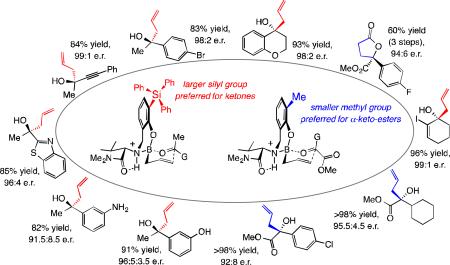
Abstract
Broadly applicable methods for efficient and catalytic additions of easy-to-handle allyl–B(pin) (pin = pinacolato) compounds to ketones and acyclic α-ketoesters have been developed. Accordingly, a large array of tertiary alcohols can be obtained in up to >98% yield and 99:1 enantiomeric ratio. At the heart of this development is rational alteration of the structures of small-molecule aminophenol-based catalysts. Notably, with ketones, increasing the size of a catalyst moiety (tBu to SiPh3) results in much higher enantioselectivity. With α-ketoesters, on the other hand, not only does the opposite hold true, as Me-substitution leads to substantially higher enantioselectivity, the sense of induction is reversed as well.
Keywords: α-ketoesters, catalysis, enantioselective synthesis, homoallylic alcohols, ketones
Tertiary homoallylic alcohols of high enantiomeric purity hold considerable value in chemical synthesis.[1] As expertly demonstrated by Shibasaki and Kanai,[2] Yamamoto,[3] Sigman[4] and Schaus,[5] a direct way to access these entities is by catalytic allyl addition to ketones; enantioselectivities are exceptional in these and several subsequent disclosures.[6] Despite such groundbreaking advances, an approach that offers the following key attributes simultaneously remains lacking: a catalyst that does not contain a toxic metal and can be prepared inexpensively and easily, a readily available set of reagents that are air and moisture stable, and broad substrate scope.
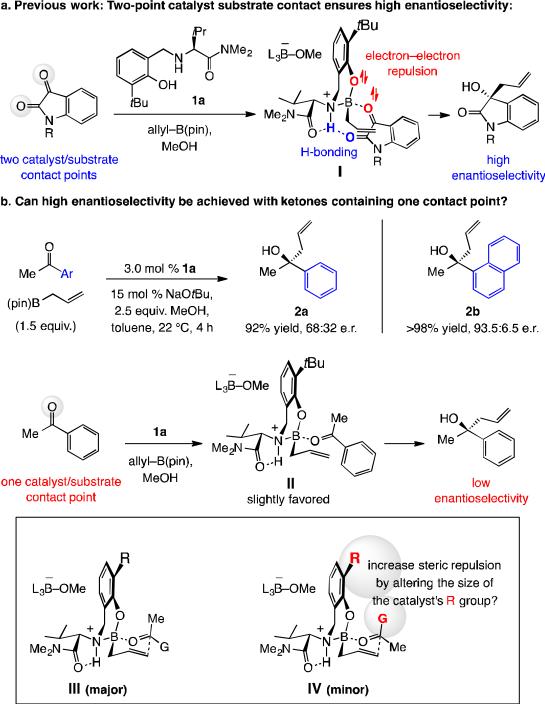
Our interest in this problem originated from an earlier discovery that easily modifiable aminophenol compounds (e.g., 1a, Scheme 1a) may be used to catalyze efficient enantioselective addition of allyl–B(pin) (pin = pinacolato) to isatins.[7] High selectivity was found to arise from structural organization caused by H-bonding between the amide carbonyl and the catalyst's ammonium group;[8] this is despite the electronic repulsion between the non-bonding aryloxide and carbonyl electrons (I; Scheme 1a). Additions to acetophenone (Scheme 1b) are therefore substantially less selective [2a in 68:32 enantiomeric ratio (e.r.)] because there is little energy difference between II and the alternative complex with a pseudoaxial phenyl group. The enhanced selectivity with the naphthyl ketone (2b, 93.5:6.5 e.r.) suggests that in modified aminophenol-based catalysts, as illustrated by III and IV (Scheme 1b), steric factors may be manipulated for achieving high enantioselectivity.
Scheme 1.
Is high enantioselectivity feasible without a second catalyst-substrate contact point?
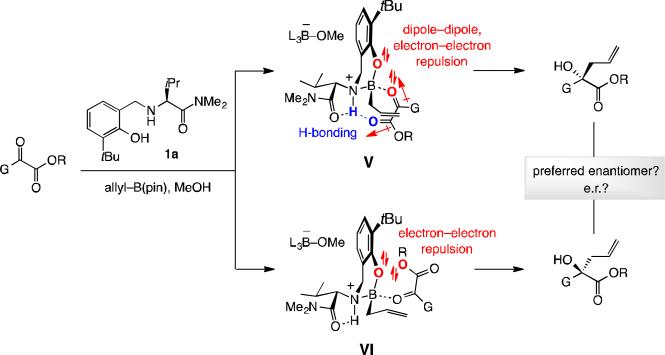
Related additions to acyclic α-ketoesters afford valuable products and are also of interest. We know of only one report that is dedicated to this class of catalytic enantioselective transformations where an (expensive) indium-based complex and (toxic) tetraallyltin is required.[9] Another disclosure, involving a Zn-based catalyst and an allylboronate compound, contains just a single example.[10] We were interested in developing a more general and practical protocol for allyl additions to α-ketoesters, aiming to investigate whether various electronic (e.g., dipolar interactions and electron–electron repulsive forces) and steric features illustrated for complexes V and VI may be manipulated such that the desired products can be obtained in appreciable enantiomeric purity.
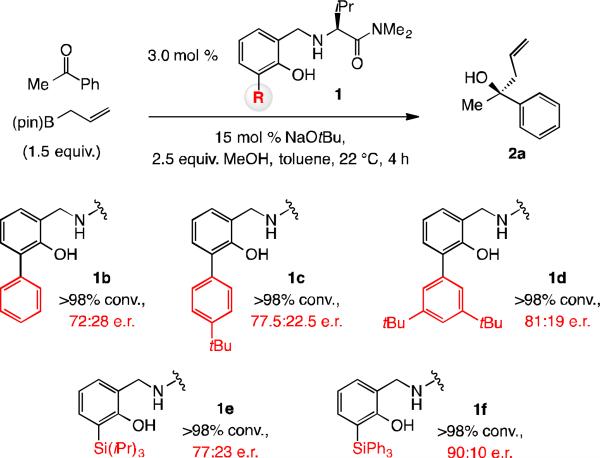
We started by examining the reactions of acetophenone and allyl–B(pin) with aminophenols 1b-f to generate tertiary alcohol 2a (Scheme 3). Phenyl-substituted 1b was selected based on the reasoning that an aryl unit extends farther (vs. a t-Bu group), thus expanding the catalyst's reach in the desired direction (cf. 1c-d). There was however no more than an incremental increase in e.r. with 1b-d (up to 81:19), leading us to envision that incorporating longer C–Si bonds at the same site could prove to be more effective. In the event, although selectivity with triisopropylsilyl-substituted 1e took was somewhat disappointing (77:23 e.r.), with triphenylsilyl variant 1f, 2a was formed in 90:10 e.r. Subsequent optimization revealed that with 3.0 mol % 1g at −30 °C for eight hours, 2a may be isolated in 89% yield and 98:2 e.r. (Scheme 4).[11]
Scheme 3.
Screening of ligands for enantioselective allyl addition to acetophenone as the model.
Scheme 4.
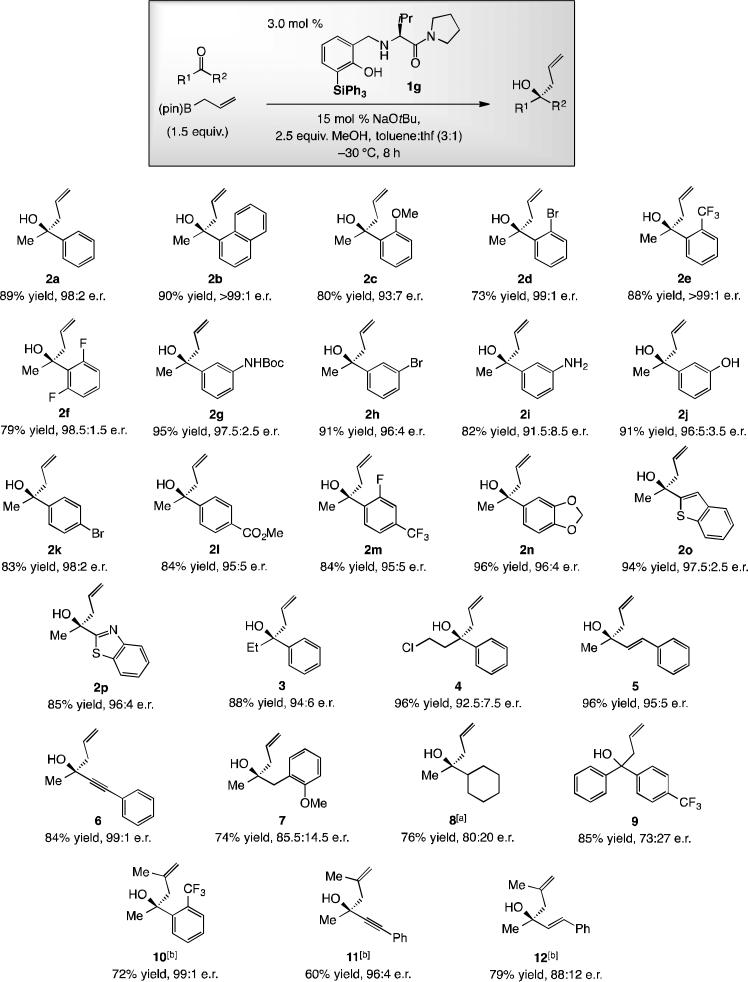
Tertiary homoallylic alcohols obtained from catalytic enantioselective additions of allyl–B(pin) compounds to acyclic ketones. The absolute stereochemistry of the major isomer of 9 has not been determined. [a] At –15 °C, 8 h. [b] At–15 °C, 12 h.
The catalytic protocol is broadly applicable, and the requisite aminophenol 1g, which is indefinitely air stable, can be prepared in ~40% overall yield from readily available starting materials.[12] Aryl-substituted ketones, including those with an electron donating (e.g., 2c; Scheme 4) or withdrawing substituent (e.g., 2e-2f) undergo efficient and highly enantioselective addition. Notably, unprotected aniline- and phenol-containing tertiary homoallylic alcohols 2i and 2j were obtained in 82% and 91% yield and 91.5:8.5 and 96.5:3.5 e.r., respectively. As represented by 2n-p (Scheme 4), ketones with an N-, O- and/or S-containing heterocyclic moiety are suitable. Products from aryl ketones that contain a larger alkyl unit (e.g., 3-4), an alkenyl group (e.g., 5) or a comparatively diminutive alkynyl moiety (e.g., 6) were accessed efficiently and in high enantiomeric purity. While reasonably efficient, enantioselectivities were lower with ketones containing two alkyl substituents (cf. 7–8). With only electronic factors distinguishing the ketone substituents, measurable enantiofacial differentiation was still observed (9 in 73:27 e.r.). Synthesis of 10–12 (Scheme 4) demonstrates that 2-substituted allylboron reagents may be used.
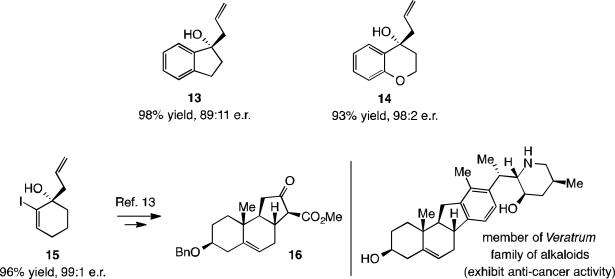
Reactions of cyclic ketones afforded products in high yield and up to 99:1 e.r., as represented by 13–15 (Scheme 5). The case of alkenyl iodide 15 is particularly notable as it has been utilized in an approach toward enantioselective synthesis of the veratrum family of alkaloid natural products (anti-cancer activity).[13]
Scheme 5.
Catalytic enantioselective additions to cyclic ketones.
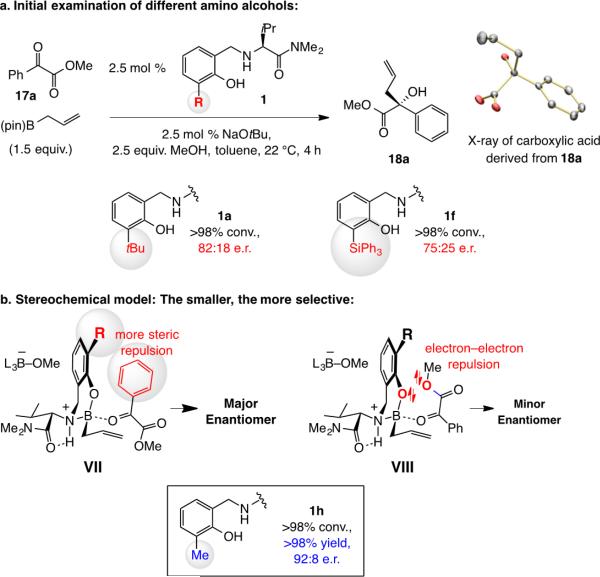
Allyl additions to α-ketoesters were next (cf. Scheme 2). It did not take long before we faced a surprise: Whereas addition of allyl–B(pin) to 17a with the tBu-substituted aminophenol (1a) afforded α-hydroxy ester 18a in 82:18 e.r. (Scheme 6a), with triphenylsilyl-substituted 1f, unlike the transformations with ketones, selectivity was lower (75:25 e.r.). Moreover, the major enantiomer is derived from the opposite sense of enantioselectivity compared to the ketone additions (cf. X-ray structure in Scheme 6a), indicating that reaction may occur via VII (Scheme 6b). The competing mode of addition is probably best represented by VIII, wherein although the net dipole–dipole repulsion is minimized there is electron–electron repulsion between the non-bonding electrons of the aryloxy and ester groups. We suspected that steric strain between the axially oriented ketone substituent and the catalyst's protruding aryloxide moiety (VII, R = tBu or SiPh3 in 1a and 1f, respectively) might be less costly than the indicated electron–electron repulsion in VIII (cf. VI, Scheme 2). Hence, an H-bonded complex such as V in Scheme 2 might not play a major role because of the dipole–dipole repulsion associated with the bound α-ketoester (unlike with structurally rigid isatins).[7a]
Scheme 2.
Another key question: which pathway, if any, would be preferred for allyl–B(pin) addition to α-ketoesters?
Scheme 6.
Unexpected sense of selectivity in reactions of α-ketoesters and identification of an optimal aminophenol.
An implication of the above hypothesis is that enantioselectivity could improve with an aminophenol that contains a smaller substituent (vs. a t-Bu or SiPh3). Indeed, when methyl-substituted ligand 1h was used, under otherwise identical conditions, 17a was generated in 92:8 e.r.[14] Reaction with the unsubstituted aminophenol did not cause further improvement;[15] this might be because the energy gained by lowering of strain in VII is not large enough to compensate for the residual steric repulsion between the methyl ester and the catalyst substituent in VIII that is no longer present either.[16]
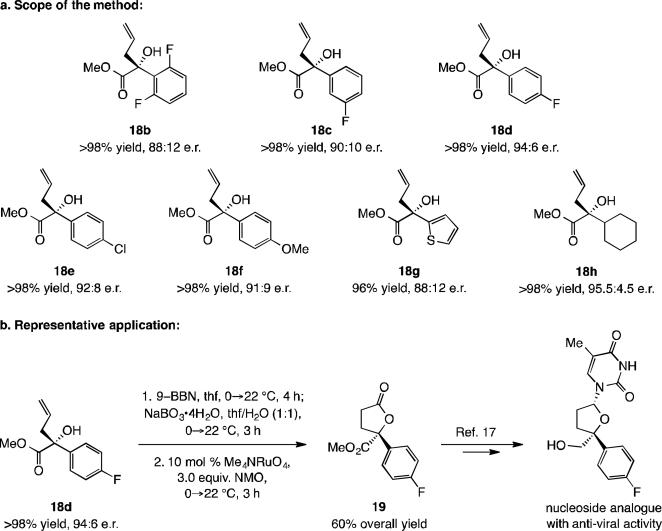
Additions to α-ketoesters have notable scope (Scheme 7). Transformations are exceptionally efficient (often >98% yield after purification) regardless of the electronic attributes of the aryl unit (e.g., 18b vs. 18f) or whether an alkyl-substituted ketoester is involved (cf. 18h). The somewhat less enantioselective additions compared to those with linear ketones (cf. Scheme 4) imply that there is greater competition between the two modes of reaction (VII vs. VIII, Scheme 6b). Utility is highlighted by concise synthesis of lactone 19 in 60% overall yield (94:6 e.r.), a compound that has been converted to antiviral nucleoside analogues.[17] Two other points are noteworthy: a) Aminophenol 1h was prepared in 76% overall yield from an inexpensive aldehyde and Boc-valine.[12] b) Reactions can be easily carried out without the need for rigorous exclusion of air and moisture in a fume hood (e.g., 18f in 94% yield, 91:9 e.r.).
Scheme 7.
Catalytic enantioselective allyl–B(pin) addition to α-ketoesters and a representative application.
To conclude, two catalytic enantioselective methods involving acyclic and cyclic ketones and acyclic α-ketoesters are presented. The transformations offer broader substrate scope (including unprotected phenols and anilines) than the previously reported approaches, and involve the use of inexpensive catalysts along with easy-to-handle and reagents that are mostly commercially available. A key aspect of the present studies is the possibility of maximizing enantioselectivity by means of rational alteration of the size of an aminophenol's aryloxy substituent based on the available stereochemical models. We show that with ketones, exchanging the tert-butyl unit with a triphenylsilyl group is needed whereas with acyclic α-ketoesters, the catalyst bearing a smaller methyl unit is superior. The advances described here offer additional evidence regarding considerable potential of the aminophenol-derived catalysts for future developments in enantioselective synthesis. Studies along these lines including reactions with more functionalized allyl–B(pin) compounds are underway.
Supplementary Material
Acknowledgements
Financial support was provided by the NIH (GM-57212). A. V. acknowledges an Olle Engkvist Byggmästare scholarship.
Footnotes
Dedicated to Professor Stuart L. Schreiber for his 60th Birthday
References & Footnotes
- 1.For recent reviews on enantioselective synthesis through additions of allyl groups to ketones and imines and their applications, see: Yus M, González-Gómez JC, Foubelo F. Chem. Rev. 2011;111:7774–7854. doi: 10.1021/cr1004474. For corresponding diastereoselective processes, see: Yus M, González-Gómez JC, Foubelo F. Chem. Rev. 2013;113:5595–5698. doi: 10.1021/cr400008h.
- 2.a Wada R, Oisaki K, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2004;126:8910–8911. doi: 10.1021/ja047200l. [DOI] [PubMed] [Google Scholar]; b Shi S-L, Xu L-W, Oisaki K, Kanai M, Shibasaki M. J. Am. Chem. Soc. 2010;132:6638–6639. doi: 10.1021/ja101948s. [DOI] [PubMed] [Google Scholar]
- 3.Wadamoto M, Yamamoto H. J. Am. Chem. Soc. 2005;127:14556–14557. doi: 10.1021/ja0553351. [DOI] [PubMed] [Google Scholar]
- 4.Miller JJ, Sigman MS. J. Am. Chem. Soc. 2007;129:2752–2753. doi: 10.1021/ja068915m. [DOI] [PubMed] [Google Scholar]
- 5.Lou S, Moquist PN, Schaus SE. J. Am. Chem. Soc. 2006;128:12660–12661. doi: 10.1021/ja0651308. [DOI] [PubMed] [Google Scholar]; b Barnett DS, Moquist PN, Schaus SE. Angew. Chem. Int. Ed. 2009;48:8679–8682. doi: 10.1002/anie.200904715. [DOI] [PMC free article] [PubMed] [Google Scholar]; c Zhang Y, Li N, Qu B, Ma S, Lee H, Gonnella NC, Gao J, Li W, Tan Z, Reeves JT, Wang J, Lorenz JC, Li G, Reeves DC, Premasiri A, Grinberg N, Haddad N, Lu BZ, Song JJ, Senanayake CH. Org. Lett. 2013;15:1710–1713. doi: 10.1021/ol400498a. [DOI] [PubMed] [Google Scholar]
- 6.a Kii S, Maruoka K. Chirality. 2003;15:68–70. doi: 10.1002/chir.10163. [DOI] [PubMed] [Google Scholar]; b Kim JG, Waltz KM, Garcia IF, Kwiatkowski D, Walsh PJ. J. Am. Chem. Soc. 2004;126:12580–12585. doi: 10.1021/ja047758t. [DOI] [PubMed] [Google Scholar]; c Thornqvist V, Manner S, Frejd T. Tetrahedron: Asymmetry. 2006;17:410–415. [Google Scholar]; d Haddad TD, Hirayama LC, Taynton P, Singaram B. Tetrahedron Lett. 2008;49:508–511. [Google Scholar]; e Huang X-R, Chen C, Lee G-H, Peng S-M. Adv. Synth. Catal. 2009;351:3089–3095. [Google Scholar]
- 7.Silverio DL, Torker S, Pilyugina T, Vieira EM, Snapper ML, Haeffner F, Hoveyda AH. Nature. 2013;494:216–221. doi: 10.1038/nature11844. For subsequent studies regarding this catalyst system, see: Wu H, Haeffner F, Hoveyda AH. J. Am. Chem. Soc. 2014;136:3780–3783. doi: 10.1021/ja500374p.van der Mei FW, Miyamoto H, Silverio DL, Hoveyda AH. Angew. Chem. Int. Ed. 2016;55:4701–4706. doi: 10.1002/anie.201600546.Koh MJ, Nguyen TT, Zhang H, Schrock RR, Hoveyda AH. Nature. 2016;531:459–465. doi: 10.1038/nature17396.Lee K, Silverio DL, Torker S, Robbins DW, Haeffner F, van der Mei FW, Hoveyda AH. Nature Chem. doi: 10.1038/nchem.2523. DOI: 10.1038/NCHEM.2523.
- 8.The presence of H-bonding is supported by quantum theory atoms-in-molecule (QTAIM)-derived bond critical point (BCP) wherein the electron density is 0.025 electrons•Bohr−3. Values of 0.005 < ρ < 0.5 electrons•Bohr−3 suggest hydrogen-bonds of varying strength. See: Parthasarathi R, Subramanian V, Sathyamurthy N. J. Phys. Chem. A. 2006;110:3349–3351. doi: 10.1021/jp060571z.
- 9.Zheng K, Qin B, Liu X, Feng X. J. Org. Chem. 2007;72:8478–8483. doi: 10.1021/jo701491r. [DOI] [PubMed] [Google Scholar]
- 10.Cui Y, Yamashita Y, Kobayashi S. Chem. Commun. 2012;48:10319–10321. doi: 10.1039/c2cc34340a. [DOI] [PubMed] [Google Scholar]
- 11.Screening studies indicated that the aminophenol with a pyrrolidine terminus (vs. NMe2) is more effective at lower temperatures, which were needed for optimal e.r. values.
- 12.See the Supporting Information for details.
- 13.Taber DF, Berry JF. J. Org. Chem. 2013;78:8437–8441. doi: 10.1021/jo401158d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.In further support of the proposed model, addition to the o-tolyl substrate afforded the product in 77:23 e.r. (74% conv., 71% yield).
- 15.Enantioselectivity did not improve at lower temperatures.
- 16.Reactions with larger carboxylic esters (e.g., tBu esters) under the same conditions led to substantial amounts of methyl ester formation through trans-esterification. Use of a more hindered alcohol (vs. MeOH) caused a noticeable decrease in the reaction rates.
- 17.Jõgi A, Paju A, Pehk T, Kailas T, Müürisep A-M, Lopp M. Tetrahedron. 2009;65:2959–2965. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.