasinghe MD, Abraham G, Anutrakulchai S, Jha V, Stenvinkel P, Roncal-Jiménez C, Lanaspa M, Correa-Rotter R, Sheik-Hamad D, Burdmann EA, Andres-Hernando A, Milagres T, Weiss I, Kanbay M, Wesseling C, Sanchez-Lozada LG, Johnson RJ: Climate Change and the Emergent Epidemic of Chronic Kidney Disease from Heat Stress in Rural Communities: The Case for Heat Stress Nephropathy [published online ahead of print May 5, 2016]. Clin J Am Soc Nephrol doi:10.2215/CJN13841215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Smith HW: From Fish to Philosopher, Boston, Little, Brown and Co, 1953 [Google Scholar]
- 8.Cartledge VA, Withers PC, Bradshaw SD: Water balance and arginine vasotocin in the cocooning frog Cyclorana platycephala (hylidae). Physiol Biochem Zool 81: 43–53, 2008 [DOI] [PubMed] [Google Scholar]
- 9.Jørgensen CB: Role of urinary and cloacal bladders in chelonian water economy: Historical and comparative perspectives. Biol Rev Camb Philos Soc 73: 347–366, 1998 [DOI] [PubMed] [Google Scholar]
- 10.Wright PA: Nitrogen excretion: Three end products, many physiological roles. J Exp Biol 198: 273–281, 1995 [DOI] [PubMed] [Google Scholar]
- 11.Jørgensen CB: 200 years of amphibian water economy: From Robert Townson to the present. Biol Rev Camb Philos Soc 72: 153–237, 1997 [DOI] [PubMed] [Google Scholar]
- 12.Schmidt-Nielsen K, Schmidt-Nielsen B, Jarnum SA, Houpt TR: Body temperature of the camel and its relation to water economy. Am J Physiol 188: 103–112, 1957 [DOI] [PubMed] [Google Scholar]
- 13.Schmidt-Nielsen K: Countercurrent systems in animals. Sci Am 244: 118–128, 1981 [DOI] [PubMed] [Google Scholar]
- 14.Warda M, Prince A, Kim HK, Khafaga N, Scholkamy T, Linhardt RJ, Jin H: Proteomics of old world camelid (Camelus dromedarius): Better understanding the interplay between homeostasis and desert environment. J Adv Res 5: 219–242, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.European Food Safety Authority (EFSA) Panel on Dietetic Products, Nutrition, and Allergies (NDA): scientific opinion on dietary reference values for water. EFSA J 8(3): 1–48, 2010
- 16.Meerman R, Brown AJ: When somebody loses weight, where does the fat go? BMJ 349: g7257, 2014 [DOI] [PubMed] [Google Scholar]
- 17.Mellanby K: Metabolic water and dessication. Nature 150:21, 1942 [Google Scholar]
- 18.Olsson KE, Saltin B: Variation in total body water with muscle glycogen changes in man. Acta Physiol Scand 80: 11–18, 1970 [DOI] [PubMed] [Google Scholar]
- 19.Kreitzman SN, Coxon AY, Szaz KF: Glycogen storage: Illusions of easy weight loss, excessive weight regain, and distortions in estimates of body composition. Am J Clin Nutr 56[Suppl]: 292S–293S, 1992 [DOI] [PubMed] [Google Scholar]
- 20.Ortiz RM: Osmoregulation in marine mammals. J Exp Biol 204: 1831–1844, 2001 [DOI] [PubMed] [Google Scholar]
- 21.Williams JB, Ostrowski S, Bedin E, Ismail K: Seasonal variation in energy expenditure, water flux and food consumption of Arabian oryx Oryx leucoryx. J Exp Biol 204: 2301–2311, 2001 [DOI] [PubMed] [Google Scholar]
- 22.Mellanby K: Human water requirements. BMJ 1: 774, 1947 [PMC free article] [PubMed] [Google Scholar]
- 23.Juul KV: The evolutionary origin of the vasopressin/V2-type receptor/aquaporin axis and the urine-concentrating mechanism. Endocrine 42: 63–68, 2012 [DOI] [PubMed] [Google Scholar]
- 24.Bankir L: Antidiuretic action of vasopressin: Quantitative aspects and interaction between V1a and V2 receptor-mediated effects. Cardiovasc Res 51: 372–390, 2001 [DOI] [PubMed] [Google Scholar]
- 25.Pouzet B, Serradeil-Le Gal C, Bouby N, Maffrand JP, Le Fur G, Bankir L: Selective blockade of vasopressin V2 receptors reveals significant V2-mediated water reabsorption in Brattleboro rats with diabetes insipidus. Nephrol Dial Transplant 16: 725–734, 2001 [DOI] [PubMed] [Google Scholar]
- 26.Nicco C, Wittner M, DiStefano A, Jounier S, Bankir L, Bouby N: Chronic exposure to vasopressin upregulates ENaC and sodium transport in the rat renal collecting duct and lung. Hypertension 38: 1143–1149, 2001 [DOI] [PubMed] [Google Scholar]
- 27.Fay MJ, Du J, Yu X, North WG: Evidence for expression of vasopressin V2 receptor mRNA in human lung. Peptides 17: 477–481, 1996 [DOI] [PubMed] [Google Scholar]
- 28.Hew-Butler T, Hummel J, Rider BC, Verbalis JG: Characterization of the effects of the vasopressin V2 receptor on sweating, fluid balance, and performance during exercise. Am J Physiol Regul Integr Comp Physiol 307: R366–R375, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hew-Butler T, Noakes TD, Soldin SJ, Verbalis JG: Acute changes in arginine vasopressin, sweat, urine and serum sodium concentrations in exercising humans: Does a coordinated homeostatic relationship exist? Br J Sports Med 44: 710–715, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spruce BA, McCulloch AJ, Burd J, Orskov H, Heaton A, Baylis PH, Alberti KG: The effect of vasopressin infusion on glucose metabolism in man. Clin Endocrinol (Oxf) 22: 463–468, 1985 [DOI] [PubMed] [Google Scholar]
- 31.Hems DA, Whitton PD: Stimulation by vasopressin of glycogen breakdown and gluconeogenesis in the perfused rat liver. Biochem J 136: 705–709, 1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rofe AM, Williamson DH: Metabolic effects of vasopressin infusion in the starved rat. Reversal of ketonaemia. Biochem J 212: 231–239, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Fujiwara Y, Hiroyama M, Sanbe A, Aoyagi T, Birumachi J, Yamauchi J, Tsujimoto G, Tanoue A: Insulin hypersensitivity in mice lacking the V1b vasopressin receptor. J Physiol 584: 235–244, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Fujiwara Y, Hiroyama M, Sanbe A, Yamauchi J, Tsujimoto G, Tanoue A: Mutual regulation of vasopressin- and oxytocin-induced glucagon secretion in V1b vasopressin receptor knockout mice. J Endocrinol 192: 361–369, 2007 [DOI] [PubMed] [Google Scholar]
- 35.Koshimizu TA, Nakamura K, Egashira N, Hiroyama M, Nonoguchi H, Tanoue A: Vasopressin V1a and V1b receptors: From molecules to physiological systems. Physiol Rev 92: 1813–1864, 2012 [DOI] [PubMed] [Google Scholar]
- 36.Rofe AM, Williamson DH: Mechanism for the ‘anti-lipolytic’ action of vasopressin in the starved rat. Biochem J 212: 899–902, 1983 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Taveau C, Chollet C, Waeckel L, Desposito D, Bichet DG, Arthus MF, Magnan C, Philippe E, Paradis V, Foufelle F, Hainault I, Enhorning S, Velho G, Roussel R, Bankir L, Melander O, Bouby N: Vasopressin and hydration play a major role in the development of glucose intolerance and hepatic steatosis in obese rats. Diabetologia 58: 1081–1090, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Sugimoto T, Saito M, Mochizuki S, Watanabe Y, Hashimoto S, Kawashima H: Molecular cloning and functional expression of a cDNA encoding the human V1b vasopressin receptor. J Biol Chem 269: 27088–27092, 1994 [PubMed] [Google Scholar]
- 39.Baertschi AJ, Friedli M: A novel type of vasopressin receptor on anterior pituitary corticotrophs? Endocrinology 116: 499–502, 1985 [DOI] [PubMed] [Google Scholar]
- 40.Itoh S, Yamada S, Mori T, Miwa T, Tottori K, Uwahodo Y, Yamamura Y, Fukuda M, Yamamoto K, Tanoue A, Tsujimoto G: Attenuated stress-induced catecholamine release in mice lacking the vasopressin V1b receptor. Am J Physiol Endocrinol Metab 291: E147–E151, 2006 [DOI] [PubMed] [Google Scholar]
- 41.Betley JN, Xu S, Cao ZF, Gong R, Magnus CJ, Yu Y, Sternson SM: Neurons for hunger and thirst transmit a negative-valence teaching signal. Nature 521: 180–185, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Caldwell HK, Lee HJ, Macbeth AH, Young WS 3rd: Vasopressin: Behavioral roles of an “original” neuropeptide. Prog Neurobiol 84: 1–24, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Johnson RJ, Nakagawa T, Sanchez-Lozada LG, Shafiu M, Sundaram S, Le M, Ishimoto T, Sautin YY, Lanaspa MA: Sugar, uric acid, and the etiology of diabetes and obesity. Diabetes 62: 3307–3315, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wolf JP, Nguyen NU, Dumoulin G, Berthelay S: Influence of hypertonic monosaccharide infusions on the release of plasma arginine vasopressin in normal humans. Horm Metab Res 24: 379–383, 1992 [DOI] [PubMed] [Google Scholar]
- 45.Zerbe RL, Robertson GL: Osmoregulation of thirst and vasopressin secretion in human subjects: Effect of various solutes. Am J Physiol 244: E607–E614, 1983 [DOI] [PubMed] [Google Scholar]
- 46.Roussel R, Fezeu L, Marre M, Velho G, Fumeron F, Jungers P, Lantieri O, Balkau B, Bouby N, Bankir L, Bichet DG: Comparison between copeptin and vasopressin in a population from the community and in people with chronic kidney disease. J Clin Endocrinol Metab 99: 4656–4663, 2014 [DOI] [PubMed] [Google Scholar]
- 47.García-Arroyo FE, Cristóbal M, Arellano-Buendía AS, Osorio H, Tapia E, Soto V, Madero M, Lanaspa MA, Roncal-Jimenez CA, Bankir L, Johnson RJ, Sanchez-Lozada LG: Rehydration with soft drink-like beverages exacerbates dehydration and worsens dehydration-associated renal injury [published online ahead of print April 6, 2016]. Am J Physiol Regul Integr Comp Physiol 10.1152/ajpregu.00354.2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Cabral PD, Hong NJ, Hye Khan MA, Ortiz PA, Beierwaltes WH, Imig JD, Garvin JL: Fructose stimulates Na/H exchange activity and sensitizes the proximal tubule to angiotensin II. Hypertension 63: e68–e73, 2014 [DOI] [PubMed] [Google Scholar]
- 49.Gelfand RA, Sherwin RS: Nitrogen conservation in starvation revisited: Protein sparing with intravenous fructose. Metabolism 35: 37–44, 1986 [DOI] [PubMed] [Google Scholar]
- 50.Roncal Jimenez CA, Ishimoto T, Lanaspa MA, Rivard CJ, Nakagawa T, Ejaz AA, Cicerchi C, Inaba S, Le M, Miyazaki M, Glaser J, Correa-Rotter R, González MA, Aragón A, Wesseling C, Sánchez-Lozada LG, Johnson RJ: Fructokinase activity mediates dehydration-induced renal injury. Kidney Int 86: 294–302, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ames BN, Cathcart R, Schwiers E, Hochstein P: Uric acid provides an antioxidant defense in humans against oxidant- and radical-caused aging and cancer: A hypothesis. Proc Natl Acad Sci U S A 78: 6858–6862, 1981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Lanaspa MA, Cicerchi C, Garcia G, Li N, Roncal-Jimenez CA, Rivard CJ, Hunter B, Andrés-Hernando A, Ishimoto T, Sánchez-Lozada LG, Thomas J, Hodges RS, Mant CT, Johnson RJ: Counteracting roles of AMP deaminase and AMP kinase in the development of fatty liver. PLoS One 7: e48801, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Lanaspa MA, Sanchez-Lozada LG, Choi YJ, Cicerchi C, Kanbay M, Roncal-Jimenez CA, Ishimoto T, Li N, Marek G, Duranay M, Schreiner G, Rodriguez-Iturbe B, Nakagawa T, Kang DH, Sautin YY, Johnson RJ: Uric acid induces hepatic steatosis by generation of mitochondrial oxidative stress: Potential role in fructose-dependent and -independent fatty liver. J Biol Chem 287: 40732–40744, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Cicerchi C, Li N, Kratzer J, Garcia G, Roncal-Jimenez CA, Tanabe K, Hunter B, Rivard CJ, Sautin YY, Gaucher EA, Johnson RJ, Lanaspa MA: Uric acid-dependent inhibition of AMP kinase induces hepatic glucose production in diabetes and starvation: Evolutionary implications of the uricase loss in hominids. FASEB J 28: 3339–3350, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Mazzali M, Hughes J, Kim YG, Jefferson JA, Kang DH, Gordon KL, Lan HY, Kivlighn S, Johnson RJ: Elevated uric acid increases blood pressure in the rat by a novel crystal-independent mechanism. Hypertension 38: 1101–1106, 2001 [DOI] [PubMed] [Google Scholar]
- 56.Sutin AR, Cutler RG, Camandola S, Uda M, Feldman NH, Cucca F, Zonderman AB, Mattson MP, Ferrucci L, Schlessinger D, Terracciano A: Impulsivity is associated with uric acid: Evidence from humans and mice. Biol Psychiatry 75: 31–37, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Tapia E, Cristóbal M, García-Arroyo FE, Soto V, Monroy-Sánchez F, Pacheco U, Lanaspa MA, Roncal-Jiménez CA, Cruz-Robles D, Ishimoto T, Madero M, Johnson RJ, Sánchez-Lozada LG: Synergistic effect of uricase blockade plus physiological amounts of fructose-glucose on glomerular hypertension and oxidative stress in rats. Am J Physiol Renal Physiol 304: F727–F736, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Kratzer JT, Lanaspa MA, Murphy MN, Cicerchi C, Graves CL, Tipton PA, Ortlund EA, Johnson RJ, Gaucher EA: Evolutionary history and metabolic insights of ancient mammalian uricases. Proc Natl Acad Sci U S A 111: 3763–3768, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Cray C, Stremme DW, Arheart KL: Postprandial biochemistry changes in penguins (Spheniscus demersus) including hyperuricemia. J Zoo Wildl Med 41: 325–326, 2010 [DOI] [PubMed] [Google Scholar]
- 60.Meisel A, Diamond H: Effect of vasopressin on uric acid excretion: Evidence for distal nephron reabsorption of urate in man. Clin Sci Mol Med 51: 33–40, 1976 [DOI] [PubMed] [Google Scholar]
- 61.Beck LH: Hypouricemia in the syndrome of inappropriate secretion of antidiuretic hormone. N Engl J Med 301: 528–530, 1979 [DOI] [PubMed] [Google Scholar]
- 62.Decaux G, Dumont I, Waterlot Y, Hanson B: Mechanisms of hypouricemia in the syndrome of inappropriate secretion of antidiuretic hormone. Nephron 39: 164–168, 1985 [DOI] [PubMed] [Google Scholar]
- 63.Saat M, Sirisinghe RG, Singh R, Tochihara Y: Effects of short-term exercise in the heat on thermoregulation, blood parameters, sweat secretion and sweat composition of tropic-dwelling subjects. J Physiol Anthropol Appl Human Sci 24: 541–549, 2005 [DOI] [PubMed] [Google Scholar]
- 64.Nguyen M, Tokura H: Observations on normal body temperatures in Vietnamese and Japanese in Vietnam. J Physiol Anthropol Appl Human Sci 21: 59–65, 2002 [DOI] [PubMed] [Google Scholar]
- 65.Edwards AM, Mann ME, Marfell-Jones MJ, Rankin DM, Noakes TD, Shillington DP: Influence of moderate dehydration on soccer performance: Physiological responses to 45 min of outdoor match-play and the immediate subsequent performance of sport-specific and mental concentration tests. Br J Sports Med 41: 385–391, 2007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Geelen G, Keil LC, Kravik SE, Wade CE, Thrasher TN, Barnes PR, Pyka G, Nesvig C, Greenleaf JE: Inhibition of plasma vasopressin after drinking in dehydrated humans. Am J Physiol 247: R968–R971, 1984 [DOI] [PubMed] [Google Scholar]
- 67.Shirreffs SM, Merson SJ, Fraser SM, Archer DT: The effects of fluid restriction on hydration status and subjective feelings in man. Br J Nutr 91: 951–958, 2004 [DOI] [PubMed] [Google Scholar]
- 68.Rosinger A: Heat and hydration status: Predictors of repeated measures of urine specific gravity among Tsimane’ adults in the Bolivian Amazon. Am J Phys Anthropol 158: 696–707, 2015 [DOI] [PubMed] [Google Scholar]
- 69.García-Trabanino R, Jarquín E, Wesseling C, Johnson RJ, González-Quiroz M, Weiss I, Glaser J, José Vindell J, Stockfelt L, Roncal C, Harra T, Barregard L: Heat stress, dehydration, and kidney function in sugarcane cutters in El Salvador--A cross-shift study of workers at risk of Mesoamerican nephropathy. Environ Res 142: 746–755, 2015 [DOI] [PubMed] [Google Scholar]
- 70.Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, Riefkohl A, Scammell MK, López-Pilarte D, Sánchez JM, Parikh CR, McClean MD: Changes in kidney function among Nicaraguan sugarcane workers. Int J Occup Environ Health 21: 241–250, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Laws RL, Brooks DR, Amador JJ, Weiner DE, Kaufman JS, Ramírez-Rubio O, Riefkohl A, Scammell MK, López-Pilarte D, Sánchez JM, Parikh CR, McClean MD: Biomarkers of kidney injury among Nicaraguan sugarcane workers. Am J Kidney Dis 67: 209–217, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cade JR, Free HJ, De Quesada AM, Shires DL, Roby L: Changes in body fluid composition and volume during vigorous exercise by athletes. J Sports Med Phys Fitness 11: 172–178, 1971 [PubMed] [Google Scholar]
- 73.Lieberman HR: Hydration and cognition: A critical review and recommendations for future research. J Am Coll Nutr 26[Suppl]: 555S–561S, 2007 [DOI] [PubMed] [Google Scholar]
- 74.Bourque CW: Central mechanisms of osmosensation and systemic osmoregulation. Nat Rev Neurosci 9: 519–531, 2008 [DOI] [PubMed] [Google Scholar]
- 75.Goforth CW, Kazman JB: Exertional heat stroke in navy and marine personnel: A hot topic. Crit Care Nurse 35: 52–59, 2015 [DOI] [PubMed] [Google Scholar]
- 76.Mohanaselvan A, Bhaskar E: Mortality from non-exertional heat stroke still high in India. Int J Occup Environ Med 5: 222–224, 2014 [PMC free article] [PubMed] [Google Scholar]
- 77.Petitti DB, Harlan SL, Chowell-Puente G, Ruddell D: Occupation and environmental heat-associated deaths in Maricopa county, Arizona: A case-control study. PLoS One 8: e62596, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dearden L: Karachi heat wave: Death toll tops 1,000 as government and electricity company trade blame. The Independent 2015. Available at: http://www.independent.co.uk/news/world/asia/pakistan-heatwave-death-toll-tops-1000-as-government-and-electricity-company-trade-blame-10344719.html. Accessed April 20, 2016 [Google Scholar]
- 79.Tasian GE, Pulido JE, Gasparrini A, Saigal CS, Horton BP, Landis JR, Madison R, Keren R; Urologic Diseases in America Project : Daily mean temperature and clinical kidney stone presentation in five U.S. metropolitan areas: A time-series analysis. Environ Health Perspect 122: 1081–1087, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Tawatsupa B, Lim LL, Kjellstrom T, Seubsman SA, Sleigh A; Thai Cohort Study Team : Association between occupational heat stress and kidney disease among 37,816 workers in the Thai Cohort Study (TCS). J Epidemiol 22: 251–260, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Abraham G, Varughese S, Thandavan T, Iyengar A, Fernando E, Naqvi SAJ, Sheriff R, Ur-Rashid H, Gopalakrishnan N, Kafle RK: Chronic kidney disease hotspots in developing countries in South Asia. Clin Kidney J 9: 135–141, 2016 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Siriwardhana EA, Perera PA, Sivakanesan R, Abeysekara T, Nugegoda DB, Jayaweera JA: Dehydration and malaria augment the risk of developing chronic kidney disease in Sri Lanka. Indian J Nephrol 25: 146–151, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Wanigasuriya K: Update on uncertain etiology of chronic kidney disease in Sri Lanka’s north-central dry zone. MEDICC Rev 16: 61–65, 2014 [DOI] [PubMed] [Google Scholar]
- 84.Consultores Ambientales Asociados: Insuficiencia Renal Crónica en Tierra Blanca Veracruz, 1998-2003, Mexico City, Mexico, 2007. Available at: http://www.greenpeace.org/mexico/Global/mexico/Docs/2013/resumen%20ejecut%20Tierra%20Blanca%202007.pdf. Accessed April 20, 2016 [Google Scholar]
- 85.Correa-Rotter R, Wesseling C, Johnson RJ: CKD of unknown origin in Central America: The case for a Mesoamerican nephropathy. Am J Kidney Dis 63: 506–520, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Wesseling C, Crowe J, Hogstedt C, Jakobsson K, Lucas R, Wegman D: Mesoamerican Nephropathy: Report from the First International Research Workshop on MeN, 2013. Available at: http://www.regionalnephropathy.org/wp-content/uploads/2013/04/Preprint-Technical-Report.pdf. Accessed December 15, 2015
- 87.Nanayakkara S, Komiya T, Ratnatunga N, Senevirathna ST, Harada KH, Hitomi T, Gobe G, Muso E, Abeysekera T, Koizumi A: Tubulointerstitial damage as the major pathological lesion in endemic chronic kidney disease among farmers in North Central Province of Sri Lanka. Environ Health Prev Med 17: 213–221, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Wijkström J, Leiva R, Elinder CG, Leiva S, Trujillo Z, Trujillo L, Söderberg M, Hultenby K, Wernerson A: Clinical and pathological characterization of Mesoamerican nephropathy: A new kidney disease in Central America. Am J Kidney Dis 62: 908–918, 2013 [DOI] [PubMed] [Google Scholar]
- 89.Roncal-Jimenez C, García-Trabanino R, Barregard L, Lanaspa MA, Wesseling C, Harra T, Aragón A, Grases F, Jarquin ER, González MA, Weiss I, Glaser J, Sánchez-Lozada LG, Johnson RJ: Heat stress nephropathy from exercise-induced uric acid crystalluria: A perspective on Mesoamerican nephropathy. Am J Kidney Dis 67: 20–30, 2016 [DOI] [PubMed] [Google Scholar]
- 90.Cirillo P, Gersch MS, Mu W, Scherer PM, Kim KM, Gesualdo L, Henderson GN, Johnson RJ, Sautin YY: Ketohexokinase-dependent metabolism of fructose induces proinflammatory mediators in proximal tubular cells. J Am Soc Nephrol 20: 545–553, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Bouby N, Bachmann S, Bichet D, Bankir L: Effect of water intake on the progression of chronic renal failure in the 5/6 nephrectomized rat. Am J Physiol 258: F973–F979, 1990 [DOI] [PubMed] [Google Scholar]
- 92.Knochel JP, Dotin LN, Hamburger RJ: Heat stress, exercise, and muscle injury: Effects on urate metabolism and renal function. Ann Intern Med 81: 321–328, 1974 [DOI] [PubMed] [Google Scholar]
- 93.Clark WF, Sontrop JM, Macnab JJ, Suri RS, Moist L, Salvadori M, Garg AX: Urine volume and change in estimated GFR in a community-based cohort study. Clin J Am Soc Nephrol 6: 2634–2641, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Sontrop JM, Dixon SN, Garg AX, Buendia-Jimenez I, Dohein O, Huang SH, Clark WF: Association between water intake, chronic kidney disease, and cardiovascular disease: A cross-sectional analysis of NHANES data. Am J Nephrol 37: 434–442, 2013 [DOI] [PubMed] [Google Scholar]
- 95.Plischke M, Kohl M, Bankir L, Shayganfar S, Handisurya A, Heinze G, Haas M: Urine osmolarity and risk of dialysis initiation in a chronic kidney disease cohort--a possible titration target? PLoS One 9: e93226, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Enhörning S, Bankir L, Bouby N, Struck J, Hedblad B, Persson M, Morgenthaler NG, Nilsson PM, Melander O: Copeptin, a marker of vasopressin, in abdominal obesity, diabetes and microalbuminuria: The prospective Malmö Diet and Cancer Study cardiovascular cohort. Int J Obes 37: 598–603, 2013 [DOI] [PubMed] [Google Scholar]
- 97.Meijer E, Bakker SJ, Halbesma N, de Jong PE, Struck J, Gansevoort RT: Copeptin, a surrogate marker of vasopressin, is associated with microalbuminuria in a large population cohort. Kidney Int 77: 29–36, 2010 [DOI] [PubMed] [Google Scholar]
- 98.Clark WF, Sontrop JM, Huang SH, Gallo K, Moist L, House AA, Weir MA, Garg AX: The chronic kidney disease Water Intake Trial (WIT): Results from the pilot randomised controlled trial. BMJ Open 3: e003666, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Stookey JD, Barclay D, Arieff A, Popkin BM: The altered fluid distribution in obesity may reflect plasma hypertonicity. Eur J Clin Nutr 61: 190–199, 2007 [DOI] [PubMed] [Google Scholar]
- 100.Stookey JD, Pieper CF, Cohen HJ: Hypertonic hyperglycemia progresses to diabetes faster than normotonic hyperglycemia. Eur J Epidemiol 19: 935–944, 2004 [DOI] [PubMed] [Google Scholar]
- 101.Asferg CL, Andersen UB, Linneberg A, Goetze JP, Jeppesen JL: Copeptin, a surrogate marker for arginine vasopressin secretion, is associated with higher glucose and insulin concentrations but not higher blood pressure in obese men. Diabet Med 31: 728–732, 2014 [DOI] [PubMed]
- 102.Saleem U, Khaleghi M, Morgenthaler NG, Bergmann A, Struck J, Mosley TH Jr., Kullo IJ: Plasma carboxy-terminal provasopressin (copeptin): A novel marker of insulin resistance and metabolic syndrome. J Clin Endocrinol Metab 94: 2558–2564, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Enhörning S, Struck J, Wirfält E, Hedblad B, Morgenthaler NG, Melander O: Plasma copeptin, a unifying factor behind the metabolic syndrome. J Clin Endocrinol Metab 96: E1065–E1072, 2011 [DOI] [PubMed] [Google Scholar]
- 104.Tenderenda-Banasiuk E, Wasilewska A, Filonowicz R, Jakubowska U, Waszkiewicz-Stojda M: Serum copeptin levels in adolescents with primary hypertension. Pediatr Nephrol 29: 423–429, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Enhörning S, Wang TJ, Nilsson PM, Almgren P, Hedblad B, Berglund G, Struck J, Morgenthaler NG, Bergmann A, Lindholm E, Groop L, Lyssenko V, Orho-Melander M, Newton-Cheh C, Melander O: Plasma copeptin and the risk of diabetes mellitus. Circulation 121: 2102–2108, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Roussel R, Fezeu L, Bouby N, Balkau B, Lantieri O, Alhenc-Gelas F, Marre M, Bankir L; D.E.S.I.R. Study Group : Low water intake and risk for new-onset hyperglycemia. Diabetes Care 34: 2551–2554, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Stookey JD, Constant F, Popkin BM, Gardner CD: Drinking water is associated with weight loss in overweight dieting women independent of diet and activity. Obesity (Silver Spring) 16: 2481–2488, 2008 [DOI] [PubMed] [Google Scholar]
- 108.Baudrand R, Campino C, Carvajal CA, Olivieri O, Guidi G, Faccini G, Vöhringer PA, Cerda J, Owen G, Kalergis AM, Fardella CE: High sodium intake is associated with increased glucocorticoid production, insulin resistance and metabolic syndrome. Clin Endocrinol (Oxf) 80: 677–684, 2014 [DOI] [PubMed] [Google Scholar]
- 109.Kim YC, Koo HS, Kim S, Chin HJ: Estimation of daily salt intake through a 24-hour urine collection in Pohang, Korea. J Korean Med Sci 29[Suppl 2]: S87–S90, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Hoffmann IS, Cubeddu LX: Salt and the metabolic syndrome. Nutr Metab Cardiovasc Dis 19: 123–128, 2009 [DOI] [PubMed] [Google Scholar]
- 111.Hulthén L, Aurell M, Klingberg S, Hallenberg E, Lorentzon M, Ohlsson C: Salt intake in young Swedish men. Public Health Nutr 13: 601–605, 2010 [DOI] [PubMed] [Google Scholar]
- 112.Libuda L, Kersting M, Alexy U: Consumption of dietary salt measured by urinary sodium excretion and its association with body weight status in healthy children and adolescents. Public Health Nutr 15: 433–441, 2012 [DOI] [PubMed] [Google Scholar]
- 113.Larsen SC, Ängquist L, Sørensen TI, Heitmann BL: 24h Urinary sodium excretion and subsequent change in weight, waist circumference and body composition. PLoS One 8: e69689, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Hu G, Jousilahti P, Peltonen M, Lindström J, Tuomilehto J: Urinary sodium and potassium excretion and the risk of type 2 diabetes: A prospective study in Finland. Diabetologia 48: 1477–1483, 2005 [DOI] [PubMed] [Google Scholar]
- 115.He FJ, Marrero NM, MacGregor GA: Salt intake is related to soft drink consumption in children and adolescents: A link to obesity? Hypertension 51: 629–634, 2008 [DOI] [PubMed] [Google Scholar]
- 116.Donovan DS, Solomon CG, Seely EW, Williams GH, Simonson DC: Effect of sodium intake on insulin sensitivity. Am J Physiol 264: E730–E734, 1993 [DOI] [PubMed] [Google Scholar]
- 117.Stenvinkel P, Bolinder J, Alvestrand A: Effects of insulin on renal haemodynamics and the proximal and distal tubular sodium handling in healthy subjects. Diabetologia 35: 1042–1048, 1992 [DOI] [PubMed] [Google Scholar]
- 118.Machnik A, Neuhofer W, Jantsch J, Dahlmann A, Tammela T, Machura K, Park JK, Beck FX, Müller DN, Derer W, Goss J, Ziomber A, Dietsch P, Wagner H, van Rooijen N, Kurtz A, Hilgers KF, Alitalo K, Eckardt KU, Luft FC, Kerjaschki D, Titze J: Macrophages regulate salt-dependent volume and blood pressure by a vascular endothelial growth factor-C-dependent buffering mechanism. Nat Med 15: 545–552, 2009 [DOI] [PubMed] [Google Scholar]
- 119.Binger KJ, Gebhardt M, Heinig M, Rintisch C, Schroeder A, Neuhofer W, Hilgers K, Manzel A, Schwartz C, Kleinewietfeld M, Voelkl J, Schatz V, Linker RA, Lang F, Voehringer D, Wright MD, Hubner N, Dechend R, Jantsch J, Titze J, Müller DN: High salt reduces the activation of IL-4- and IL-13-stimulated macrophages. J Clin Invest 125: 4223–4238, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Titze J, Machnik A: Sodium sensing in the interstitium and relationship to hypertension. Curr Opin Nephrol Hypertens 19: 385–392, 2010 [DOI] [PubMed] [Google Scholar]
- 121.Kleinewietfeld M, Manzel A, Titze J, Kvakan H, Yosef N, Linker RA, Muller DN, Hafler DA: Sodium chloride drives autoimmune disease by the induction of pathogenic TH17 cells. Nature 496: 518–522, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Johnson RJ, Nakagawa T, Sánchez-Lozada LG, Lanaspa MA, Tamura Y, Tanabe K, Ishimoto T, Thomas J, Inaba S, Kitagawa W, Rivard CJ: Umami: The taste that drives purine intake. J Rheumatol 40: 1794–1796, 2013 [DOI] [PubMed] [Google Scholar]
- 123.Bardoux P, Martin H, Ahloulay M, Schmitt F, Bouby N, Trinh-Trang-Tan MM, Bankir L: Vasopressin contributes to hyperfiltration, albuminuria, and renal hypertrophy in diabetes mellitus: Study in vasopressin-deficient Brattleboro rats. Proc Natl Acad Sci U S A 96: 10397–10402, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Bouby N, Ahloulay M, Nsegbe E, Déchaux M, Schmitt F, Bankir L: Vasopressin increases glomerular filtration rate in conscious rats through its antidiuretic action. J Am Soc Nephrol 7: 842–851, 1996 [DOI] [PubMed] [Google Scholar]
- 125.Wolfe BM, Ahuja SP: Effects of intravenously administered fructose and glucose on splanchnic secretion of plasma triglycerides in hypertriglyceridemic men. Metabolism 26: 963–978, 1977 [DOI] [PubMed] [Google Scholar]
- 126.Paula Santos U, Zanetta DM, Terra-Filho M, Burdmann EA: Burnt sugarcane harvesting is associated with acute renal dysfunction. Kidney Int 87: 792–799, 2015 [DOI] [PubMed] [Google Scholar]