Abstract
Epicardial adipose tissue is an unusual visceral fat depot with anatomical and functional contiguity to the myocardium and coronary arteries. Under physiological conditions, epicardial adipose tissue displays biochemical, mechanical and thermogenic cardioprotective properties. Under pathological circumstances, epicardial fat can locally affect the heart and coronary arteries through vasocrine or paracrine secretion of proinflammatory cytokines. What influences this equilibrium remains unclear. Improved local vascularization, weight loss, and targeted pharmaceutical interventions could help to return epicardial fat to its physiological role. This review focuses on the emerging physiological and pathophysiological aspects of the epicardial fat and its numerous and innovative clinical applications. Particular emphasis is placed on the paracrine/endocrine properties of epicardial fat and its role in the development and progression of atherosclerosis.
Anatomy of epicardial adipose tissue
The adipose tissue of the heart consists of epicardial fat (Table 1), located between the myocardium and visceral pericardium, and the pericardial fat, situated outside the visceral pericardium and on the external surface of the parietal pericardium [1–8]. Epicardial and pericardial fat are embryologically different. The epicardium comprises a population of mesothelial cells that migrate onto the surface of the heart from the area of the septum transversum [1–4]. Epicardial fat originates from the splanchnopleuric mesoderm, whereas the pericardial fat originates from the primitive thoracic mesenchyme [1–4]. Vascularization is also different between epicardial and pericardial fat because vascularization for the epicardial fat is supplied by branches of the coronary arteries whereas pericardial fat is vascularized from non-coronary sources [1–4]. Under physiological conditions, epicardial adipose tissue represents approximately 20% of the heart mass. In the adult human heart, epicardial fat is commonly found in the atrioventricular and interventricular grooves. Fat accumulation can also extend from the epicardial surface into the myocardium [5]. Interestingly, no muscle fascia divides the epicardial fat and the myocardium (Figure 1), and therefore the two tissues share the same microcirculation. Because of its anatomical proximity to the heart, and the absence of fascial boundaries, epicardial adipose tissue could interact locally with the myocardium through paracrine or vasocrine secretion of proinflammatory adipokines. Whether this interaction is through a paracrine or vasocrine mechanism is unclear [3].
Table 1.
Main anatomical and clinical characteristics of epicardial fat
| Refs | |
|---|---|
| Anatomical and functional proximity to the myocardium. | [1–5] |
| Located between the myocardium and the visceral layer of the pericardium. | [1–5] |
| More prevalent in the atrioventricular and interventricular grooves. | [1–5] |
| Originates from the splanchnopleuric mesoderm. | [1–5] |
| Vascularization is supplied by branches of the coronary arteries | [1–5] |
| Small adipocytes and mixed cellularity. | [11,12] |
| Metabolically very active and source of several adipokines | [1–4] |
| Local secretion of adipokines into the coronary circulation. | [49,50] |
| Can be measured with echocardiography, multi-detector computed tomography and cardiac magnetic resonance imaging. | [16,53–56] |
| Marker of visceral and myocardial adiposity. | [16,45,53] |
| Correlates with coronary artery disease, subclinical atherosclerosis coronary calcium score, heart morphology, insulin resistance and metabolic syndrome. | [59–75] |
| Rapidly changes and reduces with weight loss, exercise and bariatric surgery. | [83–85] |
| Decreases are related to improved cardiac morphology. | [83] |
| Ethnic, gender and age-related differences. | [15,16] |
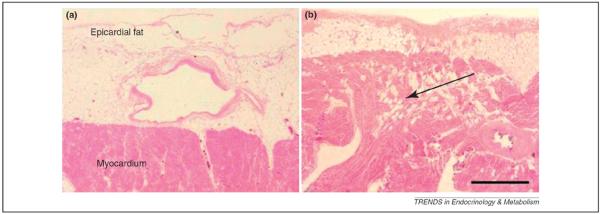
Figure 1.
Microscopic imaging of human epicardial fat. (a) Microscopic appearance of the epicardial layer in the left ventricle. (b) Microscopic appearance of the epicardial layer in the right ventricle. The arrow shows the isles of mature adipocytes. No fascial structure divides the epicardial adipose tissue from the underlying myocardium. Mature adipocytes are more frequent in the right-hand side than the left and can be seen within the subepicardiac myocardium. Scale bar, 1 mm. Figures modified from a previous version published in Iacobellis, G. et al. (2005) Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2, 536–543, Nature Publishing Group, with permission from the publisher.
A further anatomical distinction between myocardial and pericoronary epicardial fat has been suggested [9]. Pericoronary epicardial fat is directly around or on the coronary artery adventitia, whereas myocardial epicardial fat is located directly over the myocardium. Whether these two components could be functionally distinct is unclear. Microscopically, epicardial fat is predominantly composed of adipocytes, but also contains ganglia and interconnecting nerves, inflammatory, stromovascular and immune cells [10]. Epicardial adipocytes are generally smaller than those in subcutaneous and other visceral fat depots, including the pericardial depot [11,12]. The smaller cellular size can be explained by the larger number of pre-adipocytes than mature adipocytes [13]. In general, larger adipocytes are thought to release higher amounts of proinflammatory mediators and lower amounts of anti-inflammatory adipokines. Whether this is true for the epicardial fat is unclear [11–13]. Numerous ganglia and interconnecting nerves are concentrated in epicardial fat [4]. The interaction between epicardial fat and these ganglia is unknown. Whether the epicardial fat changes with aging is controversial [14–16]. Although most autopsy studies failed to find a correlation between epicardial fat and age, a more recent autopsy study detected lower epicardial fat in younger subjects [15]. No significant relationship between echocardiographic epicardial fat thickness and age was described [16].
Physiology of epicardial adipose tissue
Epicardial adipose tissue and myocardial metabolism
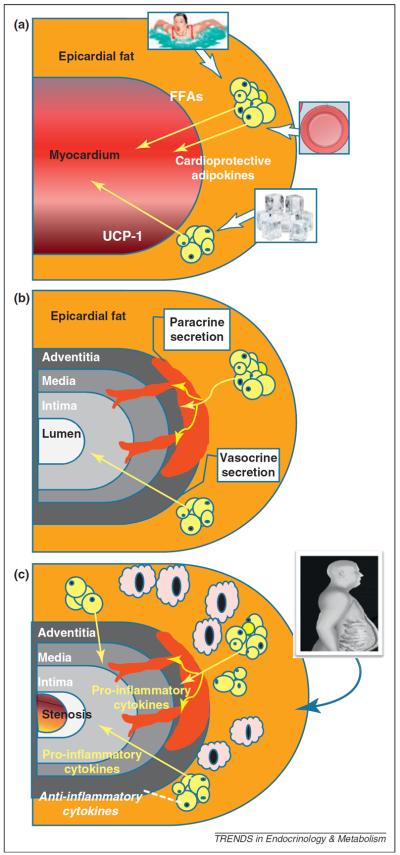
Epicardial fat is not merely a passive lipid-storage unit, but is actively involved in lipid and energy homeostasis (Table 2) [1–4]. The principal difference between epicardial adipose tissue and other visceral fat depots is its greater capacity for release and uptake free fatty acids (FFAs) and a lower rate of glucose utilization. Indeed, FFA synthesis and rates of incorporation and breakdown are significantly higher in epicardial fat than other fat depots [17]. Higher rates of lipolysis and insulin-induced lipogenesis have also been described in guinea pig epicardial fat compared to other fat depots [17]. Lipopotein lipase and acetyl-CoA carboxylase activity are consistently lower in epicardial fat than in subcutaneous fat depots. The myocardium uses and metabolizes FFAs from the coronary arterial blood, and FFA oxidation is responsible for about 50–70% of the energy production of the heart. Hence, epicardial fat has been proposed to function as a buffer to protect the heart against exposure to excessively high levels of FFAs and to provide energy for the myocardium (Figure 2) [1].
Table 2.
Known or attributed physiologic and pathophysiological functions of epicardial fat
| Physiological | Pathophysiological |
|---|---|
|
| |
| Known | Known |
| • Energy source to the myocardium | • Excess free fatty acid synthesis and release |
| • Source of anti-atherogenic and anti-inflammatory adipokines | • Modulation of intra-myocardial fat content |
| • Mechanical protection of the coronary artery | • Intrinsic inflammatory status |
| • Source and secretion of proatherogenic and proinflammatory adipokines | |
| • Correlation with coronary artery disease | |
| • Mechanical relations with bi-ventricular hypertrophy | |
| • Mechanical relations with impaired bi-ventricular diastolic relaxation and filling | |
| • Correlation with atrial fibrillation | |
|
| |
| Attributed | Attributed |
| • Protection of the myocardium against the toxicity of excess free fatty acids | • Functional relationship with the heart |
| • Causal and independent role in coronary artery disease | |
| • Coronary artery positive remodeling | • Causal and independent role in atrial fibrillation |
| • Thermoregulation of the myocardium | • Abnormal regulation of intrinsic cardiac nervous system |
| • Properties as cold-activated brown fat | |
| • Regulation of the intrinsic cardiac nervous system | |
Figure 2.
Potential physiological, pathophysiological mechanisms and vasocrine/paracrine pathways of epicardial fat. (a) Possible physiological roles attributed to the epicardial fat: release of FFAs as energy to the myocardium in condition of high metabolic demand; expression of the thermogenic protein UCP-1 in response of cold exposure; expression and secretion of cardioprotective factors in conditions of normal coronary and local circulation. (b) Putative mechanisms by which adipokines might reach the coronary artery lumen from the epicardial fat. Adipokines from periadventitial epicardial fat could traverse the coronary wall by diffusion from outside to inside, via a paracrine mechanism. Adipokines might also be released from epicardial tissue directly into the vasa vasorum and be transported downstream into the arterial wall via a vasocrine mechanism. (c) Putative pathophysiological role of epicardial fat in CAD: proinflammatory cytokines are highly expressed and secreted into the coronary lumen; anti-inflammatory adipokines are thought to be downregulated. In high-risk subjects, as well as those with metabolic syndrome and excessive visceral fat accumulation, epicardial fat increases in size and cellularity, exhibiting an elevated number of macrophages.
In humans, epicardial fat expresses fatty-acid-binding protein 4, whose expression increases in the metabolic syndrome [18]. Whether fatty-acid-binding protein 4 participates in the intracellular transport of FFAs from epicardial fat into the myocardium is plausible, but remains unexplored. Compared to subcutaneous adipose tissue, human epicardial fat appears to be rich in saturated fatty acids, including myristic acid (14:0), palmitic acid (16:0) and stearic acid (18:0), whereas unsaturated fatty acids, palmitoleic acid (16:1n-7), oleic acid (18:1n-9), linoleic acid (18:2n-6) and linolenic acid (18:3n-3) are less abundant [19]. How FFAs are transported from the epicardial fat and reach the myocardium remains to be elucidated. It has been hypothesized that FFAs could diffuse bi-directionally in interstitial fluid across concentration gradients [1–4]. Epicardial adipose tissue might also secrete vasoactive products that regulate coronary arterial tone to then facilitate FFA influx [3].
Epicardial adipose tissue as brown fat
Brown adipose tissue generates heat in response to cold temperatures and activation of the autonomic nervous system [20]. However, the presence and role of brown fat in humans is unclear. Very recently, expression of uncoupling protein-1 (UCP-1), a marker of brown fat, has been reported in human epicardial fat [21]. UCP-1 expression was significantly higher in human epicardial fat than in other fat depots, therefore suggesting that epicardial fat might function in the same way as brown fat to help to defend the myocardium and coronary artery against hypothermia (Figure 2) [21]. UCP-1 expression was fivefold higher in epicardial fat than substernal fat and was largely undetectable in subcutaneous fat [21].
Clinically, combined positron-emission tomography and computed tomography (PET–CT) has been used to identify adipose tissue with a high rate of uptake of 18F-fluorodeoxyglucose (18F-FDG), indicative of brown fat [22]. 18F-FDG uptake by the heart was reported in normoweight young men after 2 h of exposure to cold air, but uptake was blunted in obese young men and absent upon exposure to 22 °C [22]. Substantial uptake of 18F-fluorodeoxyglucose by the heart also indicates that brown adipose tissue lies in or close to the heart. Whether cold-activated 18F-FDG uptake by the heart is mediated by the epicardial fat layer is unknown. Although these recent findings are intriguing, the role of epicardial fat to serve as brown fat to the myocardium is far from being understood. Perhaps epicardial fat functions similarly to brown fat to defend the myocardium and coronary artery against hypothermia. This hypothesis could be supported by animal models. In fact, polar bears present large amounts of cardiac fat that can be used to store and supply energy to the myocardium during hibernation [17]. How and whether these observations can be translated to humans is unknown.
Between physiology and pathophysiology
Epicardial adipose tissue as an endocrine and paracrine organ
Human epicardial fat is a metabolically active organ and a source of several bioactive molecules (Table 3) that might substantially influence the myocardium and coronary arteries [1,10,23–28]. Two major mechanisms of interaction between the myocardium and the epicardial fat (i.e. paracrine and vasocrine) (Figure 2), have been suggested [3]. Paracrine release of cytokines from periadventitial epicardial fat could traverse the coronary wall by outside-to-inside diffusion. Given the dense inflammatory infiltrate within the human epicardial fat and its complex cellularity, it seems reasonable to suspect that cytokines are secreted by different cells. Adipokines secreted from epicardial adipocytes, stromal and vascular cells could diffuse in interstitial fluid across the adventitia, media, and intima, and then interact respectively with vasa vasorum, endothelial and vascular smooth muscle cells of the coronary arteries. Alternatively, adipokines and FFAs might be released from epicardial tissue directly into vasa vasorum and be transported downstream into the arterial wall, in a vasocrine signaling mechanism [3].
Table 3.
Epicardial adipose tissue bioactive molecules
| Proinflammatory, proatherogenic | Anti-inflammatory, anti-atherogenic |
|
| |
| TNF-α | Adiponectin, Adrenomedullin |
| MCP-1 | |
|
| |
| IL1, IL1β, IL-1Ra, IL6, IL8, IL10 | |
| CRP | |
| PAI-1 | |
| Prostaglandin D(2), haptoglobin, α1-glycoprotein, JNK | |
| sPLA2-IIA, fatty-acid-binding protein 4 | |
| RANTES | |
| ICAM | |
|
| |
| Insulin-mimetic, markers of visceral fat | Thermogenic |
|
| |
| Resistin | UCP 1 |
| Visfatin | |
| Omentin | |
|
| |
| Growth factors | Brown fat differentiation transcription factors |
|
| |
| NGF | PRDM16 |
| FLT1 | PGC-1α |
|
| |
| Vascular remodeling, blood pressure control, myocardial hypertrophy, adipogenesis | |
|
| |
| Angiotensin, Angiotensinogen | |
|
| |
| Leptin | |
|
| |
| Receptors | |
|
| |
| Angiotensin II type 1 receptor | |
| TLRs | |
| PPARγ | |
| GLUT-4 | |
Abbreviations: CRP, C-reactive protein; FLT1, soluble vascular endothelial growth factor receptor; GLUT-4, glucose transporter-4; ICAM, soluble intercellular adhesion molecule; IL, interleukin; IL-1Ra, interleukin-1 receptor antagonist; JNK, c-Jun N-terminal kinase; MCP-1, monocyte chemoattractant protein-1; NGF, nerve growth factor; PAI-1, plasminogen activator inhibitor-1; PGC-1α, PPAR-γ coactivator-1α; sPLA2-IIA, secretory type II phospholipase A2; PPAR-γ, peroxisome-proliferator-activated receptor γ; PRDM16, brown adipocyte differentiation transcription factor PR-domain-missing16; RANTES, regulated upon activation normal T cell and secreted; TLRs, toll-like receptors; TNF-α, tumor necrosis factor-alpha; UCP1, uncoupling protein-1.
Epicardial adipose tissue and atherosclerosis
Epicardial fat has been suggested to play a significant role in the inflammatory process within the atherosclerotic plaque (Table 2). Indeed, a substantial inflammatory infiltrate has been described in epicardial fat obtained during cardiac surgery of subjects with severe coronary artery disease (CAD) [10]. When compared to subcutaneous fat biopsied from the same subjects, epicardial fat showed thickened connective tissue septa with dense inflammatory cell infiltrates, predominantly represented by macrophages [10]. Immunohistochemical characterization of epicardial fat suggests the presence of intravascular and infiltrating inflammatory cells of diverse origin as well as lymphocytes (CD3+), macrophages (CD68+) and mast cells [10]. However, whether epicardial fat inflammation results from atherogenic inflammation in the underlying plaques, or displays an inflammatory status per se is unclear. It is reasonable to postulate that inflammatory signals from the epicardial fat could act reciprocally, due to atherogenic inflammation in the underlying plaques. Increased reactive oxygen species (ROS) in response to regional ischemia and decreased expression or post-translational modifications of antioxidant enzymes in the epicardial fat could activate inflammatory signals [29]. The presence of inflammatory cells in epicardial adipose tissue could also reflect the response to plaque rupture and lead to amplification of vascular inflammation and plaque instability [10].
The paracrine or vasocrine secretion of epicardial inflammatory adipokines, such as tumor necrosis factor alpha (TNF-α), monocyte chemoattractant protein-1 (MCP-1), interleukin-6 (IL-6), IL-1β, plasminogen activator inhibitor-1 (PAI-1), resistin, and many others (Table 3), contributes to the metabolic and inflammatory milieu that promotes atherogenesis [1–4]. Whether the atherogenic effect of the epicardial fat occurs through a vasocrine or paracrine pathways is unclear. It could be hypothesized that the vasocrine signaling might take place during atherogenesis when cell proliferation and plaque formation increase the arterial wall thickness, making paracrine diffusion more difficult. Interestingly, proinflammatory cytokines are expressed in epicardial fat close to the site of adventitial injury. Expression and secretion of inflammatory cytokines are higher in human epicardial adipose tissue than in subcutaneous fat in obese patients with critical CAD [26]. Whether the secretory activity of the epicardial fat is simply related to the total amount is unclear. A mass-dependent mechanism could also be evoked to explain elevated proinflammatory markers in combination with increased epicardial fat [1]. Recent clinical findings indicate that the relation of epicardial fat and CAD is independent of obesity and is instead driven by the excessive visceral fat accumulation [30].
The atherogenic inflammatory process within epicardial fat can also involve the innate inflammatory response. Innate immunity can be activated through toll-like receptors (TLRs) that activate the transcription of inflammatory mediators. Activation of TLRs leads to translocation of nuclear factor-κB (NF-κB) into the nucleus to initiate the transcription of IL-6, IL-1 and TNF-α, followed by the release of IL-6, TNF-α, and of resistin [31]. Epicardial fat obtained from subjects with CAD showed significantly higher NF-κB and c-Jun N-terminal kinase (JNK) activity [31]. Furthermore, greater TLR-2 and TLR-4 expression – strong evidence of the presence of activated macrophages – was found in these patients [31]. In addition, this study showed an increase of the systemic levels of lipopolysaccharides in patients with CAD [31]. Lipopolysaccharides serve as activators of TLR and thus of the inflammatory process [31]. Together, these findings suggest that macrophages and both JNK and NF-κB pathways play a significant role in the inflammatory profile of epicardial fat. The use of anti-inflammatory pharmaceutical agents targeting the fat could positively modulate epicardial fat metabolism and coronary arteries function.
Oxidative stress plays a significant role in the development and progression of atherosclerosis. Human epicardial shows higher mRNA expression in fat of proteins involved in oxidative stress, as mentioned before [29]. Interestingly, higher oxidative stress has been described in epicardial fat when compared to subcutaneous fat [29]. Specifically, in subjects with CAD, epicardial fat exhibits higher levels of ROS products and lower levels of catalase than subcutaneous fat [29]. Catalase is an antioxidant enzyme that protects cells against potentially harmful effects of the ROS H2O2 by converting it to oxygen and water. Reduced catalase expression could contribute to the higher oxidative stress in epicardial fat.
Abnormal epicardial fat can also directly or indirectly affect the endothelium. Increased expression of secretory type II phospholipase A2 (sPLA2-IIA), an enzyme involved in the retention of low-density lipoprotein in the subendothelial space, has been detected in epicardial fat [32]. Of interest, sPLA2-IIA was found in human atherosclerotic lesions, and transgenic mice overexpressing human sPLA2-IIA gene show increased susceptibility to atherosclerosis [33–35].
However, the relation of epicardial fat and oxidative stress is more complex and involves other cytokines and vasoactive factors. The functional relation of leptin, angiotensin II and NO has been clearly established [36]. Leptin inhibits the contractile response induced by angiotensin II in vascular smooth muscle cells (VSMCs) of the aorta [36]. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction is mediated via a NO-dependent mechanism [36]. Interestingly, epicardial fat express both leptin and angiotensin II [1,23]. Whether epicardial fat could affect the coronary arterial endothelium through the effects of these factors is currently unknown, but will certainly merit future studies.
Epicardial fat also induces cell-surface expression of adhesion molecules and enhances adhesion of monocytes to endothelial cells [37]. In addition to MCP-1, other inflammatory mediators, as well as growth-related oncogene α, regulated upon activation normal T cell and secreted (RANTES), and soluble intercellular adhesion molecule (ICAM), have been found in human epicardial adipose tissue [37]. These molecules participate in several stages of the atherosclerotic process including chemotaxis, foam-cell formation, smooth-muscle cell proliferation and migration, and plaque destabilization.
Abnormal glucose metabolism also contributes to the development and progression of atherosclerosis. The relation of epicardial fat with glucose metabolism has been recently addressed. Glucose transporter-4 (GLUT4) mRNA levels are lower in epicardial fat of CAD subjects and cultured epicardial adipocytes release higher levels of renitol-binding protein 4 (RBP4) than subcutaneous adipocytes, in these subjects [38]. Epicardial fat could therefore contribute to a local unfavorable lipid and glucose profile and insulin resistance.
Epicardial fat might contribute to atherosclerosis not only through the secretion of bioactive molecules but also by specific mechanical effects. For example, under physiological conditions epicardial fat could attenuate the coronary artery torsion [39]. Under pathological circumstances, the excessive amount of epicardial fat surrounding the coronary arteries could be detrimental. The presence of atherosclerotic plaque in coronary arteries leads to an asymmetric expansion of the vessel wall that is defined as positive vessel remodeling [39]. Because of its intrinsic compressibility, epicardial fat has therefore been suggested to play a permissive role in vessel expansion [39]. It has been hypothesized that coronary lesions, which are surrounded by the epicardial fat, undergo expansion more easily than those lesions surrounded by the myocardium because, owing to its extravascular resistance, the myocardium is relatively incompressible [39].
All these findings further support the role of epicardial fat in atherosclerosis. An exclusive role of epicardial fat in the development of atherosclerosis has been also hypothesized [3]. This hypothesis is based on the findings that hypercholesterolemic white rabbits develop atherosclerotic lesions only in intraepicardial portions of the left anterior descending coronary artery surrounded by fat [3]. Other studies have questioned the role of epicardial fat in the development of atherosclerosis in specific pathological circumstances, such as in patients with congenital generalized lipodystrophy who develop CAD in absence of excessive visceral fat, including epicardial fat [3]. Nevertheless, human immunodeficiency virus (HIV)-related lipodystrophy is actually associated with increased epicardial fat amount, as clinically described [40]. However, it seems more plausible that a combination of factors, including the epicardial fat, contributes to the development of atherosclerotic plaques.
Epicardial adipose tissue and intra-myocardial fat
Recent evidence has shown that obesity leads not only to increased fat depots in classical adipose tissue locations, but also to significant ectopic lipid accumulation and infiltration within and around other tissues and internal organs [41,42]. It is possible that ectopic fat deposition occurs within the heart to affect cardiovascular function. Non-adipocytes and cardiomyocytes have a very limited capacity to store excess fat. If they are exposed to high levels of plasma lipids, as typically occurs in obesity, they can undergo steatosis and loss of function, ultimately resulting in cardiac lipotoxicity. An infiltration of adipocytes from the epicardial adipose tissue to the myocardium has been suggested and corroborated by clinical studies [43,44]. In fact, there is a significant correlation between echocardiographic epicardial fat thickness and intra-myocardial lipid content, as measured by proton magnetic-resonance spectroscopy [43,44]. Modulation of epicardial fat could result in a direct quantification of changes in the myocardial fat content.
Cardioprotective effects of epicardial adipose tissue
The mechanisms that regulate the balance between protective and harmful effects of epicardial adipose tissue are not clearly understood. Epicardial fat could display cardioprotective properties through local secretion of anti-inflammatory and anti-atherogenic adipokines such as adiponectin and adrenomedullin [45,46]. Adiponectin and adrenomedullin are secreted directly from epicardial fat into the coronary circulation and their mRNA expression independently correlates with their intracoronary levels [47,48]. Chronic CAD is thought to downregulate cardioprotective adipokines in epicardial fat [47,48]. Indeed, in patients with CAD both adiponectin and adrenomedullin expression in epicardial fat is significantly reduced [47,48]. Increased intracoronary adrenomedullin levels have been reported only with improved hemodynamic conditions, such as after coronary revascularization [47,48]. Therefore, epicardial fat could exert its cardioprotective effects through adiponectin and adrenomedullin secretion only under improved hemodynamic conditions or in response to local metabolic or mechanical insults. Other emerging adipokines, such as osteocalcin and osteopontin, could positively interact with epicardial fat function and metabolism [49,50]. Specifically, in line of the potential double role of the epicardial fat, it would be of interest to study the epicardial fat expression of osteopontin and its role in modulating cardiovascular morphology and function [49]. Indeed, a double cardiac role has been attributed to osteopontin – both atherogenic and reparative in the post-infarcted heart [49].
Clinical use of epicardial adipose tissue
Imaging of epicardial adipose tissue
Epicardial fat can be easily detected and measured by imaging. Ultrasound provides a simple and readily available assessment, whereas multi-detector computed tomography (MDCT) or cardiac magnetic-resonance imaging (MRI) allow an accurate but more expensive and cumbersome measurement. Overall, objective and reproducible measurement of epicardial fat is certainly a promising tool in both the clinical and research setting. Epicardial fat thickness was first visualized and measured with transthoracic 2D echocardiography [16,51]. Echocardiographic epicardial fat measurement has several advantages, such as low cost and easy availability, but also has some limitations because it might not reflect the variability of fat thickness or total epicardial fat volume. Epicardial fat can also be measured with MRI and/or MDCT scanning, with the latter being the most used in clinical studies [52–56]. However, regardless of the imaging technique, it is important to visualize and measure epicardial and pericardial fat as two distinct fat depots [2].
Epicardial adipose tissue and the cardio-metabolic risk
Echocardiographic epicardial fat strongly and independently reflects the intra-abdominal visceral fat, and the intra-myocardial fat content, as measured by MRI [16,43]. Hence, echocardiographic epicardial fat can be considered to be a marker of visceral adiposity. Several clinical studies have shown that epicardial fat thickness is significantly and independently related to the metabolic syndrome [57,58]. When cardio-metabolic risk factors are considered separately, epicardial fat independently associates with markers of insulin resistance [59], fasting glucose [60], inflammatory markers [61,62], liver enzymes [63] and carotid intima media thickness (C-IMT) [40]. Epicardial fat, as measured with either echocardiography or MDCT, has also been associated with the presence and severity of CAD, and presence of coronary calcification, in a large number of studies [30,64–73]. Increased epicardial fat thickness has also been associated with changes in left and right ventricle mass and diastolic function [74–77]. Left ventricular hypertrophy is related to a consensual and proportionate increase in epicardial fat mass, independent of obesity and age. Mechanical and bio-molecular mechanisms could explain these correlations. Interestingly, epicardial fat has also been recently associated with atrial fibrillation [77–79]. This correlation could be explained by excess release of FFAs from the epicardial fat into the cardiomyocytes or by the mechanical effects of excess epicardial fat causing an enlarged left atrium [80], but the explanation remains unclear.
An interesting clinical application of the echocardiographic epicardial fat is its potential use as a therapeutic target during weight-loss interventions including exercise or pharmaceutical treatments [81–86]. Epicardial fat decreased after a very low calorie diet, bariatric surgery-induced weight loss, or moderate aerobic exercise [81–83]. Of interest, the decrease in epicardial fat during weight loss was quicker and larger than the decrease in common indices of body fatness [81].
Concluding remarks
Epicardial fat is a heart fat depot with unique anatomical and functional characteristics. Epicardial adipocytes are smaller in size than those of other fat depots, and with different fatty acid composition, higher rates of FFA synthesis, uptake and release, and lower rates of glucose utilization. Epicardial fat is also a source of several bioactive molecules and, given its anatomical proximity to the heart, it is thought to interact locally with the coronary arteries and the myocardium through paracrine or vasocrine pathways. A dichotomous role, both unfavorable and protective, has been attributed to epicardial fat. Under physiological and metabolically high-demand conditions, epicardial fat supplies energy and heat to the myocardium and exerts a protective modulation of the coronary arteries. Its pathological increase, and the co-occurrence of other metabolic and hemodynamic abnormalities, turn it into an adverse lipotoxic, prothrombotic and proinflammatory organ. It remains to be determined what regulates the equilibrium between its harmful and protective effects.
Future clinical and experimental studies should aim to elucidate better the function of epicardial fat in high-demand physiological conditions such as intense exercise and cold exposure. Understanding the role of epicardial fat in atherosclerosis is also a key and still unanswered question. The use of genetically-engineered animal models, such as animals which spontaneously develop atherosclerosis, diabetes or hypercholesterolemia, would significantly broaden our understanding of the role of the epicardial fat in these cardio-metabolic diseases [87]. Based on the small number of animal studies that have been published to date [9,14], rats and pigs could represent the primary choice of genetically-engineered animals for gaining insights into the function of the epicardial fat. Evaluation of novel and emerging epicardial fat adipokines and adipose-derived hormones [88] would also contribute favorably to our understanding of the mechanistic role of this fat depot in cardio-metabolic diseases. Clinically, epicardial fat and its changes can be objectively measured and monitored by imaging techniques, and this makes its assessment an appealing tool in both the clinical and research settings. Based on recent data, it is plausible that modulation of epicardial fat by improved local vascularization, weight loss, and targeted pharmaceutical interventions, might restore epicardial fat to its physiological role. Future studies addressing these important clinical questions are warranted.
References
- 1.Iacobellis G, et al. Epicardial adipose tissue: anatomic, biomolecular and clinical relationships with the heart. Nat. Clin. Pract. Cardiovasc. Med. 2005;2:536–543. doi: 10.1038/ncpcardio0319. [DOI] [PubMed] [Google Scholar]
- 2.Iacobellis G. Epicardial and pericardial fat: close, but very different. Obesity. 2009;17:625. doi: 10.1038/oby.2008.575. [DOI] [PubMed] [Google Scholar]
- 3.Sacks HS, Fain JN. Human epicardial adipose tissue: a review. Am. Heart J. 2007;153:907–917. doi: 10.1016/j.ahj.2007.03.019. [DOI] [PubMed] [Google Scholar]
- 4.Rabkin RW. Epicardial fat: properties, function and relationship to obesity. Obes. Rev. 2007;8:253–261. doi: 10.1111/j.1467-789X.2006.00293.x. [DOI] [PubMed] [Google Scholar]
- 5.Corradi D, et al. The ventricular epicardial fat is related to the myocardial mass in normal, ischemic and hypertrophic hearts. Cardiovasc. Pathol. 2004;13:313–316. doi: 10.1016/j.carpath.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 6.Iacobellis G, et al. Do cardiac and perivascular adipose tissue play a role in atherosclerosis? Curr. Diab. Rep. 2008;8:20–24. doi: 10.1007/s11892-008-0005-2. [DOI] [PubMed] [Google Scholar]
- 7.Greif M, et al. Pericardial adipose tissue determined by dual score CT is a risk factor for coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 8.Gorter PM, et al. Quantification of epicardial and peri-coronary fat using cardiac computed tomography: reproducibility and relation with obesity and metabolic syndrome in patients suspected of CAD. Atherosclerosis. 2008;197:896–903. doi: 10.1016/j.atherosclerosis.2007.08.016. [DOI] [PubMed] [Google Scholar]
- 9.Company JM, et al. Epicardial fat gene expression after aerobic exercise training in pigs with coronary atherosclerosis: relationship to visceral and subcutaneous fat. J. Appl. Physiol. 2010;109:1904–1912. doi: 10.1152/japplphysiol.00621.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Mazurek T, et al. Human epicardial adipose tissue is a source of inflammatory mediators. Circulation. 2003;108:2460–2466. doi: 10.1161/01.CIR.0000099542.57313.C5. [DOI] [PubMed] [Google Scholar]
- 11.Bambace C, et al. Adiponectin gene expression and adipocyte diameter: a comparison between epicardial and subcutaneous adipose tissue in men. Cardiovasc. Pathol. 2010 doi: 10.1016/j.carpath.2010.07.005. DOI: 10.1016/j.carpath.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 12.Eiras S, et al. Relationship between epicardial adipose tissue adipocyte size and MCP-1 expression. Cytokine. 2010;51:207–212. doi: 10.1016/j.cyto.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 13.Caprio M, et al. Antiadipogenic effects of the mineralocorticoid receptor antagonist drospirenone: potential implications for the treatment of metabolic syndrome. Endocrinology. 2011;152:113–125. doi: 10.1210/en.2010-0674. [DOI] [PubMed] [Google Scholar]
- 14.Fei J, et al. Age and sex mediated changes in epicardial fat adipokines. Atherosclerosis. 2010;212:488–494. doi: 10.1016/j.atherosclerosis.2010.06.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tansey DK, et al. Fat in the right ventricle of the normal heart. Histopathology. 2005;46:98–104. doi: 10.1111/j.1365-2559.2005.02054.x. [DOI] [PubMed] [Google Scholar]
- 16.Iacobellis G, Willens H. Echocardiographic epicardial fat: a review of research and clinical applications. J. Am. Soc. Echocardiogr. 2009;23:1311–1319. doi: 10.1016/j.echo.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 17.Marchington JM, et al. Adipose tissue in the mammalian heart and pericardium; structure, foetal development and biochemical properties. Comp. Biochem. Physiol. 1989;94B:225–232. doi: 10.1016/0305-0491(89)90337-4. [DOI] [PubMed] [Google Scholar]
- 18.Vural B, et al. Presence of fatty-acid-binding protein 4 expression in human epicardial adipose tissue in metabolic syndrome. Cardiovasc. Pathol. 2008;17:392–398. doi: 10.1016/j.carpath.2008.02.006. [DOI] [PubMed] [Google Scholar]
- 19.Pezeshkian M, et al. Fatty acid composition of epicardial and subcutaneous human adipose tissue. Metab. Syndr. Relat. Disord. 2009;7:125–131. doi: 10.1089/met.2008.0056. [DOI] [PubMed] [Google Scholar]
- 20.Richard D, Picard F. Brown fat biology and thermogenesis. Front. Biosci. 2011;16:1233–1260. doi: 10.2741/3786. [DOI] [PubMed] [Google Scholar]
- 21.Sacks HS, et al. Uncoupling Protein-1 and related mRNAs in human epicardial and other adipose tissues: epicardial fat functioning as brown fat. J. Clin. Endocrinol. Metab. 2009;94:3611–3615. doi: 10.1210/jc.2009-0571. [DOI] [PubMed] [Google Scholar]
- 22.van Marken Lichtenbelt WD, et al. Cold-activated brown adipose tissue in healthy men. N. Engl. J. Med. 2009;360:1500–1508. doi: 10.1056/NEJMoa0808718. [DOI] [PubMed] [Google Scholar]
- 23.Baker AR, et al. Human epicardial adipose tissue expresses a pathogenic profile of adipocytokines in patients with cardiovascular disease. Cardiovasc. Diabetol. 2006;13:1. doi: 10.1186/1475-2840-5-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chaldakov GN, et al. Neurotrophin presence in human coronary atherosclerosis and metabolic syndrome: a role for NGF and BDNF in cardiovascular disease? Prog. Brain Res. 2004;146:279–289. doi: 10.1016/S0079-6123(03)46018-4. [DOI] [PubMed] [Google Scholar]
- 25.Fain JN, et al. Identification of omentin mRNA in human epicardial adipose tissue: comparison to omentin in subcutaneous, internal mammary artery periadventitial and visceral abdominal depots. Int. J. Obes. (Lond.) 2008;32:810–815. doi: 10.1038/sj.ijo.0803790. [DOI] [PubMed] [Google Scholar]
- 26.Kremen J, et al. Increased subcutaneous and epicardial adipose tissue production of proinflammatory cytokines in cardiac surgery patients: possible role in postoperative insulin resistance. J. Clin. Endocrinol. Metab. 2006;91:4620–4627. doi: 10.1210/jc.2006-1044. [DOI] [PubMed] [Google Scholar]
- 27.Cheng KH, et al. Adipocytokines and proinflammatory mediators from abdominal and epicardial adipose tissue in patients with CAD. Int. J. Obes. (Lond.) 2008;32:268–274. doi: 10.1038/sj.ijo.0803726. [DOI] [PubMed] [Google Scholar]
- 28.Fain JN, et al. Human epicardial adipokine messenger RNAs: comparisons of their expression in substernal, subcutaneous, and omental fat. Metabolism. 2010;59:1379–1386. doi: 10.1016/j.metabol.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 29.Salgado-Somoza A, et al. Proteomic analysis of epicardial and subcutaneous adipose tissue reveals differences in proteins involved in oxidative stress. Am. J. Physiol. Heart Circ. Physiol. 2010;299:H202–H209. doi: 10.1152/ajpheart.00120.2010. [DOI] [PubMed] [Google Scholar]
- 30.Iacobellis G, et al. Epicardial fat thickness and CAD correlate independently of obesity. Int. J. Cardiol. 2011;146:452–454. doi: 10.1016/j.ijcard.2010.10.117. [DOI] [PubMed] [Google Scholar]
- 31.Baker AR. Epicardial adipose tissue as a source of nuclear factor-kappaB and c-Jun N-terminal kinase mediated inflammation in patients with coronary artery disease. J. Clin. Endocrinol. Metab. 2009;94:261–267. doi: 10.1210/jc.2007-2579. [DOI] [PubMed] [Google Scholar]
- 32.Dutour A, et al. Secretory type II phospholipase A2 is produced and secreted by epicardial adipose tissue and overexpressed in patients with CAD. J. Clin. Endocrinol. Metab. 2010;95:963–967. doi: 10.1210/jc.2009-1222. [DOI] [PubMed] [Google Scholar]
- 33.Ivandic B, et al. Role of group II secretory phospholipase A2 in atherosclerosis: 1. Increased atherogenesis and altered lipoproteins in transgenic mice expressing group IIa phospholipase A2. Arterioscler. Thromb. Vasc. Biol. 1999;19:1284–1290. doi: 10.1161/01.atv.19.5.1284. [DOI] [PubMed] [Google Scholar]
- 34.Menschikowski M, et al. Expression of secretory group IIA phospholipase A(2) in relation to the presence of microbial agents, macrophage infiltrates, and transcripts of proinflammatory cytokines in human aortic tissues. Arterioscler. Thromb. Vasc. Biol. 2000;20:751–762. doi: 10.1161/01.atv.20.3.751. [DOI] [PubMed] [Google Scholar]
- 35.Kugiyama K, et al. Circulating levels of secretory type II phospholipase A(2) predict coronary events in patients with coronary artery disease. Circulation. 1999;100:1280–1284. doi: 10.1161/01.cir.100.12.1280. [DOI] [PubMed] [Google Scholar]
- 36.Rodríguez A, et al. The inhibitory effect of leptin on angiotensin II-induced vasoconstriction in vascular smooth muscle cells is mediated via a nitric oxide-dependent mechanism. Endocrinology. 2007;148:324–331. doi: 10.1210/en.2006-0940. [DOI] [PubMed] [Google Scholar]
- 37.Karastergiou K, et al. Epicardial adipokines in obesity and CAD induce atherogenic changes in monocytes and endothelial cells. Arterioscler. Thromb. Vasc. Biol. 2010;30:1340–1346. doi: 10.1161/ATVBAHA.110.204719. [DOI] [PubMed] [Google Scholar]
- 38.Salgado-Somoza A, et al. Coronary artery disease is associated with higher epicardial Renitol binding protein 4 (RBP4) and lower glucose transporter (GLUT) 4 levels in epicardial and subcutaneous adipose tissue. Clin. Endocrinol. (Oxf.) 2011 doi: 10.1111/j.1365-2265.2011.04140.x. DOI: 10.1111/j.1365-2265.2011.04140.x. [DOI] [PubMed] [Google Scholar]
- 39.Prati F, et al. Eccentric atherosclerotic plaques with positive remodelling have a pericardial distribution: a permissive role of epicardial fat? A three-dimensional intravascular ultrasound study of left anterior descending artery lesions. Eur. Heart J. 2003;24:329–336. doi: 10.1016/s0195-668x(02)00426-8. [DOI] [PubMed] [Google Scholar]
- 40.Iacobellis G, et al. Relationship of subepicardial adipose tissue with carotid intima media thickness in HIV-infected patients. Am. J. Cardiol. 2007;99:1470–1472. doi: 10.1016/j.amjcard.2006.12.082. [DOI] [PubMed] [Google Scholar]
- 41.Zhou YT, et al. Lipotoxic heart disease in obese rats: implications for human obesity. Proc. Natl. Acad. Sci. U.S.A. 2000;97:1784–1789. doi: 10.1073/pnas.97.4.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Gastaldelli A, Basta G. Ectopic fat and cardiovascular disease: what is the link. Nutr. Metab. Cardiovasc. Dis. 2010;20:481–490. doi: 10.1016/j.numecd.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 43.Malavazos AE, et al. Relation of echocardiographic epicardial fat thickness and myocardial fat. Am. J. Cardiol. 2010;105:1831–1835. doi: 10.1016/j.amjcard.2010.01.368. [DOI] [PubMed] [Google Scholar]
- 44.Kankaanpaa M, et al. Myocardial triglyceride content and epicardial adipose mass in human obesity: relationship to left ventricular function and serum free fatty acid levels. J. Clin. Endocrinol. Metab. 2006;91:4689–4695. doi: 10.1210/jc.2006-0584. [DOI] [PubMed] [Google Scholar]
- 45.Iacobellis G, et al. Adiponectin expression in human epicardial adipose tissue in vivo is lower in patients with CAD. Cytokine. 2005;29:251–255. doi: 10.1016/j.cyto.2004.11.002. [DOI] [PubMed] [Google Scholar]
- 46.Silaghi A, et al. Expression of adrenomedullin in human epicardial adipose tissue: role of coronary status. Am. J. Physiol. Endocrinol. Metab. 2007;293:E1443–E1450. doi: 10.1152/ajpendo.00273.2007. [DOI] [PubMed] [Google Scholar]
- 47.Iacobellis G, et al. Epicardial adipose tissue adiponectin expression is related to intracoronary adiponectin levels. Horm. Metab. Res. 2009;41:227–231. doi: 10.1055/s-0028-1100412. [DOI] [PubMed] [Google Scholar]
- 48.Iacobellis G, et al. Epicardial adipose tissue and intracoronary adrenomedullin levels in CAD. Horm. Metab. Res. 2009;45:855–860. doi: 10.1055/s-0029-1231081. [DOI] [PubMed] [Google Scholar]
- 49.Gomez-Ambrosi J, et al. Plasma osteopontin levels and expression in adipose tissue are increased in obesity. J. Clin. Endocrinol. Metab. 2007;92:3719–3727. doi: 10.1210/jc.2007-0349. [DOI] [PubMed] [Google Scholar]
- 50.Fernández-Real JM, et al. The relationship of serum osteocalcin concentration to insulin secretion, sensitivity, and disposal with hypocaloric diet and resistance training. J. Clin. Endocrinol. Metab. 2009;94:237–245. doi: 10.1210/jc.2008-0270. [DOI] [PubMed] [Google Scholar]
- 51.Iacobellis G, et al. Epicardial fat from echocardiography: a new method for visceral adipose tissue prediction. Obes. Res. 2003;11:304–310. doi: 10.1038/oby.2003.45. [DOI] [PubMed] [Google Scholar]
- 52.Nelson AJ, et al. Validation of cardiovascular magnetic resonance assessment of pericardial adipose tissue volume. J. Cardiovasc. Magn. Reson. 2009;11:15. doi: 10.1186/1532-429X-11-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Greif M, et al. Pericardial adipose tissue determined by dual source CT is a risk factor for coronary atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2009;29:781–786. doi: 10.1161/ATVBAHA.108.180653. [DOI] [PubMed] [Google Scholar]
- 54.Sarin S, et al. Clinical significance of epicardial fat measured using cardiac multislice computed tomography. Am. J. Cardiol. 2008;102:767–771. doi: 10.1016/j.amjcard.2008.04.058. [DOI] [PubMed] [Google Scholar]
- 55.Rosito GA, et al. Pericardial fat, visceral abdominal fat, cardiovascular disease risk factors, and vascular calcification in a community-based sample: the Framingham Heart Study. Circulation. 2008;117:605–613. doi: 10.1161/CIRCULATIONAHA.107.743062. [DOI] [PubMed] [Google Scholar]
- 56.Fox CS, et al. Pericardial fat, intrathoracic fat, and measures of left ventricular structure and function: the Framingham Heart Study. Circulation. 2009;119:1586–1591. doi: 10.1161/CIRCULATIONAHA.108.828970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Iacobellis G, et al. Threshold values of high-risk echocardiographic epicardial fat thickness. Obesity. 2008;16:887–892. doi: 10.1038/oby.2008.6. [DOI] [PubMed] [Google Scholar]
- 58.Iacobellis G, et al. Echocardiographic epicardial adipose tissue is related to anthropometric and clinical parameters of metabolic syndrome: a new indicator of cardiovascular risk. J. Clin. Endocrinol. Metab. 2003;88:5163–5168. doi: 10.1210/jc.2003-030698. [DOI] [PubMed] [Google Scholar]
- 59.Iacobellis G, Leonetti F. Epicardial adipose tissue and insulin resistance in obese subjects. J. Clin. Endocrinol. Metab. 2005;90:6300–6302. doi: 10.1210/jc.2005-1087. [DOI] [PubMed] [Google Scholar]
- 60.Iacobellis G, et al. Relationship of epicardial fat thickness and fasting glucose. Int. J. Cardiol. 2008;128:424–426. doi: 10.1016/j.ijcard.2007.12.072. [DOI] [PubMed] [Google Scholar]
- 61.Malavazos AE, et al. Epicardial fat thickness: Relationship with plasma visfatin and plasminogen activator inhibitor-1 levels in visceral obesity. Nutr. Metab. Cardiovasc. Dis. 2008;18:523–530. doi: 10.1016/j.numecd.2007.09.001. [DOI] [PubMed] [Google Scholar]
- 62.Yun KH, et al. Relationship between the echocardiographic epicardial adipose tissue thickness and serum adiponectin in patients with angina. J. Cardiovasc. Ultrasound. 2009;17:121–126. doi: 10.4250/jcu.2009.17.4.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Iacobellis G, et al. Relation of epicardial fat and alanine aminotransferase in subjects with increased visceral fat. Obesity (Silver Spring) 2008;16:179–183. doi: 10.1038/oby.2007.50. [DOI] [PubMed] [Google Scholar]
- 64.Ahn SG, et al. Relationship of epicardial adipose tissue by echocardiography to cad. Heart. 2008;94:e7. doi: 10.1136/hrt.2007.118471. [DOI] [PubMed] [Google Scholar]
- 65.Jeong JW, et al. Echocardiographic epicardial fat thickness and CAD. Circ. J. 2007;71:536–539. doi: 10.1253/circj.71.536. [DOI] [PubMed] [Google Scholar]
- 66.Sade LE, et al. Relation between epicardial fat thickness and coronary flow reserve in women with chest pain and angiographically normal coronary arteries. Atherosclerosis. 2009;204:580–585. doi: 10.1016/j.atherosclerosis.2008.09.038. [DOI] [PubMed] [Google Scholar]
- 67.Eroglu S, et al. Epicardial adipose tissue thickness by echocardiography is a marker for the presence and severity of CAD. Nutr. Metab. Cardiovasc. Dis. 2009;19:211–217. doi: 10.1016/j.numecd.2008.05.002. [DOI] [PubMed] [Google Scholar]
- 68.Gorter PM, et al. Relation of epicardial and pericoronary fat to coronary atherosclerosis and coronary artery calcium in patients undergoing coronary angiography. Am. J. Cardiol. 2008;102:380–385. doi: 10.1016/j.amjcard.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 69.De Vos AM, et al. Peri-coronary epicardial adipose tissue is related to cardiovascular risk factors and coronary artery calcification in post-menopausal women. Eur. Heart J. 2008;29:777–783. doi: 10.1093/eurheartj/ehm564. [DOI] [PubMed] [Google Scholar]
- 70.Ahmadi N, et al. Increased epicardial, pericardial, and subcutaneous adipose tissue is associated with the presence and severity of coronary artery calcium. Acad. radiol. 2010;17:1518–1524. doi: 10.1016/j.acra.2010.08.017. [DOI] [PubMed] [Google Scholar]
- 71.Alexopoulos N, et al. Epicardial adipose tissue and coronary artery plaque characteristics. Atherosclerosis. 2010;210:150–154. doi: 10.1016/j.atherosclerosis.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 72.Djaberi R, et al. Relation of epicardial adipose tissue to coronary atherosclerosis. Am. J. Cardiol. 2008;102:1602–1607. doi: 10.1016/j.amjcard.2008.08.010. [DOI] [PubMed] [Google Scholar]
- 73.Bucci M, et al. Intrapericardial, but not extrapericardial, fat is an independent predictor of impaired hyperemic coronary perfusion in CAD. Arterioscler. Thromb. Vasc. Biol. 2011;31:211–218. doi: 10.1161/ATVBAHA.110.213827. [DOI] [PubMed] [Google Scholar]
- 74.Iacobellis G, et al. Relation between epicardial adipose tissue and left ventricular mass. Am. J. Cardiol. 2004;94:1084–1087. doi: 10.1016/j.amjcard.2004.06.075. [DOI] [PubMed] [Google Scholar]
- 75.Iacobellis G. Relation of epicardial fat thickness to right ventricular cavity size in obese subjects. Am. J. Cardiol. 2009;104:1601–1602. doi: 10.1016/j.amjcard.2009.07.032. [DOI] [PubMed] [Google Scholar]
- 76.Iacobellis G, et al. Relationship of epicardial adipose tissue with atrial dimensions and diastolic function in morbidly obese subjects. Int. J. Cardiol. 2007;115:272–273. doi: 10.1016/j.ijcard.2006.04.016. [DOI] [PubMed] [Google Scholar]
- 77.Shin SY, et al. Total, interatrial epicardial adipose tissues are independently associated with left atrial remodeling in patients with atrial fibrillation. J. Cardiovasc. Electrophysiol. 2011;22:647–655. doi: 10.1111/j.1540-8167.2010.01993.x. [DOI] [PubMed] [Google Scholar]
- 78.Thanassoulis G, et al. Pericardial fat is associated with prevalent atrial fibrillation: the Framingham Heart Study. Circ. Arrhythm. Electrophysiol. 2010;3:345–350. doi: 10.1161/CIRCEP.109.912055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Al Chekakie MO, et al. Pericardial fat is independently associated with human atrial fibrillation. J. Am. Coll. Cardiol. 2010;56:784–788. doi: 10.1016/j.jacc.2010.03.071. [DOI] [PubMed] [Google Scholar]
- 80.Iacobellis G. Is obesity a risk factor for atrial fibrillation? Nat. Clin. Pract. Cardiovasc. Med. 2005;2:134–135. doi: 10.1038/ncpcardio0132. [DOI] [PubMed] [Google Scholar]
- 81.Iacobellis G, et al. Substantial changes in epicardial fat thickness after weight loss in severely obese subjects. Obesity (Silver Spring) 2008;16:1693–1697. doi: 10.1038/oby.2008.251. [DOI] [PubMed] [Google Scholar]
- 82.Willens HJ, et al. Effects of weight loss after bariatric surgery on epicardial fat measured using echocardiography. Am. J. Cardiol. 2007;99:1242–1245. doi: 10.1016/j.amjcard.2006.12.042. [DOI] [PubMed] [Google Scholar]
- 83.Kim MK, et al. Aerobic exercise training reduces epicardial fat in obese men. J. Appl. Physiol. 2009;106:5–11. doi: 10.1152/japplphysiol.90756.2008. [DOI] [PubMed] [Google Scholar]
- 84.Lanes R, et al. Endothelial function, carotid artery intima-media thickness, epicardial adipose tissue, and left ventricular mass and function in growth hormone-deficient adolescents: apparent effects of growth hormone treatment on these parameters. J. Clin. Endocrinol. Metab. 2005;90:3978–3982. doi: 10.1210/jc.2005-0091. [DOI] [PubMed] [Google Scholar]
- 85.Park JH, et al. Effects of statins on the epicardial fat thickness in patients with coronary artery stenosis underwent percutaneous coronary intervention: comparison of atorvastatin with simvastatin/ezetimibe. J. Cardiovasc. Ultrasound. 2010;18:121–126. doi: 10.4250/jcu.2010.18.4.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Sacks HS, et al. Inflammatory genes in epicardial fat contiguous with coronary atherosclerosis in the metabolic syndrome and type 2 diabetes: changes associated with pioglitazone. Diabetes Care. 2011;34:730–733. doi: 10.2337/dc10-2083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiangdong L, et al. Animal models for the atherosclerosis research: a review. Protein Cell. 2011;2:189–201. doi: 10.1007/s13238-011-1016-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Frühbeck G, Gómez-Ambrosi J. Rationale for the existence of additional adipostatic hormones. FASEB J. 2001;15:1996–2006. doi: 10.1096/fj.00-0829hyp. [DOI] [PubMed] [Google Scholar]