Abstract
We examined the long-term effects of prenatal cigarette smoke exposure (PCSE) on the behavior problems and smoking behavior of 22-year-old offspring. The mothers of these offspring were interviewed about their tobacco and other drug use during pregnancy at the fourth and seventh gestational months, and at delivery. Data on the offspring are from interviews at age 22 (n=608). Behavior problems were measured by the Adult Self-Report (ASR) with the following outcome scales: total behavior problems, externalizing, internalizing, attention, anxiety/depression, withdrawn, thought, intrusive, aggression, somatic and rule breaking behavioral problems. Young adult smoking behavior was measured using self-reported average daily cigarettes, and was validated with urine cotinine. Nicotine dependence was measured with the Fagerström Tobacco and Nicotine Dependence (FTND) scale. Regression analyses tested the relations between trimester-specific PCSE and young adult’s behavioral problems and smoking behavior, adjusting for demographic and maternal psychological characteristics, and other prenatal substance exposures. Exposed young adults had significantly higher scores on the externalizing, internalizing, aggression, and somatic scales of the ASR. These young adults were also more likely to have a history of arrests. Young adults with PCSE also had a higher rate of smoking and nicotine dependence. Our previous findings of the relations between PCSE and aggressive behavior in early childhood and PCSE and smoking behavior in early adolescence extend into young adulthood.
Keywords: Prenatal cigarette smoking, Behavior problems, Young adulthood, Nicotine dependence
1. Introduction
Cigarette smoking is the leading cause of preventable morbidity and mortality (USDHHS, 2010), yet tobacco is the most commonly used substance during pregnancy. National studies show that, in spite of known adverse effects, 10 to 15% of pregnant women smoke (Allen et al., 2008; Cornelius and Day, 2000; Willford et al., 2006). Prenatal cigarette smoke exposure (PCSE) is associated with multiple adverse outcomes in offspring (Batstra et al., 2003; Cornelius and Day, 2009; Cornelius et al., 2011; Eskenazi and Trupin, 1995; Huizink and Mulder, 2006). PCSE affects central nervous system (CNS) development, predisposing the offspring to neurobehavioral deficits manifested as irritability and difficult temperament during infancy and poor self-regulation during childhood (Stroud et al., 2009). PCSE is consistently associated with behaviors such as hyperactivity and attention deficits (Button et al., 2005; Kotimaa et al., 2003; Langley et al., 2008; Thapar et al., 2003), and predicts other difficulties in self-regulation, such as aggressive behavior (Brion et al., 2010; Carter et al., 2008; Cornelius et al., 2007; Day et al., 2000; Gatzke-Kopp and Beauchaine, 2007; Huijbregts et al., 2008; Ruckinger et al., 2010), antisocial behavior (Ernst et al., 2001; Wakschlag et al., 2002) and conduct problems in childhood (Baler et al., 2008; Fergusson et al., 2007; Maughan et al., 2004) and adolescence (Wakschlag et al., 2002, 2006). However, few studies have examined the relations between PCSE and these outcomes in young adulthood.
Recent studies of the effects of PCSE on risk for smoking behavior in offspring have replicated and extended earlier reports (Kandel et al., 1994; Cornelius et al., 2000, 2005). Menezes et al. (2007) found an association between PCSE and smoking behavior in early adolescents. Agrawal et al. (2010) demonstrated that PCSE was associated with earlier age of initiation and earlier regular smoking. O’Callaghan et al. (2009) reported an association between PCSE and nicotine dependence in young adulthood. Further, these effects appear to be direct as Miles and Weden (2012) showed that the relation between PCSE and adolescent smoking was not mediated through childhood behavior problems. However, many of these studies did not control for other prenatal substance exposures and did not examine potential trimester-specific effects. Examining effects of exposures during various stages of fetal development is important because critical developmental changes occur at specific times during gestation (Miller, 2006). In addition, many previous studies did not gather prenatal exposure information during pregnancy, but rather used retrospective designs leading to potential recall bias.
Animal models demonstrate a plausible mechanism for the neuroteratogenic influence of nicotine on the fetus. Nicotine provokes alterations in neural cell replication leading to deficits in synaptic neurochemistry (Ernst et al., 2001; Slotkin, 2008), which leads to difficulties in behavioral self-regulation. Slotkin (2008) noted that prenatal nicotine exposure resulted in permanent changes in synaptic function and that prenatal exposure sensitized offspring to subsequent effects of nicotine.
Other factors can lead to neurobehavioral deficits in human offspring, and women who smoke during pregnancy are more likely to exhibit these risk factors, including psychopathology (Huijbregts et al., 2008), low SES (Martin et al., 2008), and use of other substances during pregnancy (Adams et al., 2008; Burns et al., 2008; Goldschmidt et al., in press). Therefore, it is important to control for demographic factors, maternal psychopathology and other prenatal substance exposures when testing for the teratological effects of PCSE.
The goal of this study was to examine the long-term effects of PCSE on the behavioral outcomes of a cohort followed from gestation to young adulthood. We collected trimester-specific data during pregnancy and at multiple points in the postpartum including tobacco, alcohol, marijuana and other illicit drugs during and in the postpartum. We assessed behavioral outcomes with the Adult Self Report (Achenbach and Rescorla, 2003), and measured cigarette smoking and dependence using the Fagerström Nicotine and Dependence Scale (Heatherton et al., 1991). We hypothesized that PCSE would predict 1) increased rates of behavioral problems, 2) greater levels of smoking, and 3) nicotine dependence in young adult offspring after controlling for significant covariates.
2. Material and methods
2.1. Sample selection and study design
Data collection for the initial phase of this study began in 1982 and was completed in 1985. Pregnant women who were at least 18 years of age were recruited from a prenatal clinic in their fourth month. The study was approved by the Institutional Review Board of the University of Pittsburgh and Human Experimentation Committee of the Magee-Womens Hospital. A total of 1360 women completed an initial interview. After this initial interview, two cohorts were selected. One cohort was composed of women who reported using marijuana at least two times per month and a randomly selected equal-sized sample of women who reported they used less marijuana or none at all. The other cohort was composed of women who reported they drank three or more alcoholic drinks per week during their first trimester and an equal number of women who drank less than this amount or not at all. A woman was eligible to be in either or both of the cohorts. The cohorts have been combined for these analyses, giving a total 829 women. There were no significant demographic differences between cohorts. The non-overlapping groups differed by their level of alcohol and marijuana use, but not cigarette smoking or behavior problems.
The women were interviewed again in their seventh prenatal month and at delivery. Subsequent interviews were conducted at 8 and 18 months, 3, 6, 10, 14, 16, and 22 years. Growth, cognitive development, and behavior were assessed at each follow-up using standardized and age-appropriate instruments. Demographic information and measures of offspring and maternal psychological, social, and environmental factors were gathered at each interview. This report uses data from the prenatal period and the 22-year follow-up assessment.
2.2. Sample description
The birth cohort included 763 mothers and their live-born singletons. At the 22-year phase, 608 (80%) of these offspring were interviewed. Subject loss between birth and this phase included the following: 30 refusals at the 22-year phase, 29 who had moved out of Pittsburgh, 56 lost to follow-up, 11 deaths, 3 adoptions, 18 institutionalizations, and 8 who were unable to participate due to low cognitive functioning (mental retardation, fibrous dysplasia, cerebral palsy). There were no significant differences in prenatal cigarette, alcohol, marijuana exposure or maternal demographics between those who participated in this phase (n=608) and those who were not included (n=155).
The initial sample reflected the racial and social composition of an inner city prenatal clinic population. Fifty-two percent were African-American and 48% were Caucasian. The average family income of the participants was approximately $400 per month between 1982 and 1985. The average age of the mothers at recruitment was 23 years (range: 18–42). Thirty-two percent were married, 25% worked outside the home, and 33% were primigravidas.
The average age of the offspring at the 22-year assessment was 22.8 years (range: 21–26). Fifty-seven percent were African-American and 48% were males. Their average education was 12.8 years, with 48% having some education beyond high school. Sixty percent worked full or part-time, 27% were currently attending school, and 4% were in the military. Less than 6 percent were married, 28% lived with a significant other, and 37% had at least one child.
2.3. Measures
2.3.1. Dependent measures
2.3.1.1. Young adult behavior problems
The Adult Self-Report (ASR) (Achenbach and Rescorla, 2003) is a 126-item standardized, validated measure of problem behaviors for young adult offspring. Eight syndrome scales combine into three broad-spectrum scales: internalizing, externalizing and total problems. The eight syndrome scales are: withdrawn, somatic complaints, anxious/depressed, rule-breaking behavior, aggressive behavior, intrusive behavior, thought problems, and attention problems. Higher scores indicate more problem behaviors. Reliability and validity of the ASR have been previously established (Achenbach and Rescorla, 2003). Participants complete the items by selecting answers ranging from 0 to 2 where 0 indicates not true, 1 is somewhat or sometimes true, and 2 is very true or often true.
In addition, participants were asked how many times they have been arrested in their life. This variable was dichotomized to any arrests versus no arrests.
2.3.1.2. Young adult smoking behavior
Cigarette smoking among the young adults was assessed by self-report which queried usual current number of cigarettes smoked per day. Average daily cigarette use was used as a continuous measure in our analyses. A categorical variable was also created to indicate heavy smoking. The categories of smoking were: none; >0 to <10 cigarettes per day; ≥10 cigarettes per day. The cut-point of 10 or more cigarettes is based on the review of the literature (Wakschlag et al., 2002). Urine samples were collected and analyzed for cotinine levels adjusted for creatinine to validate the self-report. If the urine sample was negative, but the participant reported use, the self-report was used (n=66). There were 13 subjects who reported not current smoking, but their biological sample was positive. These cases were excluded from the analyses of tobacco use. The Fagerström Tobacco and Nicotine Dependence scale (Heatherton et al., 1991) was used to measure dependence.
2.3.2. Independent variable
2.3.2.1. Prenatal cigarette smoke exposure
Maternal smoking during pregnancy was assessed at months 4, 7, and at birth covering the first, second, and third trimesters, respectively. Maternal smoking was measured as number of packs smoked per day (none, less than half a pack, half a pack, etc.). This was translated into average number of cigarettes smoked per day using the median of each category, assuming each pack contains 20 cigarettes. The level of smoking ranged from 0 to 3 packs/day. For the analyses, PCSE was analyzed as a continuous measure (average daily cigarettes) and as a categorical measure: No PCSE; PCSE of >0 to <10 cigarettes per day; ≥10 cigarettes per day.
2.3.3. Covariate measures
2.3.3.1. Socio-demographic characteristics
Demographic characteristics including maternal and offspring education, offspring age, race, and gender were measured.
2.3.3.2. Other prenatal exposures
For alcohol, participants separately indicated their usual, maximum, and minimum quantity and frequency of beer, wine, liquor, wine and beer coolers (Day and Robles, 1989), which was summarized as the average daily volume of alcohol (ADV). Marijuana, hashish, and sensimilla use was transformed into average daily joints (ADJ). A blunt of marijuana was converted to four joints, and a hashish cigarette or bowl was counted as three joints, based on the relative amount of delta-9-THC in each (Gold, 1989). Other illicit substances were rare and were not included in these analyses.
2.3.3.3. Maternal depression and hostility
2.3.3.3.1. Psychological measures
Maternal depression during pregnancy was assessed using the Centers for Epidemiologic Studies-Depression Scale (CES-D; Radloff, 1977). The CES-D is a 20-item self-report measure developed for use in general population samples. This measure was analyzed as a continuous variable. The CES-D correlates well with other established measures of depression (e.g., Zung, r =0.90; Beck, r=0.81), establishing its validity (Myers and Weissman, 1980). The Spielberger State-Trait Personality Inventory (STPI) was also assessed during pregnancy to measure maternal hostility (Spielberger, 1979). The CES-D and the STPI measures from the first pre-natal assessment (fourth gestational month) were used in these analyses.
2.4. Data analysis
PCSE was used as a continuous variable to test for dose–response relations and dichotomously, any/none, or ≥10 cigarettes per day, to examine the effects of heavy smoking. Relations between PCSE and behavior problems were first examined bivariately using a t-test. The hypothesis was that PCSE was associated with increased behavior problems and increased smoking and nicotine dependence. Therefore, one-sided p-values were used.
Stepwise multiple regressions were used to analyze the effects of PCSE on ASR scales and to adjust for offspring variables including gender, race, age at the time of the assessment, prenatal exposure to alcohol and marijuana, and maternal variables including education, SES, depression and hostility. Analyses were done separately for first, second, and third trimesters to assess the effects of exposure at different periods of pregnancy. Influential points and outliers were identified by using the modified Cook’s distance (Cook and Weisberg, 1982) and were excluded.
Multiple logistic analyses were performed in the analyses using categorical outcomes, lifetime history of arrests (any/none); nicotine dependent (FTND <5/≥5), and odds ratios were calculated. If, in the bivariate analyses, the rates of behavior problems for adjacent levels of PCSE were similar these categories were combined to increase the statistical power.
3. Results
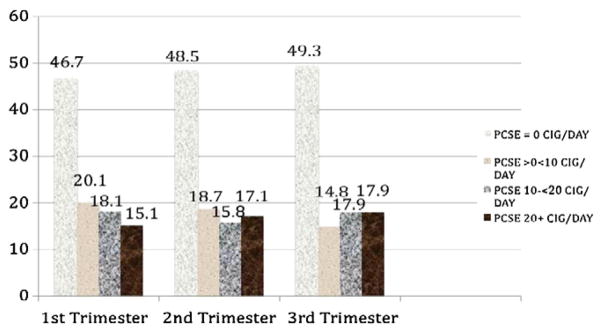
Fifty-three percent (n=324) of the women smoked in the first trimester and 33% (n=202) smoked 10 or more cigarettes per day. The rate of smoking remained stable across pregnancy. Fifty-one percent smoked cigarettes during the second and third trimesters of pregnancy and 36% (n=218) smoked 10 or more cigarettes/day during third trimester of pregnancy. The rate of smoking at each trimester of pregnancy is shown in Fig. 1. The average number of cigarettes smoked per day among smokers was 15.4 (0.5–50 cigarettes/day, sd0=11.4), 16.5 (0.5–70 cigarettes/day, sd=12.5), and 17.7 (0.5–70 cigarettes/day, sd=12.2) during first, second, and third trimesters, respectively. The modal level of smoking during pregnancy was 10 cigarettes per day.
Fig. 1.
Prevalence of maternal smoking during pregnancy.
In the first trimester, 64% of the women drank alcohol and 41% used marijuana. In contrast to cigarette smoking, alcohol and marijuana dropped substantially with 31% drinking alcohol and 19% using marijuana by the third trimester. Women who smoked 10 or more cigarettes per day during the first trimester of pregnancy were significantly more likely to be Caucasian (71% vs. 36%), have less education (11.4 years vs. 12.0 years), were less likely to work outside of the home (17% vs. 29%), and drank more alcohol (0.8 drinks/day vs. 0.4 drinks/day) than did the remainder of the cohort. There were no significant differences in maternal age, income, depression or hostility during pregnancy.
3.1. PCSE and behavioral outcomes and smoking behavior: bivariate relations
In these analyses, PCSE was categorized as 0 cigarettes/day, <10 cigarettes/day, and ≥10 cigarettes/day. First, second, and third trimester PCSE were significantly associated with the total, externalizing, and internalizing problem scales, and with the aggression, anxiety/depression, attention, and somatic problems subscales. PCSE was not significantly related to withdrawn, intrusive behaviors, thought problems, or rule breaking problem subscales at any trimester.
History of arrests was reported by 35.5% of the offspring who did not have PCSE in the first trimester, 41.1% who were exposed to <10 cigarettes/day, and 45% among offspring with PCSE of 10 or more cigarettes/day. Second and third trimester exposures were not significantly related to history of arrests.
3.2. PCSE and smoking behavior outcomes: bivariate relations
Bivariate relations were also examined with first, second, and third trimester PCSE and heavier smoking among the offspring. Heavy smoking (PCSE ≥10 cigarettes/day) during first, second, and third trimesters was significantly related to offspring heavy smoking (≥10 cigarettes/day) at age 22. PCSE in the first, second, and third trimesters was also significantly related to dependent smoking among the offspring on the FTND.
3.3. Multivariate relations: PCSE and behavioral outcomes
Offspring with first trimester PCSE had significantly higher rates on the externalizing and internalizing scales and the aggression, and somatic problems subscales using both the continuous measures of PCSE and the dichotomized measure (≥10 cigarettes/day/<10 cigarettes/day) and adjusting for covariates (Table 1). Second trimester PCSE predicted internalizing, withdrawn, aggression, and somatic problems using both continuous and dichotomous measures of PCSE (Table 1). Third trimester PCSE using both the continuous and dichotomous measures predicted significantly more aggression problems (Table 1).
Table 1.
First, second, third trimesters pcse and other significant predictors of behavior problems: adjusted analyses.
| ASR scale | First trimester PCSE (β=beta values, t=t-tests) | Other significant covariates* (β=beta values, t=t-tests) | R2 |
|---|---|---|---|
| Externalizing | 1st trimester b | Sex (Male) (β=1.46, t=1.80) | 0.04 |
| Average cigarettes/day (β=0.07, t=2.05) | Maternal education (β=−0.67, t=−2.20) | ||
| ≥10 cigarettes/day (β=1.92, t=2.20) | Maternal hostility (β=0.20, t=2.93) | ||
| Internalizing | 1st trimester | Age (β=−1.57, t=−2.43) | 0.05 |
| ≥10 cigarettes/day (β=1.96, t=1.89) | Race (Caucasian) (β=2.10, t=2.16) | ||
| Maternal education (β=−0.58, t=−1.72) | |||
| Maternal depression (β=0.15, t=2.92) | |||
| Aggression problems | 1st trimester | Maternal education (β=−0.36, t=−2.41) | 0.05 |
| Average cigarettes/day (β=0.05, t=2.58) | Maternal hostility (β=0.12, t=3.49) | ||
| ≥10 cigarettes/day (β=1.20, t=2.81) | |||
| Somatic problems | 1st trimester b | Age (β=−0.45, t=−2.43) | 0.10 |
| Average cigarettes/day (β=0.02, t=1.72) | Race (Caucasian) (β=0.79, t=2.87) | ||
| ≥10 cigarettes/day (β=0.70, t=2.33) | Sex (female) (β=−1.07, t=−4.10) | ||
| Maternal education (β= −.0.22, t=−2.23) | |||
| Prenatal alcohol exposure (β=0.57, t=1.95) | |||
| ASR scale | Second trimester PCSE (β=beta values, t=t-tests) | Other significant covariates* (β=beta values, t=t-tests) | R2 |
|
| |||
| Internalizing | 2nd trimester | 0.07 | |
| Average cigarettes/day (β=0.08, t=1.87 | Race (Caucasian) (β=3.03, t=2.86) | ||
| ≥10 cigarettes/day (β=2.08, t=1.87) | Prenatal alcohol exposure (β=5.36, t=2.05) | ||
| Prenatal marijuana exp. (β=3.90, t=2.02) | |||
| Maternal depression (β=0.14, t=2.50) | |||
| Withdrawn | 2nd trimester | Race (β=−0.65, t=−2.55) | |
| Average cigarettes/day (β=0.02, t=1.83) | Prenatal marijuana exp. (β=0.94, t=2.03) | ||
| ≥10 cigarettes/day (β=0.56, t=2.08) | Maternal depression (β=0.03, t=1.88) | ||
| Aggression problems | 2nd trimester | Age (β= −.053, t=−1.72) | 0.03 |
| Average cigarettes/day (β=0.05, t=2.59) | Maternal education (β=−0.36, t=2.36) | ||
| ≥10 cigarettes/day (β=1.14, t=2.50) | Maternal hostility (β=0.11, t=2.91) | ||
| Somatic problems | 2nd trimester b | Age (β=−0.53, t=−2.73) | 0.05 |
| Average cigarettes/day (β=0.04, t=3.50) | Race (Caucasian) (β=0.94, t=3.18) | ||
| ≥10 cigarettes/day (β=0.97, t=3.12) | Sex (female) (β=−1.06, t=−3.94) | ||
| Prenatal alcohol exposure (β=2.51, t=3.70) | |||
| ASR scale | Third trimester PCSE (β=Beta values, t=t-tests) | Other significant covariates (β=beta values, t=t-tests) | R2 |
|
| |||
| Aggression | 3rd trimester | Maternal education (β= −.0.29, t=−1.99) | 0.05 |
| Average cigarettes/day (β=0.03, t=1.94) | Prenatal alcohol exposure (β=1.89, t=2.01) | ||
| ≥10 cigarettes/day (β=1.00, t=2.43) | Maternal hostility (β=0.13, t=3.82) | ||
Significant covariates are reported with the identified PCSE exposure variables.
PCSE in the first trimester (both continuous and dichotomous) predicted a history of arrests (Table 2). Exposure to ≥10 cigarettes/day increased the odds of having a history of arrests by 65%. Second and third trimester PCSE did not significantly predict a history of arrests (Table 2).
Table 2.
PCSE and history of any arrests: adjusted analyses.
| PCSE | Young adult any arrests | Other significant covariates |
|---|---|---|
|
|
|
|
| Trimester | Adjusted odds ratio/95% C.I. | Adjusted odds ratio/95% C.I. |
| 1st trimester ave. cigarettes/day | 1.03/1.01–1.04 | Sex 3.3/2.32–4.65 |
| 1st trimester ≥10 cigarettes/day | 1.65/1.15–2.37 |
3.4. PCSE and smoking behavior outcomes: multivariate analyses
Multiple logistic regressions were run to examine relations between trimester-specific PCSE and offspring heavy smoking (≥10 cigarettes/day) and dependent smoking (FTND≥5) while controlling for the significant covariates. When the dichotomous measure of PCSE was used, first, second and third trimester PCSEs significantly predicted heavy smoking with odds ratios of 1.02, 1.03, and 1.02 per prenatal cigarette exposure per day in the first, second, and third trimesters, respectively. The odds ratios for heavy offspring smoking were 1.70, 2.22, and 1.68 for first, second, and third trimester exposures of ≥10 cigarettes/day, respectively. The odds of being a dependent smoker were 2.05 times greater for young adult offspring with third trimester PCSE (Table 3).
Table 3.
PCSE and heavy and dependent smoking behavior in the offspring: adjusted analyses.
| PCSE | Young adult smoking at ≥10 cigarettes/day | Other significant covariates |
|---|---|---|
|
|
|
|
| Trimester | Adjusted odds ratio/95% C.I. | Adjusted odds ratio/95% C.I. |
| 1st trimester ave. | 1.02/1.01–1.04 | Race 2.3/1.5–3.54 |
| cigarettes/day | Sex 1.72/1.14–2.59 | |
| 1st trimester | 1.70/1.10–2.63 | Maternal education 0.8/ |
| ≥10 cigarettes/day | 0.69–0.95 | |
| 2nd trimester ave. | 1.03/1.01–1.05 | |
| cigarettes/day | ||
| 2nd trimester ≥10 | 2.22/1.39–3.55 | |
| cigarettes/day | ||
| 3rd trimester ave. | 1.02/1.01–1.04 | |
| cigarettes/day | ||
| 3rd trimester ≥10 | 1.68/1.16–2.63 | |
| cigarettes/day | ||
| PCSE | Young adult dependent smoking, FTND≥5 | Other significant covariates |
|
|
|
|
| Trimester | Adjusted odds ratio/95% C.I. | Adjusted odds ratio/95% C.I. |
|
| ||
| 3rd trimester | 2.05/1.16–3.65 | Race 2.75/1.49–5.04 |
| ≥10 cigarettes/day | ||
FTND: Fagerström tobacco and nicotine dependence.
4. Discussion
In this study, young adult offspring with PCSE had significantly more behavioral problems across each trimester of pregnancy. To our knowledge, this is the first study of the trimester-specific effects of PCSE on young adult behavior. We have demonstrated that the effects of PCSE on behavior had a dose–response relation after controlling for significant covariates, including other prenatal substance exposures. PCSE predicted significantly increased rates of behavior problems on the total, externalizing and internalizing scales and on most of the subscales of the ASR. On a separate measure, PCSE also predicted a higher rate of arrests at age 22. These findings extend earlier results from this cohort. At age 3 (Day et al., 2000), offspring with PCSE had more aggressive behaviors and were more irritable.
There is evidence from laboratory studies to support these findings (Abbott and Winzer-Serhan, 2012; Baler et al., 2008; Ernst et al., 2001; Muneoka et al., 1997; Slotkin, 2008; Xu et al., 2001). Studies demonstrate that gestational nicotine exposure affects the central monoamineric systems in rodents (Muneoka et al., 1997; Xu et al., 2001), which could constitute an underlying cause for affective behavioral abnormalities seen in exposed human offspring (Abbott and Winzer-Serhan, 2012). Nicotine-induced inhibition of the monamine oxidase system during fetal development has also been implicated in the relation between gestational nicotine exposure and offspring aggression (Baler et al., 2008). In addition, nicotine causes alterations in neural cell replication leading to deficits in synaptic neurochemistry (Ernst et al., 2001; Slotkin, 2008) that result in behavioral self-regulation difficulties. Further, many of the effects from the animal literature parallel our findings of effects specifically in the first and second trimesters, as the rodent gestational period is the developmental equivalent to the first and second human trimesters (Dwyer et al., 2009).
We also found that the levels of PCSE at all three trimesters significantly predicted offspring smoking at 22 years of age. Although others have reported this, these studies (Agrawal et al., 2010; O’Callaghan et al., 2009; Miles and Weden, 2012) did not examine trimester-specific effects, and did not consider other prenatal substance exposures in their analyses. We have previously reported a relation between PCSE and offspring cigarette smoking at earlier ages of this cohort, showing earlier smoking experimentation at age 10 (Cornelius et al., 2000), heavier smoking at age 14 (Cornelius et al., 2005), and earlier onset of smoking and other drugs at age 16 (Goldschmidt et al., in press).
Third trimester exposure predicted nicotine dependency in the young adult offspring. There is a biological basis for the associations between third trimester PCSE and offspring nicotine dependence. Laboratory research has found that in the third trimester of gestation, brain development is highly susceptible to nicotine (Nunes-Freitas et al., 2011; Slotkin, 2008; Lotfipour et al., 2010), and exposure during this time might be more likely to predict greater levels of problematic tobacco use. In a study by Slotkin (2008), there was an upregulation of nicotinic acetylcholine receptors (nAChR) among adult rats exposed to nicotine in the immediate neonatal period, a time comparable to the third trimester in humans.
It is noteworthy that behavioral effects of PCSE were still measurable at 22 years. PCSE effects were dose–response using the continuous measure of average daily cigarettes, and also at the threshold level, using the cutoff of 10 or more cigarettes/day. Other independent predictors were also noted. Demographic predictors of several of the behavioral outcomes included male sex and African-American race, which are consistent findings with reports in the human literature (Linnet et al., 2003; McLaughlin et al., 2007; Moffitt et al., 2001). We also controlled for variables such as maternal hostility and depression that were independently related to the outcome, underscoring the importance of considering of these factors in future research on PCSE effects on offspring (Pickett et al., 2009). Finally, exposures to other prenatal substances were considered, and in some cases were independent predictors of several of the ASR syndrome scales.
This study has multiple strengths. The women attended a general prenatal clinic, not one specialized for substance use treatment. Their substance use represents that of a low-SES population. The women in the sample represented the spectrum of use from none to heavy, allowing use of linear models to analyze the data. In addition, we controlled for maternal psychological symptoms, including depression and hostility and maternal smoking, to account for the shared genetic liability for behavior problems between the mothers and their offspring. We also controlled for other trimester specific prenatal substance exposures.
This study, however, had some limitations. We did not collect biological measures of PCSE or post-partum maternal smoking. Exposure was based on maternal self-report. To increase the accuracy of the reported data, we constructed detailed questions, thoroughly tested our measures, carefully selected interviewers, and extensively trained our staff in interview techniques. The correlations between reports from each trimester of pregnancy were high, demonstrating consistency in reporting, which is an indication that maternal reports were accurate. Further, the information collected on smoking during pregnancy occurred during the early 1980s in this cohort. This was a time when, unlike the current time, women were not faced with social sanctions for smoking during pregnancy and self-reporting of smoking was not as likely to be underreported. In addition, our outcome measures of offspring behavior, with the exception of smoking behavior, were based solely on the young adults’ report of their own behavior. Finally, this is a sample of lower SES women and these findings need to be tested in a broader population to demonstrate generalizability.
5. Conclusions
PCSE has long-term effects on behavior problems and smoking patterns through young adulthood. These effects are associated with exposure at each of the three trimesters and are generally a dose–response pattern. Third trimester exposure predicted nicotine dependence. This report has extended knowledge about the effects of PCSE into an older age group, looking at trimesters separately, and controlling for other prenatal exposures in addition to controlling for significant covariates. These results make a strong case for counseling women who plan to get pregnant, as well as those who are pregnant to stop their tobacco use.
Acknowledgments
This research was supported by grants from the National Institute of Drug Abuse (PI: N. Day), the National Institute of Alcoholism and Alcohol Abuse (PI: N Day), and the National Institute on Child Health and Development (PI: N. Day; Co-PI: M. Cornelius).
Footnotes
Conflict of interest
The author declares that there are no conflicts of interest.
References
- Abbott L, Winzer-Serhan U. Smoking during pregnancy: lessons learned from epidemiological studies and experimental studies using animal models. Crit Rev Toxicol. 2012;42:279–303. doi: 10.3109/10408444.2012.658506. [DOI] [PubMed] [Google Scholar]
- Achenbach TM, Rescorla LA. Manual for the ASEBA adult forms & profiles. Burlington, VT: University of Vermont, Research Center for Children, Youth, & Families; 2003. [Google Scholar]
- Adams K, Melvin C, Raskind-Hood C. Sociodemographic, insurance, and risk profiles of maternal smokers post the 1990s: how can we reach them? Nicotine Tob Res. 2008;10:1121–9. doi: 10.1080/14622200802123278. [DOI] [PubMed] [Google Scholar]
- Agrawal A, Scherrer J, Grant J, Sartor C, Pergadia M, Duncan A, et al. The effects of maternal smoking during pregnancy on offspring outcomes. Prev Med. 2010;50:12–8. doi: 10.1016/j.ypmed.2009.12.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen A, Dietz P, Tong V, England L, Prince C. Prenatal smoking prevalence ascertained from two population-based data sources: birth certificates and PRAMS questionnaires, 2004. Public Health Rep. 2008;123:586–92. doi: 10.1177/003335490812300508. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baler R, Volkow N, Fowler J, Benveniste H. Is fetal brain monoamine oxidase inhibition the missing link between maternal smoking and conduct disorders? J Psychiatr Neurosci. 2008;33:187–95. [PMC free article] [PubMed] [Google Scholar]
- Batstra L, Hadders-Algra M, Needleman J. Effect of antenatal exposure to maternal smoking on behavioural problems and academic achievement in childhood: prospective evidence from a Dutch birth cohort. Early Hum Dev. 2003;75:21–33. doi: 10.1016/j.earlhumdev.2003.09.001. [DOI] [PubMed] [Google Scholar]
- Brion M, Victora C, Matjasevich A, Horta B, Anselmi L, Menezes A, et al. Maternal smoking and child psychological problems: Disentangling causal and noncausal effects. Pediatrics. 2010;126:e57–65. doi: 10.1542/peds.2009-2754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burns L, Mattick R, Wallace C. Smoking patterns and outcomes in a population of pregnant women with other substance use disorders. Nicotine Tob Res. 2008;10:969–74. doi: 10.1080/14622200802097548. [DOI] [PubMed] [Google Scholar]
- Button T, Thapar A, McGuffin P. Relationship between antisocial behaviour, attention-deficit hyperactivity disorder and maternal prenatal smoking. Br J Psychiatry. 2005;187:155–60. doi: 10.1192/bjp.187.2.155. [DOI] [PubMed] [Google Scholar]
- Carter S, Paterson J, Gao W. Maternal smoking during pregnancy and behaviour problems in a birth cohort of 2-year-old Pacific children in New Zealand. Early Hum Dev. 2008;84:59–66. doi: 10.1016/j.earlhumdev.2007.03.009. [DOI] [PubMed] [Google Scholar]
- Cook R, Weisberg S. Residuals and Influence in Regression. New York: Chapman and Hall; 1982. [Google Scholar]
- Cornelius M, Day N. Effects of prenatal and postnatal tobacco exposures on offspring: A review. Alcohol Health Res World. 2000;24:242–9. [Google Scholar]
- Cornelius M, Day N. Developmental consequences of prenatal tobacco exposure. Curr Opin Neurol. 2009;22:121–5. doi: 10.1097/WCO.0b013e328326f6dc. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day N. Prenatal tobacco exposure: Is it a risk factor for early tobacco experimentation? Nicotine Tob Res. 2000;2:45–52. doi: 10.1080/14622200050011295. http://dx.doi.org/10.1080/14622200050011295. [DOI] [PubMed] [Google Scholar]
- Cornelius MD, Leech SL, Goldschmidt L, Day N. Is prenatal tobacco exposure a risk factor for early adolescent smoking? A follow-up study. Neurotoxicol Teratol. 2005;27:667–76. doi: 10.1016/j.ntt.2005.05.006. http://dx.doi.org/10.1016/j.ntt.2005.05.006. [DOI] [PubMed] [Google Scholar]
- Cornelius M, Goldschmidt L, De Genna N. Smoking during teenage pregnancies: effects on behavioral problems in offspring. Nicotine Tob Res. 2007;9:739–50. doi: 10.1080/14622200701416971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornelius M, De Genna N, Leech S, Willford J. Effects of prenatal tobacco exposure on neurobehavioral outcomes of 10-year-old children of teenage mothers. Neurotoxicol Teratol. 2011;33:137–44. doi: 10.1016/j.ntt.2010.08.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Day N, Robles N. Methodological issues in the measurement of substance use. Ann N Y Acad Sci. 1989;562:8–13. doi: 10.1111/j.1749-6632.1989.tb21002.x. [DOI] [PubMed] [Google Scholar]
- Day N, Richardson G, Goldschmidt L, Cornelius M. Effects of prenatal tobacco exposure on preschooler’s behavior. J Dev Behav Pediatr. 2000;21:180–8. [PubMed] [Google Scholar]
- Dwyer J, McQuown S, Leslie F. The dynamic effects of nicotine on the developing brain. Pharmacol Ther. 2009;122:125–39. doi: 10.1016/j.pharmthera.2009.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ernst M, Moolchan E, Robinson M. Behavioral and neural consequences of prenatal exposure to nicotine. J Am Acad Child Psychiatry. 2001;40:630–42. doi: 10.1097/00004583-200106000-00007. [DOI] [PubMed] [Google Scholar]
- Eskenazi B, Trupin LS. Passive and active maternal smoking during pregnancy, as measured by serum cotinine, and postnatal smoke exposure. Il: Effects on neurodevelopment at age 5 years. Am J Epidemiol. 1995;142(9 Suppl):S19–29. doi: 10.1093/aje/142.supplement_9.s19. [DOI] [PubMed] [Google Scholar]
- Fergusson D, Horwood L, Ridder E. Conduct and attentional problems in childhood and adolescence and later substance use, abuse and dependence. Drug Alcohol Depend. 2007;88S:S14–26. doi: 10.1016/j.drugalcdep.2006.12.011. [DOI] [PubMed] [Google Scholar]
- Gatzke-Kopp L, Beauchaine T. Direct and passive prenatal nicotine exposure and the development of externalizing psychopathology. Child Psychiatry Hum Dev. 2007;38:255–69. doi: 10.1007/s10578-007-0059-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold M. Marijuana. New York: Plenum; 1989. [Google Scholar]
- Goldschmidt L, Cornelius M, Day N. Prenatal cigarette smoke exposure and early initiation of multiple substance use. Nicotine Tob Res. doi: 10.1093/ntr/ntr280. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heatherton TF, Kozlowski LT, Frecker RC, Fagerström KO. The Fagerström test for nicotine dependence: a revision of the Fagerström Tolerance Questionnaire. Br J Addict. 1991;86:1119–27. doi: 10.1111/j.1360-0443.1991.tb01879.x. [DOI] [PubMed] [Google Scholar]
- Huijbregts S, Séguin J, Zoccolillo M, Boivin M, Tremblay R. Maternal prenatal smoking, parental antisocial behavior, and early childhood physical aggression. Dev Psychopathol. 2008;20:437–53. doi: 10.1017/S0954579408000217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huizink A, Mulder E. Maternal smoking, drinking or cannabis use during pregnancy and neurobehavioral and cognitive functioning in human offspring. Neurosci Biobehav Rev. 2006;20:24–41. doi: 10.1016/j.neubiorev.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Kandel D, Wu P, Davies M. Maternal smoking during pregnancy and smoking by adolescent daughters. Am J Public Health. 1994;89(9):1377–83. doi: 10.2105/ajph.84.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kotimaa A, Mollanen I, Taanill A, Ebeling H, Smalley S, McGough J, et al. Maternal smoking and hyperactivity in 8-year-old children. Am Acad Child Adolesc Psychiatry. 2003;42:826–33. doi: 10.1097/01.CHI.0000046866.56865.A2. [DOI] [PubMed] [Google Scholar]
- Langley K, Turic D, Rice F, Holmans P, van den Bree M, Craddock N, et al. Testing for gene X environment interaction effects in attention deficit hyperactivity disorder and associated antisocial behavior. Am J Med Genet B Neuropsychiatr Genet. 2008;147B:49–53. doi: 10.1002/ajmg.b.30571. [DOI] [PubMed] [Google Scholar]
- Linnet K, Dalsgaard S, Obel C. Maternal lifestyle factors in pregnancy risk of attention deficit hyperactivity disorder and associated behaviors: Review of current evidence. Am J Psychiatry. 2003;160:1028–40. doi: 10.1176/appi.ajp.160.6.1028. [DOI] [PubMed] [Google Scholar]
- Lotfipour S, Leonard G, Perron M, Pike B, Richer L, Seguin J, et al. Prenatal exposure to maternal cigarette smoking interacts with a polymorphism in the ∝ 6 nicotinic acetylcholine receptor gene to influence drug use and striatum volume in adolescence. Mol Psychiatry. 2010;15:6–8. doi: 10.1038/mp.2009.63. [DOI] [PubMed] [Google Scholar]
- Martin L, McNamara M, Milot A, Bloch M, Hair E, Halle T. Correlates of smoking before, during, and after pregnancy. Am J Health Behav. 2008;32:272–82. doi: 10.5555/ajhb.2008.32.3.272. [DOI] [PubMed] [Google Scholar]
- Maughan B, Taylor A, Caspi A, Moffitt T. Prenatal smoking and early childhood conduct problems: testing genetic and environmental explanations of the association. Arch Gen Psychiatry. 2004;61:836–43. doi: 10.1001/archpsyc.61.8.836. [DOI] [PubMed] [Google Scholar]
- McLaughlin K, Hilt L, Nolen-Hoeksema S. Racial/ethnic differences in internalizing and externalizing symptoms in adolescents. J Abnorm Child Psychol. 2007;35:801–16. doi: 10.1007/s10802-007-9128-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menezes A, Hallal P, Horta B. Early determinants of smoking in adolescence: a prospective birth cohort study. Cad Saude Publica. 2007;23:347–54. doi: 10.1590/s0102-311x2007000200011. [DOI] [PubMed] [Google Scholar]
- Miles J, Weden M. Is the intergenerational transmission of smoking from mother to child mediated by children’s behavior problems? Nicotine Tob Res. 2012 doi: 10.1093/ntr/ntr328. http://dx.doi.org/10.1093/ntr/ntr328. [DOI] [PMC free article] [PubMed]
- Miller M. Brain Development: Normal processes and the effects of alcohol and nicotine. Oxford University Press; 2006. [Google Scholar]
- Moffitt T, Caspi A, Rutter M, Silva P. Sex differences in antisocial behavior: Conduct disorder, delinquency, and violence in the Dunedin longitudinal study. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Muneoka K, Ogaw T, Kamei K, Muraoka S, Tomiyoshi R, Mimur Y, et al. Prenatal nicotine exposure affects the development of the central serotonergic system as well as the dopaminergic system in rat offspring: involvement of route of drug administrations. Brain Res Dev Brain Res. 1997;102:117–26. doi: 10.1016/s0165-3806(97)00092-8. [DOI] [PubMed] [Google Scholar]
- Myers J, Weissman M. Use of a self-report symptom scale to detect depression in a community sample. Am J Psychiatry. 1980;137:1081–4. doi: 10.1176/ajp.137.9.1081. [DOI] [PubMed] [Google Scholar]
- Nunes-Freitas A, Ribeiro-Carvalho A, Lima C, Dutra-Taveres A, Manhaes A, Lisboa P, et al. Nicotine exposure during the third trimester equivalent of human gestation: Time course of effects on the central cholinergic system of rats. Toxicol Sci. 2011;123:144–54. doi: 10.1093/toxsci/kfr147. [DOI] [PubMed] [Google Scholar]
- O’Callaghan F, Al Mamun A, O’Callaghan M, Alati R, Najman J, Williams G, et al. Maternal smoking during pregnancy predicts nicotine disorder (dependence or withdrawal) in young adults a birth cohort study. Aust N Z J Public Health. 2009;33(4):371–7. doi: 10.1111/j.1753-6405.2009.00410.x. [DOI] [PubMed] [Google Scholar]
- Pickett K, Rathouz P, Dukic V, Kasza K, Niessner M, Wright R, et al. The complex enterprise of modeling prenatal exposure to cigarettes: What is enough? Paediatr Perinat Epidemiol. 2009;23:160–70. doi: 10.1111/j.1365-3016.2008.01010.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff L. The CES-D Scale: A self-report depression scale for research in the general population. Appl Psychol Meas. 1977;1:385–401. [Google Scholar]
- Ruckinger S, Rzehak P, Chen C, Sausenthaler S, Koletzko S, Bauer C, et al. Prenatal and postnatal tobacco exposure and behavioral problems in 10-year-old children: Results from the GINI-plus Study Group. Environ Health Perspect. 2010;118:150–4. doi: 10.1289/ehp.0901209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Slotkin T. If nicotine is a developmental neurotoxicant in animal studies, dare we recommend nicotine replacement therapy in pregnant women and adolescents? Neurotoxicol Teratol. 2008;20:1–19. doi: 10.1016/j.ntt.2007.09.002. http://dx.doi.org/10.1016/j.ntt.2007.09.002. [DOI] [PubMed] [Google Scholar]
- Spielberger CD. Preliminary manual for the State-Trait Personality Inventory (STPI) Tampa: University of South Florida; 1979. Center for Research in Behavioral Medicine and Health Psychology, PCD 4118G. [Google Scholar]
- Stroud L, Paster R, Goodwin M, Shenassa E, Buka S, Niaura R, et al. Maternal smoking during pregnancy and neonatal behavior: a large-scale community study. Pediatrics. 2009;123:e842–8. doi: 10.1542/peds.2008-2084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thapar A, Fowler T, Rice F, Scourfield J, van den Bree M, Thomas H, et al. Maternal smoking during pregnancy and attention deficit hyperactivity disorder symptoms in offspring. Am J Psychiatry. 2003;160:1985–9. doi: 10.1176/appi.ajp.160.11.1985. [DOI] [PubMed] [Google Scholar]
- US Department of Health and Human Services. Atlanta, GA: US Department of Health and Human Services, CDC; 2010. How tobacco smoke causes disease: the biology and behavioral basis for smoking-attributable disease: a report of the Surgeon General. (Available at http://www.cdc.gov/tobacco/data_statistics/sgr/2010/index.htm) [PubMed] [Google Scholar]
- Wakschlag L, Pickett K, Cook E, Benowitz N, Leventhal B. Maternal smoking during pregnancy and severe antisocial behavior in offspring: a review. Am J Public Health. 2002;92:966–74. doi: 10.2105/ajph.92.6.966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wakschlag L, Leventhal B, Pine D, Pickett K, Carter A. Elucidating early mechanisms of developmental psychopathology: the case of prenatal smoking and disruptive behavior. Child Dev. 2006;77:893–906. doi: 10.1111/j.1467-8624.2006.00909.x. [DOI] [PubMed] [Google Scholar]
- Willford J, Day N, Cornelius M. Maternal Tobacco Use during pregnancy: Epidemiology and effects on offspring. In: Michael Miller., editor. Development of the mammalian central nervous system: lessons learned from studies on alcohol and nicotine exposure. New York: Oxford University Press; 2006. pp. 315–28. [Google Scholar]
- Xu Z, Seidler F, Ali S, Slikker W, Slotkin T. Fetal and adolescent nicotine administration: effects on CNS serotonergic systems. Brain Res. 2001;914:166. doi: 10.1016/s0006-8993(01)02797-4. [DOI] [PubMed] [Google Scholar]