Abstract
Bacillus subtilis can enter three developmental pathways to form spores, biofilms or K-state cells. The K-state confers competence for transformation and antibiotic tolerance. Transition into each of these states requires a stable protein complex formed by YlbF, YmcA and YaaT. We have reported that this complex acts in sporulation by accelerating the phosphorylation of the response regulator Spo0A. Phosphorelay acceleration was also predicted to explain their involvement in biofilm formation and the K-state. This view has been challenged in the case of biofilms, by the suggestion that the three proteins act in association with the mRNA degradation protein RNaseY (Rny) to destabilize the sinR transcript. Here we reaffirm the roles of the three proteins in supporting the phosphorylation of Spo0A for all three developmental pathways and show that in their absence sinR mRNA is not stabilized. We demonstrate that the three proteins also play unknown Spo0A-P-independent roles in the expression of biofilm matrix and in the production of ComK, the master transcription factor for competence. Finally, we show that domesticated strains of B. subtilis carry a mutation in sigH, which influences the expression kinetics of the early spore gene spoIIG, thereby increasing the penetrance of the ylbF, ymcA and yaaT sporulation phenotypes.
Keywords: biofilm, K-state, sporulation, Spo0A, YmcA-YlbF-YaaT
Graphical Abstract
In Bacillus subtilis, the proteins YlbF, YmcA and YaaT form a complex needed for the formation of biofilms, for sporulation and for genetic transformation. This paper strongly supports a role for these proteins in stimulating the phosphorylation of the key transcription factor, Spo0A. The present data contradict an alternative published model that proposed a role for the complex in destabilizing the mRNA for SinR, a repressor of biofilm formation.
Introduction
Bacillus subtilis is an important model bacterium, largely because of its ability to choose among several developmental states; sporulation, biofilm formation, the K-state (which includes competence for transformation (Berka et al., 2002)) and motile or sessile modes of life (Mirouze & Dubnau, 2013). The B. subtilis genome encodes a signal integration network that permits each cell to respond to the environment and its own metabolic state by adjusting the probability that it will enter each of the above-mentioned developmental pathways. The most important of the regulatory proteins in this signal transduction network is Spo0A, which is required for all forms of development and is active as a transcription factor when phosphorylated by a multicomponent phosphorelay (Burbulys et al., 1991). In this phosphorelay, one or more of several kinases phosphorylates the response regulator protein Spo0F. The Spo0F-P phosphoryl group is then passed to the phosphotransferase protein Spo0B and then to the response regulator Spo0A. Several pathways feed pertinent information to the phosphorelay, including those that act on the kinases and those that dephosphorylate Spo0F-P or Spo0A-P. The phosphorelay thus serves as a central signal integration device that delivers the appropriate level of Spo0A-P at the right time and with an appropriate distribution within the population of cells (Ireton et al., 1993, Chastanet & Losick, 2011, Chastanet et al., 2010).
The present study explores the roles of three proteins, YlbF, YmcA and YaaT, which like Spo0A are required for the K-state, sporulation and the formation of normal biofilms. A screen for genes needed for K-state expression yielded ylbF, which was also shown to be required for spore formation (Tortosa et al., 2000). A later screen for biofilm genes identified both ylbF and ymcA (Branda et al., 2004, Kearns et al., 2005), while yaaT was revealed in a screen for sporulation genes (Hosoya et al., 2002). It was reported recently from our laboratory that individual knockouts of ylbF, ymcA and yaaT are deficient in sporulation and in establishing biofilms and the K-state. They also exhibit excessive chaining under certain conditions, suggesting that they are biased toward a sessile lifestyle (Carabetta et al., 2013). YlbF, YmcA and YaaT are associated in B. subtilis, as shown by a complete set of reciprocal immunoprecipitations (IPs) followed by mass spectrometry, using each of the three proteins as bait (Carabetta et al., 2013). This study also demonstrated that when expressed in Escherichia coli the proteins could be purified as a stable 80 kDa ternary complex, which displayed a monodisperse peak by size exclusion chromatography.
The in vitro stability of the 80kDa complex suggested strongly that the three proteins were not merely transiently associated, but rather form a stable complex in vivo. The study by Carabetta et al (2013) focused on the roles of YlbF, YmcA and YaaT in sporulation and showed that the early spore gene spoIIG, which is dependent for its transcription only on Spo0A-P, was poorly transcribed in null mutants of each of the three genes, suggesting that they play a role in the formation of Spo0A-P. Hosoya et al (2002) had previously concluded that a yaaT knockout was blocked in the phosphorelay and had demonstrated that such a mutant could be suppressed for sporulation by the sof-1 allele of spo0A. This asparagine to lysine mutation at position 12 of Spo0A, bypasses the need for Spo0F and Spo0B by facilitating the direct phosphorylation of Spo0A by one or more kinase (Spiegelman et al., 1990). The sof-1 mutation had also been reported to partially suppress ylbF inactivation for spore formation (Tortosa et al., 2000). Further support for a role of YlbF, YmcA and YaaT in Spo0A-P formation was derived from the finding that a knockout of spo0E partially bypassed the ylbF, ymcA and yaaT knockouts for spoIIG-luc expression (Carabetta et al., 2013, Hosoya et al., 2002). Since Spo0E dephosphorylates Spo0A-P (Perego, 2001) and is a major drain on the phosphorylated form of this protein, we would expect that its elimination would at least partially compensate for a decreased rate of Spo0A phosphorylation. This expectation was confirmed (Carabetta et al., 2013, Hosoya et al., 2002). These observations, and the known dependencies on Spo0A-P for biofilm formation (Chu et al., 2008), the K-state (Mirouze et al., 2012) and sporulation (Hoch, 1993), suggested that the YlbF-YmcA-YaaT complex played a positive role at one or more steps in the phosphorylation cascade that leads to the production of Spo0A-P. Support for this hypothesis was generated using sad-67, a short deletion that removes residues 63–81 from the coding sequence of spo0A thereby rendering the Spo0A protein active without phosphorylation (Ireton et al., 1993). This mutant form of Spo0A was shown to bypass the requirements for ylbF, ymcA and yaaT for expression of spoIIG (Carabetta et al., 2013). Importantly, and most directly, the purified complex was shown to stimulate the phosphorelay in vitro. Although the experimental data of Carabetta et al (2013) were obtained for sporulation, the dependence of biofilm formation and the K-state on Spo0A-P and on YlbF, YmcA and YaaT prompted extrapolation of the findings to the latter forms of development and the proposal that a general role for the protein complex was to stimulate the production of Spo0A-P. Clearly these results did not exclude the possibility that additional roles existed.
Recently, the proposed role for YlbF, YmcA and YaaT in supporting the production of Spo0A-P has been challenged for biofilm formation (DeLoughery et al., 2016). These authors confirmed some of the IP results of Carabetta et al (2013) using tagged YlbF to show association with YmcA and YaaT, and further documented the contacts among the three proteins using bacterial two-hybrid experiments. However, they did not detect an effect of the ylbF, ymcA and yaaT knockouts on the transcription of sinI, a Spo0A-P-dependent gene required for biofilm formation, nor did they detect an effect on the repression of abrB-lacZ transcription by Spo0A-P, the latter in direct contrast to the previous data for ylbF (Carabetta et al., 2013). Instead, DeLoughery et al (2016) proposed that the YlbF-YmcA-YaaT complex interacts with the ribonuclease Rny and that the absence of this interaction results in stabilization of the sinR transcript. This was proposed to result in the accumulation of SinR, a known repressor of biofilm matrix formation (Kearns et al., 2005). Consistent with the Rny model, IP data suggested an in vivo interaction of Rny with YaaT (Carabetta et al., 2013) and of Rny with YlbF (DeLoughery et al., 2016). DeLoughery et al (2016) also used bacterial two-hybrid (B2H) experiments to show an interaction of Rny with YlbF and YmcA. The Rny hypothesis predicted that in knockouts of ylbF, ymcA and yaaT, the abundance of the sinR mRNA and its translated product would be increased. Enhanced levels of sinR mRNA and of the biofilm matrix gene repressor SinR were indeed reported in knockouts of ylbF and ymcA. The Rny hypothesis was further buttressed by the similar slow growth phenotypes of ylbF, ymcA, yaaT and rny knockouts and the reported synteny of rny and ymcA. Also consistent with this hypothesis are the observations that the deletion of rny, causes severe deficiencies in sporulation, competence and biofilm formation (DeLoughery et al., 2016, Figaro et al., 2013), while the overproduction of Rny induces biofilm formation (Lehnik-Habrink et al., 2011b).
The general importance of YlbF, YmcA and YaaT for development in B. subtilis and the challenge posed by the alternative model prompted the further exploration of the roles of these three proteins in biofilm formation, the K-state and sporulation and a re-examination of the phosphorelay hypothesis. The new data, presented here, reaffirms an important role for these proteins in stimulating the production of Spo0A-P for all three forms of development. It is shown that the transcription of abrB and sinI are indeed affected by the knockouts under biofilm-forming conditions in the undomesticated strain NCIB3610 (hereafter 3610). We also show that all three knockouts are at least partially bypassed for spoIIG, sinI and abrB by the sof-1 or sad-67 alleles of spo0A and that pellicle formation, a sensitive indicator of matrix formation, is partially bypassed in ylbF and ymcA knockout strains by sad-67. Tellingly, the new data show that the knockouts do not phenocopy a deletion of rny for the stabilization of the sinR transcript. The present work also reveals additional but uncharacterized roles for YlbF, YmcA and YaaT in development beyond the acceleration of Spo0A phosphorylation.
Results
The present study investigates the roles of ylbF, ymcA and yaaT in biofilm formation by measuring the transcription of abrB and sinI, two genes that are required for the correct expression of the biofilm matrix (Kearns et al., 2005, Chu et al., 2008, Hamon et al., 2004) and which are regulated by direct binding of Spo0A-P to their promoters (Strauch et al., 1990, Shafikhani et al., 2002, Fürbass et al., 1991). Spo0A-P represses the transcription of abrB and activates that of sinI. Because AbrB represses downstream operons required for biofilm matrix formation and because SinI antagonizes the action of SinR, a repressor of these same operons (Bai et al., 1993), Spo0A-P is a necessary initiator of biofilm formation (Fig. 1). No transcription factor other than Spo0A-P has been reported to repress the transcription of abrB except for AbrB itself (Strauch et al., 1989) and only Spo0A-P appears to activate the transcription of sinI, although two repressors, AbrB and ScoC (Hpr) have been reported to bind to its promoter (Shafikhani et al., 2002). Thus, sinI and abrB transcription provide reliable readouts for the availability of Spo0A-P.
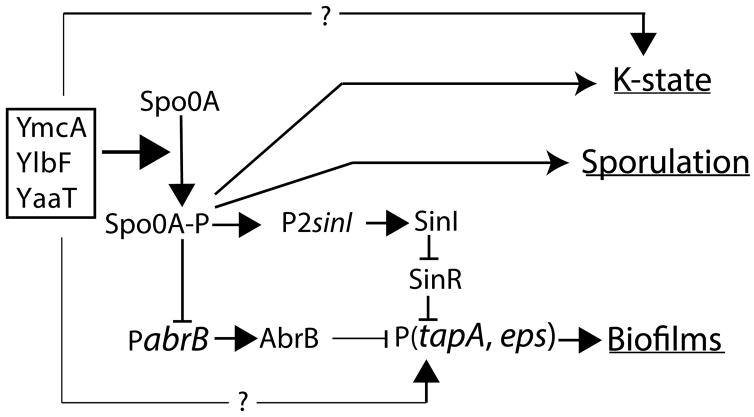
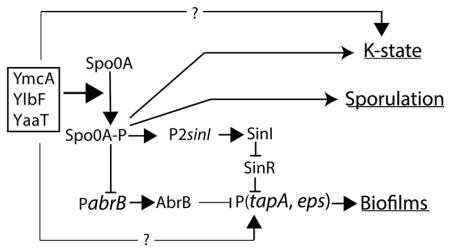
Fig. 1.
Regulation by YmcA, YlbF and YaaT. A complex of these three proteins stimulates the production of Spo0A-P by acting on the phosphorelay. Spo0A-P acts in turn to activate the transcription of sinI. SinI then sequesters SinR, preventing it from repressing the expression of the biofilm matrix operons. Spo0A-P also represses the transcription of abrB, which encodes another matrix gene repressor. Spo0A-P regulates the K-state and spore formation by acting both positively and negatively on the comK promoter and positively on early spore gene promoters. Finally, the three proteins act on matrix gene and K-state expression in at least one unknown, Spo0A-P-independent manner.
yaaT, ylbF and ymcA are needed to repress abrB transcription
It was found previously that in a ylbF knockout, abrB is incompletely repressed during growth in DSM, a medium that induces spore formation, consistent with a role for YlbF in supporting the phosphorylation of Spo0A (Carabetta et al., 2013). In order to extend these studies to biofilm formation, we have now investigated the roles of ylbF, ymcA and yaaT in the undomesticated strain 3610 growing in MSgg medium, which supports the robust expression of matrix genes (Kearns et al., 2005). To measure the transcription of abrB in real time, a fusion of its promoter to the firefly luciferase (luc) gene was used. In order to interpret these data it is important to recognize that unlike fusions to lacZ, each data point in the luciferase assay has been shown to largely reflect a transcription rate (Mirouze et al., 2011), rather than gene product accumulation.
ylbF, ymcA and yaaT knockouts mitigate the Spo0A-P-dependent decrease in the abrB transcription rate (Fig. 2A). It was reported previously that the rate of transcription of many genes fluctuates during growth, probably in response to minor changes in growth rate (Mirouze et al., 2011). Despite the swings in the rate of abrB transcription that are evident in Fig. 2A, it is clear that the knockout strains with the 3610 background exhibit a sustained increase in the rate of abrB transcription during growth in MSgg medium compared to the wild-type strain. This is also true for the transcription of abrB in 3610 strains growing in DSM, a medium that supports sporulation (Fig. 2B). In Fig. 2B, the rate of abrB transcription in the three knockouts was intermediate between the wild-type and the spo0A knockout strains, consistent with the high affinity of the abrB promoter for Spo0A-P (Fujita et al., 2005) and with the incomplete dependence of the phosphorelay on the Y-complex in vitro (Carabetta et al., 2013). As the cultures approached the stationary phase of growth, the rates of abrB transcription decreased in all the strains (Fig. 2B). This unexplained decrease is independent of Spo0A-P because it also occurred in the spo0A knockout strain. It is important to note that the ylbF, ymcA and yaaT knockouts do not affect the transcription of spo0A or the amount of Spo0A protein early in stationary phase and that YlbF, YmcA and YaaT must therefore stimulate the phosphorylation of Spo0A or the activity of Spo0A-P (Carabetta et al., 2013).
Fig. 2.
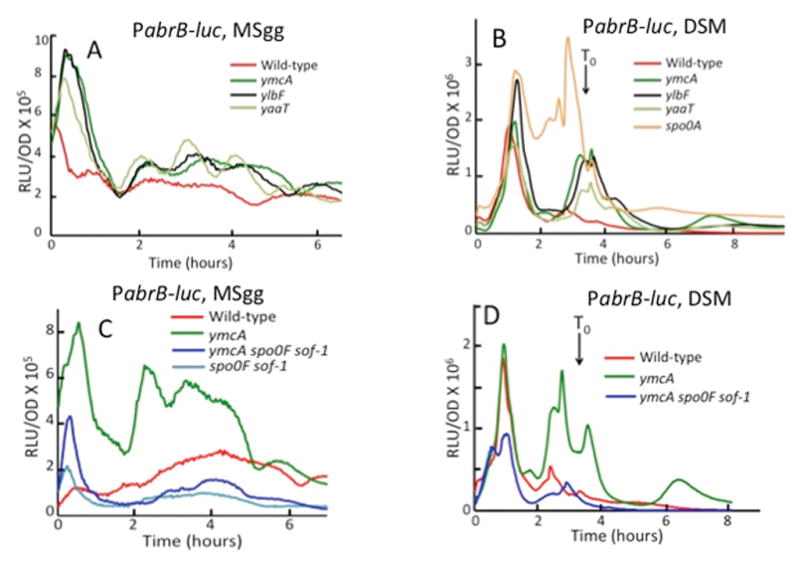
YlbF, YmcA and YaaT act negatively on the rate of abrB transcription by stimulating the production of Spo0A-P. Luciferase assays for PabrB-luc expression were performed as described in Experimental procedures. Cells were grown in MSgg (A and C) and DSM (B and D). The time at which cells enter stationary phase (T0) is indicated in B and D. The graphs are color coded as indicated. The strains used in these experiments were as follows. (A and B) wild-type (BD7623), ΔymcA (BD7823), ΔylbF (BD7824), ΔyaaT (BD7825) Δspo0A (BD7788). (C and D) wild-type (BD7623), ΔymcA (BD7647), ΔymcA Δspo0F sof-1 (BD7649), Δspo0F sof-1 (BD7646). All the strains were constructed in the 3610 background.
The transcription rate of PabrB would be expected to be sensitive to small fluctuations in the concentration of Spo0A-P during the course of growth because of the high binding affinity of Spo0A-P for this promoter (Fujita et al., 2005) and because in the absence of YlbF, YmcA or YaaT a low level production of Spo0A-P probably takes place. For this reason there was more variation from experiment to experiment in the rate curves for PabrB than for other promoters. Nevertheless, the patterns shown in Fig. 2 are reproducible: the transcription rates in the ylbF, ymcA and yaaT mutants were consistently elevated compared to the wild-type in repeated experiments. Fig. S1 shows average data for the PabrB-luc reporter plotted with the standard deviations at each time point.
If ylbF, ymcA and yaaT affect abrB transcription by stimulating the phosphorylation of Spo0A rather than the activity of Spo0A-P, we would expect that the effects of the knockouts might be suppressed by the sof-1 allele of spo0A. The sof-1 mutation is believed to relax the specificity of Spo0A so that it can be directly phosphorylated by one or another kinase at a higher rate than the wild-type Spo0A protein, bypassing the intermediate phosphorelay proteins Spo0F and Spo0B (Hoch et al., 1985, Spiegelman et al., 1990). Indeed a ymcA knockout is completely bypassed for repression of abrB transcription by the sof-1 mutation in MSgg (Fig. 2C) and in DSM (Fig. 2D). In fact, the transcription rates in the presence of sof-1 are even lower than in the wild-type strain (Fig. 2C). Because the sof-1 strains used for this experiment are deleted for spo0F these lower rates suggest that the complete phosphorelay may normally restrain the rate of Spo0A phosphorylation.
yaaT, ylbF and ymcA are needed for the activation of sinI transcription
The sinIR locus is transcribed from three promoters (Fig. 3A), producing three overlapping transcripts (Gaur et al., 1988, Shafikhani et al., 2002, Lehnik-Habrink et al., 2011b, DeLoughery et al., 2016). An upstream promoter (P1) drives expression of both sinI and sinR, to produce a transcript of ~1.2–1.5 kb. A Spo0A-P-dependent promoter (P2) also drives expression of both genes, producing a ~0.7 kb transcript and a third, presumably constitutive promoter (P3), drives the expression of only sinR to produce a transcript of ~0.4 kb (Fig. 3A). Since transcription from P2, one of the two promoters that drive the expression of sinI is dependent on the direct binding of Spo0A-P, we would expect that transcription of sinI would be decreased in ylbF, ymcA and yaaT knockouts. Three tools have been used to test this crucial prediction of the phosphorelay model; a P2sinI-luc fusion, a P2sinI-gfp fusion examined by fluorescence microscopy and quantitative real-time PCR (qRT-PCR) measurements of native sinI- and sinR-containing transcripts. Note that both P2 fusions were inserted at the native locus.
Fig. 3.
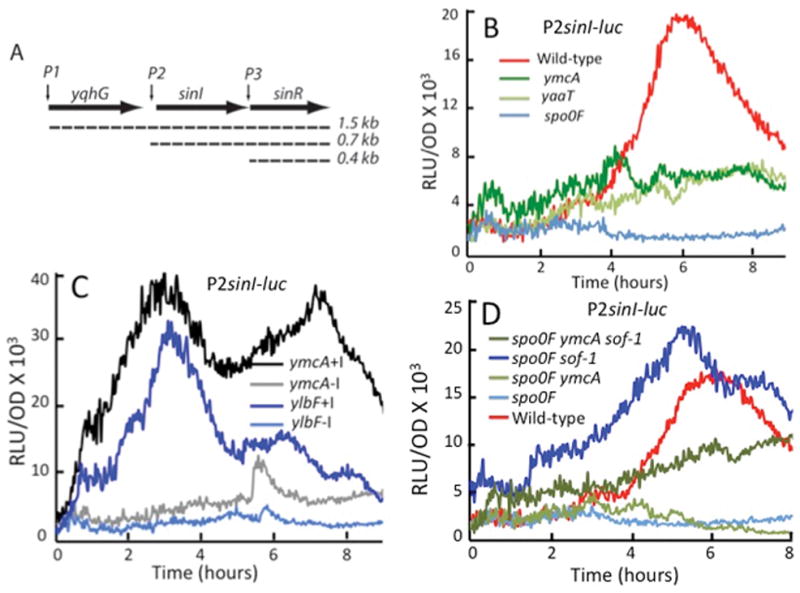
YlbF, YmcA and YaaT act positively on the rate of sinI transcription by stimulating the production of Spo0A-P. (A) A transcription schematic of the sin locus. (B) The effect of ylbF, ymcA and yaaT knockouts on transcription from a P2sinI-luc reporter construct. (C) Effects of IPTG induction of the sad-67 mutant form of spo0A on expression from the P2sinI promoter in the presence of ymcA and ylbF knockouts. The lines labeled +I and −I indicate the presence and absence of IPTG. (D) Effects of the sof-1 allele of spo0A on expression from P2sinI in the presence of spo0F and ymcA knockouts. Strains used were as follows. (B) wild-type PsinI-luc (BD7817), ΔymcA PsinI-luc (BD7821), ΔyaaT PsinI-luc (BD7820). (C) ΔymcA sad-67 PsinI-luc (BD7842), ΔylbF sad-67 PsinI-luc (BD7843). (D) wild-type PsinI-luc (BD7817), Δspo0F PsinI-luc (BD7853), Δspo0F sof-1 PsinI-luc (BD7854), Δspo0F sof-1 ΔymcA PsinI-luc (BD7855), Δspo0F ΔymcA PsinI-luc (BD7856). The bacteria were grown in MSgg and all the strains were constructed in the 3610 background.
The inactivation of ymcA, ylbF or yaaT caused decreased expression of a luc fusion to the Spo0A-P-dependent P2 promoter (Fig. 3B), as predicted by our hypothesis. This decrease is slightly less extreme than that imposed by inactivation of the phosphorelay using a spo0F knockout (compare panels B and D), suggesting that the deficit in Spo0A-P is not complete in ylbF, ymcA and yaaT knockouts, consistent with prior in vitro results (Carabetta et al., 2013). Some ylbF, ymcA and yaaT independent transcription is detectable even in the spo0F knockout, presumably due to the presence of P1. Importantly, the Spo0A-P-dependent rise in sinI transcription that takes place as cells approach stationary phase is absent in the knockout strains (Fig. 3B). The sad-67 allele of spo0A bypasses the phosphorelay by making Spo0A constitutively active and therefore independent of the need to be phosphorylated (Ireton et al., 1993). If the effect of the three knockouts on sinI transcription were indeed due to the decreased production of Spo0A-P, we would expect the sad-67 mutation to suppress the effects of ylbF, ymcA and yaaT inactivation on P2 transcription. The ylbF and ymcA knockouts were indeed suppressed when the sad-67 gene was induced by the addition of isopropyl-β-D-1-thiogalactopyranoside (IPTG, Fig. 3C (+I lines)), strongly supporting a role for YlbF, YmcA and YaaT in regulating matrix and biofilm formation via their positive effects on the production of Spo0A-P. It is worth noting that the wild-type spo0A gene is present in the sad-67 strains. Consequently, the lower than wild-type expression of sinI-luc in the ylbF and ymcA knockouts when IPTG is not added reinforces the conclusion that the these genes affect Spo0A-P phosphorylation (compare Figs. 3B and C).
In addition, the sof-1 allele of spo0A also bypasses ymcA for the expression of sinI-luc (Fig. 3D), lending additional support for the conclusion that YlbF, YmcA and YaaT contribute to biofilm formation by enhancing the production of Spo0A-P. Although suppression of a spo0F knockout by the sof-1 allele yields higher than wild-type expression, the bypass of the spo0F ymcA double knockout is partial. A possible explanation is that YmcA may assist in the phosphorylation of a kinase or in the transfer of a phosphoryl group from a kinase to Spo0A. In Fig. 3D it is evident that the levels of sinI-luc expression in spo0F and spo0F ymcA strains are the same, consistent with these two genes acting on the same pathway to produce Spo0A-P.
The conclusion that YlbF, YmcA and YaaT support the production of Spo0A-P is further buttressed by the use of qRT-PCR. The total abundance of sinI transcript sequences, which derive from P1 and P2, was reduced about five-fold in ylbF, ymcA and yaaT knockouts as well as in a knockout of spo0F (Fig. 4A). Importantly, the abundance of sinR sequences was also reduced, although more modestly, reaching statistical significance for yaaT and ylbF (Fig. 4B). The lower level of the sinR transcript would be expected if the knockouts were to decrease transcription from the P2 promoter (Fig. 3A). Importantly therefore, we have not only shown a dependence of sinI transcription on YlbF, YmcA and YaaT, but we have failed to reproduce the 2.8-fold increased abundance of sinR mRNA sequences reported by DeLoughery et al (2016) in the absence of YlbF, based on their use of Northern blots. Lehnik-Habrink et al (2011b) have shown that depletion of the rny gene product leads to stabilization of the sinR transcript. We have confirmed this result using qRT-PCR (Fig. 4B), demonstrating clearly that the effects of rny and of ylbF, ymcA and yaaT inactivation on sinR mRNA are opposite. Thus the ylbF, ymcA and yaaT knockouts do not phenocopy an rny knockout and their biofilm defects cannot be ascribed to the stabilization of sinR mRNA. Note that in these qRT-PCR experiments we have normalized our data using sequences from fusA, the gene for elongation factor G (EFG). In independent experiments, Western blotting with anti-EFG has shown that the ylbF, ymcA and yaaT knockouts do not contain elevated levels of this protein (not shown).
Fig. 4.
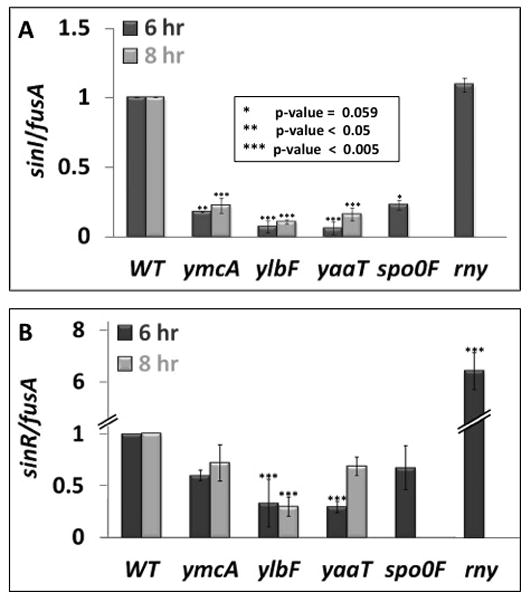
Effects of ylbF, ymcA, yaaT, spo0F and rny knockouts on the abundance of transcripts containing sinI (panel A) and sinR (panel B) sequences. The data were obtained by qRT-PCR and were normalized to the level of fusA mRNA as described in Experimental procedures. Samples for the preparation of RNA were taken from cultures growing in MSgg at the indicated times, which corresponded to late log and stationary phase. The strains used were: wild-type (BD7477), ΔymcA (BD7736), ΔylbF (BD7737), ΔyaaT (BD7731), Δspo0F (BD7593) and Δrny (BD7839). All experiments were repeated 3 independent times, and error bars represent standard deviation. Statistically significant interactions are displayed by asterisks as noted in the figure. Al the strains were constructed in the 3610 background.
As a third approach to test whether these knockouts affect transcription from the P2sinIR promoter, we employed fluorescence microscopy with a fusion of gfp to this promoter. This is a telling approach because it allows the study of P2 expression in individual cells. It has been shown that expression from this promoter is heterogeneous, presumably due to cell-to-cell variation in the levels of Spo0A-P (Chai et al., 2008). We therefore expected to see at least some cells exhibiting enhanced fluorescence as Spo0A becomes phosphorylated and we further predicted that this enhancement would be dependent on ylbF, ymcA and yaaT. Fig. 5 confirms these predictions and shows that the heterogeneous high-level expression of GFP-fluorescence seen in wild-type 3610 is absent in the knockout strains. In fact the entire distribution of fluorescence intensity is shifted to lower values in the knockouts. The histograms shown in Fig. 5 suggest that as strain 3610 grows in MSgg, the population distribution of sinI expression does not bifurcate but rather that the entire distribution shifts, with a pronounced skew toward higher intensities, presumably leading some cells to pass a threshold concentration of SinI adequate to trigger matrix gene transcription. This shift is dependent on Spo0F (and hence on Spo0A-P) as well as on YlbF, YmcA and YaaT (Fig. 5).
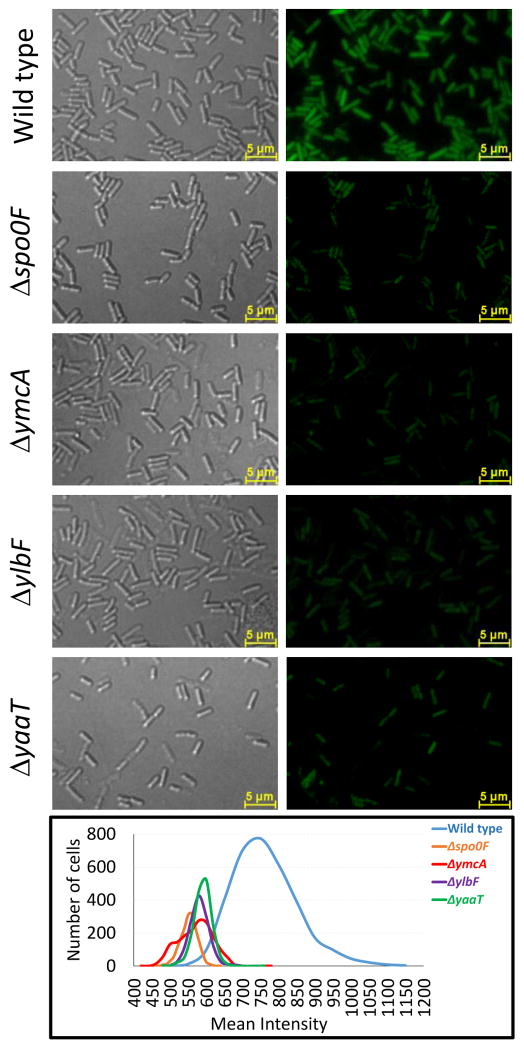
Fig. 5.
The distribution of fluorescence in cells expressing P2sinI-gfp is shifted to lower values in ylbF, ymcA, yaaT and spo0F strains. All of the representative images shown were obtained with identical microscope settings and were processed in the same way. Fluorescence intensity was measured for a population of cells and all the distributions were plotted together in the bottom panel for comparison. The strains used were wild-type P2sinI-gfp (BD7799), Δspo0F P2sinI-gfp (BD7800), ΔymcA P2sinI-gfp (BD7806), ΔylbF P2sinI-gfp (BD7807), ΔyaaT P2sinI-gfp (BD7805). Growth was in MSgg and all the strains were constructed in the 3610 background.
The data presented above show that the rate of sinI transcription, the abundance of sinI mRNA and the translation of GFP protein produced from the sinI P2 promoter are all reduced in ylbF, ymcA and yaaT knockouts. These three experimental approaches, together with the sof-1 and sad-67 suppression data, strongly indicate that YlbF, YmcA and YaaT augment the yield of Spo0A-P for the activation of sinI transcription. The results shown in Fig. 2 suggest the same for abrB repression under sporulation and biofilm forming conditions. The data in Fig. 4B clearly demonstrate that the ylbF, ymcA and yaaT knockouts do not phenocopy rny inactivation for the increase in sinR mRNA abundance shown for depletion strains by Laalami et al (2013) and by the stability study of Lehnik-Habrink et al (2011b). We conclude that YlbF, YmcA and YaaT do not exert their effects on biofilm formation by destabilizing the sinR transcript and we further conclude that there is no evidence that Rny mediates the developmental defects of knockouts of ylbF, ymcA or yaaT.
YlbF, YmcA and YaaT affect PtapA-luc expression via a SinR and Spo0A-P-independent mechanism
The tapA-sipW-tasA operon is required for the formation of biofilm matrix and is directly repressed by SinR and AbrB (Chu et al., 2006, Hamon et al., 2004, Chu et al., 2008) (Fig. 1). TapA and TasA are protein components of the matrix (Romero et al., 2010, Romero et al., 2011). As expected, transcription of the tapA operon is severely inhibited by ylbF, ymcA and yaaT knockouts (Fig. 6A). However, a sinR knockout does not completely bypass the inactivation of the three genes for tapA expression (Fig. 6B). Although appreciable bypass is evident, the levels of expression in the sinR strains carrying the knockouts are considerably less than in the sinR strain that is wild-type for ylbF, ymcA and yaaT. In fact the bypassed levels do not even reach the expression rate of the strain that is wild-type for both sinR and the three genes, particularly in the case of yaaT (compare Fig. 6A and B). This incomplete bypass can also be seen from examination of biofilm colonies on MSgg agar (Fig. 6E). The colonies of the sinR strains which are also deficient for ylbF, ymcA or yaaT are less wrinkled than the wild-type parent and much less so than the hyper-wrinkled colonies of the sinR strain that is wild-type for these three genes. If the stimulation of the phosphorelay provided the entire explanation for the roles of YlbF, YmcA and YaaT, we would expect this bypass to be complete. It is likely that the partial nature of the sinR bypass effect on colony morphology and on the expression of tapA is due to both the higher concentration of AbrB present in the ylbF, ymcA and yaaT knockouts and to a Spo0A-independent role of YlbF, YmcA and YaaT in supporting the expression of tapA. Such a role is strongly suggested by the failure of sof-1 to bypass ymcA (Fig. 6C) and by the very slight sad-67 bypass of ymcA for tapA operon expression (Fig. 6D). Although slight, this bypass is real and is evident for pellicle formation by ylbF sad-67 and ymcA sad-67 strains when IPTG is present during growth (Fig. S2). Interestingly, there is little or no bypass of pellicle formation by sad-67 in the yaaT knockout, suggesting that the YaaT protein may have a role independent of its two binding partners or may be able to function to some extent in the presence of either YlbF or YmcA. As expected, no bypass of pellicle formation by sof-1 was detected (not shown). Since both sof-1 and sad-67 efficiently bypass the loss of spo0F for tapA expression, albeit with altered kinetics, it is clear that the corresponding mutant forms of Spo0A are active (Fig. 6C and D). We conclude that while YlbF, YmcA and YaaT are required for the expression of the tapA operon and in biofilm formation by supporting the production of Spo0A-P, they have an additional important role in matrix gene expression.
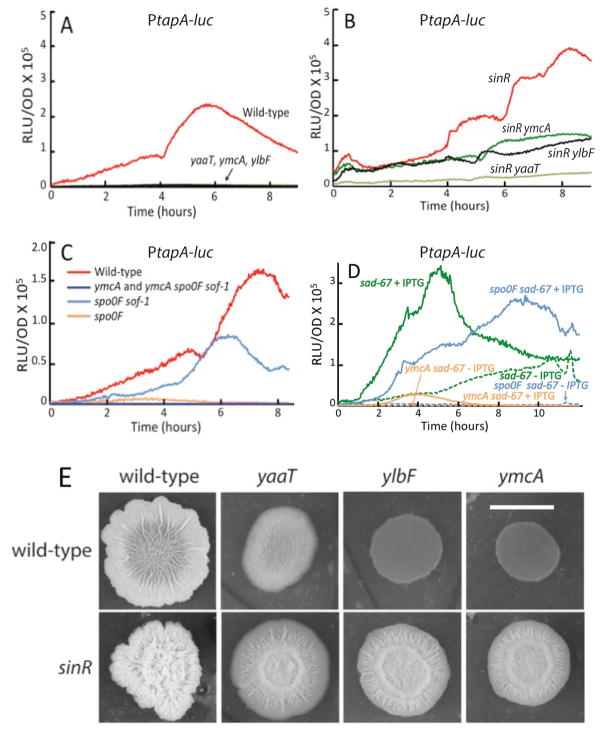
Fig. 6.
Effects of ylbF, ymcA and yaaT knockouts on expression of the tapA operon. (A–D) Luciferase assays for PtapA-luc expression were performed as described in Experimental procedures. The blue line at the bottom of panel C contains data for the ymcA and ymcA spo0F strains. (A) The effects of yaaT, ylbF and ymcA knockouts on a PtapA-luc expression reporter. (B) The effect of sinR inactivation on tapA expression in the ylbF, ymcA and yaaT knockouts. (C) and (D) show the extent of bypass of a ymcA null mutant by sof-1 and sad-67 respectively. The solid and dashed lines in Panel D show results with and without the addition of IPTG, respectively. The strains used were as follows. (A) wild-type ΔepsH PtapA-luc (BD7870), ΔymcA ΔepsH PtapA-luc (BD7872), ΔyaaT ΔepsH PtapA-luc (BD7871), ΔylbF ΔepsH PtapA-luc (BD7873). (B) wild-type ΔsinR ΔepsH PtapA-luc (BD7900), ΔymcA ΔsinRΔ epsH PtapA-luc (BD7974), ΔylbFΔ sinR ΔepsH PtapA-luc (BD7975), ΔyaaT ΔsinRΔ epsH PtapA-luc (BD7973). The strains used in panels A and B were deleted for epsH to avoid the clumping that occurs in sinR strains. We have shown that the elimination of epsH has no discernible effect on the expression of PtapA-luc (not shown). (C) wild-type PtapA-luc (BD7644), ΔymcA PtapA-luc (BD7652), ΔymcA Δspo0F sof-1 PtapA-luc (BD7654), Δspo0F sof-1 PtapA-luc (BD7651), Δspo0F Ptap -luc (BD7650). Panel (D) wild-type sad-67 PtapA-luc (BD7844), Δspo0F sad-67 PtapA-luc (BD7845), ΔymcA sad-67 PtapA-luc (BD7846). (E) Cells were grown in LB media until an OD600 of 1.0, and 5 μl were spotted on MSgg agar plates. Pictured is the colony morphology after four days of growth at room temperature. The scale bar corresponds to 1 cm and all the colonies were photographed at the same magnification. The sinR+ strains used were BD7736 (ΔymcA), BD7737 (ΔylbF), BD7731 (ΔyaaT) and BD7477 (wild-type). The sinR strains were BD7837 (ΔymcA), BD7838 (ΔylbF), BD7836 (ΔyaaT) and BD7835 (ΔsinR). Growth was in MSgg and all the strains were constructed in the 3610 background.
ylbF, ymcA and yaaT and the K-state
ComK is the master regulator of the K-state. The regulation of comK transcription involves Spo0A-P-dependent and ComK-dependent components (Mirouze et al., 2012). As cells approach stationary phase the level of Spo0A-P rises and activates expression of comK by direct binding to three operator sites upstream from the start site for transcription. This initiates a low level of ComK synthesis. As the level of Spo0A-P continues to rise, it binds to two repression sites in the comK promoter region, shutting down transcription. Thus, within a temporal window of opportunity set by the kinetics of Spo0A-P accumulation and by the binding constants for Spo0A-P, some cells in the population (~15% in the domesticated strains of B. subtilis) achieve a level of ComK sufficient to activate a positive feedback loop, mediated by the association of ComK with its own promoter. These cells enter the K-state, stop growing (Hahn et al., 2015) and become competent for transformation. The transient Spo0A-P-dependent “uptick” in comK expression can be readily measured by using a PcomK-luc reporter in a background in which comK has been inactivated.
The ylbF, ymcA and yaaT knockouts express comK poorly (Carabetta et al., 2013). If the three proteins affect comK expression by supporting the production of Spo0A-P, we would expect that in the knockouts the initial rise in this basal level expression of comK would be eliminated or perhaps delayed. If the rise were merely delayed, the subsequent decrease in transcription rate might be mitigated. The uptick, measured in a comK background, is in fact delayed in a ymcA knockout and the decline in the uptick is indeed slowed (Fig. 7A), consistent with a decreased rate of Spo0A-P formation. Both of these effects, which have been seen in five independent experiments, are consistent with a positive role for YmcA, and presumably for YlbF and YaaT as well, in the production of Spo0A-P. However, this result does not explain the K-state deficiency of the ylbF, ymcA and yaaT mutants because the area under the rate curve shown for ymcA in Fig. 7A is obviously greater than in the wild-type strain. Consequently, in a comK+ strain, a ymcA knockout should have resulted in an increase in the number of K-state cells and thus in an increase in the expression of comK. Such an increase does not take place (Fig. 7B). This contradiction demonstrates that although ymcA is needed for normal K-state regulation due to the part it plays in regulating the production of Spo0A-P, it has an additional role in the K-state. Although sof-1 can appreciably suppress the loss of spo0F for comK expression in a background that is wild-type for comK, it does not suppress ymcA for comK expression (Fig. 7B). Thus, it appears that in addition to playing a role in the proper timing of comK expression by controlling the synthesis of Spo0A-P, YmcA does something else for the K-state.
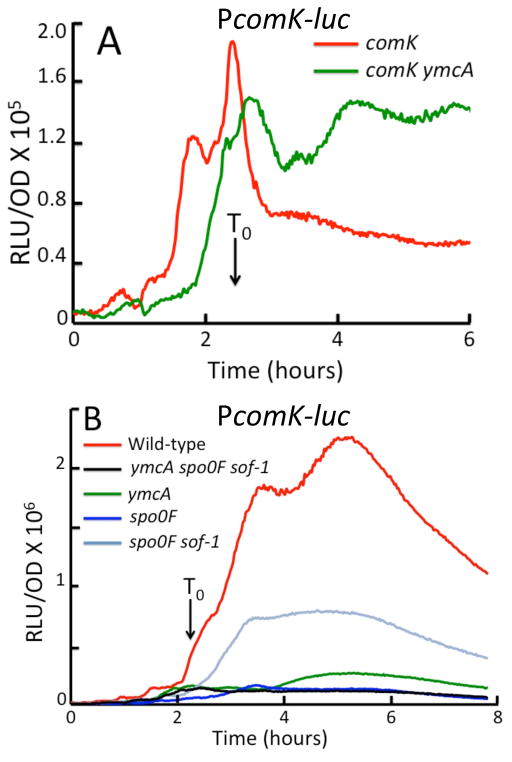
Fig. 7.
ymcA knockout effects on the expression from the comK promoter are not bypassed by sof-1. (A and B) Luciferase assays for PcomK-luc expression were performed as described in Experimental procedures. (A) shows the effect of a ymcA knockout on PcomK-luc expression in a comK knockout. (B) shows the effect of ymcA and spo0F inactivation in a comK+ background, as well as the effects of sof-1. The strains used were as follows. (A) wild-type ΔcomK PcomK-luc (BD4893), ΔymcA ΔcomK PcomK-luc (BD7642). (B) wild-type PcomK-luc (BD4773), ΔymcA Δspo0F sof- 1 PcomK-luc (BD7608), ΔymcA PcomK-luc (BD7605), Δspo0F PcomK-luc (BD7606), Δspo0F sof-1 PcomK-luc (BD7607). The strains were all derivatives of IS75 and were grown in competence medium.
ylbF, ymcA and yaaT and sporulation
Strains carrying knockouts of these three genes are deficient in sporulation and exhibit a severe block at the level of Spo0A phosphorylation, manifested as a defect in transcription from the spoIIG and spoIIE promoters. This defect was largely bypassed by the sad-67 allele of spo0A (Carabetta et al., 2013, Tortosa et al., 2000). To investigate this further, it was determined whether the sof-1 allele could also bypass ymcA for the transcription of a spoIIG-luc reporter. The sof-1 strains used for these studies carried a knockout of spo0F. The spoIIG-luc transcription rate increases sharply in the wild-type strain as the culture enters stationary phase, while the rates of the ymcA and spo0F strains are nearly zero (Fig. 8). Fig. 8 also shows that transcription in the sof-1 spo0F and ymcA sof-1 spo0F strains increases only after delays compared to the wild-type. Once these delays are completed, the slopes of all the curves are similar. These results suggest that the rates of Spo0A phosphorylation are not restored to the wild-type level by sof-1, so that the amount of Spo0A-P reaches the high threshold required for spoIIG transcription more slowly in the spo0F and ymcA strains. Although the bypass of ymcA is therefore less complete than that of the spo0F knockout, substantial suppression by sof-1 is evident, consistent with a role for YmcA in the phosphorylation of Spo0A. The delayed expression of spoIIG-luc expression in the spo0F knockout strain in the presence of sof-1 may be due to the inability of kinases to directly phosphorylate the mutant Spo0A protein as rapidly as they do Spo0F. The even longer delay in the suppressed ymcA mutant suggests that YmcA may stimulate the autophosphorylation of KinA or the transfer of the phosphoryl group from KinA-P, as suggested above to explain the partial suppression of sinI expression in the ymcA knockout by sof-1. In addition, sof-1 exhibits a substantial suppression of the sporulation deficiencies of the ylbF, ymcA and yaaT knockouts (Table 1). In the presence of the sof-1 allele, the sporulation frequency for ymcA is suppressed to 36% of the wild-type level, whereas a spo0F knockout mutation is completely bypassed. The absolute numbers of spores/ml for the ymcA spo0F sof-1 strain are about 6% of the numbers reached by the spo0F sof-1 strain but 118-fold greater than that of the ymcA strain, showing substantial but incomplete suppression of the ymcA knockout. Suppression of a yaaT knockout for sporulation by sof-1 has been reported previously (Hosoya et al., 2002). Taken together, the sof-1 and sad-67 suppression results for spoIIG transcription and spore formation provide strong evidence that YlbF, YmcA and YaaT are needed for efficient Spo0A phosphorylation..
Fig. 8.
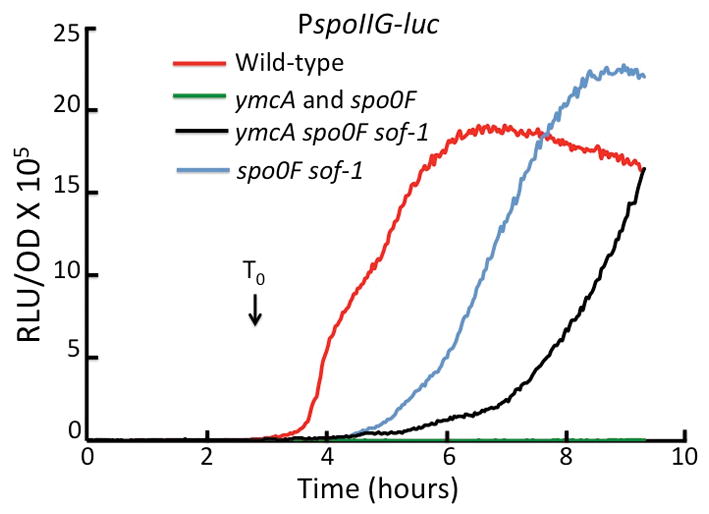
Partial bypass of ymcA and spo0F inactivation by sof-1 for spoIIG-luc expression in cultures growing in DSM. Luciferase assays for PspoIIG-luc expression were performed as described in Experimental procedures. The strains used were: wild-type (BD5813), ΔymcA (BD7624), Δspo0F (BD7710), Δspo0F sof-1 (BD7711), Δspo0F sof-1 ΔymcA (BD7712).
Table 1.
Suppression of sporulation by sof-1
| Strain | Genotype | Sporulation (%) a |
|---|---|---|
| IS75 | Wild-type (IS75) | 79.6±6 (n=7) |
| BD3032 | ΔymcA | 0.023±0.003 (n=6) |
| BD7186 | Δspo0F | 0 (n=6) |
| BD7307 | Δspo0F sof-1 | 79.5±10.8 (n=8) |
| BD7315 | ΔymcA spo0F sof-1 | 28.7±18.3 (n=8) |
All determinations were repeated at least 6 independent times, and are shown ± standard deviations. The strains were all grown for 24 hours in DSM at 37°C.
The measurements of spore formation presented to this point were carried out in domesticated strains of B. subtilis. During the course of this work, it was observed that the effects of ymcA and ylbF knockouts in 3610 on spore formation determined after 24 hours, were much milder than in the domesticated background (Fig S3). Although significantly greater, the effect of a yaaT knockout was also slightly less extreme than in the domesticated background. We have shown that the stronger penetrance of the ylbF, ymcA and yaaT knockouts in the domesticated strain is due to a T to C transition mutation in sigH, resulting in a V117A substitution present in all the sequenced domesticated strains and absent in undomesticated B. subtilis strains and their close relatives (Fig. S4). In wild-type 3610 the spoIIG transcription rate increases more sharply and reaches a higher level than in the domesticated background (Fig. S5A). When the V117A substitution was introduced into the sigH gene of strain 3610, the response of spoIIG was blunted (Fig. S5A). We interpret these kinetic differences as evidence that under sporulation conditions, the amount of Spo0A-P reaches its maximal level more rapidly in 3610 than in the domesticated strain, because it expresses a wild-type SigH. What is more, the SigH V117A change explains the difference in penetrance of the ylbF, ymcA and yaaT mutations, as demonstrated for both sporulation and spoIIG transcription (Figs. S3 and S5B–D). It is noteworthy that in the 3610 background with and without the sigH mutation, the initial rise in spoIIG transcription is followed by a pronounced decline (Fig. S5A). This decline may be due to the presence of RapP in 3610, which is known to dephosphorylate Spo0F-P (Parashar et al., 2013, Omer Bendori et al., 2015). RapP may also cause the lower maximal expression of spoIIG in 3610 carrying the sigH mutation, compared to the expression in the domesticated strain.
Most likely the commonly used domesticated strains express a crippled SigH. This may slow the production of Spo0A-P in several ways. The RNA polymerase holoenzyme with SigH transcribes kinA, spo0F and spo0A (Predich et al., 1992) and as a result the increase in these phosphorelay proteins during sporulation of the domesticated strains is probably slowed. Also, phrE, which encodes an antagonist of the RapE phosphatase, is transcribed by SigH-RNA polymerase (McQuade et al., 2001) and as a result RapE may remove phosphoryl groups from Spo0F-P more rapidly in the domesticated strain. We suggest that because the rate of Spo0A-P synthesis is challenged by the sigH mutation, the domesticated system is more dependent on the phosphorelay-accelerating roles of YlbF, YmcA and YaaT. This indirect argument provides further confirmation that these proteins accelerate the production of Spo0A-P. Despite the relatively small effects of ymcA and ylbF on the final frequency of spore formation in 3610, their kinetic effects on spoIIG transcription are substantial (Fig. S5), illustrating the importance of these genes for sporulation even in the undomesticated strain.
YlbF, YmcA and YaaT do not appear to be membrane-localized
Based on fluorescence microscopy, it has been reported that YaaT-GFP is associated with the cell membrane (Hosoya et al., 2002) and Rny is known to be an integral membrane protein (Lehnik-Habrink et al., 2011a). Deloughery et al (2016) have offered the reported membrane localization of YaaT as support for their hypothesis. We have re-examined the localization issue, using fusions of YFP to the C-termini of YlbF, YmcA and to the N-terminus of YaaT and an Rny-YFP construct (a kind gift from Jörg Stülke) as a control for membrane localization. All three of our fusion proteins are functional (Carabetta et al., 2013). Fig. S6 shows deconvolved images of cells from the four strains. Although the Rny-YFP signal is faint, it appears to be restricted to the cell periphery as expected, while the YlbF, YmcA and YaaT fusions are located in the cytoplasm, presenting a lumpy or punctate distribution. These images do not support the contention that these proteins are associated with the membrane. We cannot exclude that they do so transiently or to a limited extent. It is worth pointing out that our YaaT fusion is to the N-terminus of YaaT and that of Hosoya et al (2002) is to the C-terminus.
Bacterial two-hybrid (B2H) analysis suggests an association of YlbF, YmcA and YaaT with Spo0F, Spo0B and possibly with KinA
Our previously published B2H data (Carabetta et al., 2013), obtained in E. coli using the adenyl cyclase system (Karimova et al., 1998), detected an interaction of YmcA with itself, with Spo0F and Spo0B, but not with Spo0A and KinA. In contrast, no interactions of YlbF and YaaT with the phosphorelay proteins were detected. However, we have recently determined that a number of the restriction sites contained within the multiple cloning site of the vector pT25N-kan are not in-frame with the cya fragment, contrary to the published description (Karimova et al., 1998). This caused the T25 fragment to be out of frame with the cloned genes in our pT25 constructs. Inspection of the pT25N-ymcA plasmid revealed an overlooked deletion of a cytosine nucleotide near the 3′ end of the ymcA gene. This mutation restored the reading frame for the T25 fragment, at the same time changing the last 4 amino acid residues at the C-terminus of YmcA. The remaining intact YmcA sequence apparently still interacted with partner proteins and with itself. The self-interaction was not unexpected because YmcA has been crystallized as a dimer (Seetharaman, 2009). To determine whether interactions involving YlbF and YaaT had been missed because of our error, new T25 fusions to ymcA, ylbF and yaaT were constructed and the B2H experiments were repeated in 4 independent trials. A statistically significant increase relative to the empty vector control was interpreted as a positive interaction. No significant interactions were detected with Spo0A (Table S3), consistent with our recent in vitro observation that the purified Y-complex stimulates phosphorylation of the phosphorelay intermediates in the absence of Spo0A (not shown). However, with these corrected constructs, significant interactions were detected for all three proteins with both Spo0F and Spo0B, as well as among YlbF, YmcA and YaaT (p-values < 0.0001). The association signals for the interaction of YlbF, YmcA and YaaT with Spo0B and Spo0F were similar in strength to that observed for YmcA with YaaT. No significant interactions were detected with KinA when the cya fragment was fused to the N-terminus (T18-KinA). Switching the fragment to the C-terminus (KinA-T25) resulted in an apparently significant interaction with YmcA and YlbF (p-value = 0.048 and 0.005, respectively). However, because the KinA-T25 and T18 pair exhibited an unusually high background signal, we regard these KinA interactions as tentative. The strongest association was seen for the YmcA-YlbF heterodimer (Table S3).
Discussion
A central finding of the present study is that YlbF, YmcA and YaaT do support the production of Spo0A-P and that this role is important for biofilm formation and the K-state as well as for sporulation. A second result is that the three proteins play additional uncharacterized roles in comK expression for the K-state, in expression of the tapA operon and for biofilm formation. A third finding is the suggestive evidence from B2H experiments that YlbF, YmcA and YaaT all interact with Spo0F, Spo0B and possibly with KinA. Finally it has been shown that domesticated strains carry a mutant form of sigH, which affects the penetrance of the ylbF, ymcA and yaaT phenotypes for sporulation.
YlbF, YmcA and YaaT support the production of Spo0A-P
Any interference with the production of Spo0A-P would result in an increase in the rate of transcription from the abrB promoter and a decrease in the transcription from P2sinI (Fig. 1), thus abrogating the formation of biofilms. DeLoughery et al (2016) have reported that in the absence of YlbF or YmcA, the transcription of a sinI-lacZ fusion was unaffected and that in the absence of YlbF or YaaT, the expression of an abrB-lacZ fusion was likewise not altered. In contrast, we report here that in the absence of any one of the three proteins, sinI transcription is decreased and that of abrB is enhanced (Fig. 2, 3, 4 and 5). In the case of sinI, these effects have been documented using P2sinI-gfp and P2sinI-luc reporters and by qRT-PCR. The failure to detect these effects may lie in both the methods used by DeLoughery et al (2016) and in the kinetics of sinI and abrB transcription. Although the use of lacZ fusions is powerful, its determination is inherently less accurate than the use of fusions to luc because light output in the luciferase assay directly reports the rate of transcription whereas β-galactosidase accumulates, making the determination of rate differences between two strains less accurate when these differences are transient. With the plate reader assay, readings are taken every two minutes in real-time, rather than at widely-spaced intervals, increasing the allover accuracy of data collection. The transcription rate of sinI is quite low in MSgg and consequently inherently difficult to measure accurately. In the case of abrB, differences in the rates of transcription using lacZ are also difficult to detect because the total β-galactosidase in growing cultures is initially high and then decreases as Spo0A becomes phosphorylated. Thus, in the wild-type strain, even if transcription of abrB were to cease entirely, decreases could only result from dilution as cells divide or if the β-galactosidase were unstable. Rate differences would be particularly challenging to observe when the effect is partial, as with the ylbF, ymcA and yaaT knockouts. Whatever the cause of the discrepancies, we believe that the present results, obtained in the 3610 background with cultures growing in MSgg, are particularly robust; they have been documented for all three knockouts and in the case of sinI, using three methods. Thus, a total of five developmental promoters that are directly regulated by Spo0A-P have been shown to respond predictably to knockouts of ylbF, ymcA and yaaT: PspoIIG, PspoIIE, PabrB, PsinI and PcomK (this work, Carabetta et al (2013), Hosoya et al (2002) and Tortosa et al (2000)).
In addition to the in vivo data presented in this study that support a role for the YlbF, YmcA and YaaT in the accumulation of Spo0A-P, it has been shown that the complex of these three proteins accelerates the phosphorelay in vitro and that inactivation of spo0E, which encodes a Spo0A-P phosphatase, partially bypasses ylbF, ymcA and yaaT knockouts (Carabetta et al., 2013). An independent study concluded that yaaT is an early spore gene that supports the formation of Spo0A-P (Hosoya et al., 2002). An interesting further indication is the finding that the three knockouts increase cell chaining as cells approach stationary phase (Carabetta et al., 2013). This observation prompted the investigation of a spo0A knockout, which was also found to exhibit increased chaining, a phenotype that to our knowledge had not been reported previously. Thus, our model led to a verified prediction. We conclude that the preponderance of evidence supports a role for these proteins in accelerating the phosphorelay.
Is the Rny model correct?
An important point of difference in the two models lies in the effect of ylbF, ymcA and yaaT knockouts on the amount of sinR mRNA. In the report by DeLoughery et al (2016) it was claimed that the level of this transcript, measured in Northern blots, was increased about 2.8-fold in a ylbF mutant and by an unspecified amount in a ymcA knockout. Although a loading control (stained rRNA) is included in the relevant figure (Fig. 3 in DeLoughery et al (2016)), the signals were apparently not normalized to the load, which appears from the figure to be slightly less for the wild-type strain. The present qRT-PCR data fail to show an increase in mRNA abundance and instead show that the level of the sinR mRNA is somewhat decreased in all three knockouts compared to the wild-type. This decrease is consistent with our model, because the P2 promoter drives the transcription of both sinI and sinR. Here again, we propose that the discrepancies in our findings can be explained by the methods used. Northern blotting is inherently less sensitive than qRT-PCR and is difficult to quantitate accurately (Reue, 1998), particularly in the case of low abundance transcripts like those from the sin locus. Nevertheless, in Fig. 3C of the DeLoughery et al paper, the 700 bp sinIR transcript disappears in the absence of YlbF and YmcA, as would be expected from the phosphorelay model and consistent with the present data derived from the use of luciferase fusions, qRT-PCR and microscopy with a gfp fusion to the sinIR promoter. Lehnik-Habrink et al (2011b) have shown that when Rny is depleted, the 400 bp sinR transcript is stabilized. Both we (Fig. 4) and DeLoughery et al (2016) have confirmed this result for an rny knockout. Because the qRT-PCR data (Fig. 4) show that the sinR transcript actually decreases in abundance in the ylbF, ymcA and yaaT knockouts, as do transcripts carrying the sinI sequence, we conclude that the absence of YlbF, YmcA and YaaT does not phenocopy the rny null mutation.
It is worth pointing out that the Rny model, even if the evidence supported it, could not be generalized to the K-state. sinR is an essential gene for competence expression while a sinI knockout, which phenocopies SinR overproduction, has no obvious K-state phenotype (Bai et al., 1993). Thus, the stabilization of the sinR transcript could not explain the K-state phenotype of ylbF, ymcA and yaaT knockouts.
DeLoughery et al (2016) offer the slow growth phenotypes of the rny and ylbF, ymcA and yaaT mutants as further evidence that the knockouts phenocopy rny. Although slow growth is a rather non-specific phenotype, it is worth noting that the ylbF, ymcA and yaaT knockouts grow at room temperature on MSgg or LB agar, albeit more slowly than the wild-type. In contrast, an rny knockout strain does not form colonies at room temperature (not shown), thus exhibiting a phenotype shared with knockouts of other degradosome components (Awano et al., 2007, Luttinger et al., 1996, Purusharth et al., 2007, Wang & Bechhofer, 1996).
DeLoughery et al (2016) offer an additional argument in favor of the Rny hypothesis that relies on synteny between ymcA and rny. Although synteny is at best only a suggestive indication of common function, we believe that their analysis has overstated the synteny for these two genes, because they have limited their study to only 3 Firmicutes other than B. subtilis. We have reexamined this issue with a more complete list of bacterial species, representing all of the major families of Firmicutes (Figs. S7 and S8). ymcA and rny are quite distant from one another in the genetic maps of some of the representative Firmicutes shown in Fig. S7. In fact ymcA appears to be more consistently syntenic with mutS and mutL than with rny although we are aware of no evidence that YmcA is involved in mismatch repair. Other genes in the neighborhood are also present as often as rny, notably cotE and spoVS among the spore formers and again there is no evidence that YmcA plays a role in spore coat assembly.
Although the weight of evidence is against the Rny model as stated, the IPs of Rny by tagged YlbF and YaaT (DeLoughery et al., 2016, Carabetta et al., 2013) and the bacterial two hybrid (B2H) experiments showing association of Rny with YlbF and YmcA (DeLoughery et al., 2016) are indeed suggestive of association between these two proteins and Rny. The demonstration by DeLoughery et al (2016) that the inactivation of ylbF and ymcA, like the inactivation of rny, affect processing of the cggR-gapA transcript suggests that these interactions with Rny may have biological consequences. We emphasize that we have no evidence excluding some sort of role for YlbF, YmcA and YaaT interaction with Rny in development. For example, this interaction may be involved in the Spo0A-P-independent activities of YlbF, YmcA and YaaT that are documented in this study. One problem is that the extreme pleiotropy of the rny mutant makes it difficult to falsify a role for its interactions other than by examining each mechanistic proposal in turn. In fact it has been reported that Rny-depletion alters the abundances of 51% of the annotated protein-encoding transcripts of B. subtilis (Laalami et al., 2013).
Based on their Rny hypothesis, DeLoughery et al (2016) have suggested that the ylbF, ymcA and yaaT be renamed rcsA, rcsB and rcsC for “RNase Y-containing complex subunits A, B and C”. We do not agree with this nomenclature, in part because we are not convinced that YlbF, YmcA and YaaT activate Rny. We therefore prefer not to include “Rny” in the acronyms in the absence of evidence showing that this interaction is central to the roles of YlbF, YmcA and YaaT. Importantly, a quaternary complex of these three proteins with Rny has not been shown to exist. A stable association, suggested by the word “subunits”, has not been confirmed biochemically and there is no evidence that a quaternary complex actually exists in vivo, as implied by the Rcs nomenclature. For example it is possible that Rny associates independently with each of the three proteins or only with YlbF and YaaT. We believe that it is premature to rename these genes until more mechanistic insights are achieved.
Interactions of YlbF, YmcA and YaaT with phosphorelay components
If YlbF, YmcA and YaaT were involved in supporting the phosphorylation of Spo0A, we would expect them to interact with one or more components of the phosphorelay. In their B2H experiments, DeLoughery et al (2016) did not confirm the interactions of YmcA with Spo0F and Spo0B that we had reported (Carabetta et al., 2013). However, our two laboratories used different B2H systems; theirs was based on an interaction between the λ C1 protein and the α subunit of RNA polymerase (Deighan et al., 2008), while ours was based on the reassembly of adenyl cyclase fragments (Karimova et al., 1998). These B2H experiments have now been repeated with new constructs, correcting a sequence error in our previously used plasmid clones that led to an apparent absence of YlbF and YaaT interactions with phosphorelay components. The results shown in Table S3 extend the list of putative interactions with Spo0B and Spo0F to YlbF, YmcA and YaaT and hint that YlbF and YmcA may also contact KinA. We believe that these suggestive data must be interpreted with caution. Although the positive controls (interactions among YlbF, YmcA and YaaT) and the negative controls (with empty vectors) all behaved as expected, these interactions must be verified by independent means, most convincingly with purified proteins. We have so far not observed interactions of YlbF, YmcA and YaaT with phosphorelay proteins in our IPs. However, these interactions may occur at growth stages that were not analyzed or may be transient. Nevertheless, the consistent in vitro stimulation of the phosphorelay (Carabetta et al., 2013) argues strongly that some sort of interaction does take place.
YlbF, YmcA and YaaT have multiple roles
Several experiments show that YlbF, YmcA and YaaT play Spo0A-P-independent roles in biofilm formation, in the K-state and possibly for sporulation. Clear evidence for this in the case of biofilm formation derives from the study of suppressor mutations of spo0A and their effects on sinI, abrB and tapA transcription. ymcA and spo0F knockouts are bypassed by sad-67 and sof-1 for sinI transcription and by sof-1 for abrB transcription, consistent with a role for YmcA in the synthesis of Spo0A-P (Figs. 2D, 3C and 3D). We expected that these suppression effects would extend to tapA, which is repressed by AbrB and SinR (Fig. 1). However, sad-67 and sof-1 give little or no bypass of ymcA for tapA expression, while a spo0F knockout is largely suppressed by sof-1 (Fig. 6). These observations indicate that ylbF, ymcA and yaaT support tapA expression not only by activating the synthesis of Spo0A-P but also by an additional mechanism. The regulation of tapA is not completely understood and at least two genes, remA and remB (Winkelman et al., 2009, Winkelman et al., 2013), have been shown to contribute to matrix gene expression independently of spo0A and sinR. Also, SlrR and YmdB are needed to make matrix (Kobayashi, 2008, Chai et al., 2010, Diethmaier et al., 2011). YlbF, YmcA and YaaT may act on one or more of these proteins or on their cognate genes, in addition to stimulating the production of Spo0A-P.
Similar data are available for the K-state, suggesting the existence of Spo0A-P-dependent and -independent roles for YlbF, YmcA and YaaT in establishing the K-state. The uptick in comK basal expression, which is governed by the concentration of Spo0A-P, exhibits a sustained elevation in a ymcA knockout, as expected if the Spo0A-P concentration were to increase slowly (Fig. 7A). Thus in wild-type cells, YlbF, YmcA and YaaT help determine the window of opportunity for transitions to the K-state and thus the fraction of cells that enter this state. However, in contrast to its suppression of spo0F, sof-1 does not suppress the effect of a ymcA knockout for PcomK expression suggesting an important Spo0A-P-independent role for this protein. YlbF, YmcA and YaaT may affect ComK production in a variety of obvious ways. One possibility is that the Y- proteins increase the stability of the comK or comS mRNAs either by limiting the activity of Rny or by some other mechanism.
The evidence for a Spo0A-P-independent role for YlbF, YmcA and YaaT in sporulation is more ambiguous. The data obtained with sad-67 and sof-1 show clearly that bypass of the knockouts for spoIIG transcription takes place in the presence of these alleles of spo0A (Fig. 8 and Carabetta et al (2013)). Although suppression is nearly complete in the case of sad-67, it is incomplete for sof-1. The partial nature of the sof-1 bypass is probably due in part to a slow rate of Sof-1 protein phosphorylation, as shown by the delay in spoIIG transcription in the sof-1 spo0F strain compared with the wild-type strain (Fig. 8). But the fact that the delay is even longer in the ymcA spo0F sof-1 strain hints that something else is taking place. As suggested above in connection with the incomplete bypass of ymcA inactivation for sinI expression, YmcA may affect the autophosphorylation of one or more of the kinases that donate a phosphoryl group directly to the Sof-1 protein or the transfer of a kinase phosphoryl group, consistent with the possible interactions of YlbF, YmcA and YaaT with KinA (Table S3). Thus, the data do not point clearly to a Spo0A-P-independent role for the three proteins in sporulation, but also do not exclude such a role.
More generally, a possible role for YlbF, YmcA and YaaT beyond the acceleration of Spo0A-P production is consistent with the fact that they are encoded by the genomes of bacteria that lack spo0A (not shown). It is also clear that the lack of YaaT has a more severe effect on pellicle formation than YlbF and YmcA in the presence of sad-67 (Fig. S2), implying additional complexity. It is noteworthy that yaaT is more widely conserved among the Firmicutes than are ylbF and ymcA (not shown), suggesting that it may have an independent role even in B. subtilis. Further work is clearly required to more fully reveal the biochemistry and physiology of these important proteins. Because the pathways for biofilm formation and for the K-state are quite distinct, it is tempting to suggest that YlbF, YmcA and YaaT are involved with some underlying aspect of metabolism, in addition to their roles in Spo0A-P formation.
Domesticated B. subtilis strains carry a mutant form of sigH
The point mutation present in all sequenced domesticated strains has a clear consequence for sporulation. As a result of the V117A replacement, the induction of spoIIG expression is slowed and its maximal transcription rate is decreased. This kinetic difference may be relevant to the fitness of B. subtilis in nature, where the rapid onset of sporulation in the face of stress may be advantageous. Remarkably, an A117V mutation was selected in the PY79 background, as a suppressor of the sporulation defect of a particular allele of spo0A (Bramucci et al., 1995). Thus, the suppressor mutation restored the domesticated sigH to the “wild” state. Bramucci et al (1995) studied the effect of this sigH mutation on the transcription of spoIIE and spoIIA. In both cases they observed a elevated expression in the presence of this “wild” allele, consistent with the present data. In domesticated and undomesticated strains in which the two sigH alleles were swapped we found no difference in K-state induction and the mutation has no obvious effect on the appearance of biofilm colonies on agar (not shown) probably because less Spo0A-P is needed for these pathways than for sporulation. Because it is so widely distributed among laboratory strains, the V117A mutation may have been induced by the X-irradiation to which the parent of strain 168 was subjected (Burkholder & Giles, 1947).
Experimental procedures
Microbiological methods
Bacterial strains are listed in Table S1. The backgrounds used for all experiments were either IS75, a domesticated derivative of strain 168, or the undomesticated NCIB3610. Standard methods were used for plasmid constructions and the details are described in Supporting Information. Constructs were introduced into IS75 by transformation (Albano et al., 1987) and into 3610 by transduction using bacteriophage SPP1 (Cozy & Kearns, 2010). One exception was for the swapping of the sigH alleles, which was carried out by transformation in both backgrounds, using the pMiniMad2 plasmid as described (Cozy & Kearns, 2010, Mukherjee et al., 2013). A second exception was for the construction of certain strains with alleles of spo0A and the tapA-luc fusion. Because these two loci are linked it was convenient to combine them using transformation. In this case to avoid introducing unwanted mutations from IS75 by congression, the needed marker was first transduced into wild-type 3610 and then transferred from this intermediate strain by transformation. Pellicle and biofilm formation were assessed using MSgg medium (Kearns et al., 2005) as described (Branda et al., 2006), except that incubations were at room temperature (approximately 23°C). Spore frequency was determined as described (Carabetta et al., 2013) using Difco Spore Medium (Schaeffer et al., 1965) and K-state expression was measured in competence medium (Albano et al., 1987). All bacterial growth was at 37°C unless otherwise indicated. Antibiotic selections were carried out on Lysogeny Broth agar plates (Cozy & Kearns, 2010) containing ampicillin (100 μg ml−1), spectinomycin (100 μg ml−1), erythromycin (10 μg ml−1), tetracycline (25 μg ml−1), kanamycin (5 μg ml−1) or chloramphenicol (5 μg ml−1). In some cases selection was for erythromycin (1 μg ml−1) plus lincomycin (20 μg ml−1). Solid media were solidified by the addition of 1.5% agar. When required, IPTG was added to a concentration of 1 mM. For movement of the sof-1 allele, selection was applied for the erythromycin resistance marker associated with the accompanying Δspo0F marker and the colonies were subjected to heat treatment as described for the original suppressor screen to select for those that had inherited sof-1 (Kawamura & Saito, 1983). In each case the presence of the sof-1 mutation was confirmed by sequencing.
Luciferase assays
Experiments were carried out as described (Mirouze et al., 2011). Briefly, growth was at 37°C with shaking in the appropriate media, with the addition of luciferin (4.7 μM final concentration). Readings of optical density at 600 nm and of light output were taken at 2-minute intervals. A Perkin Elmer Envision 2104 plate reader, equipped for enhanced luminometry was used. The lids of the microtiter plates were maintained at 38°C to prevent condensation. All of the luciferase promoter fusions were inserted at their native loci by single reciprocal recombination using transformation with plasmid constructs. For each experiment growth and light output measurements were carried out in duplicate. Each plate reader experiment was repeated at least three times and representative results are shown in the figures.
qRT-PCR
Bacillus cells were grown in 10 ml of MSgg liquid media, with aeration for 6 or 8 hours at 37°C at which times they were in late log and early stationary phase respectively. Cells were harvested, and RNA was isolated using the FastRNA Pro Blue Kit (MP Biomedicals) as per manufacturer’s instructions with one modification; samples were processed in the Fastprep instrument for 3 cycles (45, 45, and 30 seconds) at a speed of 6.0 to ensure efficient cell lysis. To ensure complete removal of genomic DNA contamination, samples were treated with the TURBO DNA-free Kit (Applied Biosystems) as per manufacturer’s instructions. This step was essential because of the low abundance of the sin locus transcripts. The concentration and purity of RNA was quantified using a NanoDrop 1000 spectrophotometer (Thermo Scientific). 2 μg of RNA was copied into cDNA using the High Capacity cDNA Reverse Transcription Kit (Applied Biosystems) as per manufacturer’s instructions. For each RNA sample, a control reaction in the absence of reverse transcriptase was carried out to confirm the absence of genomic DNA contamination. Any remaining RNA was hydrolyzed by incubation with 0.1 M NaOH (final concentration) for 20 minutes at 70°C, and then neutralized by the addition of an equal volume of HCl. The reactions were cleaned using MinElute PCR purification spin columns (Qiagen) according to the manufacturer’s instructions.
For qRT-PCR, all of the cDNA samples were diluted 1:5 (considered as 1x concentration) and then further diluted 1:10 twice (0.1x and 0.01x) to use as standards. The wild-type cDNA was used for the standards and all other cDNAs were analyzed at the 0.1x concentration. Five μl of the cDNA dilutions or water (no-template control to ensure lack of contamination) were added to MicroAmp Optical tubes (Applied Biosystems) with 20 μl of a mixture of Power SYBR Green PCR master mix (Applied Biosystems) with 0.1 μM of each primer. Primers used were 5sinR-RT, 3sinR-RT, 5sinI-RT, 3sinI-RT, 5EFG-RT and 3EFG-RT (Table S2). Samples were analyzed on an ABI Prism 7700 sequence detector (Applied Biosystems) and data was analyzed (standard curve method) by using Sequence Detection Systems (SDS) software (v.1.9.1, Applied Biosystems). The fusA (EFG) transcript was used as an internal control to adjust for differing amounts of input cDNA. Experiments were performed from 3 biological replicates, and data points from individual runs were averages of triplicates.
Microscopy and image analysis
For the P2sinI-GFP fusion experiment, cells were grown in liquid MSgg media for 7 hours, at which point they were in the early stationary phase. An aliquot of cells was harvested, and diluted into PBS (81 mM Na2HPO4 + 24.6 mM NaH2PO4 + 100 mM NaCl). 1 μl of each culture was placed on an agarose pad made up in minimal medium and mounted on the microscope. Cells were imaged on a Nikon Eclipse Ti inverted microscope outfitted with an Orca Flash 4.0 Digital Camera (Hamamatsu) with a Nikon TIRF 1.45 NA Plan Neoflur 100 oil immersion objective. NIS-Elements AR (v 4.40, Nikon) was used for image acquisition and data analysis. The acquisition time for GFP fluorescence was identical for each analyzed strain. The images from all 5 strains were compared simultaneously, using the synchronizer tool. For each image, a background region of interest (“ROI”) was defined, and the average background was subtracted. The look-up tables (LUTs) were used for visualization, and all intensities were adjusted to an identical scale. Fluorescence intensity measurements were performed using the software’s automated General Analysis feature. Thresholds were established to define the objects of interest (cells), and were applied to each image. The program automatically measures the pixel intensity from the thresholded objects. At least 1200 cells per strain were counted. Similar observations were made from two independent analyses.
For YFP localization studies, cells were grown and prepared for microscopy as above. All acquisition times and parameters for the various samples were identical. Z-stacks were acquired in 27 steps using 0.2 μM slices, and images were deconvolved in NIS-Elements. Deconvolution was performed using the 3D Blind method with 10 iterations, allowing for background subtraction. Each image was enhanced in an identical fashion and for each a slice in the Z-stack from the center of the cell was selected for presentation in Fig. S6. Observations of localization were made 3 independent times.
Bacterial 2-hybrid analysis
B2H analysis was carried out exactly as described previously (Carabetta et al., 2013). The construction of plasmids for B2H analysis is described in Supporting information.
Statistical methods
All statistical analyses were performed as described in Carabetta et al (2013). In short the logarithms of RT-PCR, spore counts and B2H data values were obtained and used for subsequent analysis. The software package Stata (version 13.1) was used to perform a one-way analysis of variance (ANOVA) followed by post hoc t-tests applying the Bonferroni correction to accommodate multiple comparisons. For the B2H data, a statistically significant fold increase relative to the empty vector control was interpreted as a positive interaction.
Supplementary Material
Acknowledgments
We thank Dan Ziegler at the Bacillus Stock Center, Byoung-Mo, Carol Gross, Richard Losick, Alan Grossman and Jörg Stülke for providing strains and all the members of our laboratory for discussions and advice. We thank Nicolas Mirouze for constructing the P2sinI-luc construct, Jonathan Dworkin for a kind gift of anti-EFG antiserum and Salvatore Marras for help with the qRT-PCR. We thank an anonymous referee for pointing out the Bramucci et al paper, of which we were unaware. Finally we thank Dan Kearns for advice concerning the pMiniMad2 protocol and for providing the plasmid. This work was supported by grant NIH GM057720.
References
- Albano M, Hahn J, Dubnau D. Expression of competence genes in Bacillus subtilis. J Bacteriol. 1987;169:3110–3117. doi: 10.1128/jb.169.7.3110-3117.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Awano N, Xu C, Ke H, Inoue K, Inouye M, Phadtare S. Complementation analysis of the cold-sensitive phenotype of the Escherichia coli csdA deletion strain. J Bacteriol. 2007;189:5808–5815. doi: 10.1128/JB.00655-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bai U, Mandic-Mulec I, Smith I. SinI modulates the activity of SinR, a developmental switch protein of Bacillus subtilis, by protein-protein interaction. Genes Dev. 1993;7:139–148. doi: 10.1101/gad.7.1.139. [DOI] [PubMed] [Google Scholar]
- Berka RM, Hahn J, Albano M, Draskovic I, Persuh M, Cui X, Sloma A, Widner W, Dubnau D. Microarray analysis of the Bacillus subtilis K-state: genome-wide expression changes dependent on ComK. Mol Microbiol. 2002;43:1331–1345. doi: 10.1046/j.1365-2958.2002.02833.x. [DOI] [PubMed] [Google Scholar]
- Bramucci MG, Green BD, Ambulos N, Youngman P. Identification of a Bacillus subtilis spo0H allele that is necessary for suppression of the sporulation-defective phenotype of a spo0A mutation. J Bacteriol. 1995;177:1630–1633. doi: 10.1128/jb.177.6.1630-1633.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Branda SS, Chu F, Kearns DB, Losick R, Kolter R. A major protein component of the Bacillus subtilis biofilm matrix. Mol Microbiol. 2006;59:1229–1238. doi: 10.1111/j.1365-2958.2005.05020.x. [DOI] [PubMed] [Google Scholar]
- Branda SS, Gonzalez-Pastor JE, Dervyn E, Ehrlich SD, Losick R, Kolter R. Genes involved in formation of structured multicellular communities by Bacillus subtilis. J Bacteriol. 2004;186:3970–3979. doi: 10.1128/JB.186.12.3970-3979.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burbulys D, Trach KA, Hoch JA. Initiation of sporulation in B. subtilis is controlled by a multicomponent phosphorelay. Cell. 1991;64:545–552. doi: 10.1016/0092-8674(91)90238-t. [DOI] [PubMed] [Google Scholar]
- Burkholder PR, Giles NH., Jr Induced biochemical mutations in Bacillus subtilis. Am J Bot. 1947;34:345–348. [PubMed] [Google Scholar]
- Carabetta VJ, Tanner AW, Greco TM, Defrancesco M, Cristea IM, Dubnau D. A complex of YlbF, YmcA and YaaT regulates sporulation, competence and biofilm formation by accelerating the phosphorylation of Spo0A. Mol Microbiol. 2013;88:283–300. doi: 10.1111/mmi.12186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Chu F, Kolter R, Losick R. Bistability and biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;67:254–263. doi: 10.1111/j.1365-2958.2007.06040.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chai Y, Norman T, Kolter R, Losick R. An epigenetic switch governing daughter cell separation in Bacillus subtilis. Genes Dev. 2010;24:754–765. doi: 10.1101/gad.1915010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastanet A, Losick R. Just-in-time control of Spo0A synthesis in Bacillus subtilis by multiple regulatory mechanisms. J Bacteriol. 2011;193:6366–6374. doi: 10.1128/JB.06057-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chastanet A, Vitkup D, Yuan GC, Norman TM, Liu JS, Losick RM. Broadly heterogeneous activation of the master regulator for sporulation in Bacillus subtilis. Proc Natl Acad Sci U S A. 2010;107:8486–8491. doi: 10.1073/pnas.1002499107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chu F, Kearns DB, Branda SS, Kolter R, Losick R. Targets of the master regulator of biofilm formation in Bacillus subtilis. Mol Microbiol. 2006;59:1216–1228. doi: 10.1111/j.1365-2958.2005.05019.x. [DOI] [PubMed] [Google Scholar]
- Chu F, Kearns DB, McLoon A, Chai Y, Kolter R, Losick R. A novel regulatory protein governing biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;68:1117–1127. doi: 10.1111/j.1365-2958.2008.06201.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cozy LM, Kearns DB. Gene position in a long operon governs motility development in Bacillus subtilis. Mol Microbiol. 2010;76:273–285. doi: 10.1111/j.1365-2958.2010.07112.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deighan P, Diez CM, Leibman M, Hochschild A, Nickels BE. The bacteriophage lambda Q antiterminator protein contacts the beta-flap domain of RNA polymerase. Proc Natl Acad Sci U S A. 2008;105:15305–15310. doi: 10.1073/pnas.0805757105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DeLoughery A, Dengler V, Chai Y, Losick R. Biofilm formation by Bacillus subtilis requires an endoribonuclease-containing multisubunit complex that controls mRNA levels for the matrix gene repressor SinR. Mol Microbiol. 2016;99:425–437. doi: 10.1111/mmi.13240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Diethmaier C, Pietack N, Gunka K, Wrede C, Lehnik-Habrink M, Herzberg C, Hubner S, Stulke J. A novel factor controlling bistability in Bacillus subtilis: the YmdB protein affects flagellin expression and biofilm formation. J Bacteriol. 2011;193:5997–6007. doi: 10.1128/JB.05360-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Figaro S, Durand S, Gilet L, Cayet N, Sachse M, Condon C. Bacillus subtilis mutants with knockouts of the genes encoding ribonucleases RNase Y and RNase J1 are viable, with major defects in cell morphology, sporulation, and competence. J Bacteriol. 2013;195:2340–2348. doi: 10.1128/JB.00164-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujita M, Gonzalez-Pastor JE, Losick R. High- and low-threshold genes in the Spo0A regulon of Bacillus subtilis. J Bacteriol. 2005;187:1357–1368. doi: 10.1128/JB.187.4.1357-1368.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fürbass R, Gocht M, Zuber P, Marahiel MA. Interaction of AbrB, a transcriptional regulator from Bacillus subtilis with the promoters of the transition state-activated genes tycA and spoVG. Mol Gen Genet. 1991;225:347–354. doi: 10.1007/BF00261673. [DOI] [PubMed] [Google Scholar]
- Gaur NK, Cabane K, Smith I. Structure and expression of the Bacillus subtilis sin operon. J Bacteriol. 1988;170:1046–1053. doi: 10.1128/jb.170.3.1046-1053.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hahn J, Tanner AW, Carabetta VJ, Cristea IM, Dubnau D. ComGA-RelA interaction and persistence in the Bacillus subtilis K-state. Mol Microbiol. 2015;97:454–471. doi: 10.1111/mmi.13040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamon MA, Stanley NR, Britton RA, Grossman AD, Lazazzera BA. Identification of AbrB-regulated genes involved in biofilm formation by Bacillus subtilis. Mol Microbiol. 2004;52:847–860. doi: 10.1111/j.1365-2958.2004.04023.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hoch JA. Regulation of the phosphorelay and the initiation of sporulation in Bacillus subtilis. Annu Rev Microbiol. 1993;47:441–465. doi: 10.1146/annurev.mi.47.100193.002301. [DOI] [PubMed] [Google Scholar]
- Hoch JA, Trach K, Kawamura F, Saito H. Identification of the transcriptional suppressor sof-1 as an alteration in the spoOA protein. J Bacteriol. 1985;161:552–555. doi: 10.1128/jb.161.2.552-555.1985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hosoya S, Asai K, Ogasawara N, Takeuchi M, Sato T. Mutation in yaaT leads to significant inhibition of phosphorelay during sporulation in Bacillus subtilis. J Bacteriol. 2002;184:5545–5553. doi: 10.1128/JB.184.20.5545-5553.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ireton K, Rudner DZ, Siranosian KJ, Grossman AD. Integration of multiple developmental signals in Bacillus subtilis through the Spo0A transcription factor. Genes Dev. 1993;7:283–294. doi: 10.1101/gad.7.2.283. [DOI] [PubMed] [Google Scholar]
- Karimova G, Pidoux J, Ullmann A, Ladant D. A bacterial two-hybrid system based on a reconstituted signal transduction pathway. Proc Natl Acad Sci U S A. 1998;95:5752–5756. doi: 10.1073/pnas.95.10.5752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamura F, Saito H. Isolation and mapping of a new suppressor mutation of an early sporulation gene spo0F mutation in Bacillus subtilis. Mol Gen Genet. 1983;192:330–334. doi: 10.1007/BF00392171. [DOI] [PubMed] [Google Scholar]
- Kearns DB, Chu F, Branda SS, Kolter R, Losick R. A master regulator for biofilm formation by Bacillus subtilis. Mol Microbiol. 2005;55:739–749. doi: 10.1111/j.1365-2958.2004.04440.x. [DOI] [PubMed] [Google Scholar]
- Kobayashi K. SlrR/SlrA controls the initiation of biofilm formation in Bacillus subtilis. Mol Microbiol. 2008;69:1399–1410. doi: 10.1111/j.1365-2958.2008.06369.x. [DOI] [PubMed] [Google Scholar]
- Laalami S, Bessieres P, Rocca A, Zig L, Nicolas P, Putzer H. Bacillus subtilis RNase Y activity in vivo analysed by tiling microarrays. PLoS One. 2013;8:e54062. doi: 10.1371/journal.pone.0054062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnik-Habrink M, Newman J, Rothe FM, Solovyova AS, Rodrigues C, Herzberg C, Commichau FM, Lewis RJ, Stulke J. RNase Y in Bacillus subtilis: a Natively disordered protein that is the functional equivalent of RNase E from Escherichia coli. J Bacteriol. 2011a;193:5431–5441. doi: 10.1128/JB.05500-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lehnik-Habrink M, Schaffer M, Mader U, Diethmaier C, Herzberg C, Stulke J. RNA processing in Bacillus subtilis: identification of targets of the essential RNase Y. Mol Microbiol. 2011b;81:1459–1473. doi: 10.1111/j.1365-2958.2011.07777.x. [DOI] [PubMed] [Google Scholar]
- Luttinger A, Hahn J, Dubnau D. Polynucleotide phosphorylase is necessary for competence development in Bacillus subtilis. Mol Microbiol. 1996;19:343–356. doi: 10.1046/j.1365-2958.1996.380907.x. [DOI] [PubMed] [Google Scholar]
- McQuade RS, Comella N, Grossman AD. Control of a family of phosphatase regulatory genes (phr) by the alternate sigma factor sigma-H of Bacillus subtilis. J Bacteriol. 2001;183:4905–4909. doi: 10.1128/JB.183.16.4905-4909.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze N, Desai Y, Raj A, Dubnau D. Spo0A~P imposes a temporal gate for the bimodal expression of competence in Bacillus subtilis. PLoS Genet. 2012;8:e1002586. doi: 10.1371/journal.pgen.1002586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze N, Dubnau D. Chance and necessity in Bacillus subtilis development. Microbiol Spectrum. 2013:1. doi: 10.1128/microbiolspectrum.TBS-0004-2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mirouze N, Prepiak P, Dubnau D. Fluctuations in spo0A transcription control rare developmental transitions in Bacillus subtilis. PLoS Genet. 2011;7:e1002048. doi: 10.1371/journal.pgen.1002048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukherjee S, Babitzke P, Kearns DB. FliW and FliS function independently to control cytoplasmic flagellin levels in Bacillus subtilis. J Bacteriol. 2013;195:297–306. doi: 10.1128/JB.01654-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omer Bendori S, Pollak S, Hizi D, Eldar A. The RapP-PhrP quorum-sensing system of Bacillus subtilis strain NCIB3610 affects biofilm formation through multiple targets, due to an atypical signal-insensitive allele of RapP. J Bacteriol. 2015;197:592–602. doi: 10.1128/JB.02382-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parashar V, Konkol MA, Kearns DB, Neiditch MB. A plasmid-encoded phosphatase regulates Bacillus subtilis biofilm architecture, sporulation, and genetic competence. J Bacteriol. 2013;195:2437–2448. doi: 10.1128/JB.02030-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perego M. A new family of aspartyl phosphate phosphatases targeting the sporulation transcription factor Spo0A of Bacillus subtilis. Mol Microbiol. 2001;42:133–143. doi: 10.1046/j.1365-2958.2001.02611.x. [DOI] [PubMed] [Google Scholar]
- Predich M, Nair G, Smith I. Bacillus subtilis early sporulation genes kinA, spo0F, and spo0A are transcribed by the RNA polymerase containing sigma H. J Bacteriol. 1992;174:2771–2778. doi: 10.1128/jb.174.9.2771-2778.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Purusharth RI, Madhuri B, Ray MK. Exoribonuclease R in Pseudomonas syringae is essential for growth at low temperature and plays a novel role in the 3′ end processing of 16 and 5 S ribosomal RNA. J Biol Chem. 2007;282:16267–16277. doi: 10.1074/jbc.M605588200. [DOI] [PubMed] [Google Scholar]
- Reue K. mRNA quantitation techniques: considerations for experimental design and application. J Nutr. 1998;128:2038–2044. doi: 10.1093/jn/128.11.2038. [DOI] [PubMed] [Google Scholar]
- Romero D, Aguilar C, Losick R, Kolter R. Amyloid fibers provide structural integrity to Bacillus subtilis biofilms. Proc Natl Acad Sci U S A. 2010;107:2230–2234. doi: 10.1073/pnas.0910560107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero D, Vlamakis H, Losick R, Kolter R. An accessory protein required for anchoring and assembly of amyloid fibres in B. subtilis biofilms. Mol Microbiol. 2011;80:1155–1168. doi: 10.1111/j.1365-2958.2011.07653.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaeffer P, Millet J, Aubert JP. Catabolic repression of bacterial sporulation. Proc Natl Acad Sci USA. 1965;54:704–711. doi: 10.1073/pnas.54.3.704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seetharaman J, Abashidze M, Forouhar F, Wang D, Fang Y, Cunningham K, Ma L-C, Xia R, Liu J, Baran MC, Acton TB, Rost B, Montelione GT, Tong L, Hunt JF. Crystal structure of Protein YmcA from Bacillus subtilis. Protein Data Bank (2PIH) 2009 [Google Scholar]
- Shafikhani SH, Mandic-Mulec I, Strauch MA, Smith I, Leighton T. Postexponential regulation of sin operon expression in Bacillus subtilis. J Bacteriol. 2002;184:564–571. doi: 10.1128/JB.184.2.564-571.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spiegelman G, Van Hoy B, Perego M, Day J, Trach K, Hoch JA. Structural alterations in the Bacillus subtilis Spo0A regulatory protein which suppress mutations at several spo0 loci. J Bacteriol. 1990;172:5011–5019. doi: 10.1128/jb.172.9.5011-5019.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch M, Webb V, Spiegelman G, Hoch JA. The SpoOA protein of Bacillus subtilis is a repressor of the abrB gene. Proc Natl Acad Sci USA. 1990;87:1801–1805. doi: 10.1073/pnas.87.5.1801. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Strauch MA, Spiegelman GB, Perego M, Johnson WC, Burbulys D, Hoch JA. The transition state transcription regulator AbrB of Bacillus subtilis is a DNA binding protein. EMBO J. 1989;8:1615–1621. doi: 10.1002/j.1460-2075.1989.tb03546.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tortosa P, Albano M, Dubnau D. Characterization of ylbF, a new gene involved in competence development and sporulation in Bacillus subtilis. Mol Microbiol. 2000;35:1110–1119. doi: 10.1046/j.1365-2958.2000.01779.x. [DOI] [PubMed] [Google Scholar]
- Wang W, Bechhofer DH. Properties of a Bacillus subtilis polynucleotide phosphorylase deletion strain. J Bacteriol. 1996;178:2375–2382. doi: 10.1128/jb.178.8.2375-2382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JT, Blair KM, Kearns DB. RemA (YlzA) and RemB (YaaB) regulate extracellular matrix operon expression and biofilm formation in Bacillus subtilis. J Bacteriol. 2009;191:3981–3991. doi: 10.1128/JB.00278-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winkelman JT, Bree AC, Bate AR, Eichenberger P, Gourse RL, Kearns DB. RemA is a DNA-binding protein that activates biofilm matrix gene expression in Bacillus subtilis. Mol Microbiol. 2013;88:984–997. doi: 10.1111/mmi.12235. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.