Abstract
Posterior reversible encephalopathy syndrome (PRES) is an obstetric emergency frequently occurring in a pregnant or puerperal woman, manifested with an acute headache, consciousness impairment, seizures, and visual deficits and is associated with white matter changes predominantly affecting the posterior parietal and occipital lobes of the brain. Apart from the above-described typical location of the changes, the most common atypical location involves the brain stem and basal ganglia. Since magnetic resonance imaging (MRI) is more sensitive and specific imaging technique compared to computerized tomography, establishing the diagnosis and follow-up in patients with PRES is based mainly on MRI findings. It is particularly important not to exclude PRES as a possible diagnosis when we have the appropriate clinical presentation accompanied by the atypical radiological findings, since this clinical-radiological syndrome can often be manifested with an atypical MRI image.
Keywords: Posterior reversible encephalopathy syndrome, magnetic resonance, computed tomography, brain, vasogenic edema
INTRODUCTION
Posterior reversible encephalopathy syndrome (PRES) or reversible posterior leukoencephalopathy syndrome was first described by Hinchey, in 1996, as a reversible syndrome manifested with an acute headache, consciousness impairment, seizures, and visual deficits, associated with white matter changes predominately affecting the posterior parietal and occipital lobes of the brain but also involving the brainstem, cerebellum, and other cerebral areas [1-3].
On magnetic resonance imaging (MRI), hyperintensity on T2-weighted (T2W) and fluid-attenuated inversion recovery (FLAIR) images in the parieto-occipital and posterior frontal cortical and subcortical white matter is most commonly involved in the typical appearance of PRES. The brainstem, cerebellum, and basal ganglia are less typically included. Contrast enhancement, hemorrhage as well as restricted diffusion on MRI are the atypical imaging findings [4-6].
The exact pathogenesis of PRES is still under debate, and several different theories explain the occurrence of this syndrome. It is considered that PRES is caused by the disorder of the autoregulation of the central nervous system vasculature. Impaired cerebral vasoregulation is responsible for an increase in blood flow and elevation of the blood pressure, leading to breakdown of the blood-brain barrier and vasogenic edema. The occipito-parietal lobe is particularly susceptible to these events because of the lack of sympathetic tone in the basilar artery vasculature [7-9].
However, this theory does not show a positive correlation between the extent of cerebral edema and the level of blood pressure.
According to another theory, extreme hypertension leading to vasospasm causes local ischemia followed by impaired blood-brain barrier and edema [4,8].
This clinical and radiological entity is associated with various clinical conditions such as hypertensive encephalopathy, renal failure, acute intermittent porphyria, thrombotic thrombocytopenic purpura, pre-eclampsia, and eclampsia [6,10].
PRES is an obstetric emergency frequently occurring in a pregnant or puerperal woman usually the first 48 hours postpartum, as a consequence of eclampsia, which is characterized by the development of generalized convulsions preceded by pre-eclamptic syndrome of proteinuria and hypertension [11,12].
The significance of an early diagnosis of PRES with MRI is emphasized by the fact that its clinical presentation is often atypical. Since the posterior cortical edema can be detected by MRI early after the occurrence of initial symptoms, this technique is considered crucial for the diagnosis [13].
With an early diagnosis and the treatment of the underlying cause, the syndrome is usually completely reversible. Recognizing PRES early in eclampsia is a matter of utmost importance as an urgent control of blood pressure will result in syndrome reversion. On the other hand, delaying the appropriate treatment may lead to a permanent brain damage.
Acute ischemic stroke is considered as one of the most important differential diagnoses of PRES since it is caused by a different kind of edema. The treatment of first choice for patients developing vasogenic edema includes the reduction of blood pressure and supportive measures, whereas the treatment of patients suffering from acute stroke who developed cytotoxic edema and infarction requires a more aggressive therapy, applying the treatment protocols for other etiologies, for instance, subarachnoid hemorrhage with vasospasm [14].
Fatal outcome rarely occurs and it can be caused by increased intracranial pressure which is most commonly a consequence of the presence of progressive cerebral edema, intracerebral hemorrhage, or the underlying conditions and complications [15].
PATHOPHYSIOLOGY
There has been a controversy surrounding the mechanisms behind the PRES imaging appearance. They are still under debate and unproved. However, the most common suggestions include hypertension that causes a failure of autoregulation or forced hyperperfusion, or both. On the other hand, some studies reported the presence of vasospasm and hypoperfusion in PRES and the resemblance of the imaging appearance to a watershed distribution has been noted. Endothelial injury, as present in eclampsia, is a potential etiological factor [9,16,17].
The most widespread theory that explains the pathogenesis of PRES is the vasogenic edema theory. A constant blood flow to the brain, despite the changes of pressure in the systemic circulation, is maintained by the cerebral autoregulation. Increased perfusion pressure and the collapse of the blood-brain barrier leads to extravasation of fluid, macromolecules, and the red blood cells. Therefore, in most cases, PRES represents vasogenic and not cytotoxic edema [7,8,18].
CLINICAL PRESENTATION
PRES is usually identified in patients with eclampsia [10]. However, there are also reported cases of PRES in patients diagnosed with the following systemic conditions: Wegener’s granulomatosis, systemic lupus erythematosus, non-specific renal inflammatory conditions (glomerulonephritis and hepatorenal syndrome), hypertension, and post chemotherapy [3,19-25].
Hypertension and proteinuria during the antepartum and postpartum periods are characteristic features of pre-eclampsia. The term eclampsia is used for the onset of seizures, following pre-eclampsia, which cannot be attributed to other causes. Pre-eclampsia as a pregnancy complication most commonly occurs between 20 weeks of gestation and 48 hours postpartum. It is known as late postpartum pre-eclampsia or eclampsia when developed after 48 hours and up to 30 days after delivery [11,12,26,27].
PRES as a neuroradiological entity is relatively new. The typical features of PRES include signs and symptoms such as hypertension, headache, disturbances of vision, altered mental status as well as generalized seizures accompanied by characteristic MRI findings [1,2,10,28].
The clinical symptoms may vary in severity. Blurred vision, homonymous hemianopsia as well as cortical blindness in some cases are common manifestations of the visual disturbance. Patients can experience mild confusion and agitation but may also fall into a coma. Nausea, vomiting, and brainstem deficits are some of the possible and less common symptoms.
Pre-eclampsia is a condition characterized by a systolic blood pressure ≥140 mm Hg or a diastolic blood pressure ≥90 mm Hg or higher, proteinuria of ≥0.3 g in a 24-hour urine specimen, and a 0.3 or higher protein (mg/dL)/creatinine (mg/dL) ratio [29].
A significant fact related to PRES is the possible development of this disorder with no remarkable elevation of blood pressure. This is particularly the case during pregnancy, where there is a shift of cerebral autoregulation curve to the lower range of blood pressure. Since autoregulatory capability is completely attenuated if there is a severe endothelial injury, as it is the case in pre-eclampsia, even a moderate rise of blood pressure may lead to neurologic symptoms which culminate in eclamptic seizures [30].
In comparison to the above-mentioned predisposing conditions, a study showed that in women with pre-eclampsia and eclampsia less severe edema occurred, and the radiological evidence for cytotoxic edema, hemorrhage, and contrast enhancement was less often found. In addition, residual structural lesions were less frequently observed on follow-up imaging [31]. Hefzy et al. [32] found that in comparison to PRES in patients with eclampsia, in patients suffering from PRES associated with immunosuppressive therapy, particularly related to allogeneic bone marrow transplantation, intracranial hemorrhage is more likely to accompany the condition.
Diagnosis of PRES
A typical clinical picture and the findings on MRI of the brain are used for the diagnosis of PRES.
The clinical diagnosis of PRES is established on the presence of certain conditions such as hypertension, headache, visual disturbances, altered consciousness, and generalized seizures with characteristic MRI findings [1,2,10,28]. Imaging findings are of primary importance for PRES diagnostics.
RADIOLOGICAL CHARACTERISTICS OF PRES
Since MRI is a more sensitive and specific imaging technique than computerized tomography (CT), establishing the diagnosis and follow-up in patients with PRES is based mainly on MRI findings.
The typical MRI appearance of PRES includes T2W and FLAIR hyperintensities in the parieto-occipital and posterior frontal cortical and subcortical white matter. Moreover, the fact that PRES may also have an atypical presentation should be taken into account as highly significant for timely recognition of the condition and the application of the appropriate treatment [4].
Bartynski and Boardman [4] suggested the presence of focal or confluent vasogenic edema in the “posterior” parietal or occipital lobe region as well as in additional regions that are most commonly involved such as the frontal lobes, inferior temporal-occipital region, and cerebellum. Furthermore, the study noted that vasogenic edema in the frontal lobe generally appears to be linear along the superior frontal sulcus.
Apart from the above-described typical location of lesions, the most common atypical location involves the brain stem and includes abnormalities in the midbrain, pons, and medulla. Basal ganglia, including the thalamus and caudate nucleus, have also been identified as the atypical locations [4,33].
Atypical MRI findings include restricted diffusion on diffusion-weighted images (DWI), post-gadolinium contrast enhancement on T1W images, and hemorrhage [4,5,6].
Due to the possibility of reversing PRES, by applying an adequate treatment the deficits are usually completely resolved in a few days to weeks. However, the cases of only a partial resolution have also been reported. Therefore, PRES can cause a fatal outcome [34].
Typical radiological presentation of PRES
A typical presentation of PRES involves widespread, and usually reversible, vasogenic edema, affecting predominantly the subcortical white matter of the occipital and parietal lobes of the brain. Most commonly, the distribution of vasogenic edema in PRES appears as symmetrical and bilateral. However, there is also a possibility of asymmetrical or unilateral appearances [35].
The areas of a diffuse white matter hypodensity indicate the affected regions on CT. On MRI, the lesions are isointense or hypointense on T1W images and hyperintense on T2W images (Figure 1).
FIGURE 1.
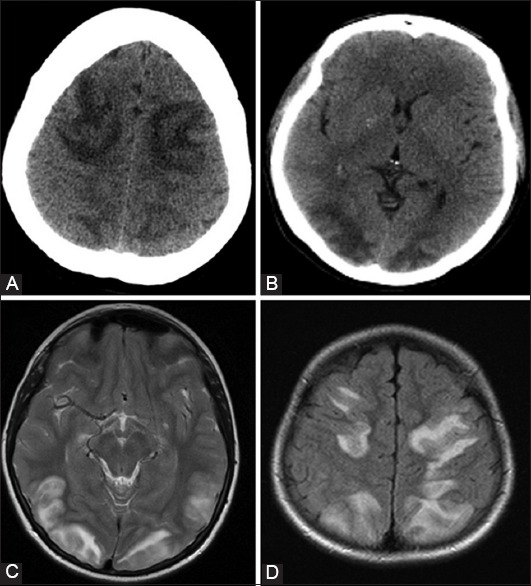
Typical presentation of posterior reversible encephalopathy syndrome. Brain computerized tomography demonstrates areas of a white matter hypodensity affecting symmetrically: Frontal lobes (A), and parieto-occipital lobes (B); Brain magnetic resonance imaging: Axial T2-weighted (C), and axial fluid-attenuated inversion recovery (D) the tomogram demonstrates typical areas of symmetrically increased signal intensities in parieto-occipital and frontal regions.
The changes in PRES are mostly visible on FLAIR images as cortical and/or subcortical hyperintensities. Usually, no enhancement can be seen following an injection of a contrast agent.
The most commonly affected regions, in descending order, are the parietal and occipital lobes, frontal lobes, inferior temporal-occipital junction, and cerebellum. This is due to a better autoregulation of the anterior circulation caused by better sympathetic innervations compared to the posterior circulation [18].
Although in 28% of the cases the lesions are asymmetrical, bilateral and symmetrical lesions are considered as a highly typical appearance of PRES. The distinction between a bilateral infarct in the posterior cerebral artery territory and PRES can be made based on the fact that PRES does not commonly affect the calcarine and occipital lobe structure.
Perfusion imaging revealed a reduced cerebral blood volume, thus indicating the mechanism involving hypoperfusion [36].
Fractional anisotropy, which shows the zones of an increase, indicates a mild and possibly reversible damage of brain tracts. This is in accordance with MR spectroscopy results showing a mild decrease of N-acetylaspartate values [36,37].
Although MR spectroscopy is not regarded as superior compared to conventional MR sequences, it can be very helpful for excluding other etiologies of changes. Increased signal intensity seen after the application of the contrast agents was present in approximately half of the cases [36].
A technique introduced for the detection of microhemorrhages in recent years is susceptibility-weighted imaging. A higher occurrence rate of microhemorrhage in PRES, associated with vasculopathy, has been revealed by this sequence [38].
Atypical radiological presentation of PRES
A number of other atypical radiological features detected by neuroimaging, including anterior, cortical, brainstem lesions, foci of permanent injury, hemorrhage into lesions, and unilateral lesions, should also be taken into account by clinicians (Figures 2 and 3) [39].
FIGURE 2.
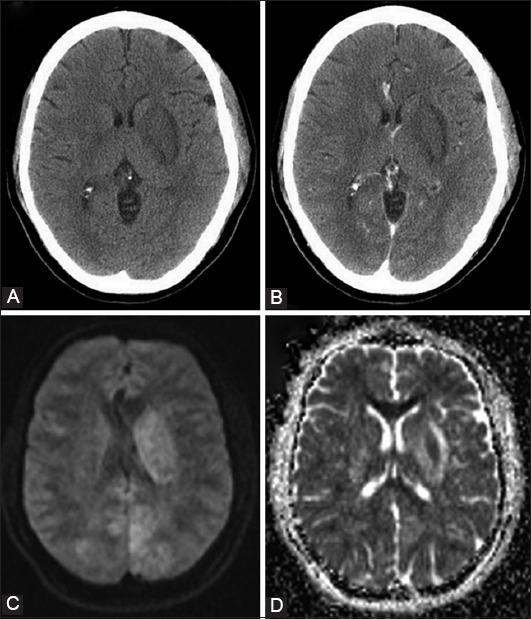
Atypical presentation of posterior reversible encephalopathy syndrome: Non-contrast (A) and post-contrast (B) brain computerized tomography indicating a hypodense lesion in the left basal ganglia with no contrast enhancement; Brain magnetic resonance imaging illustrating diffusion restriction on diffusion-weighted imaging (C) and high values on apparent diffusion coefficient (D).
FIGURE 3.
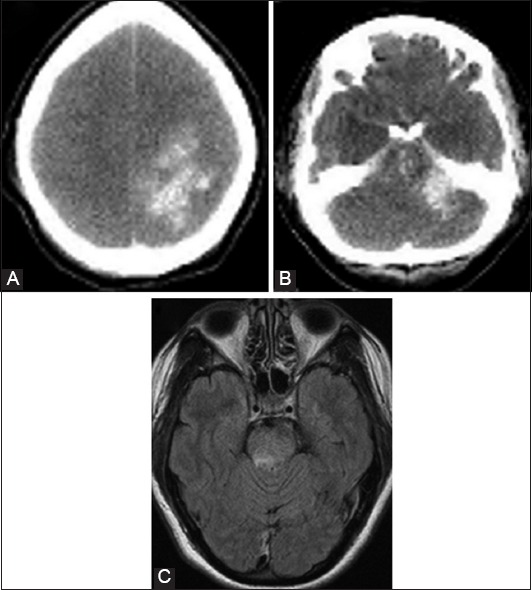
Atypical presentation of posterior reversible encephalopathy syndrome. The brain computerized tomography revealed global brain edema with a large left parietal hematoma (A), and hemorrhage in the pons (B); the axial magnetic resonance tomogram also demonstrates atypical presentation of the areas with increased signal intensities in the pons brain stem (C).
Nakagawa et al. [5] described early imaging characteristics of PRES that precede the more classic clinical presentation. Mild sulcal FLAIR hyperintensity and leptomeningeal enhancement on post contrast T1W images have been described as early findings that precede the clinical presentation [5].
Basal ganglia, brain stem, deep white matter, and splenium of the corpus callosum are rarely affected, and usually unilateral. However, this must not imply an incorrect diagnosis [4].
A progressive disorder of the cerebrovascular regulation mechanisms, in certain severe forms, may cause damage to the blood-brain barrier. In such cases, an enhanced signal in T1W images is seen on MRI after the administration of gadolinium contrast agent [40].
There is an equal possibility of the occurrence of a cytotoxic edema, whereby a decrease in diffusion coefficient would be shown, as described by Benziada-Boudour et al. [41]. Hemorrhage may occur in such cases that progressed further. Hemorrhage is becoming more widely recognized as an atypical manifestation of PRES and does not exclude the diagnosis [42]. It appears in 5% and 30% of cases according to various studies. The possible anatomical locations for its occurrence with a similar incidence are the brain parenchyma, appearing as a focal hematoma or petechial gyral bleeding, or the subarachnoid space [43,44].
DIFFERENTIAL DIAGNOSIS
The differential diagnosis of the T2W confluent white matter hyperintensities is broad, and it comprises a great number of conditions and other causes of vascular and inflammatory diseases. Various conditions that are considered in the differential diagnosis include acute cerebral ischemia, infarcts involving top of basilar syndrome, cerebral venous thrombosis, transient cerebral hyperemia, Creutzfeldt-Jakob disease, gliomatosis cerebri, infectious meningitis, encephalitis, post-infectious encephalomyelitis, vasculitis, epinephrine-induced toxic or metabolic encephalopathy, and demyelinating disorders [9].
Distinguishing PRES from acute ischemic stroke is highly significant since hypertension in ischemic stroke should not be managed aggressively while in the case of PRES it should be controlled and actively managed.
For the differentiation between vasogenic and cytotoxic edema, which represents foci of irreversible ischemia, DWI and apparent diffusion coefficient (ADC) map are of great help. These two MR techniques are sensitive to molecular diffusion of water molecules. Cytotoxic edema shows a bright signal on DWI as a result of decreased Na+K+-ATPase activity with a consequent decrease in water molecule transport. On the other hand, on DWI, vasogenic edema due to T2 shine-through effect can elicit increased signal intensity but is usually iso – or hypointense. Because of highly mobile water in the regions of vasogenic edema, there are high ADC values unlike in cytotoxic edema and that enables the exact differentiation. In cases of PRES, the signal abnormalities on DWI were not accompanied with decreased ADC values, which represent vasogenic edema [45-47].
Reversible cerebral vasoconstriction syndrome (RCVS) should also be mentioned as an important differential diagnosis. RCVS is a clinical-angiographic syndrome that occurs usually in women and is manifested by sudden attacks of severe headache, focal neurological defects, segmental narrowing and dilatation of large and medium cerebral arteries. There is a significant overlapping of clinical and radiological features between RCVS and PRES. Moreover, the two entities often occur simultaneously. RCVS may be idiopathic or occur after an administration of vasoactive drugs, migraine, in pregnancy, and puerperium. In RCVS patients, the major cerebral arteries are affected and stroke occurs, while in PRES patients the small blood vessels, arterioles, and capillaries are affected. PRES can be complicated by ischemic stroke, similar to the RCVS, which is presented with “watershed” distribution where subcortical mass is affected and the cortex is saved [48,49].
MANAGEMENT
PRESS is a possibly reversible condition. Although there have been reports of only a partial resolution and a fatal outcome of the disease, the application of an adequate therapy usually completely resolves the deficits in a few days to weeks. The recommended treatments of eclampsia differ from PRES in other clinical settings. Delivery of the baby and placenta is often sufficient. The treatment of choice should include antihypertensive drug therapy for the management of arterial hypertension, the use of magnesium sulfate or phenytoin for the prevention and control of the eclamptic seizures or the withdrawal of the offending agent [34].
Same studies recommend the discontinuation of intravenous nitroglycerin, which is a widely-used therapy for acute hypertension and hypertensive encephalopathy. Vasodilators, i.e. nitroglycerin, can widen the cerebral arteries and change the brain blood flow. This may lead to disturbances of the autoregulation. Although, nitroglycerin is an established therapy for hypertensive encephalopathy, we should be very careful with its application in cases of PRES which are initially presented as hypertensive encephalopathy, when brain imaging is not available or not typical. The distinction between hypertensive encephalopathy and hypertensive PRES is based on the radiological findings, and it is very subtle. If the neurological symptoms occurred or got worsening or the typical radiological signs appeared, nitroglycerin should be immediately discontinued [50,51].
CONCLUSION
It is of particular importance not to exclude PRES as a possible diagnosis when we have the appropriate clinical presentation which is not accompanied by the typical radiological findings since this clinical-radiological syndrome can often be manifested with atypical MRI findings. The success of the therapy lies primarily in recognizing these findings.
DECLARATION OF INTERESTS
The authors declare no conflict of interests.
REFERENCES
- 1.Oyinloye OI, Adesiyun OA, Atobatele MO, Fawole AA. Posterior reversible encephalopathy syndrome in a adult female. Ann Afr Med. 2014;13(3):138–41. doi: 10.4103/1596-3519.134422. http://dx.doi.org/10.4103/1596-3519.134422 . [DOI] [PubMed] [Google Scholar]
- 2.Wagner SJ, Acquah LA, Lindell EP, Craici IM, Wingo MT, Rose CH, et al. Posterior reversible encephalopathy syndrome and eclampsia: Pressing the case for more aggressive blood pressure control. Mayo Clin Proc. 2011;86(9):851–6. doi: 10.4065/mcp.2011.0090. http://dx.doi.org/10.4065/mcp.2011.0090 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hinchey J, Chaves C, Appignani B, Breen J, Pao L, Wang A, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334(8):494–500. doi: 10.1056/NEJM199602223340803. http://dx.doi.org/10.1056/NEJM199602223340803 . [DOI] [PubMed] [Google Scholar]
- 4.Bartynski WS, Boardman JF. Distinct imaging patterns and lesion distribution in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2007;28(7):1320–7. doi: 10.3174/ajnr.A0549. http://dx.doi.org/10.3174/ajnr.A0549 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nakagawa K, Sorond FA, Ropper AH. Ultra-early magnetic resonance imaging findings of eclampsia. Arch Neurol. 2008;65(7):974–6. doi: 10.1001/archneur.65.7.974. http://dx.doi.org/10.1001/archneur.65.7.974 . [DOI] [PubMed] [Google Scholar]
- 6.Bartynski WS. Posterior reversible encephalopathy syndrome, part 1: Fundamental imaging and clinical features. AJNR Am J Neuroradiol. 2008;29(6):1036–42. doi: 10.3174/ajnr.A0928. http://dx.doi.org/10.3174/ajnr.A0928 . http://dx.doi.org/10.3174/ajnr.A0929 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Schwartz RB, Feske SK, Polak JF, DeGirolami U, Iaia A, Beckner KM, et al. Preeclampsia-eclampsia: Clinical and neuroradiographic correlates and insights into the pathogenesis of hypertensive encephalopathy. Radiology. 2000;217(2):371–6. doi: 10.1148/radiology.217.2.r00nv44371. http://dx.doi.org/10.1148/radiology.217.2.r00nv44371 . [DOI] [PubMed] [Google Scholar]
- 8.Cipolla MJ. Cerebrovascular function in pregnancy and eclampsia. Hypertension. 2007;50(1):14–24. doi: 10.1161/HYPERTENSIONAHA.106.079442. http://dx.doi.org/10.1161/HYPERTENSIONAHA.106.079442 . [DOI] [PubMed] [Google Scholar]
- 9.Lamy C, Oppenheim C, Mas JL. Posterior reversible encephalopathy syndrome. Handb Clin Neurol. 2014;121:1687–701. doi: 10.1016/B978-0-7020-4088-7.00109-7. http://dx.doi.org/10.1016/B978-0-7020-4088-7.00109-7 . [DOI] [PubMed] [Google Scholar]
- 10.Mishra SK, Bhat R, Sudeep K, Nagappa M, Swain A, Badhe A. PRES (posterior reversible encephalopathy syndrome) and eclampsia: Review. [cited 2016 January 10];Internet J Anesth. 2008 22(1) Available from: https://www.ispub.com/IJA/22/1/13690 . [Google Scholar]
- 11.Matthys LA, Coppage KH, Lambers DS, Barton JR, Sibai BM. Delayed postpartum preeclampsia: An experience of 151 cases. Am J Obstet Gynecol. 2004;190(5):1464–6. doi: 10.1016/j.ajog.2004.02.037. http://dx.doi.org/10.1016/j.ajog.2004.02.037 . [DOI] [PubMed] [Google Scholar]
- 12.Sibai BM. Diagnosis, prevention, and management of eclampsia. Obstet Gynecol. 2005;105(2):402–10. doi: 10.1097/01.AOG.0000152351.13671.99. http://dx.doi.org/10.1097/01.AOG.0000152351.13671.99 . [DOI] [PubMed] [Google Scholar]
- 13.Huijgen W, van der Kallen B, Boiten J, Lycklama À, Nijeholt G. Unilateral reversible posterior leukoencephalopathy syndrome after coiling of an aneurysm. J Clin Neurol. 2014;10(1):59–63. doi: 10.3988/jcn.2014.10.1.59. http://dx.doi.org/10.3988/jcn.2014.10.1.59 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schneider JP, Krohmer S, Günther A, Zimmer C. Cerebral lesions in acute arterial hypertension: The characteristic MRI in hypertensive encephalopathy [Article in German] Rofo. 2006;178(6):618–26. doi: 10.1055/s-2006-926631. http://dx.doi.org/10.1055/s-2006-926631 . [DOI] [PubMed] [Google Scholar]
- 15.Golombeck SK, Wessig C, Monoranu CM, Schütz A, Solymosi L, Melzer N, et al. Fatal atypical reversible posterior leukoencephalopathy syndrome: A case report. J Med Case Rep. 2013;7:14. doi: 10.1186/1752-1947-7-14. http://dx.doi.org/10.1186/1752-1947-7-14 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bartynski WS, Zeigler Z, Spearman MP, Lin L, Shadduck RK, Lister J. Etiology of cortical and white matter lesions in cyclosporin-A and FK-506 neurotoxicity. AJNR Am J Neuroradiol. 2001;22(10):1901–14. [PMC free article] [PubMed] [Google Scholar]
- 17.Lin JT, Wang SJ, Fuh JL, Hsiao LT, Lirng JF, Chen PM. Prolonged reversible vasospasm in cyclosporin A-induced encephalopathy. AJNR Am J Neuroradiol. 2003;24(1):102–4. [PMC free article] [PubMed] [Google Scholar]
- 18.Achar SK, Shetty N, Joseph TT. Posterior reversible encephalopathy syndrome at term pregnancy. Indian J Anaesth. 2011;55(4):399–401. doi: 10.4103/0019-5049.84856. http://dx.doi.org/10.4103/0019-5049.84856 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schwartz RB, Jones KM, Kalina P, Bajakian RL, Mantello MT, Garada B, et al. Hypertensive encephalopathy: Findings on CT MR imaging, and SPECT imaging in 14 cases. AJR Am J Roentgenol. 1992;159(2):379–83. doi: 10.2214/ajr.159.2.1632361. http://dx.doi.org/10.2214/ajr.159.2.1632361 . [DOI] [PubMed] [Google Scholar]
- 20.Rippe DJ, Edwards MK, Schrodt JF, Bognanno JR, D’Amour PG, Boyko OB. Reversible cerebral lesions associated with tiazofurin usage: MR demonstration. J Comput Assist Tomogr. 1988;12(6):1078–81. doi: 10.1097/00004728-198811000-00037. http://dx.doi.org/10.1097/00004728-198811000-00037 . [DOI] [PubMed] [Google Scholar]
- 21.Vaughn DJ, Jarvik JG, Hackney D, Peters S, Stadtmauer EA. High-dose cytarabine neurotoxicity: MR findings during the acute phase. AJNR Am J Neuroradiol. 1993;14(4):1014–6. [PMC free article] [PubMed] [Google Scholar]
- 22.Ito Y, Arahata Y, Goto Y, Hirayama M, Nagamutsu M, Yasuda T, et al. Cisplatin neurotoxicity presenting as reversible posterior leukoencephalopathy syndrome. AJNR Am J Neuroradiol. 1998;19(3):415–7. [PMC free article] [PubMed] [Google Scholar]
- 23.Provenzale JM, Petrella JR, Cruz LC, Jr, Wong JC, Engelter S, Barboriak DP. Quantitative assessment of diffusion abnormalities in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2001;22(8):1455–61. [PMC free article] [PubMed] [Google Scholar]
- 24.Mukherjee P, McKinstry RC. Reversible posterior leukoencephalopathy syndrome: Evaluation with diffusion-tensor MR imaging. Radiology. 2001;219(3):756–65. doi: 10.1148/radiology.219.3.r01jn48756. http://dx.doi.org/10.1148/radiology.219.3.r01jn48756 . [DOI] [PubMed] [Google Scholar]
- 25.Covarrubias DJ, Luetmer PH, Campeau NG. Posterior reversible encephalopathy syndrome: Prognostic utility of quantitative diffusion-weighted MR images. AJNR Am J Neuroradiol. 2002;23(6):1038–48. [PMC free article] [PubMed] [Google Scholar]
- 26.Lubarsky SL, Barton JR, Friedman SA, Nasreddine S, Ramadan MK, Sibai BM. Late postpartum eclampsia revisited. Obstet Gynecol. 1994;83(4):502–5. doi: 10.1097/00006250-199404000-00003. http://dx.doi.org/10.1097/00006250-199404000-00003 . [DOI] [PubMed] [Google Scholar]
- 27.Altinkaya SO, Nergiz S, Küçük M, Yüksel H, Dayanir Y. Posterior reversible encephalopathy syndrome in obstetric patients. Report of three cases with literature review. Clin Exp Obstet Gynecol. 2014;41(6):730–3. [PubMed] [Google Scholar]
- 28.Long TR, Hein BD, Brown MJ, Rydberg CH, Wass CT. Posterior reversible encephalopathy syndrome during pregnancy: Seizures in a previously healthy parturient. J Clin Anesth. 2007;19(2):145–8. doi: 10.1016/j.jclinane.2006.07.004. http://dx.doi.org/10.1016/j.jclinane.2006.07.004 . [DOI] [PubMed] [Google Scholar]
- 29.American College of Obstetricians and Gynecologists;Task Force on Hypertension in Pregnancy. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31. doi: 10.1097/01.AOG.0000437382.03963.88. [DOI] [PubMed] [Google Scholar]
- 30.Prout RE, Tuckey JP, Giffen NJ. Reversible posterior leucoencephalopathy syndrome in a peripartum patient. Int J Obstet Anesth. 2007;16(1):74–6. doi: 10.1016/j.ijoa.2006.04.012. http://dx.doi.org/10.1016/j.ijoa.2006.04.012 . [DOI] [PubMed] [Google Scholar]
- 31.Liman TG, Bohner G, Heuschmann PU, Scheel M, Endres M, Siebert E. Clinical and radiological differences in posterior reversible encephalopathy syndrome between patients with preeclampsia-eclampsia and other predisposing diseases. Eur J Neurol. 2012;19(7):935–43. doi: 10.1111/j.1468-1331.2011.03629.x. http://dx.doi.org/10.1111/j.1468-1331.2011.03629.x . [DOI] [PubMed] [Google Scholar]
- 32.Hefzy HM, Bartynski WS, Boardman JF, Lacomis D. Hemorrhage in posterior reversible encephalopathy syndrome: Imaging and clinical features. AJNR Am J Neuroradiol. 2009;30(7):1371–9. doi: 10.3174/ajnr.A1588. http://dx.doi.org/10.3174/ajnr.A1588 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.O’Kane M, Elhalwagy H, Kumar S, Badawi C. Unusual presentation of PRES in the postnatal period. BMJ Case Rep. 2014;2014:92Bcr2013203406. doi: 10.1136/bcr-2013-203406. DOI: 10.1136/bcr-2013-203406 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Pedraza R, Marik PE, Varon J. Posterior reversible encephalopathy syndrome: A review. Crit Care Shock. 2009;12(4):135–43. [Google Scholar]
- 35.McKinney AM, Short J, Truwit CL, McKinney ZJ, Kozak OS, SantaCruz KS, et al. Posterior reversible encephalopathy syndrome: Incidence of atypical regions of involvement and imaging findings. AJR Am J Roentgenol. 2007;189(4):904–12. doi: 10.2214/AJR.07.2024. http://dx.doi.org/10.2214/AJR.07.2024 . [DOI] [PubMed] [Google Scholar]
- 36.Lee SY, Kim SH, Lee SH, Baek HJ, Shon HS, Kim SS. Serial MR spectroscopy in relapsing reversible posterior leukoencephalopathy syndrome. Neurologist. 2009;15(6):338–41. doi: 10.1097/NRL.0b013e3181914af6. http://dx.doi.org/10.1097/NRL.0b013e3181914af6 . [DOI] [PubMed] [Google Scholar]
- 37.Alexander AL, Lee JE, Lazar M, Field AS. Diffusion tensor imaging of the brain. Neurotherapeutics. 2007;4(3):316–29. doi: 10.1016/j.nurt.2007.05.011. http://dx.doi.org/10.1016/j.nurt.2007.05.011 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sonneville R, Klein IF, Wolff M. Update on investigation and management of postinfectious encephalitis. Curr Opin Neurol. 2010;23(3):300–4. doi: 10.1097/WCO.0b013e32833925ec. http://dx.doi.org/10.1097/WCO.0b013e32833925ec . [DOI] [PubMed] [Google Scholar]
- 39.Pereira PR, Pinho J, Rodrigues M, Rocha J, Sousa F, Amorim J, et al. Clinical, imagiological and etiological spectrum of posterior reversible encephalopathy syndrome. Arq Neuropsiquiatr. 2015;73(1):36–40. doi: 10.1590/0004-282X20140176. http://dx.doi.org/10.1590/0004-282X20140176 . [DOI] [PubMed] [Google Scholar]
- 40.Hamilton BE, Nesbit GM. Delayed CSF enhancement in posterior reversible encephalopathy syndrome. AJNR Am J Neuroradiol. 2008;29(3):456–7. doi: 10.3174/ajnr.A0926. http://dx.doi.org/10.3174/ajnr.A0926 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Benziada-Boudour A, Schmitt E, Kremer S, Foscolo S, Rivière AS, Tisserand M, et al. Posterior reversible encephalopathy syndrome: A case of unusual diffusion-weighted MR images. J Neuroradiol. 2009;36(2):102–5. doi: 10.1016/j.neurad.2008.08.003. http://dx.doi.org/10.1016/j.neurad.2008.08.003 . [DOI] [PubMed] [Google Scholar]
- 42.Stevens CJ, Heran MK. The many faces of posterior reversible encephalopathy syndrome. Br J Radiol. 2012;85(1020):1566–75. doi: 10.1259/bjr/25273221. http://dx.doi.org/10.1259/bjr/25273221 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lee VH, Wijdicks EF, Manno EM, Rabinstein AA. Clinical spectrum of reversible posterior leukoencephalopathy syndrome. Arch Neurol. 2008;65(2):205–10. doi: 10.1001/archneurol.2007.46. http://dx.doi.org/10.1001/archneurol.2007.46 . [DOI] [PubMed] [Google Scholar]
- 44.Donmez FY, Basaran C, Kayahan Ulu EM, Yildirim M, Coskun M. MRI features of posterior reversible encephalopathy syndrome in 33 patients. J Neuroimaging. 2010;20(1):22–8. doi: 10.1111/j.1552-6569.2008.00306.x. http://dx.doi.org/10.1111/j.1552-6569.2008.00306.x . [DOI] [PubMed] [Google Scholar]
- 45.Doelken M, Lanz S, Rennert J, Alibek S, Richter G, Doerfler A. Differentiation of cytotoxic and vasogenic edema in a patient with reversible posterior leukoencephalopathy syndrome using diffusion-weighted MRI. Diagn Interv Radiol. 2007;13(3):125–8. [PubMed] [Google Scholar]
- 46.Kastrup O, Schlamann M, Moenninghoff C, Forsting M, Goericke S. Posterior reversible encephalopathy syndrome: The spectrum of MR imaging patterns. Clin Neuroradiol. 2015;25(2):161–71. doi: 10.1007/s00062-014-0293-7. http://dx.doi.org/10.1007/s00062-014-0293-7 . [DOI] [PubMed] [Google Scholar]
- 47.Demirel I, Kavak BS, Özer AB, Bayar MK, Erhan Ö L. An intensive care approach to posterior reversible encephalopathy syndrome (PRES): An analysis of 7 cases. J Turk Ger Gynecol Assoc. 2014;15(4):217–21. doi: 10.5152/jtgga.2014.14072. http://dx.doi.org/10.5152/jtgga.2014.14072 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tan LH, Flower O. Reversible cerebral vasoconstriction syndrome: An important cause of acute severe headache. Emerg Med Int. 2012;2012:303152. doi: 10.1155/2012/303152. http://dx.doi.org/10.1155/2012/303152 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Miller TR, Shivashankar R, Mossa-Basha M, Gandhi D. Reversible cerebral vasoconstriction syndrome, part 1: Epidemiology, pathogenesis, and clinical course. AJNR Am J Neuroradiol. 2015;36(8):1392–9. doi: 10.3174/ajnr.A4214. http://dx.doi.org/10.3174/ajnr.A4214 . [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Kühn AL, Huch B, Wendt G, Dooms G, Droste DW. First description of posterior reversible encephalopathy syndrome as a complication of glycerolnitrate patch following open cardiac surgery. Acta Neurol Scand. 2011;124(3):218–20. doi: 10.1111/j.1600-0404.2010.01445.x. http://dx.doi.org/10.1111/j.1600-0404.2010.01445.x . [DOI] [PubMed] [Google Scholar]
- 51.Sheta MA, Paladugu M, Mendelson J, Holland NR. When should nitroglycerine be avoided in hypertensive encephalopathy? Hypertension. 2011;58(5):187–8. doi: 10.1161/HYPERTENSIONAHA.111.175703. http://dx.doi.org/10.1161/HYPERTENSIONAHA.111.175703 . [DOI] [PubMed] [Google Scholar]


