Abstract
Background
This prospective analysis was designed to examine the rate of RNFL loss using scanning laser polarimetry (GDx enhanced corneal compensation (GDxECC)) in progressing versus non-progressing eyes using various methods to define functional progression.
Methods
Glaucoma suspect and glaucomatous eyes with ≥3 years of follow-up participating in the Advanced Imaging for Glaucoma Study were enrolled. All eyes underwent standard automated perimetry (SAP) and GDxECC imaging every 6 months. The annual rate of RNFL loss with GDxECC was calculated using linear regression analysis. Functional progression was determined using the Early Manifest Glaucoma Trial (EMGT) criterion, SAP Visual Field Index (VFI) and Progressor software.
Results
Fifty-three eyes (30 glaucoma suspect, 23 glaucoma) of 53 patients (mean age 64.5±10.7 years, range 42–79) were enrolled. Eighteen eyes (40%) demonstrated SAP progression during the follow-up period using the Progressor criterion, 10 eyes (18.9%) using the VFI criterion, and 3 eyes (5.7%) using the EMGT criterion. The annual rate (μm/year) of mean RNFL loss was significantly greater (p<0.05) in progressing versus non-progressing eyes using Progressor (−1.24±0.99 vs −0.18±0.49), EMGT (−1.95±0.99 vs −0.46±0.78) and VFI (−1.11±0.64 vs −0.41±0.85) criteria.
Conclusion
Despite differences in the criteria used to judge functional progression, progressing eyes have a significantly greater rate of RNFL loss measured using GDxECC as compared with non-progressing eyes.
INTRODUCTION
Glaucoma is a progressive optic neuropathy disease characterised by progressive loss of retinal ganglion cells and retinal nerve fibre layer (RNFL).1 Accurate methods for detecting disease progression are therefore essential to monitor patients and evaluate the efficacy of therapy. Established methods for detection of glaucoma progression include longitudinal assessment of visual function using standard automated perimetry (SAP) or optic nerve appearance using clinical examination or optic disc photography. However, clinically significant visual-field damage may sometimes be difficult to demonstrate, even in eyes with strong evidence of structural damage. Statistical methods for evaluating glaucomatous visual-field progression have evolved considerably, yet criteria for defining progression remain inconsistent in the absence of established standards.2
Imaging may serve as a useful adjunct to optic disc photography to provide complementary information that may facilitate progression detection using rate-based changes over time, since the output data are quantitative and highly reproducible at all stages of the glaucoma continuum. Scanning laser polarimetry (SLP) is a confocal scanning laser ophthalmoscope with an integrated polarimeter that measures the amount of retardation (phase shift) of a polarised near infrared laser beam as it passes through the RNFL.3–5 The commercial polarimeter has an integrated variable corneal compensator (GDxVCC), which determines and neutralises eye specific corneal polarisation axis and magnitude using the concept of the macula as an intraocular polarimeter.6–8 Enhanced corneal compensation (GDxECC) is a modification of GDxVCC that has been recently introduced to improve the signal-to-noise ratio and eliminate artefact associated with atypical retardation pattern (ARP), thereby improving the diagnostic precision of this technology.9–16 It has been reported that the presence of ARP may confound the measurement of RNFL thickness,17 but the effect of ARP on assessment of progressive glaucomatous RNFL atrophy is presently unknown.
A recent study by Medeiros et al18 demonstrated that eyes with progressive glaucomatous optic neuropathy have greater RNFL loss than non-progressing eyes. We hypothesised that the rate of RNFL loss over time was significantly higher in eyes with functional progression irrespective of the criterion used to define visual-field progression. This prospective analysis was designed to examine the rate of RNFL loss using GDxECC in progressing versus non-progressing eyes using three criteria to define functional progression.
METHODS
Participants consisted of glaucoma suspects and patients with perimetric glaucoma who were prospectively enrolled in the Advanced Imaging for Glaucoma Study (AIGS). Informed consent was obtained from all subjects using a consent form approved by the Institutional Review Board of the University Of Miami Miller School Of Medicine, which was in agreement with the provisions of Declaration of Helsinki. Eyes meeting inclusion criteria with ≥36 months of follow-up were included. Inclusion criteria common to both groups consisted of spherical equivalent refractive error between −7.00 and +3.00 dioptres sphere, best-corrected visual acuity ≥20/40, age between 40 and 79 years, and no prior history of intraocular surgery except for uncomplicated cataract extraction. Subjects with ocular disease other than glaucoma or cataract, best-corrected visual acuity <20/40, peripapillary atrophy extending to 1.7 mm from the centre of the optic disc, unreliable SAP or with poor quality SLP images were excluded.
Glaucoma suspects consisted of patients with ocular hypertension characterised by an intraocular pressure (IOP) ≥24 mm Hg with normal optic discs and normal SAP or those with glaucomatous optic neuropathy on funduscopic examination and review of stereoscopic optic disc photographs but normal SAP. Glaucomatous optic neuropathy was defined as neuroretinal rim narrowing to the optic disc margin, notching, excavation or RNFL defect. Glaucoma patients had glaucomatous optic nerve damage and corresponding abnormal SAP defined as an abnormal glaucoma hemifield test, and pattern SD outside 95% normal limits. Patients with SAP abnormalities had at least one confirmatory visual-field examination. All patients underwent a baseline examination consisting of a complete ophthalmic examination including slit-lamp biomicroscopy, gonioscopy, Goldmann applanation tonometry, ultrasound pachymetry, dilated stereoscopic examination and photography of the optic disc, SAP and SLP imaging. Follow-up SAP and SLP imaging were performed at 6-month intervals. During the follow-up period, each patient was treated at the discretion of the attending physician.
SLP imaging and analysis
SLP imaging was performed using the commercially available GDx (Carl Zeiss Meditec, Dublin, California; Version 5.5.0 and 6.0.0) in a standardised fashion through undilated pupils. In ECC mode, the compensator is adjusted so that it combines with corneal birefringence to produce a bias retardation of approximately 55 nm with the slow axis close to the vertical axis. The software then measures a higher total retardation than the RNFL retardation alone, resulting in an improved signal-to-noise ratio. The actual bias retardation and the axis in each image are measured from the macular region and mathematically removed from the final RNFL image to determine the actual RNFL retardation.9,15,16,19
Three consecutive scans were obtained using GDxECC on the same day by the same operator. A primary scan was obtained prior to the baseline measurement to compensate for the corneal birefringence. A fixed concentric measurement band centred on the optic disc with a 3.2 mm outer and a 2.4 mm inner diameter was used to generate the peripapillary retardation measurements. Poor-quality SLP images (unfocused, poorly centred, obtained during eye movement or Q score <8) were excluded. All images had typical scan scores ≥80 indicating minimal or no ARP. GDxECC parameters investigated in this study were superior mean, inferior mean and temporal–superior–nasal–inferior–temporal mean (TSNIT; circumpapillary RNFL thickness measured under the automatically defined 3.2 mm diameter calculation circle). These parameters are provided on the standard GDxECC printout.
Assessment of progression using standard automated perimetry
SAP was performed using the Swedish Interactive Threshold Algorithm (Humphrey Field Analyzer 750 Il-i, 24–2 SITA Standard; Carl Zeiss Meditec) strategy. Only reliable test results (≤33% fixation losses, false-negative results and false-positive results) were included. All the patients were familiar with automated perimetry and had undergone a minimum of two visual-field tests prior to study enrolment.
SAP progression was defined using three methods. The first method was a linear regression analysis of sequential visual fields to measure the slope of the visual-field index (VFI; Humphrey Field Analyzer; Version 4.2). The VFI is an age-corrected index with a range from 0 to 100 calculated based on total deviation values and pattern deviation probability values. Progression was defined as a significant (p<0.05) decline in the slope of the VFI.20
The second method employed an automated pointwise linear regression analysis of SAP sensitivity values using Progressor software (Version 3.3; Medisoft, London) which generates slopes to analyse the rate of global and local sensitivity change and the associated level of significance (p values).21 Progression was defined as the presence of at least one test point with a slope of sensitivity across time of >1 dB loss per year, with p<0.01. For edge points, a stricter slope criterion of >2 dB loss per year (p<0.01) was used.22,23
The third method for assessment of progression used the Guided Progression Analysis (GPA; Humphrey Field Analyzer; Version 4.2). GPA uses statistical criteria designed for the Early Manifest Glaucoma Trial24 and compares the sensitivity values of individual points on follow-up visual fields to the sensitivity values of the same locations on a mean of two baseline exams. Progression was defined as a significant change detected in at least three points, and repeated in the same points in three consecutive follow-up tests, categorised by the GPA software as Likely Progression. All patients had visual fields and GDxEGC imaging performed on the same day.
A minimum of seven visual fields and imaging examinations were included for each patient in the progression analyses. The mean number of visual fields and GDxECC images available for progression assessment was 8.5±0.9 (range 7–11). During the follow-up period, six eyes had seven GDx images and visual fields available, 21 eyes had eight available, 22 eyes had nine available, two eyes had 10 available, and two eyes had 11 examinations available. One eye per subject was enrolled. When both eyes of the same individual were eligible for enrolment, the eye that demonstrated visual-field progression by any of the three criteria was selected for analysis.25 In patients where neither eye demonstrated progression, or both eyes progressed, one eye was randomly selected for analysis.
Statistical analysis
Statistical analysis was performed using JMP software version 8.0 (SAS) and SPSS 16 (SPSS). The rate of RNFL loss over time was calculated using a linear regression analysis. An independent-samples t test, pooled t test, χ2 test and κ statistics were performed. All tests were two-sided, and a p value of <0.05 was considered significant. The Shapiro–Wilk W test of normality was used to test the distribution of data in progressing and non-progressing eyes. The full manual of procedures for AIGS is available at http://www.aigstudy.net.
RESULTS
Fifty-three eyes (30 glaucoma suspect, 23 glaucoma) of 53 patients (mean age 64.5 ±10.7 years, range 42–79) were enrolled. The mean length of follow-up was 44.9±5.3 months (range 36–60 months). Table 1 describes the clinical characteristics of the study population.
Table 1.
Clinical characteristics of the study population at baseline (n=53 eyes).
| Mean±SD (range) | Glaucoma suspect (n = 23) | Glaucoma (n = 30) | p Value |
|---|---|---|---|
| Age (years) | 61.1±10.9 (42 to 80) | 68.9±8.9 (46 to 82) | 0.008* |
| Gender | |||
| Male | 12 | 19 | 0.57† |
| Female | 11 | 11 | |
| Race | |||
| White | 20 | 24 | 0.37† |
| Hispanic | 1 | 0 | |
| Black | 1 | 1 | |
| Asian | 1 | 5 | |
| Central corneal thickness (μm) | 559.8± 35.5 (505 to 626) | 539±45.8 (448 to 650) | 0.07* |
| Intraocular pressure (mm Hg) | 19.5± 4.4 (10 to 28) | 14.9± 3.6 (8 to 23) | <0.001* |
| Standard automated perimetry | |||
| Mean deviation (dB) | 0.02± 1.11 (−2.5 to 2.18) | −4.32± 4.95 (−15.18 to 0.09) | <0.001* |
| Pattern SD (dB) | 1.52± 0.26 (1.05 to 2.07) | 4.95± 3.98 (1.5 to 13.95) | <0.001* |
| GDx with enhanced corneal compensation retinal nerve fibre layer thickness (μm) | |||
| Temporal–superior–nasal– inferior–temporal mean |
50.74±4.92 (40.23 to 63.30) | 43.49±6.85 (30.85 to 53.33) | <0.001* |
| Superior mean | 62.04±6.66 (43.93 to 76.70) | 51.4±10.19 (33.10 to 69.51) | <0.001* |
| Inferior mean | 62.48±7.70 (49.49 to 74.37) | 51.14±9.82 (34.76 to 69.29) | <0.001* |
Independent samples t test.
χ2.
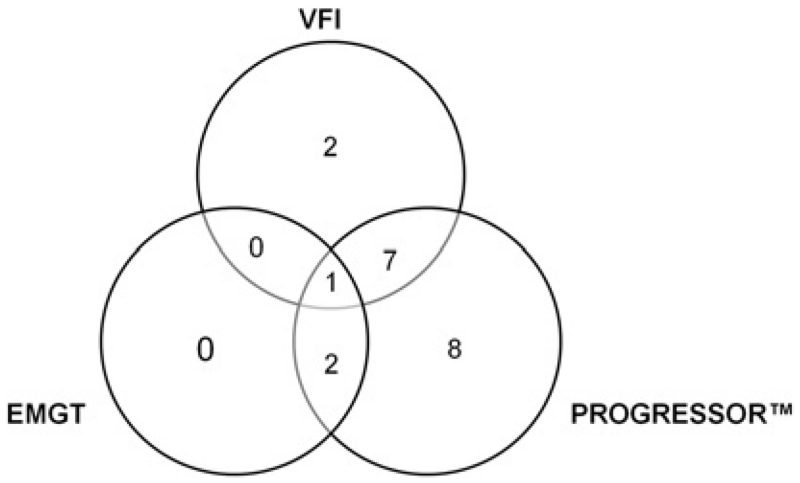
Eighteen eyes (40%) demonstrated SAP progression during the follow-up period using the Progressor criterion, 10 eyes (18.9%) using the VFI criterion and three eyes (5.7%) using the EMGT criterion. There was significant agreement between VFI and Progressor criteria (k=0.43, p=0.001) and between Progressor and EMGT criteria (k=0.21, p=0.013) but not between VFI and EMGT criteria (k=0.073, p=0.51) (figure 1). Of the total of 450 GDxECC images acquired during the follow-up period, 12 scans (2.67%) did not meet the quality criteria and were excluded from the analysis.
Figure 1.
Venn diagram showing the number of eyes classified as progressing by visual-field index (VFI), Progressor and early manifest glaucoma trial (EMGT) criteria. There was significant agreement between VFI and Progressor criteria (k=0.43, p=0.001) and between Progressor and EMGT criteria (k=0.21, p=0.013) but not between VFI and EMGT criteria (k=0.073, p=0.51).
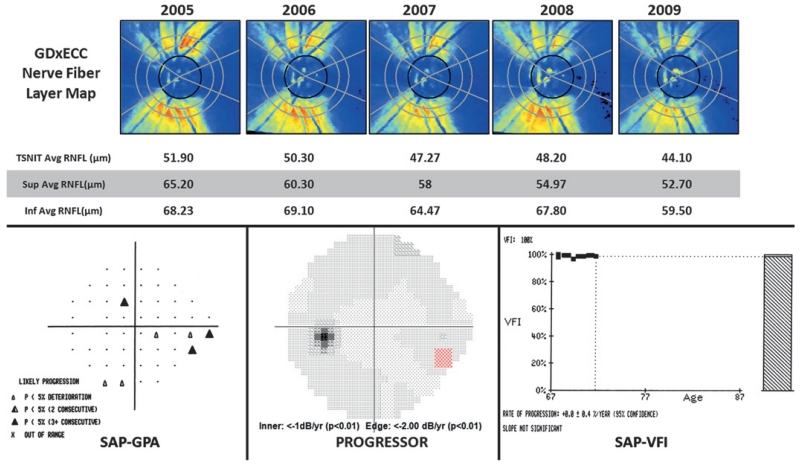
Figure 2 illustrates a 74-year-old female with progressive open-angle glaucoma over 4 years of follow-up. Table 2 illustrates the baseline RNFL thickness values in progressing and non-progressing eyes assessed using VFI, Progressor and EMGT methods of progression. Progressing and non-progressing eyes had a similar (p>0.05) baseline RNFL thickness (TSNIT mean, superior and inferior quadrants).
Figure 2.
GDx with enhanced corneal compensation (GDxECC) retardation maps (upper row) showing progressive loss of the superior retinal nerve fibre layer (RNFL) in a 74-year-old female with open-angle glaucoma over 4 years of follow-up. Standard automated perimetry Guided Progression Analysis based on EMGT criteria (SAP-GPA, bottom left) and Progressor (bottom centre) demonstrates progressive inferior nasal visual-field loss. The visual-field index (VFI, bottom right) slope shows no significant change over time. TSNIT, temporal superior nasal inferior temporal.
Table 2.
Baseline GDx with enhanced corneal compensation retinal nerve fibre layer measurements in progressing and non-progressing eyes
| Criterion | GDx with enhanced corneal compensation baseline retinal nerve fibre layer thickness (μm) |
Progressing eyes | Non-progressing eyes | p Value* |
|---|---|---|---|---|
| Standard automated perimetry visual-field index |
TSNIT mean | 46.87±8.79 | 47.76±6.40 | 0.71 |
| Superior mean | 56.99±12.81 | 57.55±9.20 | 0.87 | |
| Inferior mean | 56.45±12.49 | 57.82±9.87 | 0.71 | |
| Progressor | TSNIT mean | 45.73±7.97 | 48.56±6.05 | 0.15 |
| Superior mean | 55.07±11.42 | 58.66±8.86 | 0.21 | |
| Inferior mean | 54.69±10.40 | 59.03±10.07 | 0.15 | |
| Early manifest glaucoma trial | TSNIT mean | 44.50±11.13 | 47.78±6.61 | 0.42 |
| Superior mean | 53.03±17.40 | 57.71±9.44 | 0.43 | |
| Inferior mean | 53.23±13.98 | 57.82±10.16 | 0.46 |
Independent samples t test.
TSNIT, temporal–superior–nasal–inferior–temporal.
The Shapiro–Wilk W test of normality showed that the distribution of data was normal in progressing and non-progressing eyes for slope of RNFL loss (TSNIT, superior and inferior; W=0.87–0.96, p>0.05).
Table 3 demonstrates the mean±SD, median and 25–75% interquartile ranges for slope of RNFL loss (μm/year) measured using GDxECC in eyes judged to be non-progressing and progressing using VFI, Progressor and EMGT criteria. The annual rate of RNFL loss (μm/year) for the TSNIT mean and superior mean parameters was significantly greater (p<0.05) in progressing than in non-progressing eyes when Progressor, EMGT and VFI methods were used for progression judgement. The annual rate of RNFL loss (μm/year) for the inferior mean parameter was significantly greater (p<0.05) in progressing than in non-progressing eyes when VFI and Progressor methods were used for progression judgement.
Table 3.
Mean±SD, median and 25–75% interquartile ranges for slope of retinal nerve fibre layer loss (μm/year) measured using GDx with enhanced corneal compensation in eyes judged to be non-progressing and progressing using visual-field index, Progressor and early manifest glaucoma trial criteria.
| Progressing eyes |
Non-progressing eyes |
|||||
|---|---|---|---|---|---|---|
| Criteria | Rate of retinal nerve fibre layer loss (μm/year) |
Mean±SD | Median (25% to 75% interquartile range) |
Mean±SD | Median (25% to 75% interquartile range) |
p Value* |
| Visual-field index | TSNIT mean | −1.11±0.64 | −1.00 (−1.52 to −0.65) | −0.41±0.85 | −0.29 (−0.58 to 0.03) | 0.019 |
| Superior mean | −1.94±1.18 | −2.07 (−3.03 to −0.77) | −0.49±1.41 | −0.32 (−0.99 to 0.33) | 0.004 | |
| Inferior mean | −1.07±1.26 | −0.91 (−1.73 to −0.21) | −0.29±1.05 | −0.22 (−0.72 to 0.13) | 0.048 | |
| Progressor | TSNIT mean | −1.24±0.99 | −1.02 (−1.70 to −0.52) | −0.18±0.49 | −0.25 (−0.51 to 0.07) | <0.001 |
| Superior mean | −1.92±1.49 | −1.74 (−2.78 to −0.58) | −0.18±1.07 | −0.07 (−0.75 to 0.46) | <0.001 | |
| Inferior mean | −1.05±1.38 | −0.74 (−1.68 to −0.13) | −0.12±0.82 | −0.22 (−0.61 to 0.30) | 0.003 | |
| Early manifest glaucoma trial | TSNIT mean | −1.95±0.99 | −1.66 (−3.05 to −1.14) | −0.46±0.78 | −0.34 (−0.69 to −0.03) | 0.003 |
| Superior mean | −2.63±0.94 | −2.51 (−3.62 to −1.76) | −0.66±1.43 | −0.37 (−1.22 to 0.22) | 0.02 | |
| Inferior mean | −0.87±1.46 | −1.66 (−1.76 to 0.81) | −0.41±1.11 | −0.34 (−0.74 to 0.08) | 0.5 | |
Pooled t test.
TSNIT, temporal–superior–nasal–inferior–temporal.
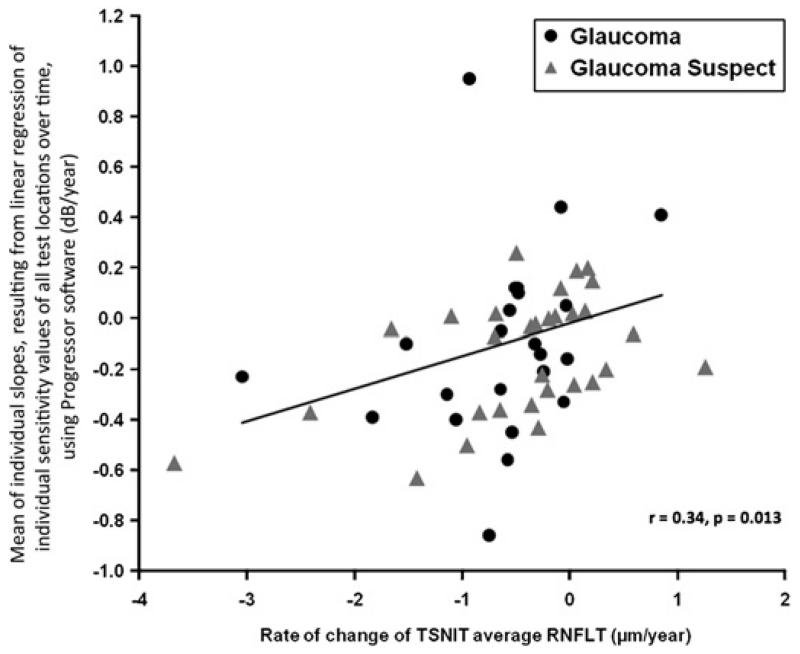
The rate of TSNIT mean retinal nerve fibre layer thickness loss over time (μm/year) correlated significantly with the mean of individual slopes, resulting from linear regression of individual sensitivity values of all test locations over time, using Progressor software (dB/year) (r=0.34, p=0.013; figure 3).
Figure 3.
Scatter plot demonstrating the relationship between the rate of temporal superior nasal inferior temporal (TSNIT) mean retinal nerve fibre layer thickness loss overtime (μm/year) and the mean of individual slopes, resulting from linear regression of individual sensitivity values of all test locations over time, using Progressor software (dB/year) in glaucoma suspect and glaucomatous eyes.
DISCUSSION
In the present study, we conducted an analysis to compare rates of progressive glaucomatous RNFL atrophy using different methods to define visual-field progression. Although event and trend analyses are complementary, there are significant differences in their approach for detection of progression.26,27 Event-based analyses identify whether progression has occurred, while trend analyses quantify the rate of progression. Previous studies28,29 have shown that different progression criteria do not always identify the same eyes as progressing and have at best only fair to moderate agreement among different algorithms. There is presently no consensus among clinicians or investigators as to the best method for defining glaucomatous visual-field progression.
Despite differences in criteria for visual-field progression, progressing eyes had a significantly faster rate of RNFL decline than non-progressing eyes. The rate of mean RNFL loss over time, represented by the GDxECC parameter TSNIT mean, was two to 11 times greater in progressing eyes than in non-progressing eyes. In our study, we found the mean rate of mean RNFL loss in progressing eyes to range from −0.87 to −2.63 μm/year, depending on the method used to define visual-field progression. These results are consistent with recent data published by Medeiros and associates using GDx18,30 and OCT.31 Although these data demonstrate that both groups were similarly matched at baseline with regard to disease severity and suggest that the greater rate of RNFL atrophy in progressing eyes was not biased by baseline differences in RNFL thickness, the current study is underpowered to detect baseline differences in progressing and non-progressing eyes. A useful progression analysis method not only identifies those individuals with disease progression but also identifies persons with progression at rapid velocity who are at a significantly high risk for functional vision loss. Clinicians should therefore be alert to identify eyes with steep rates of RNFL decline over time.26,27 Structure and function may certainly change at different rates among progressing patients,32 and within a given individual, detection of progressive change in structure and function may not occur at the same time point.18
Our study has potential limitations, including a relatively small sample size and short follow-up interval. Glaucoma is a slowly progressive disease, and many subjects were treated with ocular hypotensive therapy, thereby further reducing the risk of progression. A larger study population with greater length of follow-up is necessary to determine risk factors associated with progressive RNFL loss.
In conclusion, GDxECC may be useful for detection of glaucomatous structural progression and quantifying the velocity of progressive RNFL loss. The present study demonstrates that, despite differences in the criteria used to judge functional progression, progressing eyes have a significantly greater rate of RNFL loss (two to 11 times faster) compared with non-progressing eyes.
Acknowledgments
Funding NIH Grants R01-EY013516, Bethesda, Maryland, an unrestricted grant from Research to Prevent Blindness P30-EY14801, New York.
Footnotes
Meeting presentation: Presented in part at the annual meeting of the American Glaucoma Society, Naples, Florida, 6 March 2010.
The Advanced Imaging for Glaucoma Study Group: See http://www.aigstudy.net for the full list of authors.
Competing interests None.
Ethics approval Ethics approval was provided by the University of Miami Miller School of Medicine.
Provenance and peer review Not commissioned; externally peer reviewed.
REFERENCES
- 1.Weinreb RN, Khaw PT. Primary open-angle glaucoma. Lancet. 2004;363:1711–20. doi: 10.1016/S0140-6736(04)16257-0. [DOI] [PubMed] [Google Scholar]
- 2.Heijl A, Bengtsson B, Chauhan BC, et al. A comparison of visual field progression criteria of 3 major glaucoma trials in early manifest glaucoma trial patients. Ophthalmology. 2008;115:1557–65. doi: 10.1016/j.ophtha.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 3.Greenfield DS, Knighton RW, Huang XR. Effect of corneal polarization axis on assessment of retinal nerve fiber layer thickness by scanning laser polarimetry. Am J Ophthalmol. 2000;129:715–22. doi: 10.1016/s0002-9394(00)00353-6. [DOI] [PubMed] [Google Scholar]
- 4.Knighton RW, Huang X, Zhou Q. Microtubule contribution to the reflectance of the retinal nerve fiber layer. Invest Ophthalmol Vis Sci. 1998;39:189–93. [PubMed] [Google Scholar]
- 5.Knighton RW, Huang XR, Greenfield DS. Analytical model of scanning laser polarimetry for retinal nerve fiber layer assessment. Invest Ophthalmol Vis Sci. 2002;43:383–92. [PubMed] [Google Scholar]
- 6.Weinreb RN, Bowd C, Zangwill LM. Glaucoma detection using scanning laser polarimetry with variable corneal polarization compensation. Arch Ophthalmol. 2003;120:218–24. doi: 10.1001/archopht.121.2.218. [DOI] [PubMed] [Google Scholar]
- 7.Bagga H, Greenfield DS, Knighton RW. Scanning laser polarimetry with variable corneal compensation: identification and correction for corneal birefringence in eyes with macular disease. Invest Ophthalmol Vis Sci. 2003;44:1969–76. doi: 10.1167/iovs.02-0923. [DOI] [PubMed] [Google Scholar]
- 8.Poinoosawmy D, Tan JC, Bunce C, et al. The ability of the GDx nerve fibre analyser neural network to diagnose glaucoma. Graefes Arch Clin Exp Ophthalmol. 2001;239:122–7. doi: 10.1007/s004170100256. [DOI] [PubMed] [Google Scholar]
- 9.Reus NJ, Zhou Q, Lemij HG. Enhanced imaging algorithm for scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2006;47:3870–7. doi: 10.1167/iovs.05-0067. [DOI] [PubMed] [Google Scholar]
- 10.Garway-Heath DF, Greaney MJ, Caprioli J. Correction for the erroneous compensation of anterior segment birefringence with the scanning laser polarimeter for glaucoma diagnosis. Invest Ophthalmol Vis Sci. 2002;43:1465–74. [PubMed] [Google Scholar]
- 11.Mai TA, Reus NJ, Lemij HG. Structure–function relationship is stronger with enhanced corneal compensation than with variable corneal compensation in scanning laser polarimetry. Invest Ophthalmol Vis Sci. 2007;48:1651–8. doi: 10.1167/iovs.06-1003. [DOI] [PubMed] [Google Scholar]
- 12.Medeiros FA, Bowd C, Zangwill LM, et al. Detection of glaucoma using scanning laser polarimetry with enhanced corneal compensation. Invest Ophthalmol Vis Sci. 2007;48:3146–53. doi: 10.1167/iovs.06-1139. [DOI] [PubMed] [Google Scholar]
- 13.Morishita S, Tanabe T, Yu S, et al. Retinal nerve fibre layer assessment in myopic glaucomatous eyes: comparison of GDx variable corneal compensation with GDx enhanced corneal compensation. Br J Ophthalmol. 2008;92:1377–81. doi: 10.1136/bjo.2007.134080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Saito H, Tomidokoro A, Yanagisawa M, et al. Scanning laser polarimetry with enhanced corneal compensation in patients with open-angle glaucoma. J Glaucoma. 2008;17:24–9. doi: 10.1097/IJG.0b013e318133fb47. [DOI] [PubMed] [Google Scholar]
- 15.Sehi M, Guaqueta DC, Greenfield DS. An enhancement module to improve the atypical birefringence pattern using scanning laser polarimetry with variable corneal compensation. Br J Ophthalmol. 2006;90:749–53. doi: 10.1136/bjo.2005.086447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Toth M, Hollo G. Enhanced corneal compensation for scanning laser polarimetry on eyes with atypical polarisation pattern. Br J Ophthalmol. 2005;89:1139–42. doi: 10.1136/bjo.2005.070011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Bagga H, Greenfield DS, Feuer WJ. Quantitative assessment of atypical birefringence images using scanning laser polarimetry with variable corneal compensation. Am J Ophthalmol. 2005;139:437–46. doi: 10.1016/j.ajo.2004.10.019. [DOI] [PubMed] [Google Scholar]
- 18.Medeiros FA, Alencar LM, Zangwill LM, et al. The relationship between intraocular pressure and progressive retinal nerve fiber layer loss in glaucoma. Ophthalmology. 2009;116:1125–33. e1121–3. doi: 10.1016/j.ophtha.2008.12.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sehi M, Guaqueta DC, Feuer WJ, et al. Scanning laser polarimetry with variable and enhanced corneal compensation in normal and glaucomatous eyes. Am J Ophthalmol. 2007;143:272–9. doi: 10.1016/j.ajo.2006.09.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Bengtsson B, Heijl A. A visual field index for calculation of glaucoma rate of progression. Am J Ophthalmol. 2008;145:343–3. doi: 10.1016/j.ajo.2007.09.038. [DOI] [PubMed] [Google Scholar]
- 21.Viswanathan AC, Fitzke FW, Hitchings RA. Early detection of visual field progression in glaucoma: a comparison of PROGRESSOR and STATPAC 2. Br J Ophthalmol. 1997;81:1037–42. doi: 10.1136/bjo.81.12.1037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Strouthidis NG, Scott A, Peter NM, et al. Optic disc and visual field progression in ocular hypertensive subjects: detection rates, specificity, and agreement. Invest Ophthalmol Vis Sci. 2006;47:2904–10. doi: 10.1167/iovs.05-1584. [DOI] [PubMed] [Google Scholar]
- 23.Viswanathan AC, Crabb DP, McNaught AI, et al. Interobserver agreement on visual field progression in glaucoma: a comparison of methods. Br J Ophthalmol. 2003;87:726–30. doi: 10.1136/bjo.87.6.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Leske MC, Heijl A, Hyman L, et al. early manifest glaucoma trial: design and baseline data. Ophthalmology. 1999;106:2144–53. doi: 10.1016/s0161-6420(99)90497-9. [DOI] [PubMed] [Google Scholar]
- 25.Medeiros FA, Zangwill LM, Alencar LM, et al. Rates of progressive retinal nerve fiber layer loss in glaucoma measured by scanning laser polarimetry. Am J Ophthalmol. 2010;149:908–15. doi: 10.1016/j.ajo.2010.01.010. [DOI] [PubMed] [Google Scholar]
- 26.Chauhan BC, Garway-Heath DF, Goni FJ, et al. Practical recommendations for measuring rates of visual field change in glaucoma. Br J Ophthalmol. 2008;92:569–73. doi: 10.1136/bjo.2007.135012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Artes PH, Chauhan BC. Longitudinal changes in the visual field and optic disc in glaucoma. Prog Retin Eye Res. 2005;24:333–54. doi: 10.1016/j.preteyeres.2004.10.002. [DOI] [PubMed] [Google Scholar]
- 28.Boden C, Blumenthal EZ, Pascual J, et al. Patterns of glaucomatous visual field progression identified by three progression criteria. Am J Ophthalmol. 2004;138:1029–36. doi: 10.1016/j.ajo.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Vesti E, Johnson CA, Chauhan BC. Comparison of different methods for detecting glaucomatous visual field progression. Invest Ophthalmol Vis Sci. 2003;44:3873–9. doi: 10.1167/iovs.02-1171. [DOI] [PubMed] [Google Scholar]
- 30.Medeiros FA, Alencar LM, Zangwill LM, et al. Detection of progressive retinal nerve fiber layer loss in glaucoma using scanning laser polarimetry with variable corneal compensation. Invest Ophthalmol Vis Sci. 2009;50:1675–81. doi: 10.1167/iovs.08-2712. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Medeiros FA, Zangwill LM, Alencar LM, et al. Detection of glaucoma progression with stratus OCT retinal nerve fiber layer, optic nerve head, and macular thickness measurements. Invest Ophthalmol Vis Sci. 2009;50:5741–8. doi: 10.1167/iovs.09-3715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Heijl A, Bengtsson B, Hyman L, et al. Natural history of open-angle glaucoma. Ophthalmology. 2009;116:2271–6. doi: 10.1016/j.ophtha.2009.06.042. [DOI] [PubMed] [Google Scholar]