Abstract
The peer review processes as outlined in the Health Care Quality Improvement Act (HCQIA) is meant ensure quality standard of care through a self-policing mechanism by the medical community. This process grants immunity for people filing a peer review, which is meant to protect whistleblowers. However, it also creates a loophole that can be used maliciously to hinder competition. This is accentuated when surgeons are integrating new technologies, such as robotic surgery, into their practice. With more than 2000 da Vinci robots in use and more than 300 new units being shipped each year, robotic surgery has become a mainstay in the surgical field. The applications for robots continue to expand as surgeons discover their expanding capability. We need a better peer review process. That ensures the peer review is void of competitive bias. Peer reviewers need to be familiar with the procedure and the technology. The current process could stymie innovation in the name of competition.
Keywords: Peer Review, Health Care Quality Improvement Act (HCQIA), Robotic Surgery
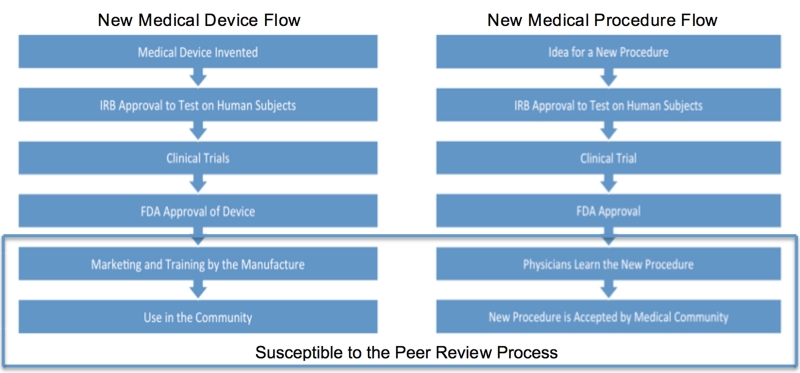
One of the difficult aspects of using a new procedure or new medical devices is to know when you are conducting research and when you are not. There are two broad categories: (1) the use of a new piece of equipment or (2) the use of a piece of established equipment for a use that it was not originally designed for (see the diagrams for the flow from idea to implementation, see Figure 1). Often procedures have planned and unplanned innovation. The key is to determine at what point the innovation is research. Asking the question, “What am I going to do with this information?”1 If the use is strictly off label then the FDA does not require IRB approval. However, if information is collected on subjects then IRB approval would be required.2 Due to the lack of federal regulation institutions may have slightly different ways to handle this. The peer review process becomes relevant in the last two stages when the medical device has migrated out to the research setting into the clinical one. With the recent boom in the use of surgical robots such as the Da Vinci many surgeons are finding new applications for the evolving field of minimally invasive robotic surgeries. We have seen robotics move from primarily gynecological and urological procedure into cardiac, gastrointestinal, thoracic, and others. Procedures are in place that address IRB protocols; however, we must address the last two steps in the process specifically pertaining to robotic surgery innovation.
Figure 1. Critical areas susceptible to the peer review in the development of medical devices and procedures.
The power afforded to the peer review process and the lack of standards for implementation may have unintended consequences. The process itself creates an opportunity for abuse, in which physicians making accusations have immunity from reprisal.3 The immunity may be used as a tool to prevent the integration of new procedures, specifically those in robotic surgery. There are no standard guidelines for what warrants a peer review investigation The Health Care Quality Improvement Act (HCQIA), which popularized the clinical peer review, gives broad guidelines for what justifies a clinical peer review, in the form of five goals.4 Although the HCQIA was designed to ensure high quality care and to help mitigate the rising cost of medical malpractice, in its current form it could be used to impede the development of new procedures in an effort to curb local competition.
Innovation in a competitive and rapidly evolving field, such as surgery, and in particular robotic surgery, requires that the community be willing to trade off procedural comfort for the chance to improve outcomes. “Innovation, invention and technology development are not simple or a single occurrences.”5 Rather innovation must continue to evolve as technology and resources become available; however innovation requires a level of risk in the medical field. Some physicians and hospitals may not be comfortable with the increased risk associated with innovative procedures, and it is their prerogative to decide whether the risk is worth the reward.
As some physicians and hospitals decide to innovate, a competitive situation could occur in which the peer review process is enacted maliciously. For example, take two surgeons who are in practice together. The more experienced surgeon is very good at preforming open resections of oropharyngeal tumors. However, the other surgeon recently learned about transoral robotic surgery (TORS) for the resection of the same types of tumors. As a result of a difference in opinion about the two procedures, they decide to separate and practice independently. Realizing that he is going to lose some of his patients the more experienced surgeon monitors the other surgeon’s outcomes closely and discovers that there is slightly increased morbidity during the first few months that the surgeon is using TORS. The more experienced surgeon logs a peer review complaint and a subsequent investigation, resulting in the termination of the other surgeon’s hospital privileges. Upon appeal of the decision, it is found that there is insufficient evidence and that the reason for logging the peer review was to maintain market share on the resection of oral tumors. Although the peer review is overturned, the other physician has difficulty finding patients and has spent many thousands of dollars on appeals while the older surgeon is immune from prosecution due to the HCQIA.
THE PEER REVIEWS PROCESS
In the peer review process doctors review the work of their peers to ensure standards of quality are being met.6 There are three main reasons that a clinical peer review is conducted:7
-
1—
Hospitals are required to conduct peer reviews to maintain accreditation. Peer reviews must be conducted on all new physicians and any physician requesting new privileges.
-
2—
Whenever there is substandard performance by a physician, a peer review can be requested by a hospital administrator or a physician’s colleague.
-
3—
Some institutions conduct peer reviews at random to ensure high levels of quality and to root out systemic errors and institute corrective actions.
Although the intention of the clinical peer review is well-placed, the process is flawed and can have significant implications for the physician under inspection. For example, hospital privileges or membership in professional organizations may be adversely affected by the decision of the board.8 In a study conducted by Dr. Kadar, it was found that reviewing physicians had poor agreement when analyzing for flaws as part of the peer review. When cases are sent to a third party for review, reviewers agreement is difficult to reproduce and is only marginally better than chance alone.9 Peer reviewers also judge more serious outcomes more harshly when compared to minor adverse outcomes even when the quality and scope of the care was identical.10 There is also little evidence to support the precision of the peer review process.11
In comparison to a new drug that requires years of research and development, many surgical procedures and techniques are developed in the surgical suite or through the use of simulators and are thus not subject to the same rigorous evaluation of safety and effectiveness. Much of what is learned is not realized until the procedure has been performed thousands of times.12 The introduction of the da Vinci robot is no exception. More that 2 million robotic procedures have been conducted in the last decade, and 125 articles on these procedures are published each month.13
THE MALICIOUS PEER REVIEW
The National Practitioner Data Base (NPDB) is the source for information about adverse actions against physicians. As of 2012 6949 adverse actions taken against physicians and 649 of them resulted in restricting physician’s hospital privileges (www.npdb.hrsa.gov). To date there are no documented malicious peer review cases tied to competition between a surgeon who predominantly conducts laparoscopic or open procedures and a surgeon who primarily uses a robotic technique. However, there have been cases in which peer reviews were used to revoke hospital privileges and limit competition. One such situation occurred in 1986 when Dr. Timothy Patrick was subjected to a bad faith peer review in an effort to prevent him from opening up a competing practice.14 More recently a radiologist named Dr. Jesse Cole had his hospital privileges revoked in an effort for the hospital to honor a contract they had established with a competing radiology service.15 There is precedence for the peer review process being used to stymie competition.
Inventors of new procedures are under extreme scrutiny while they are developing and testing their methods. The Institutional Review Board (IRB) provides oversight and guidance to mitigate this risk. However, once the procedure is FDA approved the IRB is no longer involved in the assessment of quality. At this stage the peer review process could be used as a tool to prevent the integration of new procedures into surgical mainstream. When new surgical procedures are introduced, they are often associated with a temporarily elevated level of mortality and morbidity. When learning a robotic technique it can take 10–20 procedures in order to become proficient, and outcomes are best once the surgeon had preformed in excess of 40 procedures.16 Physicians or hospitals could use these learning mistakes as ammunition during a peer review process in an effort to limit or force out a competing physician or practice.
After the procedure has been tested and approved through the FDA process it is taught to other surgeons. The FDA approval process provides extensive oversight. Once the procedure is more broadly taught we use the peer review process to ensure high quality care. The level of risk of a procedure needs to be weighed against the possible benefits. Many procedures that are in transition from research to the community have unknown risk factors due to the limited and tightly controlled microenvironment in which they were developed. This may confer a higher risk but decrease mortality and morbidity in the future. The potential benefits must be significantly better to justify additional risk. Our responsibility remains: to protect patients during that critical time when the true risk may not yet be known or fully understood.17
ROBOTIC SURGERY AND ITS ENTRY INTO MAIN STREAM
Since the da Vinci (by Intuitive Surgical) was first approved for use in 2000, the number of surgeries in which it is used has grown significantly as has the demand for fewer invasive/open procedures.18 However, the added cost associated with the use of robotic techniques ($1 MM–2.5 MM plus disposable equipment) is not always offset by enough procedures or shorter hospital stays19 and may not be possible with surgeons’ current level of skill or training.
With the evolution from open surgeries to laparoscopy to robotic-based surgeries, are surgeons that choose not to learn robotic techniques at a disadvantage? Could surgeons that find their patients shifting to surgeons or institutions with robotic capabilities exploit the peer review process? When laparoscopy was first introduced, many physicians trained to conduct open surgeries and resisted the market urge to learn laparoscopic techniques, this left many of them at a disadvantage when trying to recruit patients.
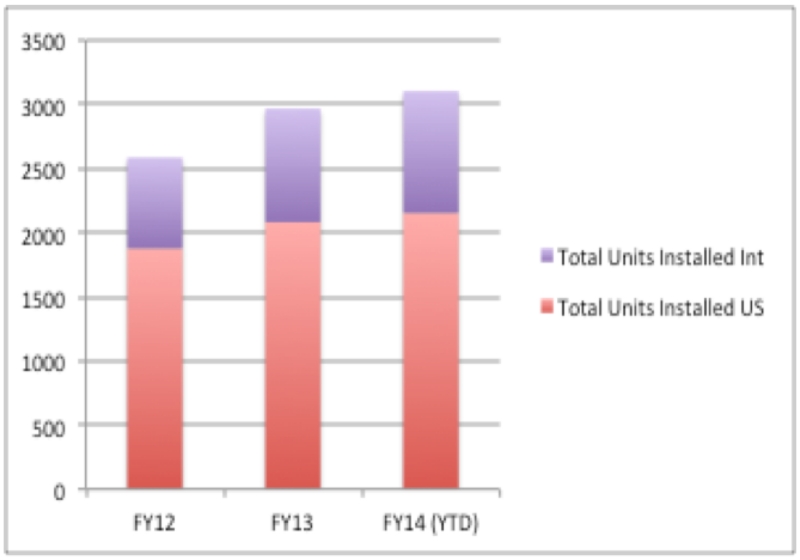
Although 2000 base units and 34320 da Vinci robots shipped in the United States in 2013 (see Fig. 2), there still may be resistance to the integration of robotic surgery. The time it takes to become a trained and proficient laparoscopic surgeon to perform robotic surgeries could be a hurdle to integrate robotic techniques into their practice.21 It’s possible they may even take measure to prevent it from becoming available to their patient population.
Figure 2. Number of da Vinci robots in the US and worldwide.
Hospitals compete for surgeons. Having a robotic surgical device demonstrates a hospital’s cutting edge program, supports talent recruitment, and increases patient demand; however, we must also consider complication rates. Being on the cutting edge of a field may result in a higher complication risk at least initially, as discussed earlier. The increased risk and marginally different outcomes may be traced back to the learning curve of the surgeon or training to use robotics in surgery. However, as surgeons become more proficient with new techniques, not only does the time in the operating room decrease, time spent between opening and closing decreases as well.22 It can therefore be hypothesized that as surgeons become more proficient, then the complication rate may become more favorable. However patients and surgeons have a very low tolerance for complication rates seen in newer procedures. This elevated complication rates may be used as evidence in the process of a peer review taken in bad faith as an attempt to limit competition within a region.
EXPANDING THE PROCEDURES FOR ROBOTIC SURGERY
With the advent of robotic surgery many of the assumptions about what could be done minimally invasively have changed drastically. The use of robotic surgical techniques provides superior access to regions of the body that are difficult to gain access to in minimally invasive ways. Robots provide the same level of access and articulation as open procedures with a minimally invasive incisions wound. However, not all physicians are trained or competent using a robotic device for procedures. What processes are in place to ensure that every physician has obtained a base line level of proficiency prior to conducting their first robotic surgery? This problem still has not been adequately addressed. Nor can it be addressed until we have a consensus about what the minimum training requirements should be. In a report by the FDA the training programs and approaches to monitoring surgeons vary greatly between institutions. We have seen robotic surgery expand from urology and gynecology to ENT, GI, Pulmonary and Cardiac surgeries. Subspecialties provide their own surgical challenges and are just getting acquainted with robotic surgical techniques.
The transition for laparoscopic techniques to robotics has been well received in some fields and is still developing in others. In the treatment of urogenital diseases significant advantages have been realized from shorter hospital stays with better outcomes for the treatment and to the management of endometrial cancers.23 There are more desirable outcomes when associated with radical prostectomy.24 There is also evidence that there is a decreasing time to mastering complex surgeries such as pyeloplasties.25 However, not every surgery clearly benefits from the robot. For example, there appears to be no advantage for lobectomys for the treatment of lung cancer.26
Surgeons who fail to embrace changes may find themselves at a disadvantage.27 In an attempt to maintain a patient population they may choose to use the peer review process as a tool to prevent new surgical techniques from being introduced into their practice market. As training programs continue to increase their use of robotics surgeons will use preferences and comfort with the technology to choose their preferred method to treat their patients. The current trend is that patients see robotic surgical procedures as beneficial over older laparoscopic techniques28 which can draw patients away from surgeons who refuse to learn robotics techniques.
RECOMMENDED CHANGES TO THE PEER REVIEW PROCESS
There are two areas in which one can address the issues associated with sham peer reviews with respect to robotic surgical innovation:
-
(1)
Minimize the number of unnecessary peer reviews
-
(2)
Carefully analyze third party review reports for bias in the reasons for initiating a case for presentation.
The most efficient way to address the threat of unnecessary peer reviews is to prevent them from occurring. The first way to do this is to have the physician in question articulate why the outcome was less than optimal. A second way is to use published data and papers to assess whether the outcome was suboptimal or medically unsound, and a third way would be to look for patterns of suboptimal care outside of the procedure in question. The HCQIA affords review boards significant power with little recourse for the physician under review.
The second point of intervention to prevent sham peer reviews from negatively affecting innovation is to establish a more consistent third party review process. The third party reviewer is systematically biased to find quality problems with the physician under review.29 Addressing this bias is complicated. Ensuring that the physicians do not have any financial attachment to the case is critical; however it is not sufficient. The reviewer should be at a different institution and have a similar but different patient population. The reviewers should consist of at least three physicians, all with highly credible academic practice and have previous reviews endorsed in a similar setting by the subjected physician. Each time a review process starts, a new pool of experts should be created with education in law pertaining to peer review and their reviews are judged:
-
(1)
A physician well versed in the research aspect of care
-
(2)
An expert in the current practices
-
(3)
A physician with a basic knowledge of the procedure and a strong understanding of the pathophysiology.
All reviewers should be required to explain what they would have done differently and why the alternative course is supported by literature. It is naive to think that reviews would be done without financial compensation; however, its implementation is complicated.
We must go back and be reminded of the peer review process, it was designed to improve care. Conducting a peer review systematically and leveraging people who care about the process will have a profound effect on the success of the program. The use of a five-step process helps ensure the integrity of the peer review program:30
-
(1)
Identifying a reason for the review
-
(2)
Conducting the review
-
(3)
Reaching a consensus
-
(4)
Creating an action plan
-
(5)
Improving performance.
When a physician or institution uses the peer review process for his or her own benefit it only serves to weaken its legitimacy.
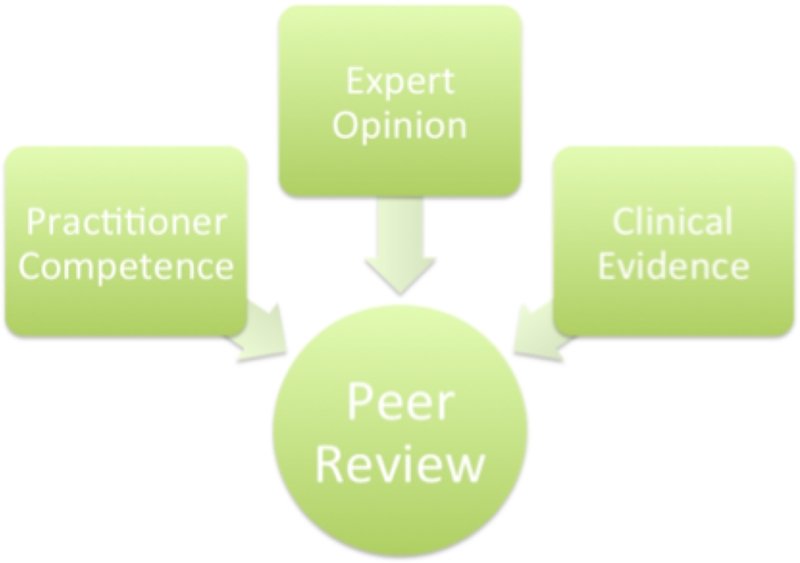
As more new surgeons are trained using robotic surgical techniques, the possible use of peer reviews to hinder the integration of the robotic surgeries will wane as fewer and fewer surgeons refuse to learn. The issue of malicious peer reviews may present an obstacle to innovation in any area, not just robotic surgery. We need a better peer review process The goal of every institution should be to eliminate malicious peer review and to foster learning from the ones conducted. We believe that this paper can be a starting point by which programs can try to develop their own rules and regulations governing this invaluable process improvement tool. The cornerstones of which should include: (1) clinical evidence, (2) expert opinion (practitioners that are preforming the same procedures), and (3) the practitioner in questions clinical competence (see Fig. 3).
Figure 3. The three steps necessary to improve the peer review process.
CONCLUSION
Surgical training has typically been one of apprenticeship and often lacks a single standardized methodology. The problem is further complicated by the fact that there is a lack of concrete method to judge a surgeon’s proficiency.31 In a comprehensive review, laparoscopic box model training appeared to improve a surgeon’s performance, particularly if they had little to no previous surgical experience.32 All of these factors lead to a stratification of knowledge among surgeons. Surgeons that were trained and became proficient in more traditional techniques may not have the skillset to adequately judge the merits of a peer review case involving robotic procedures. This in no way diminishes the knowledge and skill and continued need for open procedures but simply implies that when conducting a peer review, the reviews must be a more precisely defined group of peers.
Although there are inherent flaws in the peer review process, it is still a valuable tool for instructors, doctors, and hospitals to maintain a high level of care, however the committees must exercise caution when presented with third party reviewers, particularly when a perceived conflict of interest exists.
REFERENCES
- 1.LIJ Feinstein Institute Surgical Innovation versus Research Activities Subject to IRB Review. 2009 Available from: http://www.feinsteininstitute.org/wp-content/uploads/2013/02/Surgical-Innovation.pdf.
- 2.U.S. Department of Health and Human Services Food and Drug Administration Center for Devices and Radiological Health (CDRH) Center for Biologics Evaluation and Research Information Sheet Guidance for IRBs, Clinical Investigators, and Sponsors. 2006 Available from: http://www.fda.gov/downloads/RegulatoryInformation/Guidances/UCM127067.pdf.
- 3.Livingston DH, Harwell JD. Peer review. Am. J. Surg. 2001;182:103–109. doi: 10.1016/s0002-9610(01)00679-1. [DOI] [PubMed] [Google Scholar]
- 4.U.S. Government Printing Office Health Care Quality improvement Act of 1986, HR 5540; House of Representatives, 99th Cong, 2nd Sess; September 1986; Available from: http://www.gpo.gov/fdsys/pkg/STATUTE-100/content-detail.html. [Google Scholar]
- 5.Fogarty T. Will the United States maintain its position as a world leader in medical technology? Cleve. Clin. J. Med. 2008;76(Supp. 6):S55–S60. doi: 10.3949/ccjm.75.suppl_6.s55. [DOI] [PubMed] [Google Scholar]
- 6.Newton GE. Maintaining the balance: Reconciling the social and judicial costs of medical peer review protection. Ala L Rev. 2001;723:723–742. [Google Scholar]
- 7.Vyas D, Hozain A. Clinical peer review in the United States: History, legal development and subsequent abuse. World J. Gastroenterol. 2014;20(21):6357–6363. doi: 10.3748/wjg.v20.i21.6357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.AMA Medical Peer Review. Available from: http://www.ama-assn.org/ama/pub/physician-resources/legal-topics/medical-peer-review.page.
- 9.Kadar N. Systemic bias in peer review: Suggested causes, potential remedies. J. Laparoendosc. Adv. Surg. Tech. A. 2010;20(2):123–128. doi: 10.1089/lap.2009.0345. [DOI] [PubMed] [Google Scholar]
- 10.Weingart S, Mukamal K, Davis R, Davies D, Palmer R, Cahalane M, Hamel M, Phillips R, Iezzoni L. Physician-reviews’ perceptions and judgments about quality of care. Int. J. Qual. Health Care. 2001;12(5):357–365. doi: 10.1093/intqhc/13.5.357. [DOI] [PubMed] [Google Scholar]
- 11.Goldman RL. The reliability of peer assessments of quality of care. JAMA. 1992;267(7):958. [PubMed] [Google Scholar]
- 12.Mastroianni A. Liability, regulation and policy in surgical innovation: The cutting edge of research and therapy. Health Matrix Clevel. 2006;16(2):351–442. [PubMed] [Google Scholar]
- 13.Intuitive Clinical Evidence. Available from: http://www.intuitivesurgical.com/company/clinical-evidence/
- 14.U.S. Supreme Court Certiorari to the United States Court of appeals for the ninth circuit. 1988 Available from: http://caselaw.lp.findlaw.com/cgi-bin/getcase.pl?court=us&vol=486&invol=94.
- 15.Dr. Jesse Cole Wins $4 Million Lawsuit Against St. James Healthcare, The Montana Standard, 9 November. 2010 Available from: http://wwww.mtstandard.com.
- 16.Schatlo B, Martinez R, Alaid A, von Eckardstein K, Akhavan-Sigari R, Hahn A, Stockhammer F, Rohde V. Unskilled unawareness and the learning curve in robotic spine surgery. Acta Neurochir. 2015;157(10):1819–1823. doi: 10.1007/s00701-015-2535-0. [DOI] [PubMed] [Google Scholar]
- 17.Miller M, Siegler M, Angelos P. Ethics in surgical innovation. World J. Surg. 2014;38:1638–1643. doi: 10.1007/s00268-014-2568-1. [DOI] [PubMed] [Google Scholar]
- 18.Epstein A, Groeneveld P, Harhay M, Yang F, Polsky D. Impact of minimally invasive surgery on medical spending and employee absenteeism. JAMA Surg. 2013;148(7):641–647. doi: 10.1001/jamasurg.2013.131. [DOI] [PubMed] [Google Scholar]
- 19.Barbash G, Clied S. New technology and health care costs—The case of robotic-assisted surgery. N Engl. J. Med. 2010;363(8):701–704. doi: 10.1056/NEJMp1006602. [DOI] [PubMed] [Google Scholar]
- 20.Intuitive Surgical, Inc. Trended Quarterly and Annual Condensed Consolidated Income Statement and Other Metrics, 2012-2014. [Google Scholar]
- 21.Patel A, Patel M, Lytle N, Toro JP, Medbery RL, Bluestein S, Perez SD, Sweeney JF, Davis SS, Lin E. Can we become better robot surgeons through simulator practice? Surg Endosc. 2013;28(3):847–53. doi: 10.1007/s00464-013-3231-x. [DOI] [PubMed] [Google Scholar]
- 22.Angus AA, Sahi SL, McIntosh BB. Learning curve and early clinical outcomes for a robotic surgery novice performing robotic single site cholecystectomy. Int. J. Med. Robot. 2014;10:203–207. doi: 10.1002/rcs.1540. [DOI] [PubMed] [Google Scholar]
- 23.Hoekstra AV, Jairam-Thodla A, Rademaker A, Singh DK, Buttin BM, Lurain JR, Schink JC, Lowe MP. The impact of robotics on practice management of endometrial cancer: Transitioning from traditional surgery. Int. J. Med. Robot. 2009;5(4):392–397. doi: 10.1002/rcs.268. [DOI] [PubMed] [Google Scholar]
- 24.Trabulsi EJ, Zola JC, Gomella LG, Lallas CD. Transition from pure laparoscopic to robotic-assisted radical prostatectomy: A single surgeon institutional evolution. Urol. Oncol. 2010;28(1):81–85. doi: 10.1016/j.urolonc.2009.07.002. [DOI] [PubMed] [Google Scholar]
- 25.Samarasekera D, Stein RJ. Robotic-assisted laparoscopic approaches to the ureter: Pyeloplasty and ureteral reimplantation. Indian J. Urol. 2014;30(3):293–299. doi: 10.4103/0970-1591.128503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lee BE, Korst RJ, Kletsman E, Rutledge JR. Transitioning from video-assisted thoracic surgical lobectomy to robotics for lung cancer: Are there outcomes advantages? J. Thorac. Cardiovasc. Surg. 2014;147(2):724–729. doi: 10.1016/j.jtcvs.2013.10.002. [DOI] [PubMed] [Google Scholar]
- 27.Krummel TM. Inventing our future: Training the next generation of surgeon innovators. J. Pediatr. Surg. 2009;44(1):21–35. doi: 10.1016/j.jpedsurg.2008.10.005. [DOI] [PubMed] [Google Scholar]
- 28.Boys JA, Alicuben ET, Demeester MJ, Worrell SG, Oh DS, Hagen JA, Demeester SR. Public perceptions on robotic surgery, hospitals with robots, and surgeons that use them. Surg. Endosc. 2015 doi: 10.1007/s00464-015-4368-6. [DOI] [PubMed] [Google Scholar]
- 29.Kadar N. Systemic bias in peer review: Suggested causes, potential remedies. J. Laparoendosc. Adv. Surg. Tech. A. 2010;20(2):123–128. doi: 10.1089/lap.2009.0345. [DOI] [PubMed] [Google Scholar]
- 30.Salud A, Shapiro D, Rampulla T, Reddin K. Peer review: Innovating change. Perm. J. 2012;16(1):72–73. doi: 10.7812/tpp/12.996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Barrie J, Jayne DG, Wright J, Murray CJ, Collinson FJ, Pavitt SH. Attaining surgical competency and its implications in surgical clinical trial design: A systematic review of the learning curve in laparoscopic and robot-assisted laparoscopic colorectal cancer surgery. Ann. Surg. Oncol. 2014;21(3):829–840. doi: 10.1245/s10434-013-3348-0. [DOI] [PubMed] [Google Scholar]
- 32.Gurusamy KS, Nagendran M, Toon CD, Davidson BR. Laparoscopic surgical box model training for surgical trainees with limited prior laparoscopic experience. Cochrane Database of Systematic Reviews. 2014;(3) doi: 10.1002/14651858.CD010478.pub2. Article Id. CD010478. [DOI] [PMC free article] [PubMed] [Google Scholar]