Abstract
Background and Purpose
Agmatine, a putative neurotransmitter, plays a vital role in learning and memory. Although it is considered an endogenous ligand of imidazoline receptors, agmatine exhibits high affinity for α‐adrenoceptors, NOS and NMDA receptors. These substrates within the locus coeruleus (LC) are critically involved in learning and memory processes.
Experimental Approach
The hippocampus and LC of male Wistar rat were stereotaxically cannulated for injection. Effects of agmatine, given i.p. or intra‐LC, on acquisition, consolidation and retrieval of inhibitory avoidance (IA) memory were measured. The NO donor S‐nitrosoglutathione, non‐specific (L‐NAME) and specific NOS inhibitors (L‐NIL, 7‐NI, L‐NIO), the α2‐adrenoceptor antagonist (yohimbine) or the corresponding agonist (clonidine) were injected intra‐LC before agmatine. Intra‐hippocampal injections of the NMDA antagonist, MK‐801 (dizocilpine), were used to modify the memory enhancing effects of agmatine, SNG and yohimbine. Expression of tyrosine hydroxylase (TH) and eNOS in the LC was assessed immunohistochemically.
Key Results
Agmatine (intra‐LC or i.p.) facilitated memory retrieval in the IA test. S‐nitrosoglutathione potentiated, while L‐NAME and L‐NIO decreased, these effects of agmatine. L‐NIL and 7‐NI did not alter the effects of agmatine. Yohimbine potentiated, whereas clonidine attenuated, effects of agmatine within the LC. The effects of agmatine, S‐nitrosoglutathione and yohimbine were blocked by intra‐hippocampal MK‐801. Agmatine increased the population of TH‐ and eNOS‐immunoreactive elements in the LC.
Conclusions and Implications
The facilitation of memory retrieval in the IA test by agmatine is probably mediated by interactions between eNOS, NO and noradrenergic pathways in the LC.
Abbreviations
- 7NI
7‐nitroindazole
- IA
inhibitory avoidance
- LC
locus coeruleus
- L‐NIL
L‐N6‐(1‐iminoethyl) lysine hydrochloride
- L‐NIO
N5‐(1‐iminoethyl)‐L‐ornithine dihydrochloride
- SNG
S‐nitrosoglutathione
- NMDA
N‐methyl‐D‐aspartate
- NO
Nitric oxide
- NOS
Nitric oxide synthase
Tables of Links
| TARGETS |
|---|
| Enzymes a |
| eNOS, endothelial NOS |
| iNOS, inducible NOS |
| nNOS, neuronal NOS |
| TH, tyrosine hydroxylase |
| GPCRs b |
| α2‐adrenoceptors |
These Tables list key protein targets and ligands in this article which are hyperlinked to corresponding entries in http://www.guidetopharmacology.org, the common portal for data from the IUPHAR/BPS Guide to PHARMACOLOGY (Southan et al., 2016) and are permanently archived in the Concise Guide to PHARMACOLOGY 2015/16 (a,bAlexander et al., 2015a, 2015b).
Introduction
Agmatine is a putative neurotransmitter that plays a crucial role in learning and memory (Liu et al., 2008, 2009; Liu and Bergin, 2009). Agmatine improves scopolamine‐ and streptozotocin‐induced impairment of learning and memory (Utkan et al., 2012; Moosavi et al., 2014), and spatial training has been found to increase agmatine levels (Leitch et al., 2011; Rushaidhi et al., 2013). Agmatine immunoreactivity has been detected in the locus coeruleus (LC) (Otake et al., 1998), which may serve as a site of action for its facilitatory effect on inhibitory avoidance (IA) memory (Arteni et al., 2002; Lu et al., 2010). However, the molecular mechanism underlying the facilitatory effect of agmatine on IA memory in the LC remains unknown.
NO has been recognized as a critical neuronal messenger in the CNS (Koriyama, 2013). NO is present abundantly in the LC (Santamarta et al., 2014) and increases the firing rate of LC neurons (Torrecilla et al., 2007). Data suggest that NO activates noradrenergic neurons of LC via cGMP‐dependent protein kinase and a nonselective cationic channel (Pineda et al., 1996b; Koriyama, 2013). Additionally, blockade of somatodendritic α2‐adrenoceptors has been shown to stimulate noradrenergic neurons in the LC, leading to memory facilitation (Chen et al., 1992). Moreover, noradrenergic projections from the LC to the dentate gyrus (DG) (Hansen and Manahan‐Vaughan, 2015) may underlie potentiation after LC activation (Rajkumar et al., 2013), and hippocampal activity is influenced by increased release of noradrenaline from the terminals of activated LC cells (Bari and Aston‐Jones, 2013).
Interestingly, agmatine stimulates the firing rate of LC neurons via NOS‐dependent mechanisms (Ruiz‐Durántez et al., 2002). Further, several physiological effects of agmatine, for example, anticonvulsant (Payandemehr et al., 2013), anti‐anxiety (Taksande et al., 2014), apoptosis and memory loss (Zarifkar et al., 2010; Moosavi et al., 2014), morphine withdrawal syndrome (Li et al., 2012) and antinociception (Aglawe et al., 2014), are found to involve interaction with the NO system. Agmatine may produce unique isoform‐specific effects on NOS as this compound inhibited the inducible NOS (iNOS, Ahn et al., 2011) and neuronal NOS (nNOS, Demady et al., 2001) but activated endothelial NOS (eNOS) (Mun et al., 2010). Whether these selective effects on NOS isoforms mediate memory facilitation remains unknown.
Using the IA model, together with pharmacological tools (NOS inhibitors, NO donor, α2‐adrenoceptor agonist clonidine and antagonist yohimbine) and local, intra‐LC injections, we investigated whether the facilitatory effect of agmatine on memory was mediated by interactions between the NOS and α2‐adrenoceptors, within the LC. Further, we also investigated whether these effects were dependent on adrenergic afferents from the LC to the hippocampus. Our results demonstrate that agmatine enhanced retrieval of IA memory via a eNOS‐α2‐adrenoceptor pathway in the LC. We also establish a requirement for a LC‐hippocampus pathway in this phenomenon. Together, these results establish a novel molecular mechanism underlying memory enhancement by agmatine.
Methods
Animals
All animal care and experimental procedures were approved by the Institutional Animal Ethical Committee and executed strictly according to the guidelines of Committee for the Purpose of Control and Supervision of Experiments on Animals, Government of India. All studies involving animals are reported in accordance with the ARRIVE guidelines for reporting experiments involving animals (Kilkenny et al., 2010; McGrath and Lilley, 2015). Adult male Wistar rats (230–250 g; National Institute of Nutrition, Hyderabad, India) were housed under controlled room temperature (25 ± 2°C) and maintained at 12:12 h light/dark cycle, (light on at 07:00 h). Food and water were available ad libitum.
Surgery
The rats were anaesthetized with ketamine (90 mg·kg−1; Ketmin® 50, Themis Medicare Ltd., Mumbai, India) and xylazine (10 mg·kg−1; Xylaxin®, Indian Immunologicals Ltd., Hyderabad, Telangana, India) combination injected i.p. and placed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA, USA). A 24 gauge stainless steel guide cannula prepared in‐house (Kokare et al., 2011) was implanted bilaterally 1 mm above the LC or hippocampus, using stereotaxic co‐ordinates (LC: −9.8 mm posterior, ±1.2 mm lateral and −7.1 mm ventral; and hippocampus: −4.3 mm posterior, ±3.0 mm lateral and −2.2 mm ventral; Paxinos and Watson, 1998). Following surgery, the rats were allowed 7 days to recover and then used in the behavioural tests, as described later. All test protocols were conducted between 09:00 and 13:00 h.
Inhibitory avoidance test
The step‐through inhibitory avoidance apparatus consisted of a box divided by a guillotine door into two equal (20 × 20 × 30 cm) compartments, one being the light and the other dark compartment. Stainless steel grids (2.5 mm in diameter) were placed at 1 cm intervals on the floor of the dark compartment to produce foot shock. Electric shock (50 Hz, 1 mA and 3 s) was delivered to the grid floor of the dark compartment by an insulated stimulator. All animals were allowed to habituate to the experimental room for at least 30 min before the experiments. The rat was gently placed in the light compartment, the guillotine door was opened after 20 s, and the animal was allowed to enter the dark compartment. The latency to cross into the dark compartment by animal was recorded. Once the animal crossed with all four paws to the next compartment, the guillotine door was closed, and the rat was taken into its home cage. Animals that took more than 100 s to cross to the dark compartment were eliminated from the experiments. The acquisition trial was initiated after 30 min. The animal was again placed in the light compartment. The guillotine door was opened after 20 s, and as soon as the animal crossed to the dark compartment, the door was closed, and a foot shock (50 Hz, 1 mA, and 3 s) was immediately delivered to the grid floor of the dark compartment. After 20 s, the rat was taken back into its home cage. The acquisition trial was repeated after 5 min. If the rat did not enter the dark compartment within 120 s, a successful acquisition of passive avoidance response was recorded. However, if the rat entered the dark compartment before 120 s, the door was closed, and the animal received a similar shock again. The animal was then removed from the apparatus.
A retention test was performed after 24 h of the last acquisition trial. The animal was placed in the light compartment, and the door was opened after 20 s to measure the step‐through latency for entering into the dark compartment. The maximum cut‐off time for step‐through latency was 300 s. No electric shock was applied during this session (Harooni et al., 2008).
Immunohistofluorescence method
Rats (n = 5) treated with aCSF (composition in mM, 20 NaCl, 4.8 KCl, 1.2 KH2PO4, 1.2 MgSO4, 25 NaHCO3, 2.5 CaCl2 and 10 D‐glucose; 0.5μL) or agmatine (20 ng per rat, intra‐LC) were anaesthetized after the IA test, transcardially perfused, and their brains were removed, sectioned in a coronal plane (30 μm) on a cryostat (Leica, Wetzlar, Germany) and examined by immunostaining (see later). The LC sections were processed for immunolabelling with antibody against rabbit TH, a key enzyme in the biosynthesis of noradrenaline (Millipore, Billerica, MA, USA; dilution 1:1000) and mouse eNOS (BD transduction lab, Lexington, KY, USA; dilution 1:500), according to the protocol already described (Shelkar et al., 2015). Sections were incubated in AlexaFluor‐568‐conjugated anti‐rabbit IgG or AlexaFluor‐568‐conjugated anti‐mouse IgG (Invitrogen, Carlsbad, CA, USA; 1:500 each) for 2 h. They were rinsed in PBS and observed under a Leica DM‐2500 fluorescence microscope using suitable filter sets, and the images were captured, adjusted for brightness and contrast, and merged using adobe photoshop cs4 software (Adobe Systems, Inc., San Jose, CA, USA).
Morphometric analysis
The TH‐ or eNOS‐ immunoreactive (ir) elements (% area) and TH‐ir or eNOS‐ir expressing cells from the digitized images of TH‐ and eNOS‐immunolabelled sections were analyzed using image j (National Institute of Health (NIH), Bethesda, MO) software. Ten readings were taken from both the sides of each brain, and the data from five brains in each group were collected and mean ± SEM was calculated. The details of the method have been described by Shelkar et al., (2015).
Treatment groups
Dose‐dependent effect of agmatine (intra‐LC or i.p.) on acquisition, consolidation and retrieval of IA memory
The dose‐dependent effects of agmatine on acquisition (before training), consolidation (immediately after training) and retrieval (24 h after training) of memory were investigated in the IA test. Separate groups of rats were injected intracranially (intra‐LC) with aCSF or agmatine (5, 10 and 20 ng per rat; n = 8 per group) or systemically (i.p.) with saline or agmatine (10, 20 and 40 mg·kg−1; n = 5 per group). Injections were performed 15 min (for intra‐LC) or 30 min (for i.p.) before the acquisition trials, after the acquisition trials or before retrieval testing and thereafter step‐through latency was measured.
Dose related intra‐LC effects of the NO donor (SNG), non‐specific (L‐NAME) and specific NOS inhibitors (L‐NIL, 7‐NI, L‐NIO), α2‐adrenoceptor antagonist (yohimbine) or agonist (clonidine) on retrieval of IA memory
Separate groups of rats (n = 8 per group) were injected either with aCSF, SNG (1.4, 2.8 and 5.6 pg per rat, intra‐LC), L‐NAME (26.9, 53.8 and 107.6 pg per rat, intra‐LC), iNOS inhibitor, L‐NIL (208, 416 and 832 pg per rat, intra‐LC), nNOS inhibitor, 7‐NI (10, 20 and 40 ng per rat, intra‐LC), eNOS inhibitor, L‐NIO (60, 123 and 246 pg per rat, intra‐LC), yohimbine (0.5, 1 μg per rat, intra‐LC) or clonidine (0.3, 1 μg per rat, intra‐LC) 15 min before memory retrieval and step‐through latency were measured.
Influence of the NO donor (SNG) and NOS inhibitors within the LC on IA memory facilitation induced by agmatine
Rats (n = 8 per group) were injected with aCSF or an ineffective dose of SNG (2.8 pg per rat, intra‐LC) 10 min before aCSF or agmatine (5 ng per rat, intra‐LC). Separately, rats were injected with aCSF or L‐NAME (107.6 pg per rat, intra‐LC), L‐NIL (832 pg per rat, intra‐LC), 7‐NI (20 ng per rat, intra‐LC) or L‐NIO (246 pg per rat, intra‐LC) before 10 min of aCSF or agmatine (10 ng per rat, intra‐LC). Retrieval was tested after 15 min of agmatine injection.
Influence of α2‐adrenoceptor antagonist and agonist on agmatine related memory facilitation within the LC
Separate groups of rats (n = 8 per group) were treated either with aCSF or a sub‐effective dose of yohimbine (0.5 μg per rat, intra‐LC) 10 min before aCSF or agmatine (5 ng per rat, intra‐LC). In a separate group, aCSF or clonidine (1 μg per rat, intra‐LC) was injected 10 min before aCSF, or effective dose of agmatine (10 ng per rat, intra‐LC) and retrieval was tested 15 min after agmatine injection.
Influence of eNOS inhibitor (L‐NIO) on yohimbine induced memory facilitation within the LC
Rats (n = 8 per group) were treated with either aCSF or L‐NIO (246 pg per rat, intra‐LC) 10 min before aCSF or yohimbine (1 μg per rat, intra‐LC) treatment and after 15 min retrieval was tested.
Influence of clonidine on the memory facilitating effect of NO donor (SNG) within the LC
In a separate group (n = 8 per group), aCSF or SNG (5.6 pg per rat, intra‐LC) was administered after 10 min of aCSF or clonidine (1 μg per rat, intra‐LC) and 15 min thereafter retrieval was tested.
Influence of intra‐hippocampus NMDA antagonist MK‐801 on memory facilitation induced by intra‐LC agmatine or the NO donor
Different groups of rats (n = 8 per group) were treated either with aCSF or NMDA antagonist, MK‐801 (1 ng per rat, intra‐hippocampus), 10 min before the injection of aCSF or agmatine (10 ng per rat, intra‐LC) or SNG (5.6 pg per rat, intra‐LC) and 15 min later retrieval was tested.
Data analysis
The data and statistical analysis comply with the recommendations on experimental design and analysis in pharmacology (Curtis et al., 2015). Data were expressed as mean ± SEM. The step‐through latencies were analysed by one‐way ANOVA followed by Dunnett's test or Newman–Keuls's multiple comparisons test. The morphometric data drawn from the immunolabelled sections were analysed by unpaired t‐test. Post hoc test was used only when a significant effect was found, i.e. P < 0.05. P < 0.05 was considered to be statistically significant in all the analyses.
Materials
Agmatine sulphate, S‐nitrosoglutathione (SNG), 7‐nitroindazole (7‐NI), L‐N6‐(1‐iminoethyl) lysine hydrochloride (L‐NIL), N ω‐nitro‐L‐arginine methyl ester hydrochloride (L‐NAME), yohimbine and clonidine were purchased from Sigma‐Aldrich Co., St. Louis, MO, USA. N5‐(1‐iminoethyl)‐L‐ornithine dihydrochloride (L‐NIO) and MK‐801 were purchased from Tocris Biosciences, Bristol, UK. For i.p. injections, agmatine sulphate was dissolved in 0.9% saline. For intracranial injections, agmatine sulphate, SNG, L‐NIL, L‐NIO, L‐NAME, yohimbine and clonidine were dissolved in aCSF, while 7‐NI and MK‐801 were dissolved in DMSO. All dilutions were made immediately before the experiments and directly infused in a volume of 0.5 μL into each side of the LC (intra‐LC) or the hippocampus (intra‐hippocampus), to avoid peripheral effects.
Results
Agmatine facilitates retrieval of IA memory
Injections of agmatine given i.p. or intra‐LC, immediately before retrieval produced enhanced step‐through latencies of aversive IA memory in a dose‐dependent manner, compared to the saline treated group [F(3, 19) = 6.278, P < 0.05]. Post hoc analysis revealed that pre‐retrieval agmatine, at the highest dose used i.p. (40 mg·kg−1), significantly increased step‐through latency, compared with the saline treated group (Figure 1C). However, agmatine showed no effect on acquisition and consolidation of aversive IA memory compared with the saline/aCSF treated control animals (Figure 1). One‐way ANOVA revealed that, agmatine (10, 20 and 40 mg·kg−1, i.p.) had no significant effect on acquisition and consolidation of IA memory (Figure 1A and B).
Figure 1.
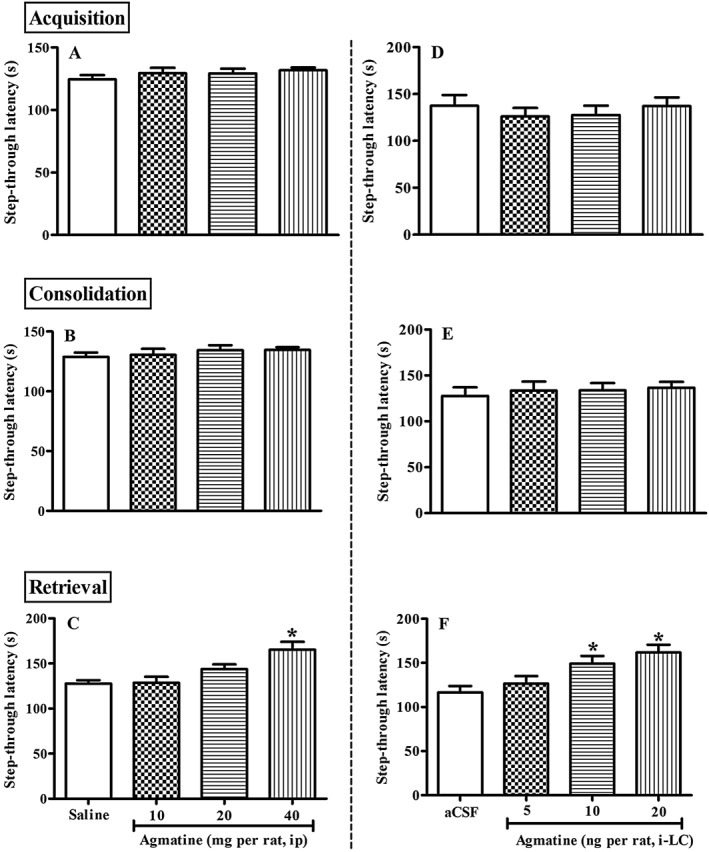
Dose‐dependent effect of agmatine [i.p. or intra‐locus coeruleus (intra‐LC)] on acquisition (A and D), consolidation (B and E) or retrieval (C and F) of IA memory. Saline or agmatine (10, 20, 40 mg·kg−1, i.p.; n = 5 per group) was administered either 30 min before training (acquisition, A), immediately after training (consolidation, B) or 24 h after training (retrieval, C), and step‐through latencies observed in the inhibitory avoidance (IA) test. Separately, aCSF (0.5 μL per LC) or agmatine (5, 10 20 ng per rat, intra‐LC; n = 8 per group) was administered 15 min before training (D), immediately after training (E) or before test (F), and step‐through latency observed in the IA test. Each bar represents mean ± SEM of step‐through latency. *P < 0.05; significantly different from respective control; one‐way ANOVA followed by Newman–Keuls's test.
Similarly, intra‐LC agmatine (5, 10 and 20 ng per rat, intra‐LC) administration 15 min before acquisition and consolidation did not show any significant increase in step‐through latency (Figure 1D and E). However, pre‐retrieval administration of agmatine (5, 10 and 20 ng per rat, intra‐LC) significantly increased the step‐through latency [F(3, 31) = 6.103, P < 0.05]. Post hoc test revealed that agmatine, in a dose‐dependent manner, increased step‐through latency, compared with the aCSF treated group [10 and 20 ng, P < 0.05; Figure 1F].
The NO donor, SNG potentiates effect of agmatine on IA memory within the LC
Pre‐retrieval administration of SNG (1.4, 2.8 and 5.6 pg per rat, intra‐LC) significantly increased the step‐through latencies in a dose‐dependent manner [F(3, 31) = 11.25, P < 0.05; Table S1]. Notably, a dose of SNG (2.8 pg per LC) which was ineffective when given alone, did potentiate [F(3, 31) = 10.68, P < 0.05] the response to a low dose of agmatine (5ng per rat), which given alone was also ineffective (Figure 2A).
Figure 2.
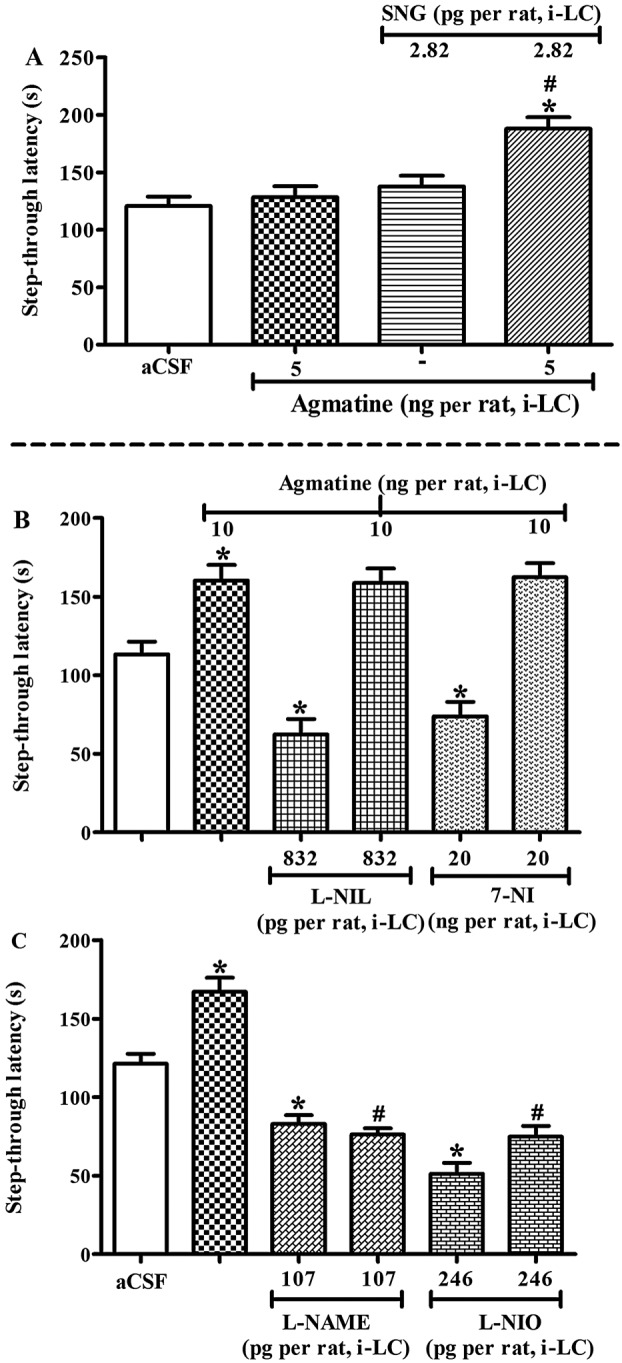
Effect of nitrergic agents on agmatine‐induced memory facilitation within the locus coeruleus (LC) in the inhibitory avoidance (IA) test. All the drugs were administered before a retrieval test. (A) Concomitant effect of sub‐effective doses of S‐nitrosoglutathione (SNG) and agmatine on step‐through latency in the IA test. Rats were either treated with agmatine (5 ng per rat, intra‐LC, n = 8) or SNG (2.8 pg per rat, intra‐LC, n = 8) alone or in combination before the IA test. (B) Effect of L‐NIL or 7‐NI, and (C) L‐NAME or L‐NIO on agmatine induced memory facilitation in the IA test. Animals were either treated with aCSF (0.5 μL per rat, intra‐LC), L‐NAME (107.6 pg per rat, intra‐LC, n = 8), L‐NIO (246 pg per rat, intra‐LC, n = 8), L‐NIL (832 pg per rat, intra‐LC, n = 8) or 7‐NI (20 ng per rat, intra‐LC, n = 8) 15 min before aCSF or agmatine (10 ng per rat, intra‐LC) and observed in the IA test. Each bar represents mean ± SEM of step‐through latency (n = 8). *P < 0.05; significantly different from respective aCSF treatment; # P < 0.05; significantly different from respective agmatine treatment; one‐way ANOVA followed by Newman–Keuls's test.
Effect of nitrergic agents on agmatine‐induced memory facilitation within the LC in the IA test
We found that pre‐retrieval administration of NO inhibitors L‐NAME (107.6 pg per rat, intra‐LC), L‐NIO (246 pg per rat, intra‐LC), L‐NIL (832 pg per rat, intra‐LC) and 7‐NI (20 ng per rat, intra‐LC) significantly decreased the step‐through latencies in the IA test (Table S1). However, only L‐NAME (107.6 pg per rat, intra‐LC) and L‐NIO (246 pg per rat, intra‐LC) significantly attenuated (P < 0.05 each) the step‐through latencies produced by agmatine (10 ng per rat, intra‐LC) in the IA test [F(5, 47) = 40.19, P < 0.05; Figure 2C]. In contrast, although L‐NIL (832 pg per rat, intra‐LC; P < 0.05) and 7‐NI (20 ng per rat, intra‐LC; P < 0.05) given alone significantly decreased the step‐through latency; Table S1), both of these inhibitors failed to attenuate the effect of agmatine in the IA test (Figure 2B).
Blockade of α2‐adrenoceptors by yohimbine potentiates, whereas their activation by clonidine attenuates, the memory facilitating effect of agmatine in the IA test
Pre‐retrieval concomitant administration of sub‐effective doses of yohimbine (0.5 μg per rat, intra‐LC) and agmatine (5 ng per rat, intra‐LC) significantly increased the step‐through latencies, compared with agmatine alone (5 ng per rat, intra‐LC) treated group [F(3, 31) = 16.80, P < 0.05; Figure 3A]. Post hoc analysis revealed that yohimbine (0.5 μg per rat, intra‐LC) significantly potentiated the step‐through latencies produced by agmatine (5 ng per rat, intra‐LC; P < 0.05) that were per se ineffective. By contrast, pre‐retrieval administration of clonidine (0.5 μg per rat, intra‐LC) significantly attenuated effect of agmatine (10 ng per rat, intra‐LC) [F(3, 31) = 11.10, P < 0.05; Figure 3B]. Post hoc analysis revealed that clonidine (0.5 μg per rat, intra‐LC) significantly attenuated step‐through latency when given alone, as well as decreasing the memory facilitation effects of agmatine (P < 0.05).
Figure 3.
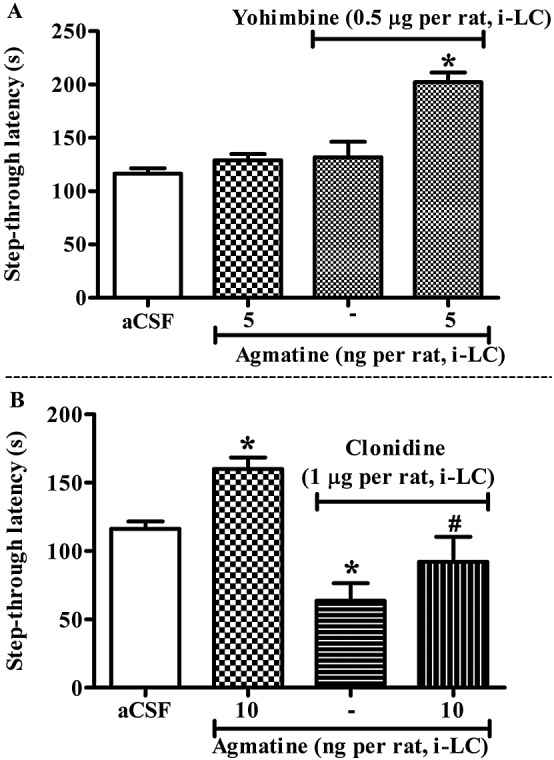
Effect of the α2‐adrenoceptor antagonist, yohimbine (A) or agonist, clonidine (B) on the memory facilitating effect of agmatine in the inhibitory avoidance (IA) test. (A) Yohimbine (0.5 μg per rat, intra‐LC) significantly potentiated the effect of agmatine (5 ng per rat, intra‐LC) on step‐through latencies (P < 0.05). (B) On the contrary, pre‐test administration of clonidine (0.5 μg per rat, intra‐LC) significantly attenuated the effect of agmatine (10 ng per rat, intra‐LC) [F(3, 31) = 11.10, P < 0.05]. Each bar represents the means ± SEM of step‐through latency. *P < 0.05; significantly different from aCSF; # P < 0.05; significantly different from agmatine; one‐way ANOVA followed by Newman–Keuls's test.
eNOS inhibition significantly attenuated memory facilitating effect of the α2‐ adrenoceptor antagonist yohimbine within the LC
Prior administration of the eNOS inhibitor, L‐NIO (246 pg per rat, intra‐LC) significantly decreased the effect of yohimbine (1 μg per rat, intra‐LC) on step‐through latencies in the IA test [F(3, 31) = 55.90, P < 0.05; Figure 4A]. Post hoc test reveals that L‐NIO (246 pg per rat, intra‐LC) per se significantly (P < 0.05) attenuated, while yohimbine (1 μg per rat, intra‐LC) per se increased (P < 0.05) the step‐through latencies in the IA test.
Figure 4.
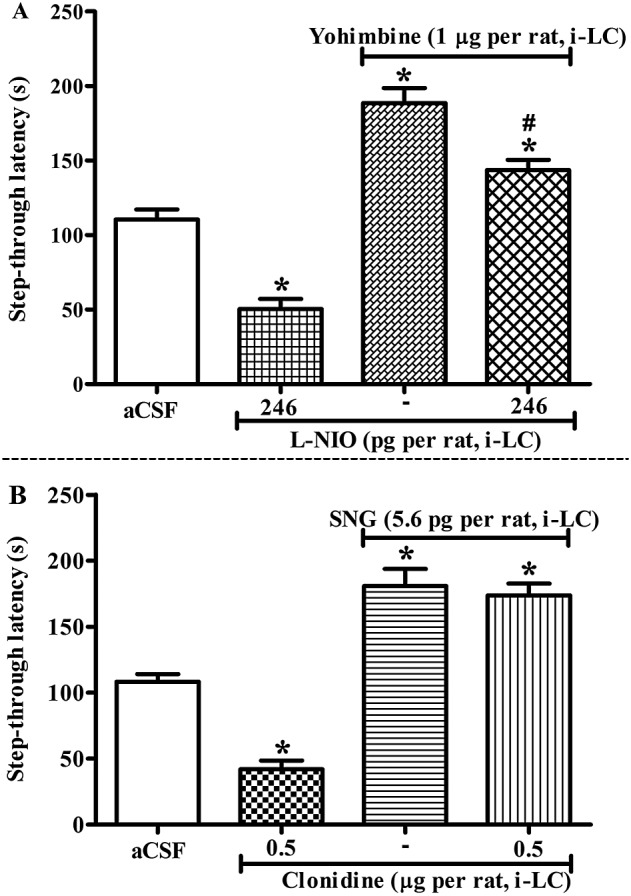
(A) Influence of L‐NIO on the memory facilitating effect of yohimbine in IA test. All the drugs were administered before retrieval test. Animals were injected with aCSF or L‐NIO (246 pg per rat, intra‐LC) 15 min prior to aCSF or yohimbine (1 μg per rat, intra‐LC) and, 15 min thereafter, the step‐through latencies were tested in the IA task. (B) Influence of clonidine on SNG (5.6 pg per rat, intra‐LC) induced memory facilitation. Rats were treated with aCSF or clonidine (0.5 μg per rat, intra‐LC), 15 min prior to aCSF or SNG (5.6 pg per rat, intra‐LC) and a retention test was performed. Each bar represents mean ± SEM of step‐through latency. *P < 0.05; significantly different from aCSF; # P < 0.05 vs yohimbine; one‐way ANOVA followed by Newman–Keuls's test.
The α2‐adrenoceptor agonist clonidine failed to attenuate memory facilitating effect of the NO donor SNG within the LC
Given alone, clonidine (0.5 μg per rat, intra‐LC) significantly decreased (P < 0.05), while SNG (5.6 pg per rat, intra‐LC) increased (P < 0.05) step‐through latencies in the IA test [F(3, 31) = 73.20, P < 0.05; Figure 4B]. However, pretreatment with clonidine (0.5 μg per rat, intra‐LC) prior to SNG (5.6 pg per rat, intra‐LC), did not affect the increased step‐through latencies produced by SNG.
NMDA receptor blockade in the hippocampus attenuated memory facilitating effect of agmatine and SNG in the IA test
Pre‐retrieval administration of the NMDA receptor blocker MK‐801 alone (1 ng per rat, intra hippocampus) significantly decreased step‐through latencies in the IA test (Figure 5). Furthermore, this intra hippocampus administration of MK‐801 also decreased the memory facilitating effect of agmatine (10 ng per rat, intra‐LC) or SNG (5.6 pg per LC) in the IA test [F(5, 47) = 33.25, P < 0.05].
Figure 5.
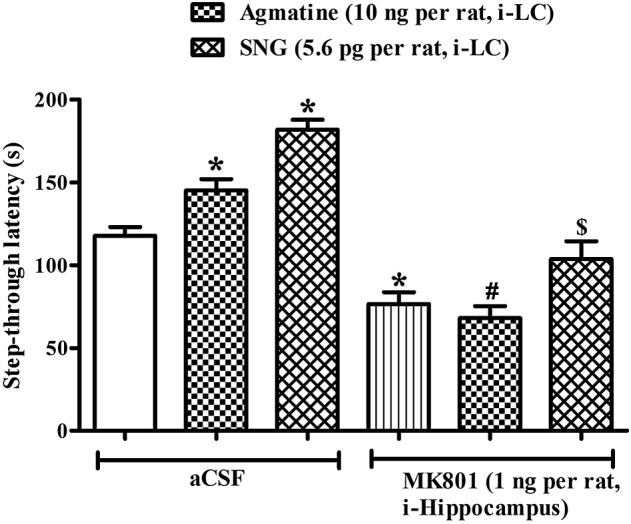
Effect of MK801 (1 ng per rat, intra‐hippocampus) on the memory‐facilitating effect of agmatine (10 ng per rat, intra‐LC) and SNG (5.6 pg per rat, intra‐LC). Rats were treated with either aCSF (0.5 μL per LC) or MK801 (1 ng per rat, intra‐hippocampus) 15 min before agmatine (10 ng per rat, intra‐LC) or SNG (5.6 pg per rat intra‐LC), and thereafter 15 min step‐through latencies were observed in the IA test. Each bar represents mean step‐through latencies ±SEM. *P < 0.05; significantly different from aCSF, # P < 0.05 vs agmatine, $ P < 0.05; significantly different from SNG; one‐way ANOVA followed by Newman–Keuls's test.
Agmatine increased the expression of TH and eNOS containing elements in the LC
The changes in the immunoreactive profile for TH (arrows; Figure 6A–C) and eNOS (arrowheads; Figure 6D–F) in the LC following aCSF (A and D, respectively for TH and eNOS) or agmatine (B and E, respectively for TH and eNOS) treatment have been summarized in the Figure 6. The data from the morphometric analysis are summarized in Figure 6C, C′, F and F′. Unpaired t‐test revealed that agmatine treatment increased the population of TH‐ (P < 0.05) as well as eNOS‐ir (P < 0.05) elements and cells in the LC, compared with that in the respective aCSF‐treated rats.
Figure 6.
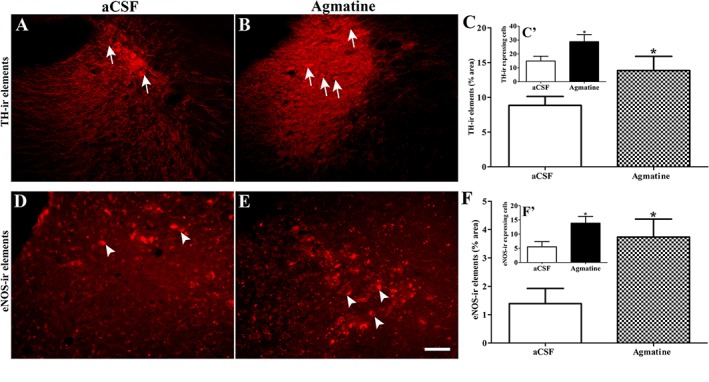
Coronal sections showing TH immunoreactive (TH‐ir; A–C, arrow) and endothelial NO immunoreactive (eNOS‐ir; D–F, arrow head) elements in the locus coeruleus (LC) of aCSF (A and D, respectively for TH and eNOS) or agmatine‐treated (B and E, respectively for TH and eNOS) animals. Scale bar = 50 μm. Agmatine was found to significantly increase the TH‐ir (B) as well as eNOS‐ir (E) elements and cells in the LC, compared with the respective aCSF treated rats (A and D). Bar graph represents the semiquantitative morphometric analysis of TH‐ir elements and TH‐ir expressing cells (C and C′, respectively) and eNOS‐ir elements eNOS‐ir expressing cells (F and F′, respectively) in the LC of aCSF or agmatine treated rats. *P < 0.05; significantly different from aCSF treated rats; unpaired t‐test.
Discussion
The present study revealed that agmatine injected i.p. or intra‐LC facilitated retrieval of IA memory and that this effect of agmatine was mediated by interactions between α2‐adrenoceptors and NO in the LC. Agmatine is known to facilitate memory in animal models (Arteni et al., 2002; Liu and Bergin, 2009; Utkan et al., 2012; but see Rastegar et al., 2011). In particular, agmatine facilitates various aspects of IA memory. Specifically, Arteni et al., 2002 reported that i.p. injection of agmatine facilitated consolidation of memory perhaps through the activation of the LC but had no effect on acquisition and retrieval of memory in IA learning. Additionally, Lu et al. (2010) found that pre‐training and pre‐test administration of agmatine facilitated memory formation and retrieval while post‐training administration of agmatine had no effect on memory consolidation. In contrast to these previous studies, we found that neither pre‐training nor post‐training but only pre‐retrieval administration of agmatine, either i.p. or intra‐LC, increased step‐through latencies in the IA task. As the behavioural effects of agmatine have been shown to be task and delay‐dependent (Liu and Collie, 2009), we would attribute this discrepancy between our present results and those of other groups to differences in route and time of administration. For instance, Lu et al., (2010) administered agmatine 60 min before the IA test, compared to 15 min in our study and used different behavioural models. However, despite these discrepancies, our data are, overall, also showing a facilitation of IA memory by agmatine.
Role of NO in agmatine‐mediated enhancement of memory
The involvement of NO in the IA task is supported by the improvement in learning of this task by the NO precursor, L‐arginine (Telegdy and Kokavszky, 1997). Our studies further support this involvement because the NO donor SNG injected into the LC significantly increased the step‐through latency in the IA task and the non‐specific (L‐NAME) as well as specific eNOS (L‐NIO), iNOS (L‐NIL) and nNOS (7‐NI) inhibitors administered alone significantly impaired the retrieval of IA memory. These effects of NO in the LC, on IA memory are corroborated by electrophysiological studies, as NO as well as the NO donor, sodium nitroprusside, increased the firing rate of LC neurons (Pineda et al., 1996a; Torrecilla et al., 2007). Furthermore, the increased firing rate of LC neurons was reduced by central administration of non‐specific NOS inhibitors, such as L‐NAME and L‐NA (Torrecilla et al., 2007).
In terms of the actions of agmatine, Morrissey and Klahr (1997) have demonstrated endothelial NO synthesis in response to agmatine exposure. Additionally, neuronal firing initiated by agmatine was blocked by NOS inhibitors in the LC but showed no interaction with α2‐adrenoceptors or imidazoline receptors (Ruiz‐Durántez et al., 2002). We found that the NO donor SNG injected into the LC significantly potentiated the effects of agmatine on step‐through latency in the IA test. Interestingly, the memory facilitating effect of agmatine was attenuated by a non‐specific NOS inhibitor, L‐NAME and a selective eNOS inhibitor, L‐NIO but not by a specific iNOS inhibitor, L‐NIL or nNOS inhibitor, 7‐NI. In parallel, agmatine also increased the eNOS expression in LC. Thus, our present results support the earlier electrophysiological data (Ruiz‐Durántez et al., 2002), suggesting that agmatine elicits its memory facilitation effect in the LC through a mechanism dependent on eNOS‐derived NO.
It is known that agmatine inhibits iNOS and nNOS (Demady et al., 2001) but not eNOS. It was initially suggested that agmatine might function as an alternative substrate for eNOS (Ishikawa et al., 1995) and thus increase NO output in cultures of endothelial cells (Morrissey and Klahr, 1997). Many protective effects of agmatine are found to be mediated by eNOS in brain (Yang et al., 2007; Jung et al., 2010; Mun et al., 2010). Although the exact mechanism is still not known it is most likely that agmatine activates protein kinase β/Akt to phosphorylate eNOS and elevate cGMP level, as shown by Santhanam et al. (2007). Soluble guanylate cyclase is present in LC neurons (Xu et al., 1998) and cGMP has been detected in the LC (Vulliemoz et al., 1999; Pablos et al., 2015). However, further studies are required to fully evaluate the effect of agmatine on cGMP levels in this tissue.
Noradrenergic signalling underlies agmatine enhancement of IA memory
There is good evidence for the involvement of the noradrenergic system in the retrieval of memory (Sara and Devauges, 1989). Presynaptic α2‐adrenoceptors function as autoreceptors in the LC (Elsworth et al., 2007) and the α2‐adrenoceptor agonist clonidine, per se, impaired the memory retention (Jafari‐Sabet et al., 2013). We also found that clonidine administered within the LC inhibited memory retrieval. Further, the α2‐adrenoceptor antagonist yohimbine, per se, enhanced IA retrieval as well as potentiating the memory facilitating effect of agmatine. These results are in agreement with previous studies, which reported that yohimbine increased extrasynaptic noradrenaline and enhanced memory (Sara and Devauges, 1989). Moreover, local application of yohimbine to the LC dose‐dependently enhanced memory (Chen et al., 1992). From all these reports, the memory facilitating effect of yohimbine can be attributed to inhibition of presynaptic α2‐autoreceptors, which regulate noradrenaline release. Indeed, we found significant increase in TH immunoreactivity in the LC after treatment with agmatine. However, it will be interesting to see the effect of agmatine directly on NE release in LC. Although our study demonstrated the involvement of α2‐adrenoceptors, in memory facilitation by agmatine, an earlier study suggested that agmatine did not have direct agonist or antagonist properties at α2‐adrenoceptors of LC neurons (Pineda et al., 1996a). This is why we have postulated that agmatine produced its effects indirectly, via a mechanism involving NO.
There is a tonic regulation of noradrenergic neurons by NO (Torrecilla et al., 2007) and the anticonvulsant effect of agmatine is said to possibly involve both α2‐adrenoceptor and NO‐related mechanisms (Demehri et al., 2003; Halaris and Plietz, 2007). Several α2‐adrenoceptor agonists including dexmedetomidine are known to stimulate endothelial NO production (Joshi et al., 2007; Xia et al., 2015). Exogenous arginine or agmatine activated eNOS to produce NO via α2‐adrenoceptors, suggesting interactions between α2‐adrenoceptors and NO (Joshi et al., 2007). In this context, we found that the α2‐adrenoceptor antagonist yohimbine attenuated the memory‐impairing effect of the selective eNOS inhibitor, L‐NIO, within the LC. On the contrary, the NO donor SNG attenuated the memory impairment effect of the α2‐adrenoceptor agonist clonidine. This suggests that the NO‐induced increase in noradrenaline levels is probably not regulated by α2‐adrenoceptors. Indeed, data suggest that NO activates noradrenergic neurons of the LC via a cGMP‐dependent protein kinase and a non‐selective cation channel. It is also proposed that these effects occur at the postsynaptic level and that there may be a tonic regulation of LC neuronal firing by the cGMP pathway (Pineda et al., 1996b). Further, injection of a cGMP derivative or a NO donor into the LC causes excitation of LC neurons (Aghajanian et al., 1994). These studies suggest that regulation of cGMP by NO within the LC might be an important action for several physiological outcomes. Thus, our study demonstrated that agmatine facilitated IA memory retrieval via a eNOS‐NO‐noradrenaline pathway within the LC, rather than directly acting on α2‐adrenoceptors.
The hippocampus is extensively innervated by noradrenergic fibres projecting exclusively from the LC, involved in the hippocampal learning and memory processes (Mello‐Carpes and Izquierdo, 2013). It is also proposed that enhancement of the survival and plasticity of newborn neurons by pharmacological activation of the LC‐noradrenergic system may provide a way to facilitate certain types of hippocampal learning and memory (Rizk et al., 2006). In this context, we found that the NMDA receptor antagonist MK‐801 injected within the hippocampus blocked the memory facilitation produced by agmatine, NO donor SNG or yohimbine administered within the LC, suggesting a role for the noradrenergic afferents from the LC to the hippocampus in the memory retrieval effect.
However, the IA task represents short‐term memory and therefore it should not be extrapolated to other behavioural models of learning and memory. Although many more studies are needed to elucidate this issue, our present data demonstrates an important role for NO and the α2‐adrenoceptors in the LC in the memory‐related effects of agmatine and proposes that NO and α2‐adrenoceptors within the LC are involved in the memory‐related effects of agmatine.
We can conclude that the facilitation of memory retrieval in the IA task by agmatine is mediated by a pathway involving eNOS, NO and noradrenaline, within the LC. The result of the present study proposes NO and α2‐adrenoceptors as important substrates for therapeutic intervention in memory related disorders.
Author contributions
G.P.S., S.G.G. and S.S.C. performed the experiments, analyzed the data and prepared the manuscript. S.M.D. and R.R.U. designed the experiments, provided the drugs and other facility and critically corrected the manuscript.
Conflict of interest
The authors declare no conflicts of interest.
Declaration of transparency and scientific rigour
This Declaration acknowledges that this paper adheres to the principles for transparent reporting and scientific rigour of preclinical research recommended by funding agencies, publishers and other organisations engaged with supporting research.
Supporting information
Table S1 Dose‐dependent effect of nitrergic and α2‐adrenoceptor drugs on inhibitory avoidance (IA) memory retrieval.
Figure S1 The diagram shows the representative site of injection in the locus coeruleus (LC; −9.8 mm to bregma). In the image, filled circles indicate the injection placement sites within the LC. The squares represent injection sites outside the targeted area. 4 V, fourth ventricle.
Supporting info item
Shelkar, G. P. , Gakare, S. G. , Chakraborty, S. , Dravid, S. M. , and Ugale, R. R. (2016) Interactions of nitric oxide with α2‐adrenoceptors within the locus coeruleus underlie the facilitation of inhibitory avoidance memory by agmatine. British Journal of Pharmacology, 173: 2589–2599. doi: 10.1111/bph.13531.
References
- Aghajanian GK, Kogan JH, Moghaddam B (1994). Opiate withdrawal increases glutamate and aspartate efflux in the locus coeruleus: an in vivo microdialysis study. Brain Res 636: 126–130. [DOI] [PubMed] [Google Scholar]
- Aglawe MM, Taksande BG, Kuldhariya SS, Chopde CT, Umekar MJ, Kotagale NR (2014). Participation of central imidazoline binding sites in antinociceptive effect of ethanol and nicotine in rats. Fundam Clin Pharmacol 28: 284–293. [DOI] [PubMed] [Google Scholar]
- Ahn SK, Hong S, Park YM, Lee WT, Park KA, Lee JE (2011). Effects of agmatine on hypoxic microglia and activity of nitric oxide synthase. Brain Res 1373: 48–54. [DOI] [PubMed] [Google Scholar]
- Alexander SPH, Fabbro D, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015a). The Concise Guide to PHARMACOLOGY 2015/16: Enzymes. Br J Pharmacol 172: 6024–6109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alexander SPH, Davenport AP, Kelly E, Marrion N, Peters JA, Benson HE et al. (2015b). The Concise Guide to PHARMACOLOGY 2015/16: G Protein‐Coupled Receptors. Br J Pharmacol 172: 5744–5869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arteni NS, Lavinsky D, Rodrigues AL, Frison VB, Netto CA (2002). Agmatine facilitates memory of an inhibitory avoidance task in adult rats. Neurobiol Learn Mem 78: 465–469. [DOI] [PubMed] [Google Scholar]
- Bari A, Aston‐Jones G (2013). Atomoxetine modulates spontaneous and sensory‐evoked discharge of locus coeruleus noradrenergic neurons. Neuropharmacology 64: 53–64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen MF, Chiu TH, Lee EH (1992). Noradrenergic mediation of the memory‐enhancing effect of corticotropin‐releasing factor in the locus coeruleus of rats. Psychoneuroendocrinology 17: 113–124. [DOI] [PubMed] [Google Scholar]
- Curtis MJ, Bond RA, Spina D, Ahluwalia A, Alexander SP, Giembycz MA et al. (2015). Experimental design and analysis and their reporting: new guidance for publication in BJP. Br J Pharmacol 172: 3461–3471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Demady DR, Jianmongkol S, Vuletich JL, Bender AT, Osawa Y (2001). Agmatine enhances the NADPH oxidase activity of neuronal NO synthase and leads to oxidative inactivation of the enzyme. Mol Pharmacol 59: 24–29. [DOI] [PubMed] [Google Scholar]
- Demehri S, Homayoun H, Honar H, Riazi K, Vafaie K, Roushanzamir F et al. (2003). Agmatine exerts anticonvulsant effect in mice: modulation by α2‐adrenoceptors and nitric oxide. Neuropharmacology 45: 534–542. [DOI] [PubMed] [Google Scholar]
- Elsworth JD, Morrow BA, Nguyen VT, Mitra J, Picciotto MR, Roth RH (2007). Prenatal cocaine exposure enhances responsivity of locus coeruleus norepinephrine neurons: role of autoreceptors. Neuroscience 147: 419–427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaris A, Plietz J (2007). Agmatine: metabolic pathway and spectrum of activity in brain. CNS Drugs 21: 885–900. [DOI] [PubMed] [Google Scholar]
- Hansen N, Manahan‐Vaughan D (2015). Locus coeruleus stimulation facilitates long‐term depression in the dentate gyrus that requires activation of β‐adrenergic receptors. Cereb Cortex 25: 1889–1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harooni HE, Naghdi N, Sepehri H, Rohani AH (2008). Intra hippocampal injection of testosterone impaired acquisition, consolidation and retrieval of inhibitoryavoidance learning and memory in adult male rats. Behav Brain Res 188: 71–77. [DOI] [PubMed] [Google Scholar]
- Ishikawa T, Misonou T, Ikeno M, Sato K, Sakamaki T (1995). N omega‐hydroxyagmatine: a novel substance causing endothelium‐dependent vasorelaxation. Biochem Biophys Res Commun 214: 145–151. [DOI] [PubMed] [Google Scholar]
- Jafari‐Sabet M, Banafshe HR, Khodadadnejad MA (2013). Modulation of muscimol state-dependent memory by α2-adrenoceptors of the dorsal hippocampal area. Eur J Pharmacol 710: 92–99. [DOI] [PubMed] [Google Scholar]
- Joshi MS, Ferguson TB Jr, Johnson FK, Johnson RA, Parthasarathy S, Lancaster JR Jr (2007). Receptor‐mediated activation of nitric oxide synthesis by arginine in endothelial cells. Proc Natl Acad Sci U S A 104: 9982–9987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung HJ, Yang MZ, Kwon KH, Yenari MA, Choi YJ, Lee WT et al. (2010). Endogenous agmatine inhibits cerebral vascular matrix metalloproteinases expression by regulating activating transcription factor 3 and endothelial nitric oxide synthesis. Curr Neurovasc Res 7: 201–212. [DOI] [PubMed] [Google Scholar]
- Kilkenny C, Browne W, Cuthill IC, Emerson M, Altman DG (2010). Animal research: reporting in vivo experiments: The ARRIVE guidelines. Br J Pharmacol 160: 1577–1579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kokare DM, Shelkar GP, Borkar CD, Nakhate KT, Subhedar NK (2011). A simple and inexpensive method to fabricate a cannula system for intracranial injections in rats and mice. J Pharmacol Toxicol Methods 64: 246–250. [DOI] [PubMed] [Google Scholar]
- Koriyama Y (2013). Role of protein S‐nitrosylation in central nervous system survival and regeneration. Yakugaku Zasshi 133: 843–848. [DOI] [PubMed] [Google Scholar]
- Leitch B, Shevtsova O, Reusch K, Bergin DH, Liu P (2011). Spatial learning‐induced increase in agmatine levels at hippocampal CA1 synapses. Synapse 65: 146–153. [DOI] [PubMed] [Google Scholar]
- Li F, Wu N, Su R, Chen Y, Lu X, Liu Y et al. (2012). Imidazoline receptor antisera‐selected/Nischarin regulates the effect of agmatine on the development of morphine dependence. Addict Biol 17: 392–408. [DOI] [PubMed] [Google Scholar]
- Liu P, Bergin DH (2009). Differential effects of i.c.v. microinfusion of agmatine on spatial working and reference memory in the rat. Neuroscience 159: 951–961. [DOI] [PubMed] [Google Scholar]
- Liu P, Collie ND (2009). Behavioral effects of agmatine in naive rats are task‐ and delay‐dependent. Neuroscience 163: 82–96. [DOI] [PubMed] [Google Scholar]
- Liu P, Collie ND, Chary S, Jing Y, Zhang H (2008). Spatial learning results in elevated agmatine levels in the rat brain. Hippocampus 18: 1094–1098. [DOI] [PubMed] [Google Scholar]
- Liu P, Jing Y, Collie ND, Chary S, Zhang H (2009). Memory‐related changes in L‐citrulline and agmatine in the rat brain. Hippocampus 19: 597–602. [DOI] [PubMed] [Google Scholar]
- Lu W, Dong HJ, Gong ZH, Su RB, Li J (2010). Agmatine inhibits morphine‐induced memory impairment in the mouse step‐down inhibitory avoidance task. Pharmacol Biochem Behav 97: 256–261. [DOI] [PubMed] [Google Scholar]
- McGrath JC, Lilley E (2015). Implementing guidelines on reporting research using animals (ARRIVE etc.): new requirements for publication in BJP. Br J Pharmacol 172: 3189–3193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello‐Carpes PB, Izquierdo I (2013). The nucleus of the solitary tract → nucleus paragigantocellularis → locus coeruleus → CA1 region of dorsal hippocampus pathway is important for consolidation of object recognition memory. Neurobiol Learn Mem 100: 56–63. [DOI] [PubMed] [Google Scholar]
- Moosavi M, Zarifkar AH, Farbood Y, Dianat M, Sarkaki A, Ghasemi R (2014). Agmatine protects against intracerebroventricular streptozotocin‐induced water maze memory deficit, hippocampal apoptosis and Akt/GSK3β signaling disruption. Eur J Pharmacol 736: 107–114. [DOI] [PubMed] [Google Scholar]
- Morrissey JJ, Klahr S (1997). Agmatine activation of nitric oxide synthase in endothelial cells. Proc Assoc Am Physicians 109: 51–57. [PubMed] [Google Scholar]
- Mun CH, Lee WT, Park KA, Lee JE (2010). Regulation of endothelial nitric oxide synthase by agmatine after transient global cerebral ischemia in rat brain. Anat Cell Biol 43: 230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otake K, Ruggiero DA, Regunathan S, Wang H, Milner TA, Reis DJ (1998). Regional localization of agmatine in the rat brain: an immunocytochemical study. Brain Res 787: 1–14. [DOI] [PubMed] [Google Scholar]
- Pablos P, Mendiguren A, Pineda J (2015). Contribution of nitric oxide‐dependent guanylate cyclase and reactive oxygen species signaling pathways to desensitization of μ‐opioid receptors in the rat locus coeruleus. Neuropharmacology 99: 422–431. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C (1998). The rat brain in stereotaxic coordinates. Academic Press: London. [DOI] [PubMed] [Google Scholar]
- Payandemehr B, Rahimian R, Bahremand A, Ebrahimi A, Saadat S, Moghaddas P et al. (2013). Role of nitric oxide in additive anticonvulsant effects of agmatine and morphine. Physiol Behav 118: 52–57. [DOI] [PubMed] [Google Scholar]
- Pineda J, Ruiz‐Ortega JA, Martín‐Ruiz R, Ugedo L (1996a). Agmatine does not have activity at alpha 2‐adrenoceptors which modulate the firing rate of locus coeruleus neurons: an electrophysiological study in rat. Neurosci Lett 219: 103–106. [DOI] [PubMed] [Google Scholar]
- Pineda J, Kogan JH, Aghajanian GK (1996b). Nitric oxide and carbon monoxide activate locus coeruleus neurons through a cGMP‐dependent protein kinase: involvement of a nonselective cationic channel. J Neurosci 16: 1389–1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajkumar R, Suri S, Deng HM, Dawe GS (2013). Nicotine and clozapine cross‐prime the locus coeruleus noradrenergic system to induce long‐lasting potentiation in the rat hippocampus. Hippocampus 23: 616–624. [DOI] [PubMed] [Google Scholar]
- Rastegar K, Roosta H, Zarifkar A, Rafati A, Moosavi M (2011). The effect of intra‐CA1 agmatine microinjection on water maze learning and memory in rat. Iran Red Crescent Med J 13: 316–322. [PMC free article] [PubMed] [Google Scholar]
- Rizk P, Salazar J, Raisman‐Vozari R, Marien M, Ruberg M, Colpaert F et al. (2006). The alpha 2‐adrenoceptor antagonist dexefaroxan enhances hippocampal neurogenesis by increasing the survival and differentiation of new granule cells. Neuropsychopharmacology 31: 1146–1157. [DOI] [PubMed] [Google Scholar]
- Ruiz‐Durántez E, Ruiz‐Ortega JA, Pineda J, Ugedo L (2002). Effect of agmatine on locus coeruleus neuron activity: possible involvement of nitric oxide. Br J Pharmacol 135: 1152–1158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rushaidhi M, Jing Y, Zhang H, Liu P (2013). Participation of hippocampal agmatine in spatial learning: an in vivo microdialysis study. Neuropharmacology 65: 200–205. [DOI] [PubMed] [Google Scholar]
- Santhanam AV, Viswanathan S, Dikshit M (2007). Activation of protein kinase B/Akt and endothelial nitric oxide synthase mediates agmatine‐induced endothelium‐dependent relaxation. Eur J Pharmacol 572: 189–196. [DOI] [PubMed] [Google Scholar]
- Santamarta MT, Llorente J, Mendiguren A, Pineda J (2014). Involvement of neuronal nitric oxide synthase in desensitisation of μ‐opioid receptors in the rat locus coeruleus. J Psychopharmacol 28: 903–914. [DOI] [PubMed] [Google Scholar]
- Sara SJ, Devauges V (1989). Idazoxan, an alpha‐2 antagonist, facilitates memory retrieval in the rat. Behav Neural Biol 51: 401–411. [DOI] [PubMed] [Google Scholar]
- Shelkar GP, Kale AD, Singh U, Singru PS, Subhedar NK, Kokare DM (2015). Alpha‐melanocyte stimulating hormone modulates ethanol self‐administration in posterior ventral tegmental area through melanocortin‐4 receptors. Addict Biol 20: 302–315. [DOI] [PubMed] [Google Scholar]
- Southan C, Sharman JL, Benson HE, Faccenda E, Pawson AJ, Alexander SPH et al. (2016). The IUPHAR/BPS Guide to PHARMACOLOGY in 2016: towards curated quantitative interactions between 1300 protein targets and 6000 ligands. Nucl Acids Res 44 (Database Issue): D1054–D1068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taksande BG, Kotagale NR, Gawande DY, Bharne AP, Chopde CT, Kokare DM (2014). Neuropeptide Y in the central nucleus of amygdala regulates the anxiolytic effect of agmatine in rats. Eur Neuropsychopharmacol 24: 955–963. [DOI] [PubMed] [Google Scholar]
- Telegdy G, Kokavszky R (1997). The role of nitric oxide in passive avoidance learning. Neuropharmacology 36: 1583–1587. [DOI] [PubMed] [Google Scholar]
- Torrecilla M, Ruiz‐Ortega JA, Ugedo L, Pineda J (2007). Excitatory regulation of noradrenergic neurons by L‐arginine/nitric oxide pathway in the rat locus coeruleus in vivo. Naunyn Schmiedebergs Arch Pharmacol 375: 337–347. [DOI] [PubMed] [Google Scholar]
- Utkan T, Gocmez SS, Regunathan S, Aricioglu F (2012). Agmatine, a metabolite of L‐arginine, reverses scopolamine‐induced learning and memory impairment in rats. Pharmacol Biochem Behav 102: 578–584. [DOI] [PubMed] [Google Scholar]
- Vulliemoz Y, Whittington RA, Virag L (1999). The nitric oxide‐cGMP system of the locus coeruleus and the hypnotic action of alpha‐2 adrenergic agonists. Brain Res 849: 169–174. [DOI] [PubMed] [Google Scholar]
- Xia R, Xu J, Yin H, Wu H, Xia Z, Zhou D et al. (2015). Intravenous infusion of dexmedetomidine combined isoflurane inhalation reduces oxidative stress and potentiates hypoxia pulmonary vasoconstriction during one‐lung ventilation in patients. Mediators Inflamm 2015: 238041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu ZQ, de Vente J, Steinbusch H, Grillner S, Hökfelt T (1998). The NO‐cGMP pathway in the rat locus coeruleus: electrophysiological, immunohistochemical and in situ hybridization studies. Eur J Neurosci 10: 3508–3516. [DOI] [PubMed] [Google Scholar]
- Yang MZ, Mun CH, Choi YJ, Baik JH, Park KA, Lee WT et al. (2007). Agmatine inhibits matrix metalloproteinase‐9 via endothelial nitric oxide synthase in cerebral endothelial cells. Neurol Res 29: 749–754. [DOI] [PubMed] [Google Scholar]
- Zarifkar A, Choopani S, Ghasemi R, Naghdi N, Maghsoudi AH, Maghsoudi N et al. (2010). Agmatine prevents LPS‐induced spatial memory impairment and hippocampal apoptosis. Eur J Pharmacol 634: 84–88. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Table S1 Dose‐dependent effect of nitrergic and α2‐adrenoceptor drugs on inhibitory avoidance (IA) memory retrieval.
Figure S1 The diagram shows the representative site of injection in the locus coeruleus (LC; −9.8 mm to bregma). In the image, filled circles indicate the injection placement sites within the LC. The squares represent injection sites outside the targeted area. 4 V, fourth ventricle.
Supporting info item


