Abstract
Kidney cancer, or renal cell carcinoma (RCC), is a disease of increasing incidence that is commonly seen in the general practice of nephrology. However, RCC is under-recognized by the nephrology community, such that its presence in curricula and research by this group is lacking. In the most common form of RCC, clear cell renal cell carcinoma (ccRCC), inactivation of the von Hippel–Lindau tumor suppressor is nearly universal; thus, the biology of ccRCC is characterized by activation of hypoxia-relevant pathways that lead to the associated paraneoplastic syndromes. Therefore, RCC is labeled the internist’s tumor. In light of this characterization and multiple other metabolic abnormalities recently associated with ccRCC, it can now be viewed as a metabolic disease. In this review, we discuss the basic biology, pathology, and approaches for treatment of RCC. It is important to distinguish between kidney confinement and distant spread of RCC, because this difference affects diagnostic and therapeutic approaches and patient survival, and it is important to recognize the key interplay between RCC, RCC therapy, and CKD. Better understanding of all aspects of this disease will lead to optimal patient care and more recognition of an increasingly prevalent nephrologic disease, which we now appropriately label the nephrologist’s tumor.
Keywords: cancer, renal carcinoma, chronic renal disease
Renal cell carcinoma (RCC) is a kidney disease with an incidence that continues to increase, and it has the highest mortality rate among genitourinary tract cancers1,2 but lacks effective therapy. RCC comprises 15 distinct histologic subtypes; clear cell renal cell carcinoma (ccRCC) is the most common and will be the major focus of this review. Despite advanced sensitive imaging techniques and disease awareness, approximately one third to one fifth of patients present with metastatic disease,3 which is associated with a poor prognosis. In addition, although new targeted therapeutics are more effective than the old nonspecific immune–stimulating agents, resistance to these treatments can develop within months to years. However, what remains poorly recognized among nephrologists is that, despite the frequent lethality of RCC in itself, postnephrectomy CKD and its complications are a very pressing issue specifically needing the nephrologist’s attention. Thus, RCC remains a significant health burden, necessitating greater emphasis and research by nephrologists and cancer biologists.
RCC remains an enigmatic disease because of its unique biologic characteristics, idiosyncratic behavior, and unusual clinical presentation. RCC is known as the internist’s tumor, because its diagnosis is elusive and frequently suggested by systemic (i.e., medical) rather than urologic (i.e., surgical) manifestations. Indeed, there are many surprising peculiarities of RCC metabolism, such that this cancer can legitimately be labeled a metabolic disease, which underscores the need for additional multidisciplinary investigative approaches. In this review, we will briefly discuss the basic genetics and biology of RCC, highlighting the most unique aspects, and current clinical approaches to the diagnosis and management of this malignancy.
Basic Biology of RCC: A Metabolic Disease
ccRCC comprises 70% of all RCC and for the reasons to be discussed, is one of the most lethal subtypes. The loss of von Hippel–Lindau (VHL) tumor suppressor function is common in ccRCC,4–6 which to a large degree, dictates the biologic behavior. The behavior of ccRCC is related to apparent hypoxia and dysregulation of the hypoxia–inducible factor (HIF) pathway and target genes. Loss of VHL tumor suppressor results in stabilization of HIF, with the net result that these VHL tumor–inactive cells and tissues act as if they are hypoxic when, in fact, they may not be.7 (The biology of VHL inactivation and HIF stabilization has been extensively reviewed previously.8,9) This pseudohypoxic state is the cause of many abnormal biologic responses, and it is the origin of some of the paraneoplastic syndromes seen with this disease, such as increased hemoglobin resulting from high erythropoietin levels and the finding of high levels of tumor angiogenesis as the result of increased vascular endothelial growth factor (VEGF) levels. At the same time, however, identification of this hypoxia pathway opened up new avenues for targeted therapeutics, which can disrupt one or several biochemical pathways that are not normally active and not involved in normal tissue homeostasis.
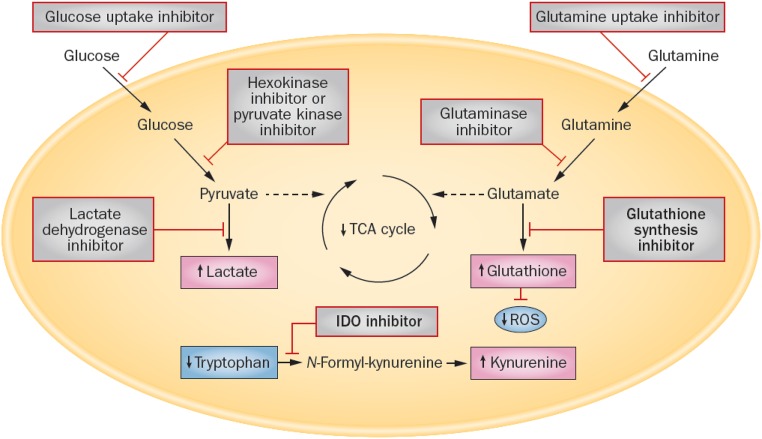
The HIF/hypoxia pathway was one of the first pathways identified in ccRCC, but subsequent research has shown that RCC has a number of metabolic abnormalities responsible for paraneoplastic syndromes. For example, the Warburg effect (aerobic glycolysis), arginine synthetic abnormalities (caused by arginosuccinate synthetase-1 deficiency), and glutamine pathway reprogramming are all pronounced in RCC. The recent elucidation of the metabolic abnormalities that occur in RCC10,11 (Figure 1) is not only useful for understanding how this tumor can be so difficult to treat, but also, these data turn out to be a gold mine for therapeutic exploration. Indeed, targeted therapy using pathway-specific inhibitors (which in some cases, are compounds that were discarded after their discovery) would show high specificity with fewer adverse effects; such an approach will be discussed below.
Figure 1.
New therapeutic targets arise from metabolic reprogramming in RCC. Novel targets for therapeutic intervention on the basis of published work10,85 are shown in bold. Kidney cancer cells exhibit increased glucose uptake, glycolysis, and lactate production, with an associated decrease in pyruvate entering the tricarboxylic acid (TCA) cycle. These pathways can be targeted by inhibiting glucose uptake or the enzymes hexokinase, pyruvate kinase, and lactate dehydrogenase. Kidney cancer cells also exhibit alterations in glutamine metabolism to generate glutathione and reduce reactive oxygen species (ROS). This pathway can be targeted by inhibiting glutamine uptake, glutaminase, or enzymes involved in glutathione synthesis (γ-glutamylcysteine synthetase and glutathione synthetase). Kidney cancer cells also exhibit increased tryptophan metabolism, which results in increased levels of kynurenine, an immunosuppressive metabolite. The production of kynurenine can be inhibited by targeting indoleamine 2,3-diaxygenase (IDO). Reprinted from ref. 11 with permission.
Molecular Genetics of RCC
Our understanding of the molecular genetics of RCC has significantly improved in recent years. VHL is the most common mutation in RCC, and is present in 44% of patients, although some studies have reported VHL gene inactivation in >90% of patients with sporadic RCC.12 Mutations of the PBRM1 gene (Polybromo 1) occur in 30%–40% of patients with sporadic RCC.13–15 PBRM1 is located on chromosome 3p21 near VHL and codes for BAF180, a subunit of the PBAF SWI/SNF chromatin remodeling complex.15 Interestingly, more than one half of patients with RCC and VHL mutations also have PBRM1 mutations.16 The association of this mutation with clinical outcomes is, however, still unclear.
However, mutations in BRCA–associated protein-1 (BAP1), although less common, are linked to poor clinical outcomes. BAP1 mutations are seen in 6%–15% of patients with RCC14,17 and linked to a median survival rate of 4.6 years compared with 10.6 years in patients without the mutation.18 BAP1 is a nuclear deubiquitinase that targets histone H2A and plays a role in chromatin remodeling.17 The mechanisms by which BAP1 mutations promote tumor growth are not well established.
Mutations in SET domain containing 2 (SETD2) occur in ≤11% of RCC.19 SETD2 is a tumor suppressor in proximal epithelial tubular cells.20 Mutations in SETD2 are associated with advanced tumor stage and reduced survival as shown by The Cancer Genome Atlas (TCGA) cohort, with a median survival of 62.7 months compared with 78.2 months in patients without the mutation.14 Data from the TCGA cohort show that 28% of patients with RCC have mutations of the PI(3)K/AKT/mammalian target of rapamycin (mTOR) pathway, including PTEN, PIK3CA, AKT, TSC1, RHEB, and mTOR.21 Whether these mutations predict benefit from mTOR-targeted therapy remains, however, to be determined.
Hereditary RCC Syndromes
There are several hereditary RCC syndromes that account for 2%–3% of patients with RCC. Among them is included VHL disease, an autosomal dominant syndrome that predisposes affected subjects to the development of benign and malignant tumors.22 Nearly 75% of patients with VHL disease will develop ccRCC,23 although only 1.6% of patients with ccRCC are associated with VHL disease.24 Other hereditary RCCs include chromosome 3 translocations,25,26 hereditary leiomyomatosis,27 hereditary papillary renal cancer,28 and Birt–Hogg Dube syndrome.29,30
The Biology and Rationale of Current Therapeutics
Prior therapeutic approaches exploited the high level of immunogenicity of RCC and used immunotherapy with IFN and IL-2, but they were associated with severe and unpleasant adverse effects with very modest success. More recently, therapies targeting newly elucidated biochemical pathways have a better response as well as fewer adverse effects. However, the marked inter- and intratumoral heterogeneity in ccRCC31 has made it difficult to study this disease as a single entity with respect to therapeutic response.
VEGF Inhibitors
VEGF is a critical angiogenic factor expressed in several cell types, including progenitor endothelial cells, endothelial cells, podocytes, fibroblasts, macrophages, and certain tumor types.32 Angiogenesis inhibition as a therapeutic strategy for cancer has rapidly evolved to become standard treatment for several solid tumors (in particular, metastatic RCC). The use of VEGF and its receptors as targets to block angiogenesis have dramatically improved the clinical outcomes for patients with RCC.
Hypertension as an adverse effect of VEGF inhibition was described for bevacizumab, the first agent approved for clinical use,33 and now subsequently, tyrosine kinase inhibitors, which also block the VEGF signaling pathways, such as axitinib, cidarinib, sorafenib, sunitinib, and pazopanib.34,35 The incidence of hypertension with VEGF inhibition is 11%–43%35 and seems dose dependent.36 Proteinuria, a common complication of bevacizumab, is dose dependent, more often subnephrotic,36 and occurs in 41%–63% of patients.33 Such complications are generally managed by the addition of antihypertensive or antiproteinuric medications. With the development of nephrotic-range proteinuria, dose reduction or transient interruption can be made. Medication cessation is generally not recommended unless AKI or thrombotic microangiopathy occurs.37 Nephrology referral should be considered for the management of these adverse effects.
mTOR Inhibitors
mTOR is a major intracellular pathway upregulated in RCC. First generation mTOR inhibitors that selectively inhibit the mTOR complex-1 have shown activity against RCC.38 These drugs, however, do not have activity against mTOR complex-2 or PI3K, and treatment of tumor cells with rapamycin results in increased activity of PI3K through feedback loops containing IGF-1.39 Increased PI3K activity stimulates proliferation and angiogenesis in the tumor and has been linked to mTOR inhibitor resistance.40 Second generation mTOR inhibitors are ATP mimetics that target the mTOR kinase domain and likely to overcome the problem of resistance to first generation mTOR inhibitors by blocking mTOR as well as PI3K and other PI3K–related kinases.41
Newer and Pipeline Therapies
More recently, on the basis of the immunogenic nature of RCC, the immune checkpoint inhibitors, such as nivolumab (anti–PD-1), have joined the list of active therapies for advanced RCC.42 New research on metabolic reprogramming10 has identified indoleamine 2,3-dioxygenase inhibitors, which act in the tryptophan metabolism pathway, as well as PPARα inhibition43 and attenuation of exogenous arginine (because of arginosuccinate synthetase-1 deficiency in ccRCC)44 as potential new targets after evaluated in clinical trials.
Epidemiology of RCC
According to the US National Cancer Institute, >65,000 new kidney cancers are diagnosed annually. Because of increased detection of small renal masses (SRMs) with newer and more sensitive imaging techniques, there has been a significant stage migration, because >60% are early–stage T1 kidney cancers (<7 cm in size and confined to the kidney) compared with only 43% two decades ago. Given that the 5-year survival rate exceeds 90% for T1 tumors and approaches 100% for T1a tumors (<4 cm), a cure for localized RCC presenting as an SRM is a reasonable goal in the majority of patients; management of these patients has begun to shift toward kidney function preservation for >350,000 kidney cancer survivors in the United States. Only a very small fraction of these patients are referred for nephrology consultation, despite 25% of patients with RCC having CKD before nephrectomy and the reasonable likelihood of additional patients developing CKD from the tumor and its treatment. In fact, a patient with kidney cancer and a T1 tumor is much more likely to die of complications related to CKD than those related to their cancer.45–47
With the possible exception of an adipose-rich angiomyolipoma, there are no clinical or radiologic features that can accurately predict the histologic subtype of a renal mass. Establishing the diagnosis has traditionally relied on the removal of the renal mass using radical nephrectomy (RN); however, newer renal sparing approaches are being used by urologists. Biopsies of renal masses have been avoided because of the notion that tumor seeding could be a complication of this diagnostic procedure, but this myth is gradually being refuted.48 Since the advent of coaxial biopsy techniques, no cases of tumor seeding have been reported. Recent studies have also shown that approximately 25% of SRMs are benign neoplasms, such as oncocytoma or angiomyolipoma, which would not require additional therapy49,50; hence, the renal mass biopsy, performed by non-nephrologists, is gaining importance in the work up of SRMs.
CKD Prevalence and Risk of RCC
It is important to note that the relationship between CKD and RCC is bidirectional. Kidney disease, such as cystic disease and ESRD, has been highly associated with RCC.51 For example, the increased risk for RCC was 100-fold for patients with ESRD,51 and it was increased for patients with CKD (stage 3/4) as well.52 The incidence of renal cancers rose incrementally with higher CKD stages, and the strongest association was with ccRCC.52 Remarkably, the incidence of renal cancers also varied according to periods of renal function (transplant) versus nonfunction (graft failure requiring dialysis) among 202,195 renal transplant candidates.53 Incident rates of renal cancer rose when renal function was lost and fell after transplantation, and this pattern recurred during subsequent transplantations. Furthermore, this variation was seen only with localized renal cancers and not seen with those that extended beyond the kidney.53 The mechanism for these findings is largely unknown, but proposed explanations include uremia–related chronic inflammation, oxidative stress, compromised immune function, dialysis, medications, and/or common comorbid diseases further increasing cancer risk.52,54,55 For example, type 2 diabetes mellitus (a risk factor for both RCC and CKD) increases cancer risk for many organs, including the kidney, where hyperinsulinemia (as a growth factor), insulin resistance, and obesity–related inflammatory cytokines are plausible mechanisms.55 The biologic basis for increased cancer risk among those with CKD or ESRD is not clear, but understanding these factors may not only help determine treatment of renal causes but will likely lower the risk of or even prevent renal cancers.
Approach and Management of RCC
Management of renal cancers is evolving, because an increasing proportion of RCC is SRMs, which are found incidentally with favorable survival outcomes. Patients with SRMs are no longer dying primarily of kidney cancer, but rather, the RCC and CKD populations both have common risk factors, which predispose them to cardiovascular events and cardiovascular disease–related death.46 Therefore, a greater focus of SRM treatment (T1a RCCs) should center on maximizing kidney function preservation, cardiovascular risk reduction, and long–term CKD care (Figure 2). A multidisciplinary team approach is necessary, involving the nephrologist, oncologist, pathologist, and urologist.
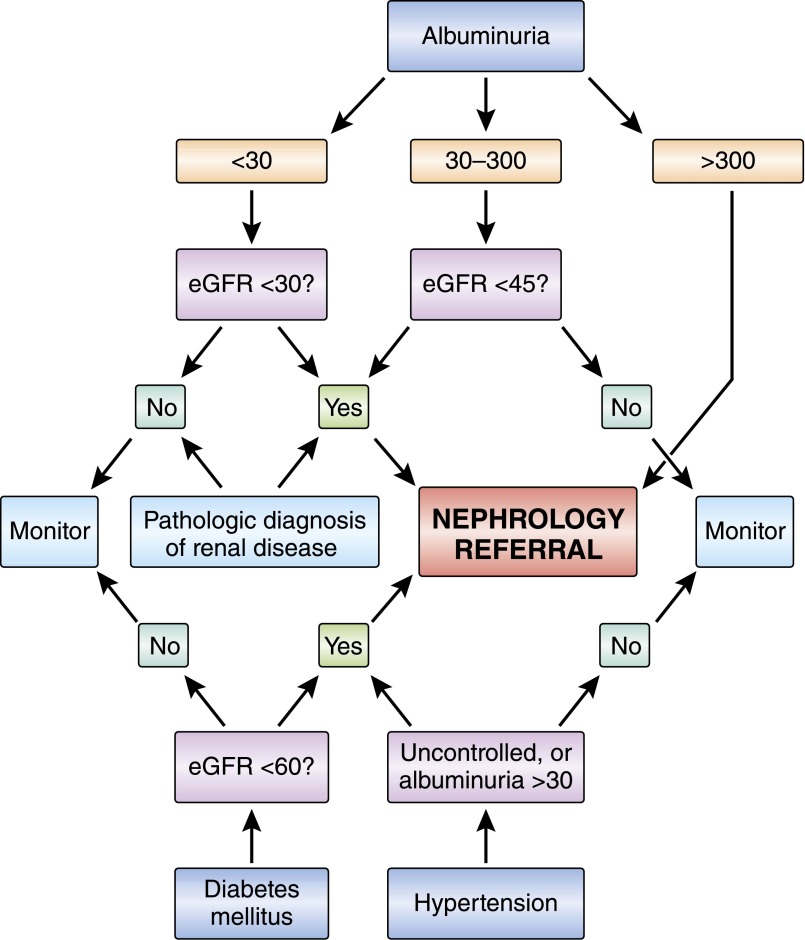
Figure 2.
Renal characteristics determine the need for nephrology referral in RCC patients. Several clinical parameters can be used to determine the need for nephrology evaluation before nephrectomy. This flowchart will assure that patients with high risk of postnephrectomy decline in renal function are evaluated by a nephrologist before nephrectomy is performed. Albumin is in mg/24 h; eGFR is in ml/min per 1.73m2.
Presurgical CKD Risk and Prevention
Before either surgical procedure or ablative therapy, it is important to identify patients who are at high risk for CKD and cardiovascular events. Screening patients at risk for postablative or postsurgical AKI or progressive CKD can be done by estimating GFR and measuring albuminuria (Kidney Disease Improving Global Outcomes CKD Staging). Preoperative optimization of glycemic and BP control, particularly for those with preexisting CKD, could minimize renal function decline after tumor resection (Figure 3). Furthermore, prevention of AKI through avoidance of nephrotoxins and renal hypoperfusion reduces risk for GFR loss postoperatively.56 Differential kidney function measurement typically performed with nuclear scintigraphy may help predict GFR decline with nephrectomy, although it tends to underestimate GFR in the preserved kidney before nephrectomy. Postsurgical differential GFR is more likely reflective of intraoperative renal damage related to ischemia and resected tumor size.57
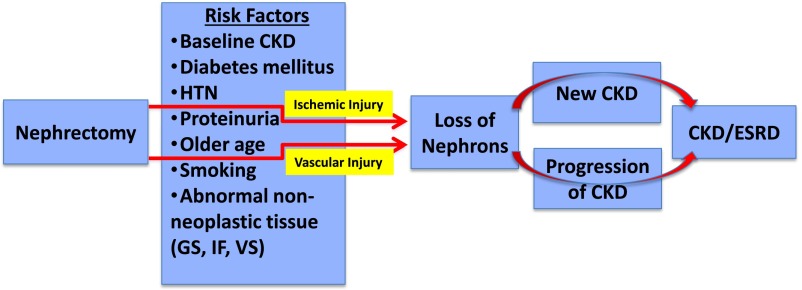
Figure 3.
New-onset CKD or progression of CKD and ESRD may develop after nephrectomy because of nephron loss in patients with underlying risk factors. Nephrectomy performed for RCC is associated with nephron loss caused by tissue removal as well as ischemic and vascular injuries. This loss of nephrons is associated with either new-onset CKD or progression of CKD in patients who have various risk factors as noted. GS, glomerulosclerosis; HTN, hypertension; IF, interstitial fibrosis; VS, vascular sclerosis.
The high prevalence of CKD (10%–52%)58–61 among those with SRMs may be explained by common risk factors, including older age, men, tobacco use, diabetes mellitus, and hypertension.62 A higher burden of diabetes mellitus and hypertension was observed among not only those with preexisting CKD59 but also, those with RCC compared with cancer–free case–matched controls.63 After surgical resection, the prevalence of CKD rose from 10%–24% to 16%–52%.58–60 Postoperative risk of new diagnosis or progression of CKD was also related to these same risk factors64,65 but also included obesity,65,66 decreased GFR, larger tumor size60,63 and corresponding renal volume reduction,67 hypoalbuminemia,59 and postoperative AKI.68 Diabetes mellitus and preexisting CKD likewise increased risk for progression to ESRD (4.05% for RCC versus 0.68% for control with hazard ratio [HR], 5.63; 95% confidence interval [95% CI], 4.37 to 7.24) over a 10-year follow-up period in an incident cohort (RCC, n=2940; control, n=23,520).63 Albuminuria is associated with cancers and has even been described as a paraneoplastic phenomenon; its presence in RCC seems to herald an unfavorable prognosis, but it is typically seen with higher-grade or -stage tumors.69 Thus, patients with RCC and underlying CKD or risk factors for development of CKD after surgery should have nephrology input before nephrectomy.
Clinicopathogic/Pathologic Considerations
The pathologic evaluation of tumor nephrectomies has traditionally focused on the renal mass, but recent studies have shown the high incidence of non–neoplastic renal diseases in the affected kidneys; these issues have been reviewed70,71 and are summarized in Table 1. As a result, the College of American Pathologists required the evaluation of the non–neoplastic kidney parenchyma for the synoptic reporting of kidney cancer starting on January 1, 2010. Even with this implementation, it is unclear whether pathologists are compliant with this requirement, and another recent study found that >60% of the diagnoses were still initially missed.72 A 2012 survey of European genitourinary pathologists found that >25% do not even examine the non-neoplastic part of the kidney in nephrectomy specimens.73 Accurate assessment of the non-neoplastic parenchyma in kidney resection specimens provides an opportunity to identify patients with glomerular, tubulointerstitial, or vascular diseases who will require additional medical management. For this reason, urologists and nephrologists should request that their pathologist specifically review the non-neoplastic parenchyma, especially if their patient with RCC has diabetes, hypertension, or proteinuria.
Table 1.
Incidence of non–neoplastic kidney diseases in tumor nephrectomy specimens
| Reference | Patients, n | GS, IF/TA, VS | Diabetic Nephropathy | Other | Conclusions |
|---|---|---|---|---|---|
| Bijol et al.86 | 110 | Normal ± VS: 42 (38%); any scarring (severe): 24 (22%)/14 (12.7%); ESRD: 2 (2%) | Early: 15 (13.7%); moderate: 4 (3.6%); advanced: 3 (2.7%); total: 22 (20%) | Chronic TMA: 5 (4.6%); collapsing FSGS: 2 (1.8%); IgAN: 2 (1.8%); TBMN: 2 (1.8%); atheroemboli: 2 (1.8%); AIN: 4 (3.6%); amyloidosis: 1 | >20% global GS predicts >0.5 mg/dL Scr increase |
| Henriksen et al.87 | 246 | N/A | Moderate to advanced: 19 (7.7%) | TMA: 3; FSGS: 1 (<1%); SCN: 1 | 88% of diagnoses not initially identified |
| Gautam et al.88 | 49 RN only | Each 10% GS increase resulted in a 9% eGFR decrease | N/A | N/A | |
| Lifshitz et al.89 | 150 PN only | Higher GS correlated with lower eGFR | N/A | N/A | Presence of VS was an independent predictor of GFR decline |
| Salvatore et al.72 | 381 | >5% GS, >10% IF/TA, and severe VS predicted Scr increase | 28 (7.3%) | Hypertensive nephropathy: 11; noncollapsing FSGS: 7 (1.8%); collapsing FSGS: 2 (0.5%); GIN: 2; TMA: 1; pyelonephritis: 1; atheroemboli: 1; MN: 1; amyloidosis: 1 | |
| Sejima et al.90 | 100 RN only | >14% GS predicted worse cardiovascular disease–specific survival | N/A | N/A |
GS, glomerulosclerosis; IF/TA, interstitial fibrosis/tubular atrophy; VS, vascular sclerosis; TMA, thrombotic microangiopathy; IgAN, IgA nephropathy; TBMN, thin basement membrane nephropathy; AIN, acute interstitial nephritis; Scr, serum creatinine; N/A, not applicable/not studied; SCN, sickle cell nephropathy; GIN, granulomatous interstitial nephritis; MN, membranous nephropathy.
Tumor Resection and Ablative Therapies
RN remains the conventional therapy for large renal masses and nonlocalized tumors. Nephron-sparing procedures, including partial nephrectomy (PN) and ablative therapies, are emerging as mainstream therapies for SRMs, because accumulating evidence has shown comparable oncologic and overall survival and greater renal preservation.64,74,75 Moreover, many cohort studies showed a survival advantage and greater GFR preservation with PN.75 Correspondingly, a meta-analysis evaluated findings across 36 studies (mostly retrospective cohort studies) including 40,000 patients (31,000 RN and 9300 PN) and found that treatment of SRMs with PN conferred a 19% risk reduction for all-cause mortality and a 29% risk reduction for cancer-specific mortality.75 This included one randomized, controlled trial the European Organization for Research and Treatment of Cancer (EORTC) study of 541 patients with solitary unilateral SRMs (≤5 cm). The conclusions were somewhat contradictory to the meta-analysis, revealing slightly better overall 10-year survival for RN (81.1%) than PN (75.7%) with an HR of 1.5 (95% CI, 1.03 to 2.16), which disappeared when considering only those diagnosed with RCC.46 Oncologic outcomes were not different.46
In the same meta-analysis, risk reduction of 61% for CKD was associated with PN75; however, such renal benefits were not seen in the EORTC Trial.74 Surgically induced CKD has not been found to have the same cardiovascular morbidity or mortality typically as that seen with those who have underlying medical CKD.76 Among cohort studies, the favorable overall survival for PN has been attributed to greater GFR preservation after nephrectomy, where increasing degree of GFR loss with nephrectomy resulted in a dose–dependent worsening risk of death and cardiovascular disease.47 This is consistent with the incremental rise in mortality risk seen in CKD from cardiovascular disease, which directly correlates with worsening GFR.77 The hard end point of ESRD was examined in a Canadian cohort of 11,937 patients undergoing PN or RN. ESRD risk was no different between PN and RN in the overall cohort spanning from 1995 to 2010; however, when only a contemporary cohort (2003–2010) was considered, the benefit of PN over RN became apparent with an HR of 0.44 (95% CI, 0.25 to 0.95) using a multivariable proportional hazards model. They also observed a lower risk of new-onset CKD (HR, 0.48; 95% CI, 0.41 to 0.57).78 This discrepancy was attributed to changes in clinical practice patterns, where those with lower risk lesions were also being considered for PN in the modern cohort. Data for tumor staging were not available to definitively support these presumptions.78 Renal outcomes are further examined in Table 2. Despite the possible benefits of PN, the adoption rate has remained low, especially among many community hospital urologists, who continue to perform more RNs compared with those in academic centers.79 This likely reflects lack of training in PN in those practicing at community hospitals.
Table 2.
Renal outcomes after PN versus RN
| Reference/Year | Study | N | Renal Outcomes Post-Nx | Comments |
|---|---|---|---|---|
| Lau et al.91 2000 | Case control | RN: 164; PN: 164 | RN: RR, 3.7 (95% CI, 1.2 to 11.2) for CKD (Cr>2.0 mg/dl) compared with PN | Matched for tumor grade/stage/size, age, and sex; 10-yr follow-up |
| McKiernan et al.92 2002 | Case control | RN: 173; PN: 117 | RN: greater risk for CKD (Cr>2.0 mg/dl) compared with PN; RN: post–Nx mean Cr (1.5 mg/dl); PN: post–Nx mean Cr (1.0 mg/dl) | Controlled for age, DM, HTN, smoking, and kidney function; 25-mo median follow-up |
| Huang et al.61 2006 | Cohort study | RN: 262; PN: 385 | RN: HR, 3.8 (95% CI, 2.75 to 5.32) for GFR<60; HR, 11.8 (95% CI, 6.24 to 22.4) for GFR<45 | Matched for age and baseline GFR; 26% with CKD before Nx |
| Malcolm et al.65 2009 | Cohort study | RN: 499; PN: 250 | RN: GFR<60 (44.7%) post-Nx; PN: GFR<60 (16.0%) post-Nx | Proteinuria: RN: 22.2%, PN: 13.2%; Cr>2 mg/dl: RN: 14.2%, PN: 8.4% |
| Barlow et al.58 2010 | Cohort study | RN: 172; PN: 102 | RN: CKD (71.4%) post-Nx; PN: CKD (17.1%) post-Nx; RN: higher risk of new-onset GFR<60; higher percentage of GFR decrease; higher CKD upstaging | Controlled for multiple risk factors and baseline kidney function; 24% with CKD before Nx; CKD independent risk factor for progression |
| Jeon et al.60 2009 | Cohort study | RN: 129; PN: 96 | RN: CKD (66.7%) post-Nx; PN: CKD (11.5%) post-Nx; PN: HR, 0.11 (95% CI, 0.06 to 0.22) for CKD compared with RN | Controlled for multiple risk factors and baseline GFR |
| Klarenbach et al.93 2011 | Population dataset | 1151 | RN: HR, 1.75 (95% CI, 1.02 to 2.99) for composite of ESRD, increased CKD, and acute dialysis compared with PN | Proteinuria-adjusted HR, 2.4 (95% CI, 1.47 to 3.88) for primary outcome |
| Süer et al.94 2011 | Cohort study | RN: 383; PN: 105 | RN: HR, 6.45 (95% CI, 1.02 to 11.06) for GFR<60; RN: HR, 13.5 (95% CI, 3.02 to 21.06) for GFR<45 compared with PN; all tumor sizes | Local recurrence: RN: 1.3%, PN: 5.7%; metastatic disease: no difference; GFR<60 post-Nx: RN: 68.0%, PN: 18.9%; GFR<45 post-Nx: RN: 37.2%, PN: 2.9%; dialysis post-Nx: RN: 2.6%, PN: 0% |
| Sun et al.95 (SEER) 2012 | Cohort study | RN: 840; PN: 840 | RN: HR, 1.9 (95% CI, 1.48 to 2.45) for GFR<60; HR, 1.5 (95% CI, 1.16 to 1.86) for AKI; HR, 1.8 (95% CI, 1.48 to 2.27) for CKD compared with PN | No difference in ESRD incidence; HR, 1.8 (95% CI, 1.03 to 3.27) |
| Kaushik96 2013 | Cohort study | RN: 206; PN: 236 | RN: HR, 4.23 (95% CI, 1.80 to 9.93) for stage 4 CKD compared with PN | Older age, larger tumor size, and higher percentage of oncocytoma in RN group; higher mortality (HR, 1.75; 95% CI, 1.08 to 2.83) in RN group |
| Kim et al.59 2013 | Case control | RN: 605; PN: 1071 | RN: OR, 11.89 (95% CI, 7.98 to 17.70) for GFR<60 compared with PN | Controlled for age, sex, and preoperative Cr |
| Choi et al.97 2014 | Cohort study | RN: 1502; PN: 952 | PN: HR, 0.23 (95% CI, 0.19 to 0.28) for GFR<60 compared with RN | Controlled for age, DM, HTN, and baseline kidney function; preoperative GFR was worse |
| Scosyrer et al.74 2014 | RCT | RN: 273; PN: 268 | RN: GFR<60 (85.7%); GFR<45 (49%); GFR<30 (10%); GFR<15 (1.5%); PN: GFR<60 (64.7%); GFR<45 (27.1%); GFR<30 (6.3%); GFR<15 (1.6%) | No difference between RN and PN for GFR<30 and <15; limited by small numbers; few patients with lower GFR in the study |
| Takagi et al.98 2014 | Case control | RN: 59; PN: 113 | RN: 32.2% function loss on renal scan; PN: 9.6% function loss on renal scan | RN: 40% total renal volume loss on CT; PN: 6% total renal volume loss on CT |
| Woldu et al.99 2014 | Cohort study | 1306; RN: 766; PN: 540 | RN: GFR post-Nx (−1.89/yr decline); PN: GFR post-Nx (−1.17/yr decline); PN: HR, 2.3 (95% CI, 1.6 to 3.3) for freedom from GFR<45 compared with RN | GFR<30: RN: 6.0%, PN: 3.5%; lower-stage CKD associated with greater GFR decline |
| Yap et al.78 2015 | Cohort study | RN: 9830; PN: 2107 | PN: HR, 0.44 (95% CI, 0.25 to 0.75) for ESRD (HR, 0.48 (95% CI, 0.27 to 0.82) with propensity scoring); HR for new-onset CKD | Used multivariable proportional hazards model and propensity scoring; median follow-up of 57 mo |
Nx, nephrectomy; RR, relative risk; Cr, creatinine; DM, diabetes mellitus; HTN, hypertension; RCT, randomized, controlled trial; SEER, surveillance epidemiology and end results; OR, odds ratio; CT, computed tomography.
Nonsurgical therapies, including radiofrequency ablation and cryoablation, have favorable oncologic and overall survival outcomes. Although associated with slightly higher disease progression than observed for surgical treatment (PN), ablative therapies resulted in fewer procedural complications, shorter duration of hospitalization, and greater GFR preservation.64,74,75 When excluding those with high risk for recurrence, oncologic outcomes were equivalent with PN.80 These alternative therapies also enable treatment for those with high operative risk.81 Furthermore, the use of active surveillance when tumor size was monitored closely and regularly, with surgical treatment as required, has also been shown to be effective and comparable with surgical resection, particularly for the elderly (age >75 years old) and in select populations.82
Surveillance of localized SRMs (T1 staging) after definitive treatment is recommended by the American Urological Association at baseline, within 3–12 months with abdominal imaging (computed tomography or magnetic resonance imaging) after RN or PN, and yearly (computed tomography, magnetic resonance imaging, or ultrasonography) for 3 years (time of highest recurrence risk) after PN, with chest radiography also suggested yearly for 3 years or longer if clinically indicated. For those who have received ablative therapies, abdominal imaging and chest radiography are suggested yearly for 5 years. Renal biopsy is suggested before and 6 months after ablation if there are imaging findings83 of concern. Surveillance for recurrent disease is principally for T1b tumors, given that the 5-year recurrence–free survival approaches 97%–98% for T1a tumors with RN or PN.46 Additionally, for all, monitoring for CKD (eGFR<60 ml/min per 1.73 m2 with or without albuminuria ≥30 mg/g for >3 months) is also advised.83 The national comprehensive cancer network guidelines suggest more frequent imaging (every 3–6 months) and longer surveillance (5 years) for stage 1 disease.84
Summary
Kidney cancer is a disease that is under-recognized and understudied by the nephrology community. Because it is a cancer commonly seen in the general nephrology practice and because its incidence is increasing, it is of great importance that the practicing nephrologist has a working knowledge of its biology and treatment. In this brief review, we have summarized these facets of this interesting and increasingly prevalent disease; we are hopeful that our writings will stimulate interest among the nephrology community in research and new treatment approaches for RCC, which is now more appropriately labeled the nephrologist’s tumor.
Disclosures
None.
Acknowledgments
This work was supported by the National Institutes of Health (NIH) grant P30 CA008748 (to E.A.J.), the Byrne Research Fund (E.A.J.), NIH grants 1R03CA181837-01 (to R.H.W.) and 1R01DK082690-01A1 (to R.H.W.), the Medical Service of the US Department of Veterans’ Affairs (R.H.W.), and Dialysis Clinics, Inc. (R.H.W.).
Footnotes
Published online ahead of print. Publication date available at www.jasn.org.
References
- 1.Cairns P: Renal cell carcinoma. Cancer Biomark 9: 461–473, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Weiss RH, Lin P-Y: Kidney cancer: Identification of novel targets for therapy. Kidney Int 69: 224–232, 2006 [DOI] [PubMed] [Google Scholar]
- 3.Janzen NK, Kim HL, Figlin RA, Belldegrun AS: Surveillance after radical or partial nephrectomy for localized renal cell carcinoma and management of recurrent disease. Urol Clin North Am 30: 843–852, 2003 [DOI] [PubMed] [Google Scholar]
- 4.Rechsteiner MP, von Teichman A, Nowicka A, Sulser T, Schraml P, Moch H: VHL gene mutations and their effects on hypoxia inducible factor HIFα: Identification of potential driver and passenger mutations. Cancer Res 71: 5500–5511, 2011 [DOI] [PubMed] [Google Scholar]
- 5.Gnarra JR, Tory K, Weng Y, Schmidt L, Wei MH, Li H, Latif F, Liu S, Chen F, Duh FM, Lubensky I, Duan DR, Florence C, Pozzatti R, Walther MM, Bander NH, Grossman HB, Brauch H, Pomer S, Brooks JD, Isaacs WB, Lerman MI, Zbar B, Ling W, Ling W, Linehan WM: Mutations of the VHL tumour suppressor gene in renal carcinoma. Nat Genet 7: 85–90, 1994 [DOI] [PubMed] [Google Scholar]
- 6.Herman JG, Latif F, Weng Y, Lerman MI, Zbar B, Liu S, Samid D, Duan DS, Gnarra JR, Linehan WM, Baylin SB: Silencing of the VHL tumor-suppressor gene by DNA methylation in renal carcinoma. Proc Natl Acad Sci U S A 91: 9700–9704, 1994 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wettersten HI, Weiss RH: Potential biofluid markers and treatment targets for renal cell carcinoma. Nat Rev Urol 10: 336–344, 2013 [DOI] [PubMed] [Google Scholar]
- 8.Schödel J, Grampp S, Maher ER, Moch H, Ratcliffe PJ, Russo P, Mole DR: Hypoxia, hypoxia-inducible transcription factors, and renal cancer [published online ahead of print August 19, 2015]. Eur Urol 10.1016/j.eururo.2015.08.007 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Baldewijns MM, van Vlodrop IJ, Vermeulen PB, Soetekouw PM, van Engeland M, de Bruïne AP: VHL and HIF signalling in renal cell carcinogenesis. J Pathol 221: 125–138, 2010 [DOI] [PubMed] [Google Scholar]
- 10.Wettersten HI, Hakimi AA, Morin D, Bianchi C, Johnstone ME, Donohoe DR, Trott JF, Aboud OA, Stirdivant S, Neri B, Wolfert R, Stewart B, Perego R, Hsieh JJ, Weiss RH: Grade-dependent metabolic reprogramming in kidney cancer revealed by combined proteomics and metabolomics analysis. Cancer Res 75: 2541–2552, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Minton DR, Nanus DM: Kidney cancer: Novel targets in altered tumour metabolism in kidney cancer. Nat Rev Urol 12: 428–429, 2015 [DOI] [PubMed] [Google Scholar]
- 12.Nickerson ML, Jaeger E, Shi Y, Durocher JA, Mahurkar S, Zaridze D, Matveev V, Janout V, Kollarova H, Bencko V, Navratilova M, Szeszenia-Dabrowska N, Mates D, Mukeria A, Holcatova I, Schmidt LS, Toro JR, Karami S, Hung R, Gerard GF, Linehan WM, Merino M, Zbar B, Boffetta P, Brennan P, Rothman N, Chow WH, Waldman FM, Moore LE: Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res 14: 4726–4734, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Duns G, Hofstra RM, Sietzema JG, Hollema H, van Duivenbode I, Kuik A, Giezen C, Jan O, Bergsma JJ, Bijnen H, van der Vlies P, van den Berg E, Kok K: Targeted exome sequencing in clear cell renal cell carcinoma tumors suggests aberrant chromatin regulation as a crucial step in ccRCC development. Hum Mutat 33: 1059–1062, 2012 [DOI] [PubMed] [Google Scholar]
- 14.Hakimi AA, Ostrovnaya I, Reva B, Schultz N, Chen YB, Gonen M, Liu H, Takeda S, Voss MH, Tickoo SK, Reuter VE, Russo P, Cheng EH, Sander C, Motzer RJ, Hsieh JJ ccRCC Cancer Genome Atlas (KIRC TCGA) Research Network investigators : Adverse outcomes in clear cell renal cell carcinoma with mutations of 3p21 epigenetic regulators BAP1 and SETD2: A report by MSKCC and the KIRC TCGA research network. Clin Cancer Res 19: 3259–3267, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Varela I, Tarpey P, Raine K, Huang D, Ong CK, Stephens P, Davies H, Jones D, Lin ML, Teague J, Bignell G, Butler A, Cho J, Dalgliesh GL, Galappaththige D, Greenman C, Hardy C, Jia M, Latimer C, Lau KW, Marshall J, McLaren S, Menzies A, Mudie L, Stebbings L, Largaespada DA, Wessels LF, Richard S, Kahnoski RJ, Anema J, Tuveson DA, Perez-Mancera PA, Mustonen V, Fischer A, Adams DJ, Rust A, Chan-on W, Subimerb C, Dykema K, Furge K, Campbell PJ, Teh BT, Stratton MR, Futreal PA: Exome sequencing identifies frequent mutation of the SWI/SNF complex gene PBRM1 in renal carcinoma. Nature 469: 539–542, 2011 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Guo G, Gui Y, Gao S, Tang A, Hu X, Huang Y, Jia W, Li Z, He M, Sun L, Song P, Sun X, Zhao X, Yang S, Liang C, Wan S, Zhou F, Chen C, Zhu J, Li X, Jian M, Zhou L, Ye R, Huang P, Chen J, Jiang T, Liu X, Wang Y, Zou J, Jiang Z, Wu R, Wu S, Fan F, Zhang Z, Liu L, Yang R, Liu X, Wu H, Yin W, Zhao X, Liu Y, Peng H, Jiang B, Feng Q, Li C, Xie J, Lu J, Kristiansen K, Li Y, Zhang X, Li S, Wang J, Yang H, Cai Z, Wang J: Frequent mutations of genes encoding ubiquitin-mediated proteolysis pathway components in clear cell renal cell carcinoma. Nat Genet 44: 17–19, 2012 [DOI] [PubMed] [Google Scholar]
- 17.Peña-Llopis S, Vega-Rubín-de-Celis S, Liao A, Leng N, Pavía-Jiménez A, Wang S, Yamasaki T, Zhrebker L, Sivanand S, Spence P, Kinch L, Hambuch T, Jain S, Lotan Y, Margulis V, Sagalowsky AI, Summerour PB, Kabbani W, Wong SW, Grishin N, Laurent M, Xie XJ, Haudenschild CD, Ross MT, Bentley DR, Kapur P, Brugarolas J: BAP1 loss defines a new class of renal cell carcinoma. Nat Genet 44: 751–759, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kapur P, Peña-Llopis S, Christie A, Zhrebker L, Pavía-Jiménez A, Rathmell WK, Xie XJ, Brugarolas J: Effects on survival of BAP1 and PBRM1 mutations in sporadic clear-cell renal-cell carcinoma: A retrospective analysis with independent validation. Lancet Oncol 14: 159–167, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Hakimi AA, Chen YB, Wren J, Gonen M, Abdel-Wahab O, Heguy A, Liu H, Takeda S, Tickoo SK, Reuter VE, Voss MH, Motzer RJ, Coleman JA, Cheng EH, Russo P, Hsieh JJ: Clinical and pathologic impact of select chromatin-modulating tumor suppressors in clear cell renal cell carcinoma. Eur Urol 63: 848–854, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Duns G, van den Berg E, van Duivenbode I, Osinga J, Hollema H, Hofstra RM, Kok K: Histone methyltransferase gene SETD2 is a novel tumor suppressor gene in clear cell renal cell carcinoma. Cancer Res 70: 4287–4291, 2010 [DOI] [PubMed] [Google Scholar]
- 21.Cancer Genome Atlas Research Network : Comprehensive molecular characterization of clear cell renal cell carcinoma. Nature 499: 43–49, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Maher ER, Yates JR, Harries R, Benjamin C, Harris R, Moore AT, Ferguson-Smith MA: Clinical features and natural history of von Hippel-Lindau disease. Q J Med 77: 1151–1163, 1990 [DOI] [PubMed] [Google Scholar]
- 23.Richard S, Gardie B, Couvé S, Gad S: Von Hippel-Lindau: How a rare disease illuminates cancer biology. Semin Cancer Biol 23: 26–37, 2013 [DOI] [PubMed] [Google Scholar]
- 24.Neumann HP, Bender BU, Berger DP, Laubenberger J, Schultze-Seemann W, Wetterauer U, Ferstl FJ, Herbst EW, Schwarzkopf G, Hes FJ, Lips CJ, Lamiell JM, Masek O, Riegler P, Mueller B, Glavac D, Brauch H: Prevalence, morphology and biology of renal cell carcinoma in von Hippel-Lindau disease compared to sporadic renal cell carcinoma. J Urol 160: 1248–1254, 1998 [PubMed] [Google Scholar]
- 25.Bodmer D, Eleveld MJ, Ligtenberg MJ, Weterman MA, Janssen BA, Smeets DF, de Wit PE, van den Berg A, van den Berg E, Koolen MI, Geurts van Kessel A: An alternative route for multistep tumorigenesis in a novel case of hereditary renal cell cancer and a t(2;3)(q35;q21) chromosome translocation. Am J Hum Genet 62: 1475–1483, 1998 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bonné AC, Bodmer D, Schoenmakers EF, van Ravenswaaij CM, Hoogerbrugge N, van Kessel AG: Chromosome 3 translocations and familial renal cell cancer. Curr Mol Med 4: 849–854, 2004 [DOI] [PubMed] [Google Scholar]
- 27.Linehan WM, Pinto PA, Bratslavsky G, Pfaffenroth E, Merino M, Vocke CD, Toro JR, Bottaro D, Neckers L, Schmidt LS, Srinivasan R: Hereditary kidney cancer: Unique opportunity for disease-based therapy. Cancer 115[Suppl]: 2252–2261, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Lubensky IA, Schmidt L, Zhuang Z, Weirich G, Pack S, Zambrano N, Walther MM, Choyke P, Linehan WM, Zbar B: Hereditary and sporadic papillary renal carcinomas with c-met mutations share a distinct morphological phenotype. Am J Pathol 155: 517–526, 1999 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Toro JR, Glenn G, Duray P, Darling T, Weirich G, Zbar B, Linehan M, Turner ML: Birt-Hogg-Dubé syndrome: A novel marker of kidney neoplasia. Arch Dermatol 135: 1195–1202, 1999 [DOI] [PubMed] [Google Scholar]
- 30.Murakami Y, Wataya-Kaneda M, Tanaka M, Takahashi A, Tsujimura A, Inoue K, Nonomura N, Katayama I: Two Japanese cases of birt-hogg-dubé syndrome with pulmonary cysts, fibrofolliculomas, and renal cell carcinomas. Case Rep Dermatol 6: 20–28, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Gerlinger M, Rowan AJ, Horswell S, Larkin J, Endesfelder D, Gronroos E, Martinez P, Matthews N, Stewart A, Tarpey P, Varela I, Phillimore B, Begum S, McDonald NQ, Butler A, Jones D, Raine K, Latimer C, Santos CR, Nohadani M, Eklund AC, Spencer-Dene B, Clark G, Pickering L, Stamp G, Gore M, Szallasi Z, Downward J, Futreal PA, Swanton C: Intratumor heterogeneity and branched evolution revealed by multiregion sequencing. N Engl J Med 366: 883–892, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ferrara N, Gerber HP, LeCouter J: The biology of VEGF and its receptors. Nat Med 9: 669–676, 2003 [DOI] [PubMed] [Google Scholar]
- 33.Zhu X, Wu S, Dahut WL, Parikh CR: Risks of proteinuria and hypertension with bevacizumab, an antibody against vascular endothelial growth factor: Systematic review and meta-analysis. Am J Kidney Dis 49: 186–193, 2007 [DOI] [PubMed] [Google Scholar]
- 34.Chen HX, Cleck JN: Adverse effects of anticancer agents that target the VEGF pathway. Nat Rev Clin Oncol 6: 465–477, 2009 [DOI] [PubMed] [Google Scholar]
- 35.Hutson TE, Figlin RA, Kuhn JG, Motzer RJ: Targeted therapies for metastatic renal cell carcinoma: An overview of toxicity and dosing strategies. Oncologist 13: 1084–1096, 2008 [DOI] [PubMed] [Google Scholar]
- 36.Yang JC, Haworth L, Sherry RM, Hwu P, Schwartzentruber DJ, Topalian SL, Steinberg SM, Chen HX, Rosenberg SA: A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349: 427–434, 2003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Perazella MA, Izzedine H: New drug toxicities in the onco-nephrology world. Kidney Int 87: 909–917, 2015 [DOI] [PubMed] [Google Scholar]
- 38.Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S, Grünwald V, Thompson JA, Figlin RA, Hollaender N, Kay A, Ravaud A RECORD‐1 Study Group : Phase 3 trial of everolimus for metastatic renal cell carcinoma : Final results and analysis of prognostic factors. Cancer 116: 4256–4265, 2010 [DOI] [PubMed] [Google Scholar]
- 39.Jacinto E, Loewith R, Schmidt A, Lin S, Rüegg MA, Hall A, Hall MN: Mammalian TOR complex 2 controls the actin cytoskeleton and is rapamycin insensitive. Nat Cell Biol 6: 1122–1128, 2004 [DOI] [PubMed] [Google Scholar]
- 40.Tamburini J, Chapuis N, Bardet V, Park S, Sujobert P, Willems L, Ifrah N, Dreyfus F, Mayeux P, Lacombe C, Bouscary D: Mammalian target of rapamycin (mTOR) inhibition activates phosphatidylinositol 3-kinase/Akt by up-regulating insulin-like growth factor-1 receptor signaling in acute myeloid leukemia: Rationale for therapeutic inhibition of both pathways. Blood 111: 379–382, 2008 [DOI] [PubMed] [Google Scholar]
- 41.Cho DC, Cohen MB, Panka DJ, Collins M, Ghebremichael M, Atkins MB, Signoretti S, Mier JW: The efficacy of the novel dual PI3-kinase/mTOR inhibitor NVP-BEZ235 compared with rapamycin in renal cell carcinoma. Clin Cancer Res 16: 3628–3638, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Quinn DI, Lara PN Jr.: Renal-cell cancer—targeting an immune checkpoint or multiple kinases. N Engl J Med 373: 1872–1874, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Abu Aboud O, Donohoe D, Bultman S, Fitch M, Riiff T, Hellerstein M, Weiss RH: PPARα inhibition modulates multiple reprogrammed metabolic pathways in kidney cancer and attenuates tumor growth. Am J Physiol Cell Physiol 308: C890–C898, 2015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Perroud B, Ishimaru T, Borowsky AD, Weiss RH: Grade-dependent proteomics characterization of kidney cancer. Mol Cell Proteomics 8: 971–985, 2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Hollingsworth JM, Miller DC, Daignault S, Hollenbeck BK: Five-year survival after surgical treatment for kidney cancer: A population-based competing risk analysis. Cancer 109: 1763–1768, 2007 [DOI] [PubMed] [Google Scholar]
- 46.Van Poppel H, Da Pozzo L, Albrecht W, Matveev V, Bono A, Borkowski A, Colombel M, Klotz L, Skinner E, Keane T, Marreaud S, Collette S, Sylvester R: A prospective, randomised EORTC intergroup phase 3 study comparing the oncologic outcome of elective nephron-sparing surgery and radical nephrectomy for low-stage renal cell carcinoma. Eur Urol 59: 543–552, 2011 [DOI] [PubMed] [Google Scholar]
- 47.Weight CJ, Larson BT, Fergany AF, Gao T, Lane BR, Campbell SC, Kaouk JH, Klein EA, Novick AC: Nephrectomy induced chronic renal insufficiency is associated with increased risk of cardiovascular death and death from any cause in patients with localized cT1b renal masses. J Urol 183: 1317–1323, 2010 [DOI] [PubMed] [Google Scholar]
- 48.Volpe A, Finelli A, Gill IS, Jewett MA, Martignoni G, Polascik TJ, Remzi M, Uzzo RG: Rationale for percutaneous biopsy and histologic characterisation of renal tumours. Eur Urol 62: 491–504, 2012 [DOI] [PubMed] [Google Scholar]
- 49.McKiernan J, Yossepowitch O, Kattan MW, Simmons R, Motzer RJ, Reuter VE, Russo P: Partial nephrectomy for renal cortical tumors: Pathologic findings and impact on outcome. Urology 60: 1003–1009, 2002 [DOI] [PubMed] [Google Scholar]
- 50.Kutikov A, Fossett LK, Ramchandani P, Tomaszewski JE, Siegelman ES, Banner MP, Van Arsdalen KN, Wein AJ, Malkowicz SB: Incidence of benign pathologic findings at partial nephrectomy for solitary renal mass presumed to be renal cell carcinoma on preoperative imaging. Urology 68: 737–740, 2006 [DOI] [PubMed] [Google Scholar]
- 51.Denton MD, Magee CC, Ovuworie C, Mauiyyedi S, Pascual M, Colvin RB, Cosimi AB, Tolkoff-Rubin N: Prevalence of renal cell carcinoma in patients with ESRD pre-transplantation: A pathologic analysis. Kidney Int 61: 2201–2209, 2002 [DOI] [PubMed] [Google Scholar]
- 52.Lowrance WT, Ordoñez J, Udaltsova N, Russo P, Go AS: CKD and the risk of incident cancer. J Am Soc Nephrol 25: 2327–2334, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Yanik EL, Clarke CA, Snyder JJ, Pfeiffer RM, Engels EA: Variation in cancer incidence among patients with ESRD during kidney function and nonfunction intervals [published online ahead of print November 12, 2015]. J Am Soc Nephrol doi:ASN.2015040373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jørgensen L, Heuch I, Jenssen T, Jacobsen BK: Association of albuminuria and cancer incidence. J Am Soc Nephrol 19: 992–998, 2008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Hartmann A, Jenssen T, Holdaas H: Diabetes, chronic kidney disease and cancer risk. Nephrol Dial Transplant 27: 3018–3020, 2012 [DOI] [PubMed] [Google Scholar]
- 56.Thadhani R, Pascual M, Bonventre JV: Acute renal failure. N Engl J Med 334: 1448–1460, 1996 [DOI] [PubMed] [Google Scholar]
- 57.Bachrach L, Negron E, Liu JS, Su YK, Paparello JJ, Eggener S, Kundu SD: Preoperative nuclear renal scan underestimates renal function after radical nephrectomy. Urology 84: 1402–1406, 2014 [DOI] [PubMed] [Google Scholar]
- 58.Barlow LJ, Korets R, Laudano M, Benson M, McKiernan J: Predicting renal functional outcomes after surgery for renal cortical tumours: A multifactorial analysis. BJU Int 106: 489–492, 2010 [DOI] [PubMed] [Google Scholar]
- 59.Kim SH, Lee SE, Hong SK, Jeong CW, Park YH, Kim YJ, Kang SH, Hong SH, Choi WS, Byun SS: Incidence and risk factors of chronic kidney disease in korean patients with t1a renal cell carcinoma before and after radical or partial nephrectomy. Jpn J Clin Oncol 43: 1243–1248, 2013 [DOI] [PubMed] [Google Scholar]
- 60.Jeon HG, Jeong IG, Lee JW, Lee SE, Lee E: Prognostic factors for chronic kidney disease after curative surgery in patients with small renal tumors. Urology 74: 1064–1068, 2009 [DOI] [PubMed] [Google Scholar]
- 61.Huang WC, Levey AS, Serio AM, Snyder M, Vickers AJ, Raj GV, Scardino PT, Russo P: Chronic kidney disease after nephrectomy in patients with renal cortical tumours: A retrospective cohort study. Lancet Oncol 7: 735–740, 2006 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Chow WH, Dong LM, Devesa SS: Epidemiology and risk factors for kidney cancer. Nat Rev Urol 7: 245–257, 2010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hung PH, Tsai HB, Hung KY, Muo CH, Chung MC, Chang CH, Chung CJ: Increased risk of end-stage renal disease in patients with renal cell carcinoma: A 12-year nationwide follow-up study. Medicine (Baltimore) 93: e52, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Li L, Lau WL, Rhee CM, Harley K, Kovesdy CP, Sim JJ, Jacobsen S, Chang A, Landman J, Kalantar-Zadeh K: Risk of chronic kidney disease after cancer nephrectomy. Nat Rev Nephrol 10: 135–145, 2014 [DOI] [PubMed] [Google Scholar]
- 65.Malcolm JB, Bagrodia A, Derweesh IH, Mehrazin R, Diblasio CJ, Wake RW, Wan JY, Patterson AL: Comparison of rates and risk factors for developing chronic renal insufficiency, proteinuria and metabolic acidosis after radical or partial nephrectomy. BJU Int 104: 476–481, 2009 [DOI] [PubMed] [Google Scholar]
- 66.Stiles KP, Moffatt MJ, Agodoa LY, Swanson SJ, Abbott KC: Renal cell carcinoma as a cause of end-stage renal disease in the United States: Patient characteristics and survival. Kidney Int 64: 247–253, 2003 [DOI] [PubMed] [Google Scholar]
- 67.Song C, Bang JK, Park HK, Ahn H: Factors influencing renal function reduction after partial nephrectomy. J Urol 181: 48–53, 2009 [DOI] [PubMed] [Google Scholar]
- 68.Cho A, Lee JE, Kwon GY, Huh W, Lee HM, Kim YG, Kim DJ, Oh HY, Choi HY: Post-operative acute kidney injury in patients with renal cell carcinoma is a potent risk factor for new-onset chronic kidney disease after radical nephrectomy. Nephrol Dial Transplant 26: 3496–3501, 2011 [DOI] [PubMed] [Google Scholar]
- 69.Vaglio A, Buzio L, Cravedi P, Pavone L, Garini G, Buzio C: Prognostic significance of albuminuria in patients with renal cell cancer. J Urol 170: 1135–1137, 2003 [DOI] [PubMed] [Google Scholar]
- 70.Henriksen KJ, Meehan SM, Chang A: Nonneoplastic kidney diseases in adult tumor nephrectomy and nephroureterectomy specimens: Common, harmful, yet underappreciated. Arch Pathol Lab Med 133: 1012–1025, 2009 [DOI] [PubMed] [Google Scholar]
- 71.Bonsib SM, Pei Y: The non-neoplastic kidney in tumor nephrectomy specimens: What can it show and what is important? Adv Anat Pathol 17: 235–250, 2010 [DOI] [PubMed] [Google Scholar]
- 72.Salvatore SP, Cha EK, Rosoff JS, Seshan SV: Nonneoplastic renal cortical scarring at tumor nephrectomy predicts decline in kidney function. Arch Pathol Lab Med 137: 531–540, 2013 [DOI] [PubMed] [Google Scholar]
- 73.Algaba F, Delahunt B, Berney DM, Camparo P, Compérat E, Griffiths D, Kristiansen G, Lopez-Beltran A, Martignoni G, Moch H, Montironi R, Varma M, Egevad L: Handling and reporting of nephrectomy specimens for adult renal tumours: A survey by the European Network of Uropathology. J Clin Pathol 65: 106–113, 2012 [DOI] [PubMed] [Google Scholar]
- 74.Scosyrev E, Messing EM, Sylvester R, Campbell S, Van Poppel H: Renal function after nephron-sparing surgery versus radical nephrectomy: Results from EORTC randomized trial 30904. Eur Urol 65: 372–377, 2014 [DOI] [PubMed] [Google Scholar]
- 75.Kim SP, Thompson RH, Boorjian SA, Weight CJ, Han LC, Murad MH, Shippee ND, Erwin PJ, Costello BA, Chow GK, Leibovich BC: Comparative effectiveness for survival and renal function of partial and radical nephrectomy for localized renal tumors: A systematic review and meta-analysis. J Urol 188: 51–57, 2012 [DOI] [PubMed] [Google Scholar]
- 76.Lane BR, Campbell SC, Demirjian S, Fergany AF: Surgically induced chronic kidney disease may be associated with a lower risk of progression and mortality than medical chronic kidney disease. J Urol 189: 1649–1655, 2013 [DOI] [PubMed] [Google Scholar]
- 77.Go AS, Chertow GM, Fan D, McCulloch CE, Hsu CY: Chronic kidney disease and the risks of death, cardiovascular events, and hospitalization. N Engl J Med 351: 1296–1305, 2004 [DOI] [PubMed] [Google Scholar]
- 78.Yap SA, Finelli A, Urbach DR, Tomlinson GA, Alibhai SM: Partial nephrectomy for the treatment of renal cell carcinoma (RCC) and the risk of end-stage renal disease (ESRD). BJU Int 115: 897–906, 2015 [DOI] [PubMed] [Google Scholar]
- 79.Bjurlin MA, Walter D, Taksler GB, Huang WC, Wysock JS, Sivarajan G, Loeb S, Taneja SS, Makarov DV: National trends in the utilization of partial nephrectomy before and after the establishment of AUA guidelines for the management of renal masses. Urology 82: 1283–1289, 2013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Olweny EO, Park SK, Tan YK, Best SL, Trimmer C, Cadeddu JA: Radiofrequency ablation versus partial nephrectomy in patients with solitary clinical T1a renal cell carcinoma: Comparable oncologic outcomes at a minimum of 5 years of follow-up. Eur Urol 61: 1156–1161, 2012 [DOI] [PubMed] [Google Scholar]
- 81.Wang S, Qin C, Peng Z, Cao Q, Li P, Shao P, Ju X, Meng X, Lu Q, Li J, Wang M, Zhang Z, Gu M, Zhang W, Yin C: Radiofrequency ablation versus partial nephrectomy for the treatment of clinical stage 1 renal masses: A systematic review and meta-analysis. Chin Med J (Engl) 127: 2497–2503, 2014 [PubMed] [Google Scholar]
- 82.Sun M, Becker A, Tian Z, Roghmann F, Abdollah F, Larouche A, Karakiewicz PI, Trinh QD: Management of localized kidney cancer: Calculating cancer-specific mortality and competing risks of death for surgery and nonsurgical management. Eur Urol 65: 235–241, 2014 [DOI] [PubMed] [Google Scholar]
- 83.Donat SM, Diaz M, Bishoff JT, Coleman JA, Dahm P, Derweesh IH, Herrell SD 3rd, Hilton S, Jonasch E, Lin DW, Reuter VE, Chang SS: Follow-up for clinically localized renal neoplasms: AUA Guideline. J Urol 190: 407–416, 2013 [DOI] [PubMed] [Google Scholar]
- 84.Motzer RJ, Jonasch E, Agarwal N, Beard C, Bhayani S, Bolger GB, Chang SS, Choueiri TK, Costello BA, Derweesh IH, Gupta S, Hancock SL, Kim JJ, Kuzel TM, Lam ET, Lau C, Levine EG, Lin DW, Michaelson MD, Olencki T, Pili R, Plimack ER, Rampersaud EN, Redman BG, Ryan CJ, Sheinfeld J, Shuch B, Sircar K, Somer B, Wilder RB, Dwyer M, Kumar R National comprehensive cancer network : Kidney cancer, version 3.2015. J Natl Compr Canc Netw 13: 151–159, 2015 [DOI] [PubMed] [Google Scholar]
- 85.Ganti S, Taylor SL, Abu Aboud O, Yang J, Evans C, Osier MV, Alexander DC, Kim K, Weiss RH: Kidney tumor biomarkers revealed by simultaneous multiple matrix metabolomics analysis. Cancer Res 72: 3471–3479, 2012 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Bijol V, Mendez GP, Hurwitz S, Rennke HG, Nosé V: Evaluation of the nonneoplastic pathology in tumor nephrectomy specimens: Predicting the risk of progressive renal failure. Am J Surg Pathol 30: 575–584, 2006 [DOI] [PubMed] [Google Scholar]
- 87.Henriksen KJ, Meehan SM, Chang A: Non-neoplastic renal diseases are often unrecognized in adult tumor nephrectomy specimens: A review of 246 cases. Am J Surg Pathol 31: 1703–1708, 2007 [DOI] [PubMed] [Google Scholar]
- 88.Gautam G, Lifshitz D, Shikanov S, Moore JM, Eggener SE, Shalhav AL, Chang A: Histopathological predictors of renal function decrease after laparoscopic radical nephrectomy. J Urol 184: 1872–1876, 2010 [DOI] [PubMed] [Google Scholar]
- 89.Lifshitz DA, Shikanov SA, Razmaria AA, Eggener SE, Liao C, Chang A, Shalhav AL: Clinical and histologic predictors of renal function decline after laparoscopic partial nephrectomy. J Endourol 25: 1435–1441, 2011 [DOI] [PubMed] [Google Scholar]
- 90.Sejima T, Yumioka T, Yamaguchi N, Iwamoto H, Masago T, Morizane S, Honda M, Takenaka A: Characterization of mild and severe post-radical nephrectomy renal functional deterioration utilizing histopathological evaluation of non-neoplastic nephrectomized renal parenchyma [published online ahead of print October 8, 2015]. Int J Clin Oncol [DOI] [PubMed] [Google Scholar]
- 91.Lau WK, Blute ML, Weaver AL, Torres VE, Zincke H: Matched comparison of radical nephrectomy vs nephron-sparing surgery in patients with unilateral renal cell carcinoma and a normal contralateral kidney. Mayo Clin Proc 75: 1236–1242, 2000 [DOI] [PubMed] [Google Scholar]
- 92.McKiernan J, Simmons R, Katz J, Russo P: Natural history of chronic renal insufficiency after partial and radical nephrectomy. Urology 59: 816–820, 2002 [DOI] [PubMed] [Google Scholar]
- 93.Klarenbach S, Moore RB, Chapman DW, Dong J, Braam B: Adverse renal outcomes in subjects undergoing nephrectomy for renal tumors: a population-based analysis. Eur Urol 59: 333–339, 2011 [DOI] [PubMed] [Google Scholar]
- 94.Süer E, Burgu B, Gökce Mİ, Türkölmez K, Bedük Y, Baltaci S: Comparison of radical and partial nephrectomy in terms of renal function: A retrospective cohort study. Scand J Urol Nephrol 45: 24–29, 2011 [DOI] [PubMed] [Google Scholar]
- 95.Sun M, Bianchi M, Hansen J et al. : Chronic kidney disease after nephrectomy in patients with small renal masses: a retrospective observational analysis. Eur Urol 62: 696–703, 2012 [DOI] [PubMed] [Google Scholar]
- 96.Kaushik D, Kim SP, Childs MA, Lohse CM, Costello BA, Cheville JC, Boorjian SA, Leibovich BC, Thompson RH: Overall survival and development of stage IV chronic kidney disease in patients undergoing partial and radical nephrectomy for benign renal tumors. Eur Urol 64: 600–606, 2013 [DOI] [PubMed] [Google Scholar]
- 97.Choi SK, Song C: Risk of chronic kidney disease after nephrectomy for renal cell carcinoma. Korean J Urol 55: 636–642, 2014 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Takagi T, Mir MC, Sharma N, Remer EM, Li J, Demirjian S, Kaouk JH, Campbell SC: Compensatory hypertrophy after partial and radical nephrectomy in adults. J Urol 192: 1612–1618, 2014 [DOI] [PubMed] [Google Scholar]
- 99.Woldu SL, Weinberg AC, Korets R, Ghandour R, Danzig MR, RoyChoudhury A, Kalloo SD, Benson MC, DeCastro GJ, McKiernan JM: Who really benefits from nephron-sparing surgery? Urology 84: 860–867, 2014 [DOI] [PubMed] [Google Scholar]